SUMMARY
Although many similarities in arthropod CNS development exist, differences in axonogenesis and the formation of midline cells, which regulate axon growth, have been observed. For example, axon growth patterns in the ventral nerve cord of Artemia franciscana differ from that of Drosophila melanogaster. Despite such differences, conserved molecular marker expression at the midline of several arthropod species indicates that midline cells may be homologous in distantly related arthropods. However, data from additional species are needed to test this hypothesis. In this investigation, nerve cord formation and the putative homology of midline cells were examined in distantly related arthropods, including: long- and short-germ insects (D. melanogaster, Aedes aeygypti, and Tribolium castaneum), branchiopod crustaceans (A. franciscana and Triops longicauditus), and malacostracan crustaceans (Porcellio laevis and Parhyale hawaiensis). These comparative analyses were aided by a cross-reactive antibody generated against the Netrin (Net) protein, a midline cell marker and regulator of axonogenesis. The mechanism of nerve cord formation observed in Artemia is found in Triops, another branchiopod, but is not found in the other arthropods examined. Despite divergent mechanisms of midline cell formation and nerve cord development, Net accumulation is detected in a well-conserved subset of midline cells in branchiopod crustaceans, malacostracan crustaceans, and insects. Notably, the Net accumulation pattern is also conserved at the midline of the amphipod P. hawaiensis, which undergoes split germ-band development. Conserved Net accumulation patterns indicate that arthropod midline cells are homologous, and that Nets function to regulate commissure formation during CNS development of Tetraconata.
INTRODUCTION
Recent studies indicate that neuroblasts and the neurons that they produce are homologous in a variety of arthropod species. For example, morphological data suggest that various arthropods have homologous neuroblasts, as well as neurons with comparable cell-body locations and axonal projections (Thomas et al. 1984; Whitington et al. 1993; Whitington 1996; Ungerer and Scholtz 2008). Furthermore, the examination of molecular marker expression in neuroblasts and neurons in a number of insect and crustacean species has provided support for arthropod CNS homology (Duman-Scheel and Patel 1999; Wheeler and Skeath 2005; Wheeler et al. 2005; Browne et al. 2006). Despite these similarities, several differences in arthropod neurogenesis have been noted. For example, ventral nerve cord formation in the brine shrimp Artemia franciscana differs from that of the fruit fly Drosophila melanogaster. In Drosophila, although the longitudinals are pioneered during early CNS development, the commissures are well-established before completion of the longitudinal connectives, and the majority of commissural axons turn rostrally or caudally into one of the longitudinal axon tracts after they have crossed the midline (reviewed by Doe and Goodman 1993). In Artemia, two pairs of terminally located neurons originating in the anterior head region pioneer the longitudinal connectives along the entire length of the larval trunk. Most commissural axons in Artemia do not cross the midline until after the longitudinals are well established (Blanchard 1987; Duman-Scheel et al. 2007). It is presently unknown how prevalent the mechanism for nerve cord formation observed in Artemia is among various arthropod species.
The development of midline cells that regulate axon guidance has also diverged within the arthropods. Midline cells regulate the guidance of axons at the ventral midline of the Drosophila ventral nerve cord, as well as the spinal cord of vertebrate organisms. These midline cells, including the floor plate cells of higher vertebrates and the midline glia in Drosophila, secrete guidance molecules that regulate the growth of commissural axons (reviewed by Tessier-Lavigne and Goodman 1996; Kaprielian et al. 2001). Although there is evidence supporting homology of insect and crustacean midline cells (Manzanares et al. 1996; Duman-Scheel and Patel 1999; Gerberding and Scholtz 1999, 2001; Browne et al. 2006; Duman-Scheel et al. 2007), homology of midline cells in arthropods has been questioned, as the formation of these cells varies in different arthropods (discussed by Gerberding and Scholtz 1999). In Drosophila, the midline forms from the right and left mesectoderm anlagen which fuse after gastrulation (reviewed by Doe and Goodman 1993). In the branchiopod crustacean Leptodora kindti, the midline differentiates from a uniform ectodermal layer following germ-band growth (Gerberding 1997). Midline formation in Artemia (Freeman 1989; Manzanares et al. 1996) is fairly comparable to Leptodora (Gerberding 1997), indicating that the means of generating midline cells may be conserved within the branchiopods, though it differs from Drosophila. Furthermore, in the amphipod crustacean Parhyale hawaiensis, cells contributing to the developing ventral midline form from a single tightly associated cluster of cells present at the posterior end of the early germ-band. These cells converge and extend anteriorly in a single column of midline cells that divide the developing left and right hemisegments during germ-band formation (Gerberding et al. 2002; Browne et al. 2006).
When investigating the homology of midline cells and the different mechanisms for generating a nerve cord in arthropods, it is important to consider that the germ-band of certain crustaceans, including P. hawaiensis, is temporarily split during its elongation. The split initiates in anterior thoracic segments (Browne et al. 2005). Eventually, the two lateral sides will fuse. However, if nerve cord formation initiates while the germ-band is split, it is possible that axonogenesis and its regulation by midline cells may differ from other arthropods. It is therefore interesting to follow nerve cord formation in Parhyale relative to the timing of the split germ-band. Examination of midline cells and midline cell markers at the split germ-band midline will provide further insight into the homology of midline cells in arthropods.
The differences in nerve cord development, midline cell formation, and segmentation described above suggest that arthropod midline cells may not be homologous. To investigate this possibility, Duman-Scheel et al. (2007) studied netrin (Net) accumulation in A. franciscana and compared it to that of D. melanogaster. Net proteins are Laminin-related diffusible molecules that regulate midline axonal guidance in both invertebrate and vertebrate organisms (reviewed by Tessier-Lavigne and Goodman 1996; Kaprielian et al. 2001). The Drosophila NetA and B proteins are expressed at the midline and are necessary for proper commissure formation in flies. The Drosophila Net receptor Frazzled, homolog of the vertebrate Deleted in Colorectal Cancer Net receptor, guides axons in response to Net signaling (Kolodziej et al. 1996) and also controls Net distribution in flies (Hiramoto et al. 2000). Previous studies indicated that deletion of netA and B results in defective guidance of commissural axons in fruit flies (Harris et al. 1996; Mitchell et al. 1996). More recent data suggest that Drosophila Nets function as short-range guidance cues that promote midline crossing (Brankatschk and Dickson 2006).
Despite differences between Drosophila and Artemia nerve cord development, as well as observed differences in the way that midline cells are formed in various arthropods, comparison of the A. franciscana Net accumulation pattern to that of D. melanogaster revealed a conserved set of Net-positive cells at the midline of these two arthropod species. However, it was found that accumulation of afrNet at the midline and on commissural axons occurs at a relatively later time point in Artemia as compared with Drosophila. Despite temporal changes in accumulation patterns, detection of afrNet in a subset of midline cells that closely resemble Net-expressing cells at the Drosophila midline provides evidence for homology of midline cells in arthropods, as well as a conserved role for Net in nerve cord formation in these species (Duman-Scheel et al. 2007). However, data must be collected from additional species, which requires the identification of molecular markers for cells of many insect and crustacean nervous systems, a rather time-consuming endeavor.
The use of cross-reactive antibodies has allowed for the collection of molecular marker expression data from multiple arthropod species in an efficient manner. Cross-reactive antibodies are antibodies raised to an epitope of a protein from a particular species that recognize a conserved epitope in other species. In recent years, cross-reactive antibodies to Engrailed (Patel et al. 1989), Even-skipped (Patel et al. 1994), Ubx/AbdA (Averof and Patel 1997), Pax3/7 (Davis and Patel 2005), and Distal-less (Panganiban et al. 1995) have served as useful tools that have led to advances in the understanding of evolution and development. Here, the characterization of a cross-reactive Net antibody is described. This antibody provides an efficient way of analyzing the homology of midline cells in conjunction with axonogenesis in a variety of insect and crustacean species. In this investigation, Net accumulation patterns are examined with respect to nerve cord development in a number of distantly related insects and crustaceans, including both long- and short-germ insects, as well as branchiopod and malacostracan crustaceans. Because the Net antibody stains axons, it was possible to follow axon projection patterns with an aim of understanding if the Artemia mechanism of nerve cord formation is found in other arthropods. Furthermore, inclusion of P. hawaiensis in this investigation permits examination of midline cell homology and nerve cord formation in a crustacean with a split germ-band.
MATERIALS AND METHODS
Animal sources and culturing conditions
San Francisco Bay Brand A. franciscana were obtained from Marine Depot (Anaheim, CA, USA), hatched, and maintained as described previously (Duman-Scheel et al. 2007). Triops longicauditus were purchased from Carolina Biological Supply Company (Burlington, NC, USA) and were hatched and maintained according the instructions provided. Porcellio laevis were acquired from Carolina Biological Supply Company and maintained as described previously (Duman-Scheel and Patel 1999). P. hawaiensis were cultured as described (Browne et al. 2005). Tribolium castaneum were obtained from Carolina Biological Supply Company and cultured according to the instructions provided. Aedes aegypti eggs were provided by Dr. David Severson (University of Notre Dame) and were cultured in water until the desired developmental stages were attained.
The following Drosophila strains were utilized in this investigation: Net deficiencies Df(1)KA9 and Df(1)NP5 (Harris et al. 1996; Mitchell et al. 1996), NetAΔ and NetBΔ mutants (Brankatschk and Dickson 2006), UAS-NetA, and UAS-NetB (Mitchell et al. 1996), hsFLP22, and P{GAL4-ey.H}SS5. Information about these stocks is provided fat Drosophila Flybase. Ectopic expression of NetA and NetB was performed using the Flp-out method (Struhl and Basler 1993).
Immunohistochemistry
In general, fixation and staining were completed as described in previous studies (see Patel et al. 1989, 1994, and Duman-Scheel and Patel 1999 for general methodology and Duman-Scheel et al. 2007 for specific details pertaining to Net staining). More details for the processing of each different species have been described: D. melanogaster (Patel 1994), Tribolium castaneum (Patel et al. 1989), A. aegypti (the Anopholes protocol described by Goltsev et al. 2004 applies), P. laevis (Duman-Scheel and Patel 1999), P. hawaiensis (Browne et al. 2005), T. longicauditus (Duman-Scheel and Patel 1999), and A. franciscana (Patel et al. 1989 and Duman-Scheel et al. 2007). After fixation, Triops, Artemia, and Porcellio were sonicated (Patel et al. 1989, 1994) or hand dissected to allow for better penetration of antibodies. For staining, animals were rinsed briefly in PBS+0.2% Triton-X, blocked in PBS+0.2% Trition X+5% NGS, and stained with the appropriate antibodies. Both primary and secondary antibody incubations were completed at 4°C overnight. The anti-afrNet antibody (Duman-Scheel et al. 2007), which was found to cross-react to additional species, was used at a concentration of 1:200. Anti-acetylated tubulin (Zymed, San Francisco, CA, USA) was used at a concentration of 1:100, and HRP-conjugated secondary antibodies (Jackson Immunoresearch, West Grove, PA, USA) were used at a final concentration of 1:200.
In situ hybridization
In situ hybridization in Drosophila was performed with Drosophila NetA and NetB riboprobes according to the procedure described by Patel (1996).
RESULTS
The afrNet antibody specifically recognizes Drosophila NetA and NetB proteins
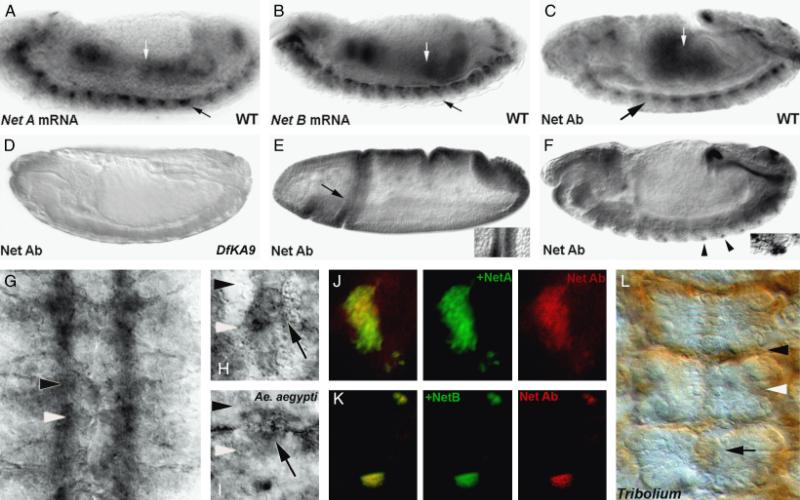
An antibody raised to the well-conserved C-terminus of the afrNet protein (Duman-Scheel et al. 2007) was tested for cross-reactivity to Net proteins in other arthropods. Staining with this antibody in Drosophila revealed an accumulation pattern comparable to the previously described patterns of Drosophila NetA and NetB (Harris et al. 1996; Mitchell et al. 1996, Figs. 1, A–H). The apparent cross-reactivity of this antibody was further characterized. Net accumulation marked by the antibody in wild-type embryos (Fig. 1C) corresponds to the Drosophila NetA (Fig. 1A) and NetB (Fig. 1B) expression patterns. With the exception of occasional background in the head region, staining was not observed in homozygous embryos bearing the Df(1)NP5 (not shown) or Df(1)KA9 (Fig. 1D) deficiencies, both of which delete the two Drosophila Net genes (Harris et al. 1996; Mitchell et al. 1996). These data provide evidence that the anti-afrNet antibody is cross-reactive, yet specific to Net proteins.
Fig. 1.
Anti-afrNetrin is a cross-reactive antibody. The anti-afrNet antibody was characterized in Drosophila melanogaster (fly embryos are shown in all panels except I, Aedes aegypti and L, Tribolium castaneum). Net A (A) and Net B (B) mRNA are detected in the gut (white arrows) and CNS (black arrows) of St. 14 D. melanogaster embryos. Expression is detected in these tissues with the cross-reactive anti-afrNet antibody in a slightly older embryo (C). Net protein staining is not detected in Df(1)KA9 homozygous mutant embryos (D) which lack both the NetA and NetB genes. In wild-type Drosophila embryos, the antibody recognizes staining corresponding to NetA (St. 6 cephalic fold staining marked by arrow in E is magnified at right) and NetB (lateral neuron staining marked by arrowheads in F is magnified at right) specific patterns. Furthermore, the antibody can detect ectopic Net protein expression when either NetA (J) or NetB (K) are expressed ectopically in third instar eye disc clones (ectopic expression clones marked by GFP at center, Net staining shown in red at right; overlay shown at left). These data suggest that the Net Ab recognizes Net proteins specifically, and that it can recognize both Drosophila NetA and NetB. The cross-reactive Net Ab recognizes Net protein accumulation on CNS axons (G) and midline glia in wild-type fly embryos (high magnification of single segment of St. 15 embryo is shown in H). A similar staining pattern is observed in the A. aegypti (mosquito) embryo (high magnification of midline glia in a single segment is shown in I). Comparable Net accumulation patterns are also observed in the short-germ beetle T. castaneum (marked by arrow in L; three segments shown). These data indicate that the anti-afrNet antibody is a cross-reactive antibody that specifically recognizes expression of Net proteins in multiple insects, which possess a conserved set of Net-positive midline cells. In this figure, the locations of the anterior commissures are marked with black arrowheads, while posterior commissure locations are marked with white arrowheads. Anterior is oriented left in (A–F) and up in (G–I) and (L).
Several pieces of evidence suggest that the anti-afrNet antibody recognizes both the Drosophila NetA and NetB proteins. First, although Net staining is not observed in flies lacking both genes (Figs. 1D), it is observed in single mutants lacking either NetA or NetB (data not shown). These data suggest that the antibody recognizes NetB in NetAΔ mutants, or NetA in NetBΔ mutants. Also, when NetA is ectopically expressed in eye disc clones, ectopic expression is detectable with the cross-reactive antibody (Fig. 1J). Likewise, when NetB is ectopically expressed in eye disc clones, the ectopic expression is detectable with the cross-reactive antibody (Fig. 1K). Finally, the afrNet antibody recognizes elements of the combined Drosophila NetA and NetB accumulation patterns that are specific to each individual Net protein. For example, Drosophila NetA accumulation, detected in St. 6, precedes that of NetB (Harris et al. 1996). The anti-afrNet antibody detects this early staining pattern (Fig. 1E). Furthermore, the afrNet antibody detects lateral neurons that specifically express NetB and not NetA (Harris et al. 1996; Fig. 1F).
Detailed Drosophila (Harris et al. 1996; Mitchell et al. 1996) CNS expression patterns have been described previously and are included here, both for characterization of the afrNet antibody, and also to serve as a basis for comparative studies presented in this investigation. Net accumulation initiates in a cluster of midline cells as the first growth cones are extending toward the midline and continues throughout the period of ventral nerve cord formation. Drosophila Net accumulation at the midline is found in cells of the MGM and MGA clusters, as well as in the Vum neurons (Harris et al. 1996; Mitchell et al. 1996). In flies, Net expression is also detected in cells posterior to the commissures, and it has been suggested that these cells may correspond to the MNB cluster (Mitchell et al. 1996). A comparable pattern of expression is observed with the cross-reactive antibody in flies (Fig. 1H), which also recognizes Net accumulation on axons of the ventral nerve cord (Fig. 1G), as is typical for Net proteins (Harris et al. 1996).
Net is detected at the midline of other insects
The cross-reactive antibody was used to detect Net accumulation patterns in additional species of insects. For comparative purposes, Net accumulation patterns were studied in a short-germ insect, the beetle T. castaneum. As opposed to the long germ-band found in Dipterans, which contains the primordia of all the segments of the adult, in Tribolium as well as other short-germ insects and crustaceans, most segments are added sequentially over time from the posterior (reviewed by Davis and Patel 2002). Despite these differences, analysis of nerve cord formation through Nomarksi optics, and with an anti-acetylated tubulin antibody indicate that nerve cord development in beetles is similar to that of Drosophila (not shown). Net accumulation in Tribolium is detected as neurogenesis initiates and is observed throughout neurogenesis. Net is detected in midline glia, in lateral cells that likely correspond to neurons, and on axons in Tribolium (Fig. 1L).
Net accumulation was examined in a second Dipteran, the mosquito A. aegypti. Net accumulation patterns observed at the midline of A. aegypti (Fig. 1I) are similar to that of Drosophila (Fig. 1H) and T. castaneum (Fig. 1L). Net accumulation is detected as neurogenesis initiates and is observed throughout neurogenesis. Net is detected in midline glia, lateral cells that likely correspond to neurons, and on axons in A. aegypti (Fig. 1I). Thus, analyses with the cross-reactive Net antibody reveal similar Net accumulation patterns in distantly related long- and short-germ insects.
Nerve cord development and Net accumulation in T. longicauditus are comparable to that of A. franciscana
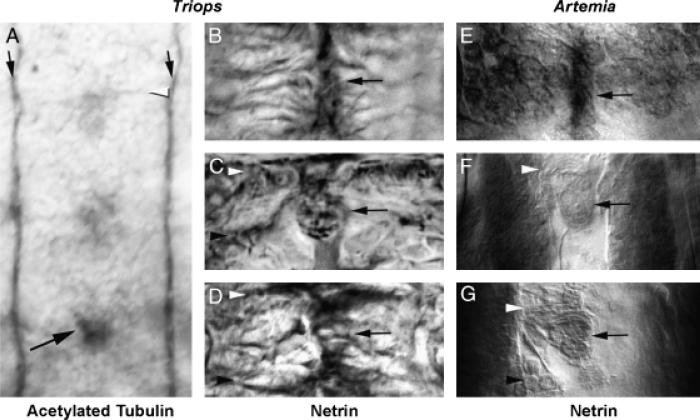
The cross-reactive antibody was used to study nerve cord formation and Net accumulation patterns in several crustacean species. Analysis of nerve cord formation and Net accumulation in branchiopod crustaceans is of particular interest, as a divergent mechanism of nerve cord formation (Blanchard 1987) and temporal changes in the Net accumulation pattern in the branchiopod A. franciscana (Duman-Scheel et al. 2007) have been previously reported. In Artemia, two pairs of terminally located neurons originating in the anterior head region pioneer the longitudinal connectives along the entire length of the larval trunk. Most commissural axons in Artemia do not cross the midline until after the longitudinals are well established (Blanchard 1987; Duman-Scheel et al. 2007). To determine whether this mechanism for nerve cord generation is conserved in other branchiopod crustaceans, nerve cord formation was examined in a second brachiopod, T. longicauditus. Anti-acetylated tubulin staining was used to trace axon movement duringe arly Triops neurogenesis (Fig. 2A). This staining revealed a mechanism of nerve cord formation that is comparable to that of Artemia. In Triops, as in Artemia, longitudinal axons originating anteriorly extend along the entire length of the embryo (Fig. 2A). Thus, in Triops, as in Artemia, the longitudinals are established along the trunk of the embryo before formation of the commissural axons. However, in comparison with Artemia, which have well-established thick longitudinal connectives at the time of commissural axon extension (Duman-Scheel et al. 2007, Fig. 2, F and G), the Triops longitudinals are much thinner when the first commissural axons extend toward the midline (Fig. 2A).
Fig. 2.
A conserved mechanism of ventral nerve cord formation and midline Netrin accumulation in branchiopod crustaceans. Axonogenesis in Triops longicauditus was examined with antiacetylated tubulin staining (A). The temporal gradient of CNS development is evident in the 2-day-old animal shown in A, in which commissure formation (white arrowhead) is initiating in anterior segments (top), but not in the most posterior segment (bottom). In contrast, the longitudinal connectives (black arrows) have already been pioneered along the length of the entire trunk. A comparable mechanism of nerve cord formation is found in Artemia (E–G, Blanchard 1987; Duman-Scheel et al. 2007), suggesting that this mechanism for generating a nerve cord is conserved in brachiopods. The black arrow in A marks an acetylated tubulin positive cell at the midline that is observed in all of the arthropods analyzed in this investigation. Net accumulation in Triops (B–D) is detected by the cross-reactive Net Ab and resembles that of Artemia (E–G). Panels B, C, and D (Triops) are temporally comparable to panels E, F, and G, respectively, in Artemia. In branchiopods, Net accumulation is observed at the midline before commissure formation (B and E), continues as neurogenesis progresses (C, F), and is ultimately found in a subset of midline cells (D, G) that resemble those found in insects (Fig. 1). In this figure, anterior commissures are marked by white arrowheads, while posterior commissures are marked with black arrowheads in various panels; midline Net-positive cells are marked by black arrows in B–G. Anterior is oriented up in all figures.
Because of the generally well-conserved mechanism of nerve cord formation found in Triops and Artemia, it was predicted that midline Net accumulation in Triops would be comparable to that of Artemia (Fig. 2, E–G), which is observed after the longitudinals are established along the trunk of the animal (Duman-Scheel et al. 2007). This is also the case in Triops, where midline Net-positive cells (Fig. 2, B–D) are observed after the longitudinals are established (Fig. 2A). Midline Net accumulation in Triops persists throughout neurogenesis and is detected in a conserved subset of Net-positive midline cells (Fig. 2, B–D).
Midline Net accumulation is detected in the split germ-band crustacean P. hawaiensis
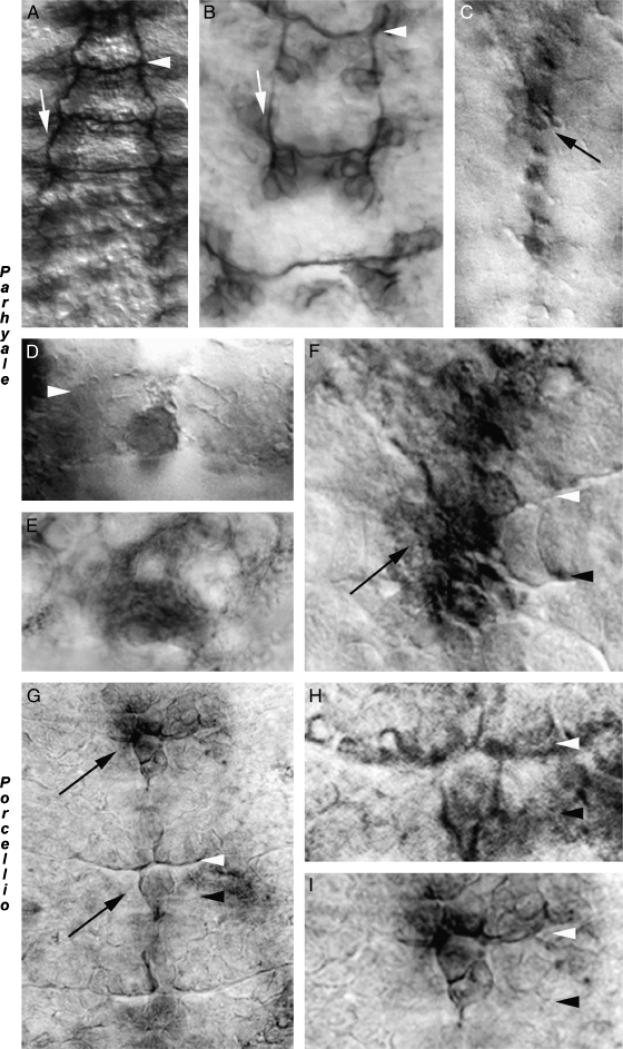
The germ-band of certain crustaceans, including the amphipod crustacean P. hawaiensis, is temporarily split during its elongation. The split initiates in anterior thoracic segments (Browne et al. 2005). Neurogenesis initiates during the split in Parhyale, raising the question of how axon guidance proceeds during this period of lateral separation. Acetylated tubulin staining (Fig. 3, A and B) revealed that the mechanism of nerve cord formation observed in Artemia (Blanchard 1987; Duman-Scheel et al. 2007) and Triops (Fig. 2A) is not found in Parhyale, a malacostracan crustacean. With the exception that an anterior–posterior gradient of CNS maturation is observed in crustaceans, in which segments form sequentially over time, nerve cord formation in Parhyale (Fig. 3A) is more comparable to Drosophila (reviewed by Doe and Goodman 1993) than it is to that of branchiopods (Fig. 2). Furthermore, it was determined that commissural axon growth to the midline initiates in split germ-band segments during the period of lateral separation (Fig. 3B).
Fig. 3.
Netrin accumulation in malacostracan crustaceans: detection of midline netrin accumulation in split germ band embryos. The mechanism of ventral nerve cord formation in Paryhale hawaiensis, which has a split germ band during development, was examined with anti-acetylated tubulin staining (A, B). While the anterior–posterior gradient of nerve cord formation is evident (A), the mechanism for pioneering the longitudinals observed in branchiopods is not seen in Parhyale, as evidenced by the gaps in the longitudinals (white arrows) apparent in B. Commissure formation commences despite the split germ band found in Parhyale (see lower segment in B). Net accumulation marked by the cross-reactive Ab is observed in midline cells in Parhyale (C–F). Midline Net accumulation initiates as the first commissural axons are extending (D), and is even found in split germ band segments (E), where Net-positive projections extending laterally from midline cells are observed. Net eventually accumulates in a pattern comparable to other arthropods and is detected on axons (F). Midline Net accumulation is also detected in Porcellio (G–I), another malacostracan. Higher magnification views of the middle (H) and upper (I) segments from the embryo in G are shown. In this figure, white arrowheads mark the anterior commissures, and black arrowheads mark the posterior commissures. Midline Net-positive cells are marked by black arrows. Anterior is oriented up in all figures.
Net-positive cells are detected in Parhyale as the first commissural axons are extending toward the midline (Fig. 3, C–F). Net-positive midline cells are detected in split germ-band segments during the period of lateral separation (Fig. 3E). These Net-positive midline cells in split germ-band segments appear to have extensions (Fig. 3E) comparable to what have previously been described in Orchestia, another split germ-band crustacean (Gerberding 1997). Net accumulates in midline cells throughout the period of neurogenesis and can be detected on axons (Fig. 3F).
To further explore whether Net accumulation at the midline is conserved in malacostracans, nerve cord formation and Net accumulation were analyzed in the terrestrial isopod P. laevis. Acetylated tubulin staining (not shown) revealed that the mechanism of nerve cord formation observed in P. laevis is comparable to Parhyale and insects. Net accumulation during commissure formation is also observed in Porcellio (Fig. 3, G–I). Despite lateral separation in Parhyale, the mechanism of nerve cord formation observed in two malacostracan species is comparable to insects and differs substantially from branchiopod crustaceans, yet Net accumulation patterns in midline cells are comparable in all of these arthropods.
DISCUSSION
A cross-reactive antibody provides evidence of a conserved role for Nets in arthropod nerve cord development
Given the divergent mechanisms for generating midline cells and the ventral nerve cord that have been observed in arthropods, one might expect that the expression or function of axon guidance molecules may have changed during arthropod evolution. Identification of molecular markers within the Drosophila CNS helped to establish flies as a model organism for studying CNS development (reviewed by Doe and Goodman 1993). Likewise, an initial step toward gaining an understanding of how CNS development has evolved in arthropods is the identification and comparative analysis of CNS markers among arthropods. However, cloning and expression analysis of various CNS development genes from multiple arthropods can be a time consuming process. Occasionally, an antibody raised to a protein in one arthropod organism is found to recognize homologs of the same protein in multiple organisms. Such rare cross-reactive antibodies permit efficient data collection from multiple organisms.
In this investigation, an antibody raised to the well-conserved C-terminus of the afrNet protein (Duman-Scheel et al. 2007) proved to be a cross-reactive antibody (Fig. 1) that specifically recognizes Net proteins in multiple arthropod species. Examination of Net accumulation patterns in D. melanogaster, T. castaneum, A. aegypti, A. franciscana, T. longicauditus, P. hawaiensis, and P. laevis revealed a conserved Net accumulation pattern that is consistent with a role for Net during ventral nerve cord formation in these arthropods. This conserved accumulation pattern exists despite the divergent mechanisms of nerve cord development and midline cell formation found in these organisms. It is difficult to know if the cross-reactive antibody is recognizing one or more Net proteins in various arthropods. Characterization of the antibody in Drosophila indicates that it recognizes both the NetA and NetB proteins (Fig. 1), but not other proteins. It is therefore possible that the antibody specifically recognizes multiple Net proteins in other species.
It was previously thought that midline expression of chemoattractive Net proteins guides commissural axons at long-range in Drosophila (Harris et al. 1996; Mitchell et al. 1996). However, Brankatschk and Dickson (2006) recently demonstrated that Nets act not as long-range chemoattractants, but as short-range cues that promote midline crossing of axons in flies. Furthermore, Hiramoto and Hiromi (2006) also proposed that Drosophila Nets may mediate association or fasciculation with commissural axons, and that Net may mediate axon–axon recognition via a Net receptor expressed by longitudinal pioneer neurons that has not yet been identified. Given the conservation of Net accumulation observed among arthropods, it is likely that these roles are conserved among various arthropod species. Although recent advances in RNA inhibition studies would allow functional analysis of Net genes in other arthropods, the sophisticated genetic tools that have shaped the current understanding of Net function in Drosophila are presently not available in other arthropod species. Development of additional arthropod species as genetic model organisms will permit more sophisticated analyses of Net function outside of Drosophila.
Homology of midline cells in arthropods
The mechanisms for generating midline cells have diverged among arthropods (see “Introduction”). These observed differences raise the question of whether arthropod midline cells are homologous. Despite these differences, several studies support the homology of arthropod midline cells (Manzanares et al. 1996; Duman-Scheel and Patel 1999; Gerberding and Scholtz 1999, 2001; Browne et al. 2006; Duman-Scheel et al. 2007). For example, Gerberding and Scholtz (1999, 2001) suggested that the MNB of Drosophila, the neuroglioblast of the grasshopper Schistocerca americana, and the d0 cell of the amphipod Orchestia cavimana are homologous. Furthermore, conserved Net accumulation patterns observed in the Drosophila and Artemia (Harris et al. 1996; Mitchell et al. 1996; Duman-Scheel et al. 2007) ventral nerve cords provided evidence that Net-positive midline glia are homologous in Drosophila and Artemia. In this investigation, Net accumulation is found in a spatially conserved set of cells during nerve cord formation in additional insects (Fig. 1, I and L), branchiopods (Fig. 2), and malacostracans (Fig. 3). These data suggest that Net-positive midline cells are conserved in distantly related arthropods. These homologies occur despite the divergent mechanisms for generating midline cells that exist in different arthropod species. Thus, this study supports the hypothesis that divergent developmental mechanisms have generated homologous midline cells in Tetraconata.
Conserved mechanisms of nerve cord formation and Net accumulation in branchiopods
The arthropod ventral nerve cord is another example of a homologous structure that can be generated by divergent developmental mechanisms. In Drosophila, the commissures are well established before completion of the longitudinal connectives which thicken after axons cross the midline and then extend rostrally or caudally (reviewed by Doe and Goodman 1993).
By contrast, in Artemia, two pairs of terminally located neurons originating at the anterior grow toward the posterior, thereby pioneering the longitudinal connectives along the entire length of the larval trunk (Blanchard 1987). The Artemia longitudinals are well established before most commissural axons extend across the midline. In comparison to flies, although the Net accumulation pattern in Artemia is well conserved, Net accumulation in Artemia is delayed until after the longitudinals are well established. Although different mechanisms of nerve cord formation and a temporal delay in Net accumulation at the midline are notable, the nerve cords produced are comparable (Duman-Scheel et al. 2007). Thus, divergent developmental mechanisms produce homologous structures.
One goal of this investigation was to determine whether the mechanism of Artemia nerve cord formation and delayed onset of Net accumulation is found in other arthropods. Although nerve cord development was examined in a number of species, the Artemia mechanism was found only in T. longicauditus, another branchiopod (Fig. 2A). These data indicate that this mechanism of generating a nerve cord is conserved in branchiopods, but may not be found in other arthropod lineages. The temporal delay of midline Net accumulation with respect to formation of the longitudinals previously observed in Artemia (Duman-Scheel et al. 2007) was also noted in Triops (Fig. 2, B–D). Thus, the temporal changes in the Net accumulation pattern that accompany divergent mechanisms of nerve cord formation are conserved in branchiopods. However, in comparison with Artemia (Duman-Scheel et al. 2007, Fig. 2, E and G), the Triops longitudinals are not as well established and thickened at the time of Net accumulation at the midline (Fig. 2, B–D). Furthermore, while the delay in Net accumulation in Artemia occurs over a nearly 2-week period (Duman-Scheel et al. 2007), such an extreme temporal delay is not observed in Triops (the embryo pictured in Fig. 2, B–D is 2 days old).
Net is expressed at the midline in split germ-band crustaceans
It was hypothesized that the Artemia mechanism for nerve cord formation might be observed in split germ-band crustaceans. Given the spatial separation of the right and left sides during the split, it would seem likely that the longitudinals might form while the germ-band is separated, and that commissures would then form following fusion of the germ-band. However, this is not the case, as commissural axons begin to extend toward the midline during the period of lateral separation in Parhyale (Fig. 3B). Furthermore, Net accumulation initiates in midline cells when the two lateral sides are split (Fig. 3E). Gerberding (1997) also noted the presence of midline cells in another split germ-band amphipod crustacean, O. cavimana. In Orchestia, midline cells stretch extensively and bridge the large distance between the split germ-band. Although Net-positive Parhyale midline cells don't appear to stretch as extensively as those of Orchestia, Net-positive extensions are associated with these midline cells (Fig. 3E). These extensions may help to guide commissural axons across the split germ-band. This is an interesting possibility given that Drosophila Nets were recently shown to promote crossing of axons after they reach the midline, rather than guidance of the axons to the midline (Brankatschk and Dickson 2006). It is possible that early accumulation of Net at the midline and on extensions of midline cells may be necessary for axon guidance to the midline in certain arthropods, such as amphipods with split germ-bands.
Acknowledgments
We thank Kevin Mitchell for providing Drosophila net DNA, and Barry Dickson, Krishna Bhat, Greg Bashaw, and Drosophila Flybase for providing Drosophila fly stocks. This project was funded by NIH Award R15 NS 048904-0 to M. D. S. The Albion Foundation for Undergraduate Research and Scholarly Activity (FURSCA) funded S. C., W. S., and P. B., and W. E. B. is funded by NSF Award IOS-0721662.
REFERENCES
- Averof M, Patel NH. Crustacean appendage evolution associated with changes in Hox gene expression. Nature. 1997;388:682–686. doi: 10.1038/41786. [DOI] [PubMed] [Google Scholar]
- Blanchard CE. Pioneer neurons and the early development of the nervous system in Artemia. In: Sorgeloss P, Bengtson DA, Declair W, Jaspers E, editors. Artemia Research and its Applications. I. Universa Press; Wetteren, Belgium: 1987. pp. 5–32. [Google Scholar]
- Brankatschk M, Dickson BJ. Netrins guide Drosophila commissural axons at short range. Nat. Neurosci. 2006;9:188–194. doi: 10.1038/nn1625. [DOI] [PubMed] [Google Scholar]
- Browne WE, Price AL, Gerberding M, Patel NH. Stages of embryonic development in the amphipod crustacean, Parhyale hawaiensis. Genesis. 2005;42:124–149. doi: 10.1002/gene.20145. [DOI] [PubMed] [Google Scholar]
- Browne WE, Schmid BGM, Wimmer EA, Martindale MQ. Expression of otd orthologs in the amphipod crustacean, Parhyale hawaiensis. Dev. Genes Evol. 2006;216:581–595. doi: 10.1007/s00427-006-0074-7. [DOI] [PubMed] [Google Scholar]
- Davis GK, Patel NH. Short, long and beyond: molecular and embryological approaches to insect segmentation. Annu. Rev. Entomol. 2002;47:669–699. doi: 10.1146/annurev.ento.47.091201.145251. [DOI] [PubMed] [Google Scholar]
- Davis GK, Patel NH. Pax3/7 genes reveal conservation and divergence in the arthropod segmentation hierarchy. Dev. Biol. 2005;285:169–184. doi: 10.1016/j.ydbio.2005.06.014. [DOI] [PubMed] [Google Scholar]
- Doe CQ, Goodman C. Embryonic development of the Drosophila central nervous system. In: Martinez Arias A, editor. The Development of Drosophila Melanogaster. Cold Spring Harbor Laboratory Press; Plainview, NY: 1993. pp. 1091–1130. [Google Scholar]
- Duman-Scheel M, Clark SM, Grunow ET, Hasley AO, Hill BL, Simanton WL. Delayed onset of midline netrin expression in Artemia franciscana coincides with commissural axon growth and provides evidence for homology of midline cells in distantly related arthropods. Evol. Dev. 2007;9:131–140. doi: 10.1111/j.1525-142X.2007.00144.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duman-Scheel M, Patel NH. Analysis of molecular marker expression reveals neuronal homology in distantly related arthropods. Development. 1999;126:2327–2334. doi: 10.1242/dev.126.11.2327. [DOI] [PubMed] [Google Scholar]
- Freeman JA. Segment morphogenesis in Artemia larvae. In: Warner AH, Macrae TH, Bagshaw JC, editors. Cell and Molecular Biology of Artemia Development. Plenum Press; New York: 1989. pp. 77–90. [Google Scholar]
- Gerberding M. Germ band formation and early neurogenesis of Leptodora kindti (Cladocera): first evidence for neuroblasts in the entomostracan crustaceans. Invert. Reprod. Dev. 1997;32:63–73. [Google Scholar]
- Gerberding M, Browne WE, Patel NH. Celll ineage analysis of the amphipod crustacean Parhyale hawaiensis reveals an early restriction of cell fates. Development. 2002;129:5789–5801. doi: 10.1242/dev.00155. [DOI] [PubMed] [Google Scholar]
- Gerberding M, Scholtz G. Cell linage of the midline cells in the amphipod crustacean Orchestia cavmana (Crustacea, Malacostraca) during formation and separation of the germ band. Dev. Genes Evol. 1999;209:91–102. doi: 10.1007/s004270050231. [DOI] [PubMed] [Google Scholar]
- Gerberding M, Scholtz G. Neurons and glia in the midline of the higher crustacean Orchestia cavimana are generated via an invariant cell lineage that comprises a median neuroblast and glial progenitors. Dev. Biol. 2001;235:397–409. doi: 10.1006/dbio.2001.0302. [DOI] [PubMed] [Google Scholar]
- Goltsev Y, Hsiong W, Lanzaro G, Levine M. Different combinations of gap repressors for common stripes in Anopheles and Drosophila embryos. Dev. Biol. 2004;275:435–446. doi: 10.1016/j.ydbio.2004.08.021. [DOI] [PubMed] [Google Scholar]
- Harris R, Sabatelli LM, Seeger MA. Guidance cues at the Drosophila CNS midline: identification and characterization of two Drosophila Netrin/UNC-6 homologs. Neuron. 1996;17:217–228. doi: 10.1016/s0896-6273(00)80154-3. [DOI] [PubMed] [Google Scholar]
- Hiramoto M, Hiromi Y. Robo directs axon crossing of segmental boundaries by suppressing responsiveness to relocalized Netrin. Nat. Neurosci. 2006;9:58–66. doi: 10.1038/nn1612. [DOI] [PubMed] [Google Scholar]
- Hiramoto M, Hiromi Y, Giniger E, Hotta Y. The Drosophila netrin receptor frazzled guides axons by controlling netrin distribution. Nature. 2000;406:886–889. doi: 10.1038/35022571. [DOI] [PubMed] [Google Scholar]
- Kaprielian Z, Runko E, Imondi R. Axon guidance at the midline choice point. Dev. Dyn. 2001;221:154–181. doi: 10.1002/dvdy.1143. [DOI] [PubMed] [Google Scholar]
- Kolodziej PA, et al. Frazzled encodes a Drosophila member of the DCC immunoglobulin subfamily and is required for CNS and motor axon guidance. Cell. 1996;87:197–204. doi: 10.1016/s0092-8674(00)81338-0. [DOI] [PubMed] [Google Scholar]
- Manzanares M, Williams TA, Marco R, Garesse R. Segmentation in the crustacean Artemia: engrailed staining studied with an antibody raised against the Artemia protein. Roux's Arch. Dev. Biol. 1996;205:424–431. doi: 10.1007/BF00377222. [DOI] [PubMed] [Google Scholar]
- Mitchell KJ, et al. Genetic analysis of Netrin genes in Drosophila: netrins guide CNS commissural axons and peripheral motor axons. Neuron. 1996;17:203–215. doi: 10.1016/s0896-6273(00)80153-1. [DOI] [PubMed] [Google Scholar]
- Panganiban G, Sebring A, Nagy L, Carroll S. The development of crustacean limbs and the evolution of arthropods. Science. 1995;270:1363–1366. doi: 10.1126/science.270.5240.1363. [DOI] [PubMed] [Google Scholar]
- Patel NH. Imaging neuronal subsets and other cell types in whole-mount Drosophila embryos and larvae using antibody probes. Methods Cell Biol. 1994;44:445–487. doi: 10.1016/s0091-679x(08)60927-9. [DOI] [PubMed] [Google Scholar]
- Patel N. In situ hybridization to whole mount Drosophila embryos. In: Krieg P, editor. A Laboratory Guide to RNA: Isolation, Analysis, and Synthesis. Wiley-Liss; New York: 1996. pp. 357–370. [Google Scholar]
- Patel NH, Condron BG, Zinn K. Pair-rule expression patterns of even-skipped are found in both short- and long-germ beetles. Nature. 1994;367:429–434. doi: 10.1038/367429a0. [DOI] [PubMed] [Google Scholar]
- Patel NH, Kornberg TB, Goodman CS. Expression of engrailed during segmentation in grasshopper and crayfish. Development. 1989;107:201–212. doi: 10.1242/dev.107.2.201. [DOI] [PubMed] [Google Scholar]
- Struhl G, Basler K. Organizing activity of wingless protein in Drosophila. Cell. 1993;72:527–540. doi: 10.1016/0092-8674(93)90072-x. [DOI] [PubMed] [Google Scholar]
- Tessier-Lavigne M, Goodman CS. The molecular biology of axon guidance. Science. 1996;274:1123–1133. doi: 10.1126/science.274.5290.1123. [DOI] [PubMed] [Google Scholar]
- Thomas JB, Bastiani MJ, Bate M, Goodman CS. From grasshopper to Drosophila: a common plan for neuronal development. Nature. 1984;310:203–207. doi: 10.1038/310203a0. [DOI] [PubMed] [Google Scholar]
- Ungerer P, Scholtz G. Filling the gap between identified neuroblasts and neurons in crustaceans adds new support for Tetraconata. Proc. R. Soc. B. 2008;275:369–376. doi: 10.1098/rspb.2007.1391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wheeler SR, Carrico ML, Wilson BA, Skeath JB. The Tribolium columnar genes reveal conservation and plasticity in neural precursor patterning along the embryonic dosal–ventral axis. Dev. Biol. 2005;279:491–500. doi: 10.1016/j.ydbio.2004.12.031. [DOI] [PubMed] [Google Scholar]
- Wheeler SR, Skeath JB. The identification and expression of achaete-scute genes in the branchiopod crustacean Triops longicauditus. Gene Expr. Patterns. 2005;5:695–700. doi: 10.1016/j.modgep.2005.02.005. [DOI] [PubMed] [Google Scholar]
- Whitington PM. Evolution of neural development in thearthro-pods. Semin. Cell Dev. Biol. 1996;7:605–614. [Google Scholar]
- Whitington PM, Leach D, Sandeman R. Evolutionary change in neural development within the arthropods: axonogenesis in the embryos of two crustaceans. Development. 1993;118:449–461. doi: 10.1242/dev.118.2.449. [DOI] [PubMed] [Google Scholar]