Abstract
Adult neurogenesis is often studied by labeling new cells with the thymidine analog bromodeoxyuridine (BrdU) and using immunohistochemical methods for their visualization. Using this approach, considerable variability has been reported in the number of new cells produced in the dentate gyrus of adult rodents. We examined whether immunohistochemical methods, including BrdU antibodies from different vendors (Vector, BD, Roche, Dako, Novocastra, Accurate) and DNA denaturation pretreatments, alter the quantitative and qualitative patterns of BrdU labeling. We also compared the sensitivity and specificity of BrdU with two other thymidine analogs, iododeoxyuridine (IdU) and chlorodeoxyuridine (CldU). We found that the number of BrdU-labeled cells in the dentate gyrus of adult rats was dependent on the BrdU antibody used but was unrelated to differences in antibody penetration. Even at a higher concentration, some antibodies stained fewer cells (Vector, Novocastra). A sensitive BrdU antibody (BD) was specific for dividing cells; all BrdU-labeled cells stained for Ki67, an endogenous marker of cell proliferation. We also observed that DNA denaturation pretreatments affected the number of BrdU-labeled cells and staining intensity for a marker of neuronal differentiation, NeuN. Finally, we found that IdU and CldU, when used at molarities comparable to those that label the maximal number of cells with BrdU, are less sensitive. These data suggest that antibody and thymidine analog selection, as well as the staining procedure employed, can affect the number of newly generated neurons detected in the adult brain thus providing a potential explanation for some of the variability in the adult neurogenesis literature.
Keywords: BrdU, IdU, CldU, hippocampus, dentate gyrus, subventricular zone
INTRODUCTION
Altman and colleagues first reported that new neurons are produced in the adult mammalian brain over forty years ago (Altman and Das, 1965; 1966; 1967). However, the study of adult neurogenesis has become widespread only during the past decade, largely due to the use of bromodeoxyurdine (BrdU) as an in vivo marker of proliferating cells (Cameron and Gould, 1996; Kuhn et al., 1996). BrdU is a thymidine analog that becomes integrated into cells undergoing DNA synthesis – this label is then visualized using immunohistochemistry (Abrous et al., 2005; Christie and Cameron, 2006; Wojtowicz and Kee, 2006; Kuhn and Cooper-Kuhn, 2007). BrdU labeling is often combined with immunolabeling for cell-type specific markers to determine the identity of newly born cells. Using BrdU labeling, it has become accepted that new neurons are produced throughout adulthood in the dentate gyrus (Gage, 2002). This phenomenon has been demonstrated in all mammalian species examined, ranging from rodents to primates, including humans (Eriksson et al., 1998; Gould et al., 1999; Lindsey and Tropepe, 2006). Adult neurogenesis in the dentate gyrus has been studied most extensively in rodents and although there is consensus regarding its existence, controversy remains about its magnitude and regulation, largely due to different laboratories reporting variable results.
One potential reason for these inconsistencies may be the wide variations in experimental design with BrdU studies. The number of BrdU-labeled cells in the dentate gyrus is affected by the age (Kuhn et al., 1996; Cameron and McKay, 1999; Leuner et al., 2007) and strain of the animal (Kempermann et al., 1997), the dose and regimen of BrdU injections, and the post-BrdU injection survival time (Christie and Cameron, 2006; Wojtowicz and Kee, 2006; Kuhn and Cooper-Kuhn, 2007). However, even in instances when these variables remain constant, the reported number of BrdU-labeled cells ranges dramatically across studies. For example, injecting age-matched control male Sprague-Dawley rats with 200 mg/kg of BrdU followed by a 2h survival time has led to reports of BrdU-labeled cells in the bilateral dentate gyrus ranging from under 3000 to over 5000 (Fornal et al., 2007; Dayer et al., 2003). This raises the possibility that some quantitative differences in BrdU labeling within the dentate gyrus arise from variations in histological procedures. At present, numerous BrdU antibodies are in use and their relative sensitivity and specificity remain unknown which could lead to imprecise and conflicting results (Saper and Sawchenko, 2003; Rhodes and Trimmer, 2006). Moreover, BrdU antibodies recognize BrdU only in single-stranded DNA (Gratzner et al., 1982; Vanderlaan and Thomas, 1985). As a result, denaturation steps are necessary for antibody/antigen binding to occur. Denaturation techniques vary greatly, sometimes involving incubation in solvents and/or application of high heat (Kuhn et al., 1996; Cameron and Gould, 1996; Gould et al., 1999; Leuner et al., 2006a; Wojtowicz and Kee, 2006; Tang et al., 2007). These methodological variations may have important consequences for histological quality and thus impact quantitative measurement and phenotypic assessment of newborn cells. Moreover, several recent reports have used iododeoxyuridine (IdU) and chlorodeoxyuridine (CldU), thymidine analogs with a structure similar to BrdU, to label newly generated neurons (Bauer and Patterson, 2005; Burns and Kuan, 2005; Vega and Peterson, 2005; Thomas et al., 2007; Dupret et al., 2007). These analogs can be detected individually by different antibodies that have been reported to recognize IdU or CldU exclusively (Burns and Kuan, 2005; Vega and Peterson, 2005; Thomas et al., 2007). However, these studies did not use IdU and CldU at molarities comparable to those of BrdU which labels the maximal number of cells (i.e., 200–300 mg/kg; Cameron and McKay, 2001). Thus, it remains unclear whether these analogs are as sensitive as BrdU at detecting newly generated neurons in the dentate gyrus.
Here, we systematically compared multiple commonly used BrdU antibodies and report large differences in their sensitivity. We also evaluated the number of labeled cells in the dentate gyrus following injection with the thymidine analogs IdU and CldU. At doses equimolar to that of BrdU which labels the maximal number of cells (Cameron and McKay, 2001), IdU and CldU labeled fewer cells and could not be discriminated because of antibody cross-reactivity. Finally, we examined different DNA denaturation pretreatments and demonstrate that in addition to affecting the number of BrdU-labeled cells, denaturation methods produce variations in the staining quality of the neuronal marker, NeuN. On the basis of the data presented here, optimal procedures for the detection of new neurons are suggested.
MATERIALS AND METHODS
Animals
For all experiments, adult male Sprague-Dawley rats (8–10 weeks of age) were used (Taconic Farms, Germantown, NY). Rats were acclimated to the colony for at least 5d prior to injection with BrdU, IdU, or CldU. Rats were group housed (2–3/cage), provided with unlimited access to food and water, and maintained on a reverse 12:12 light-dark cycle with lights on at 19:00 h. All procedures were conducted in accordance with Princeton University AALAC and The National Institutes of Health Guide for the Care and Use of Laboratory Animals.
Preparation and Injection of Thymidine Analogs
The dose of BrdU used for all experiments was 200 mg/kg. This dose was selected because it labels the maximal number of cells in the dentate gyrus (Cameron and McKay, 2001). For purposes of comparison, IdU and CldU doses were equimolar to the BrdU dose. Solutions of BrdU (Sigma-Aldrich, Milwaukee, WI, Cat. No. B5002, Lot No. 076K1307) were prepared in sterile saline with 0.007M NaOH at 20 mg/ml. IdU and CldU were less soluble than BrdU and, at the high doses used for this study, required dilution in a larger volume. IdU (MP Biomedicals, Irvine, CA, Cat. No. 100357, Lot No. 2742J) was prepared at 6 mg/ml and CldU (Sigma-Aldrich, Cat. No. C6891, Lot No. 106K1306) at 10 mg/ml, both in sterile saline with 0.007M NaOH. The pH of all thymidine analog solutions was approximately 7.0.
Rats (n= 6/group) were injected i.p. at approximately 13:00 h with one of the thymidine analogs and perfused after a 2h survival time. An additional group of rats (n = 6) were given BrdU injections on the same day and time but perfused after a 3 week survival time.
Histological procedures
Rats were anesthetized with an overdose of sodium pentobarbital and transcardially perfused with 4% paraformaldehyde in 0.1 M phosphate buffer (pH 7.5). Brains were dissected from the skulls and post-fixed for at least 2d. Forty µm thick coronal sections throughout the entire rostrocaudal extent of the dentate gyrus were cut with a Vibratome from half brains into a bath of 0.1 M phosphate buffered saline (PBS; pH 7.5). Each immunohistochemical reaction was performed simultaneously from all rats within a given experiment to maximize the reliability of comparison across groups.
Immunoperoxidase staining for BrdU, CldU, and IdU
A 1:12 series of sections from rats sacrificed at the 2h (BrdU, IdU, or CldU) or 3 week (BrdU) post-injection survival times were mounted onto glass slides and allowed to dry overnight. For pretreatment, 0.1 M citric acid (pH 6.0) was microwaved (GE, Model No. JEM25GY001) at power level 10 for ~3 min. Slides were then placed into the heated citric acid, microwaved for 5 min at power level 2, removed from the microwave, and allowed to cool for 20 min. Slides were then rinsed 3x in PBS, incubated in trypsin for 10 min, rinsed, denatured in 2N HCl:PBS for 30 min, and rinsed again. For BrdU staining, slides were incubated overnight at 4°C in one of the following antibodies (for each, technical information was provided by the manufacturer): 1) Vector anti-BrdU (Burlingame, CA, Cat. No. VPB209, Lot. No. 103009, 103010), clone 85-2C8, generated using mice immunized with a conjugate of BrdU and helix pomatia haemocyanin, 2) BD BioSciences anti-BrdU (San Jose, CA, Cat. No. 347580, Lot No. 63272, 70048), clone B44, derived from hybridization of mouse Sp2/0-Ag14 myeloma cells with spleen cells from mice immunized with iodouridine-conjugated ovalbumin, 3) Roche anti-BrdU (Mannheim, Germany, Cat. No. 11170376001, Lot. No. 93596620), prepared using mice immunized with a BrdU-bovine serum albumin conjugate. Lymphocytes isolated from the spleen were fused with Ag8.653 myeloma cells to create the BMC9318 clone, 4) Dako anti-BrdU (Glostrup, Denmark, Cat. No. M0744, Lot. No. 00031732), clone Bu20a, generated using mice immunized with BrdU conjugated to bovine serum albumin, 5) Novocastra Vision Biosystems anti-BrdU (Newcastle Upon Tyne, UK, Cat. No. NCL-BrdU, Lot. No. 103013), clone 85-2C8, derived from hybridization of mouse P3-X63.Ag8 myeloma cells with spleen cells from mice immunized with a conjugate of BrdU and helix pomatia haemocyanin, or 6) Accurate anti-BrdU (Westbury, NY, Cat. No. OBT0030, Lot. No. H8365), clone BU1/75 ICR1, raised in rat. All antibodies were monoclonal, react with BrdU in single-stranded DNA, and do not cross-react with thymidine. For IdU or CldU staining, slides were incubated in mouse monoclonal antibody against BrdU (BD or Accurate). Antibodies were diluted 1:200, or 1:100 where noted, in PBS with 0.5% Tween-20.
A 1:12 series of sections from a separate group of rats sacrificed 2h after BrdU injection were: 1) slide mounted and subjected to the denaturation pretreatment described above (HCl), 2) slide mounted, outlined with a PAP pen, rinsed 3x with PBS, then placed in a food steamer (Oster, Model No. 5712) preheated to ~99°C and covered with 1 ml of 10 mM sodium citrate buffer. After 15 min, the lower steamer rack was removed and allowed to cool for 2 min and the slides rinsed (Steam heating; Tang et al., 2007), 3) free-floating, rinsed 3× in TBS, incubated in 50% formamide/2×SSC (0.3M NaCl, 0.03M sodium citrate) for 2h at 65°C, rinsed in 2×SSC for 5 min, incubated in 2N HCl:TBS at 37°C for 30 min, rinsed in 0.1M boric acid (pH 8.5) for 10 min and then in TBS, incubated in TBS/0.25% Triton X-100/3% normal horse serum for 30 min, and rinsed in TBS (HCl + formamide; Kuhn et al., 1996). After pretreatments, sections were incubated overnight at 4°C in the BD mouse monoclonal antibody against BrdU diluted 1:200 in PBS (HCl, Steam heating) or TBS (HCl + formamide) with 0.5% Tween-20.
Following overnight incubation in primary antibodies, sections were rinsed, incubated with biotinylated horse anti-mouse (1:200; Vector, Cat. No. BA2000, Lot. No. S0721, T0205) or biotinylated goat anti-rat (1:200; Chemicon, Temecula, CA, Cat. No. AP183B, Lot. No. 0512017268, 0701049350) for 60 min, rinsed, incubated with avidin–biotin complex (1:100; Vector, Cat No. PK6100) for 60 min, rinsed, and reacted in 0.01% diaminobenzidine with 0.003% H2O2 (Sigma-Aldrich, Cat. No. D4293). Free floating tissue was mounted onto glass slides and allowed to dry for 2h. All slides were counterstained with cresyl violet, dehydrated, cleared with Citrisolv (Fisher Scientific, Fair Lawn, NJ), and coverslipped with Permount (Fisher Scientific) or with glycerol:PBS (3:1) for antibody penetration analysis.
Immunofluorescent co-labeling of BrdU and Ki67
Free-floating 40 µm thick sections from rats sacrificed at the 2h timepoint were rinsed 3× in TBS, pretreated with 2N HCl:TBS for 30 min, rinsed, then incubated at 4°C in rabbit polyclonal anti-Ki67:TBS (1:500; Novocastra Vision BioSystems, Cat. No. NCLKi67p, Lot. No. 301114) and mouse monoclonal anti-BrdU:TBS (1:200; BD) with 0.5% Tween-20. The rabbit polyclonal anti-Ki67 antibody was generated using prokaryotic recombinant fusion protein corresponding to a 1,086-bp Ki67 motif-containing cDNA (manufacturer's technical information). A double band at 245–395 kD is detected by Western blot analysis (Key et al.,1993). Specific staining of dividing cells in the adult rat dentate gyrus by this antibody has been demonstrated previously (Dayer et al., 2003). Following overnight incubation, sections were rinsed and incubated for 1h in the dark with goat anti-rabbit Alexa 488:TBS (1:250; Invitrogen Molecular Probes, Carlsbad, CA, Cat. No. A11034, Lot No. 51726A) and goat anti-mouse Alexa 568:TBS (1:250; Invitrogen Molecular Probes, Cat. No. A11031, Lot No. 39053A). Sections were rinsed, mounted onto glass slides, dried for at least 2h in the dark, and coverslipped with glycerol:TBS (3:1). As a control, brain sections from a rat that did not receive a BrdU injection was processed as described above.
Immunofluorescent labeling of NeuN
Forty µm thick sections from rats sacrificed at the 3 week timepoint were rinsed 3x in TBS and then subjected to one of the following pretreatments: 1) incubated in 2N HCl:TBS for 30 min (HCl), 2) sections were transferred with a brush onto a slide outlined with a PAP pen containing a pool of 10 mM sodium citrate buffer, such that the sections floated in the incubation solution. The slides were then transferred to the preheated steamer and heat-treated for 15 min at ~99°C. The lower steamer rack was then removed and allowed to cool for 2 min. Subsequent steps were performed free floating (Steam heating; Tang et al., 2007), 3) incubated in 50% formamide/2×SSC for 2h at 65°C, rinsed in 2×SSC for 5 min, incubated in 2N HCl at 37°C for 30 min, rinsed in 0.1M boric acid (pH 8.5) for 10 min and then in TBS, and incubated in TBS/0.25% Triton X-100/3% normal horse serum for 30 min (HCl+ formamide; Kuhn et al., 1996).
After pretreatments, sections were rinsed 3× in TBS and then incubated at 4°C in mouse anti-NeuN (Chemicon, Cat. No. MAB377, Lot. No. 0607036763) diluted 1:500 in TBS with 25 µl/ml of 10% Tween-20. This antibody was raised against purified cell nuclei from mouse brain and has been shown to recognize 2–3 bands at 46–48 kDa by Western blot (manufacturer's technical information; Lind et al., 2005). The next day, tissue was rinsed, incubated in secondary goat anti-mouse Alexa 488:TBS (1:500; Invitrogen Molecular Probes, Cat. No. A11029, Lot. No. 54421A) for 30 min in the dark, rinsed, mounted onto glass slides, dried for at least 2h in the dark, and coverslipped with glycerol:TBS (3:1).
Data analysis
Slides were coded prior to data collection. The code was broken after the analysis of each experiment was complete.
BrdU, IdU, and CldU peroxidase analysis
The number of BrdU-, IdU-, or CldU-labeled cells on every 12th unilateral section throughout the entire rostrocaudal extent of the dentate gyrus (granule cell layer, subgranular zone, and hilus) were counted at 1000× with an Olympus BX-50 light microscope using a modified version of the optical fractionator method (West et al., 1991; Ngwenya et al., 2005). The estimated total number (N) of BrdU-, IdU-, or CldU-labeled cells was calculated according to the following formula: N = ∑Q− × (t/h) × (1/asf) × (1/ssf) where ∑Q− is the sum of counted labeled cells (excluding cells in the outermost focal plane), t is the mean section thickness, h is the height of the optical disector, asf is the area sampling fraction, and ssf is the section sampling fraction. Optical fractionator rules typically require the use of guard zones at the upper and lower surfaces of the section. However, tissue sections shrink substantially (to ~15 µm) in the z-dimension when mounted on slides, dried, processed for immunohistochemical staining, counterstained, dehydrated, and cleared. Consequently, we used a modified optical fractionator technique in which we did not use guard zones making the height of the counting frame equal to the section thickness. Since we used an exhaustive sampling scheme, asf was equal to one. Thus, the simplified formula for the estimated total number of labeled cells was: N = ∑Q− × (1/ssf) which is the total number of labeled cells counted multiplied by the reciprocal of the section sampling fraction (1/24).
Brightfield photographs were taken with an Olympus U-PMTUC camera attached to the microscope using ImagePro software (Media Cybernetics, Bethesda, MD). Images were cropped and optimized by adjusting brightness and color balance in Adobe Photoshop 7.0 (San Jose, CA). Photographs were resized and arranged in Canvas 8 (Deneba Software, Miami, FL).
For purposes of comparison, the density of BrdU-labeled cells was also determined in the SVZ (Kuhn et al., 1996; Mirescu et al., 2004). This analysis included a substantial part of the SVZ, but excluded the anterior portion. BrdU-labeled cells in the SVZ present on every 12th coronal section of the dentate gyrus were counted (−1.8 to −4.8 mm from Bregma; Paxinos and Watson, 1998) and expressed as the number of cells per mm3. The volume of the analyzed area was determined using Cavalieri’s principle on video-projected images with cross-sectional area measurements performed using ImagePro software (Gundersen et al., 1999).
Antibody penetration analysis
Analyses of antibody penetration were performed using the z-axis encoder on an Olympus BX-60 microscope attached to a computer equipped with StereoInvestigator software (MicroBrightField, Williston, VT), section thickness and depth of labeling penetration (minimum and maximum) from the surface of the section were measured for all labeled cells in the granule cell layer and subgranular zone at 1000×. Cells that had a maximum depth penetration greater than half of the section thickness were also quantified in order to determine the percentage of labeled cells that penetrated to the center of the section.
BrdU and Ki67 immunofluorescent analysis
Analyses were done using the 40× objective on a Zeiss Axiovert confocal microscope (Zeiss, Oberkochen, Germany) with Argon 458/488 and HeNe 543 lasers. A sequence configuration to scan each channel separately was employed in order to avoid cross-talk between fluorescent labels. For each brain, the percentage of Ki67-labeled cells in the dentate gyrus (granule cell layer and subgranular zone) that expressed BrdU was determined from 40–50 randomly selected Ki67-labeled cells. In addition, the percentage of BrdU-labeled cells in the dentate gyrus that expressed Ki67 was determined from 8–40 randomly selected BrdU-labeled cells per brain. For both analyses, optical stacks of 1 µm thick sections were obtained through putatively double-labeled cells. To verify double-labeling throughout their extent, cells were examined in orthogonal planes. Confocal images were acquired and stacked using Zeiss 510LSM software, cropped, contrast adjustments made in Adobe Photoshop 7.0, then resized and arranged in Canvas 8.
NeuN immunofluorescent analysis
One µm thick optical sections were scanned from the dentate gyrus using the 40× objective on a confocal microscope with an Argon laser and Zeiss 510LSM software. Ten frames were obtained per rat per pretreatment condition, while holding the scanning parameters constant. Outlines of all NeuN stained cell bodies in the scans were traced using the measurement tool in the LSM software and the mean intensity for each cell body was determined (0–255 grayscale). The outline was then moved to an adjacent region void of any NeuN positive cell bodies and a background optical intensity measure was recorded. Every labeled cell was analyzed in this manner. Signal was defined as the mean intensity value for the cell bodies. Background was defined as the mean intensity value for the regions containing no stained cell bodies. The signal:noise ratio was calculated according to the following formula: (Signal - Background) / Standard deviation of the Background. Fluorescent images were acquired using the LSM software, cropped in Adobe Photoshop 7.0, then resized and arranged in Canvas 8. No other digital manipulations were employed.
Statistical analysis
Means ± SEMs were determined for each variable (BrdU, IdU, or CldU cell counts; signal:noise ratios; antibody penetration depth). Data were subjected to either a two-tailed, unpaired t-test or analysis of variance (one or two way) followed by Newman-Keuls post hoc comparisons, when appropriate. Statistical significance was accepted at P < 0.05.
RESULTS
Commonly used BrdU antibodies are not equivalently sensitive
Using antibodies from Vector, BD, and Roche, we compared the number of newborn cells in the dentate gyrus from rats perfused 2h after BrdU injection and found large differences across groups (F(2,15) = 75.81, P < 0.0001; Fig. 1A). BrdU antibodies from BD and Roche labeled more cells than Vector (P < 0.001 for each post hoc comparison), with no difference observed between BD and Roche. In a second comparison, we extended our analysis to include three additional BrdU antibodies (Dako, Novocastra, Accurate), again using a 2h post-BrdU survival time. For comparison, we also included the BD antibody. Again, we observed differences in the number of BrdU-labeled cells in the dentate gyrus depending on the BrdU antibody used (F(3,20) = 6.17, P < 0.005; Fig. 1B). Novocastra labeled the fewest number of cells compared to the other antibodies (P < 0.05 for each post hoc comparison), which did not differ from one another.
Figure 1.
BrdU antibodies do not label the same number of cells in the dentate gyrus. (A) BD and Roche antibodies detected significantly more BrdU-labeled cells in the dentate gyrus with a 2h post-BrdU survival time compared to Vector. The number of BrdU-labeled cells did not differ between BD and Roche. (B) In a second comparison, we extended our analysis to include three other BrdU antibodies (Dako, Novocastra, Accurate) in addition to BD. Two hours following BrdU administration, Novocastra detected the fewest number of BrdU-labeled cells compared to all other antibodies, while the remaining antibodies did not differ from each other. (C) Similar results were obtained 3 weeks following BrdU administration. Vector and Novocastra detected the fewest number of BrdU-labeled cells relative to all other antibodies examined, with the remaining antibodies not differing significantly from each other. Bars represent mean ± SEM, * P < 0.05.
To address the possibility that the differences in the number of BrdU-labeled cells is due to differential permeability of antibodies into slide-mounted sections, we assessed whether a high staining (BD) and a low staining (Vector) antibody penetrate throughout the section thickness equally. For both antibodies, labeled cells were distributed throughout the section with the majority of labeling (~80 %) found in the center of the ~15 µm section height. There was no difference in the minimum (t10 = 0.44, P > 0.05) or maximum (t10 = 1.84, P > 0.05) depth of labeling penetration (Supplementary Fig. 1A). Since there is considerable collapse when sections are mounted on slides, dried, processed for immunohistochemical staining, counterstained, and dehydrated, it may be difficult to detect complete label penetration. As such, we performed an additional analysis on slide mounted sections that were stained using peroxidase methods and coverslipped with glycerol:PBS. Without the counterstain and dehydration steps, section collapse was less with a final section thickness of ~25 µm. We again found no differences in antibody penetration (minimum: t10 = 0.35, P > 0.05; maximum: t10 = 0.33, P > 0.05) between BD and Vector antibodies (Supplementary Fig. 1B), with the majority (~80 %) of labeling evident in the center of the section. These data suggest that variations in the number of BrdU-labeled cells are likely due to differences in antibody sensitivity rather than antibody permeability.
To assess whether these differences were specific to a short post-BrdU injection survival time, we compared all six BrdU antibodies on tissue from rats injected with BrdU and perfused after 3 weeks. Again, we found large differences in the number of BrdU-labeled cells in the dentate gyrus across groups (F(5,30) = 8.01, P < 0.0001; Fig. 1C). Vector and Novocastra labeled the fewest cells compared to all other antibodies (P < 0.05 for each post hoc comparison). BD, Roche, Dako, and Accurate antibodies stained more cells in the dentate gyrus and did not differ significantly from each other.
Qualitatively, labeling intensity in the dentate gyrus was greatest in tissue stained with antibodies that labeled the most cells (Fig. 2; Supplementary Fig. 2). BrdU-labeled cells could be visualized at a total magnification of 100× for BD, Dako, Accurate, and Roche, whereas Vector and Novocastra required visualization using at least 400×. Staining was not observed in the dentate gyrus with any of the antibodies on tissue from a rat that did not receive a BrdU injection.
Figure 2.
BrdU antibodies, analogs, and pretreatments produce variable histology. (A, B) BrdU-labeled cells (arrows) in the dentate gyrus from sections stained with Vector (A) or BD (B) antibodies 3 weeks after BrdU administration. High magnification examples of BrdU-labeled cells in the granule cell layer (gcl) 2h (C, D) and 3 weeks (F, G) after BrdU administration as detected with Vector (C, F) or BD (D, G). At both the 2h and 3 week timepoints, more BrdU-labeled cells were detected with the BD antibody. (E, H) High magnification examples of IdU- and CldU-labeled cells in the gcl 2h after equimolar delivery of the analogs as detected with the BD antibody. IdU and CldU labeled fewer cells than BrdU and could not be discriminated because of antibody cross-reactivity. (I-K) Immunofluorescence double-labeling of BrdU (I) and Ki67 (J). Merged image shown in (K). All BrdU-labeled cells detected with the BD antibody stained for Ki67 indicating specificity for dividing cells. (L–P) Confocal images of NeuN staining from sections pretreated with HCl alone (L), HCl + formamide (M, N) or Steam heating (O, P). HCl + formamide and Steam heating pretreatments were variable in their staining patterns, some had high background (M, O) whereas others had no nuclear staining (N, P). Scale bars, 10 µm.
Similar differences were observed in BrdU-labeled cells in the subventricular zone (SVZ) across antibodies following a 2h post-BrdU survival time. Vector labeled the fewest cells compared to BD and Roche (F(2,15) = 6.02, P < 0.01; Supplementary Fig. 3A). In a second comparison, Novocastra labeled the fewest cells (F(3,20) = 3.43, P < 0.05; Supplementary Fig. 3B), but differed significantly only from Dako.
Sensitive BrdU antibodies are specific
To rule out the possibility that BrdU antibodies that label a high number of cells do so nonspecifically, we evaluated the specificity of a high staining BrdU antibody (BD). Tissue from rats injected with BrdU followed by a 2h survival time was co-labeled with Ki67. Since Ki67 is an endogenous marker expressed during most phases of the cell cycle including S-phase (Kee et al., 2002; Eisch and Mandyam, 2007), all BrdU-labeled cells should co-express Ki67. However, not all Ki67 positive cells should co-express BrdU since BrdU only labels cells undergoing DNA synthesis. Indeed, we found that 100% of the BrdU-labeled cells in the dentate gyrus stained for Ki67, whereas only 47.8 ± 8.1% of the Ki67-labeled cells stained for BrdU (Fig. 2I–K). Tissue from a rat that did not receive a BrdU injection showed Ki67, but not BrdU, labeling. These data suggest that the high staining BD antibody specifically labels cells that are in S-phase at the time of the BrdU injection.
Less sensitive antibodies are not made equally sensitive by increasing their concentration
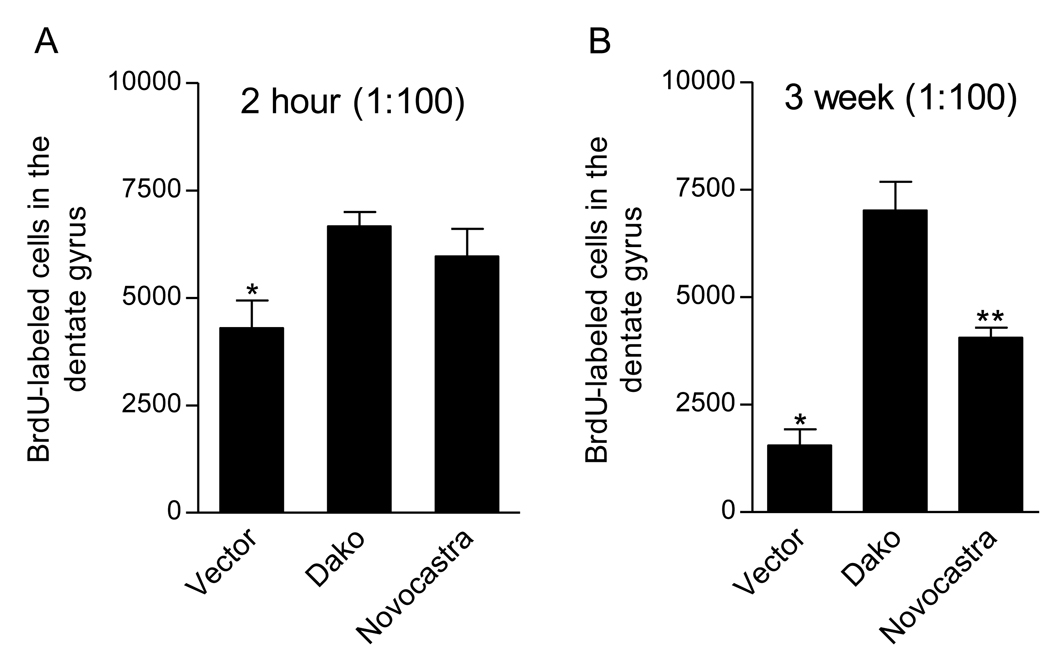
We examined whether the less sensitive BrdU antibodies (Vector, Novocastra) could be made more sensitive by increasing their concentration from 1:200 to 1:100. We also assessed whether the Dako antibody, which labels a large number of new cells at 1:200 (Fig. 1), would label even more cells with an increased concentration of 1:100. We found that Dako at 1:100 did not label more cells in the dentate gyrus or SVZ than at 1:200 (dentate gyrus: compare Fig. 3 with Fig. 1; SVZ: compare Supplementary Fig. 4 with Supplementary Fig. 3). However, at both the 2h (F(2,15) = 4.72, P < 0.05; Fig. 3A) and 3 week (F(2,15) = 35.34, P < 0.0001; Fig. 3B) post-BrdU survival times, there were differences in the number of BrdU-labeled cells in the dentate gyrus across antibodies at the 1:100 dilution. Vector again labeled the fewest cells at both timepoints (P < 0.05 for each post hoc comparison). In contrast, increasing the concentration of the Novocastra antibody amplified the number of BrdU-labeled cells at both 2h and 3 weeks; however at 3 weeks, the number of cells labeled by Novocastra did not reach the number labeled by Dako (P < 0.001). Thus, increasing the concentration of the less sensitive antibodies did not equivalently enhance their sensitivity.
Figure 3.
Less sensitive BrdU antibodies are not made more sensitive by increasing their concentration. At both the 2h (A) and 3 week (B) survival times, a 1:100 concentration of the Vector antibody labeled fewer cells in the dentate gyrus compared to the Dako antibody. In contrast, a 1:100 concentration of the Novocastra BrdU antibody amplified the number of BrdU-labeled cells at both 2h and 3 weeks. However, at 3 weeks, the number of cells labeled by Novocastra was lower than that labeled by Dako. Bars represent mean ± SEM, * P < 0.05 vs. Dako, ** P < 0.05 vs. Dako and Vector.
At the 1:100 dilution, similar differences were observed in the SVZ across antibodies following a 2h post-BrdU survival time. Vector labeled the fewest cells compared to Dako and Novocastra (F(2,15) = 9.55, P < 0.005; Supplementary Fig. 4). Increasing the concentration of the Novocastra antibody amplified the number of BrdU-labeled cells to a number comparable to that labeled by Dako.
Denaturation methods affect the number of BrdU-labeled cells and staining for NeuN
To test whether methods for DNA denaturation affect the number of BrdU-labeled cells, we stained tissue using the BD antibody in combination with three different pretreatment methods – HCl alone (Cameron and McKay, 2001; Leuner et al., 2006a), HCl + formamide (Kuhn et al., 1996) or Steam heating (Tang et al., 2007). Qualitatively, BrdU peroxidase staining resulting from Steam heating was less defined compared to HCl alone or HCl + formamide. Quantitatively, we found that while HCl alone and HCl + formamide resulted in similar numbers of BrdU-labeled cells in the dentate gyrus, Steam heating revealed fewer labeled cells (F(2,15) = 5.08, P < 0.05; Fig. 4A). Pretreatment methods had no effect on the density of BrdU-labeled cells in the SVZ (F(2,15) = 2.04, P > 0.05; Supplementary Fig. 5).
Figure 4.
Denaturation pretreatment methods affect the number of BrdU-labeled cells and staining for the mature neuronal marker, NeuN, in the dentate gyrus. (A) Fewer BrdU-labeled cells were detected with Steam heating on tissue stained using peroxidase methods. (B) Tissue stained for NeuN immunofluorescence using the HCl alone pretreatment had a substantially higher signal:noise ratio than tissue stained for NeuN using either HCl + formamide or Steam heating pretreatments. Bars represent mean ± SEM, * P < 0.05.
We also examined whether pretreatment methods impact phenotypic assessment of newborn cells by calculating a signal:noise ratio for immunofluorescence staining of the mature neuronal marker, NeuN. Compared to tissue stained for NeuN using the HCl alone protocol, the signal:noise ratio was substantially reduced when HCl + formamide or Steam heating pretreatment methods were used (F(2,15) = 8.83, P < 0.005; Fig. 4B). Qualitatively, HCl + formamide and Steam heating were variable in their NeuN staining patterns – some sections had high background whereas others had no nuclear staining (Fig. 2L–P).
The thymidine analogs IdU and CldU are neither as sensitive nor as specific as BrdU
Using doses of IdU and CldU equivalent to a saturating dose of BrdU (Cameron and McKay, 2001), we evaluated the sensitivity and specificity of IdU and CldU and compared it to that of BrdU in separate groups of rats that were injected with these analogs and perfused after a 2h survival. BD and Accurate antibodies were selected for this comparison, since prior studies have reported that these antibodies are specific to IdU and CldU, respectively (Vega and Peterson, 2005; Thomas et al., 2007).
Overall, BrdU-labeled cells were more abundant in the dentate gyrus (F(2,26) = 55.06; P < 0.0001; Fig. 5) and SVZ (F(2,26) = 25.73, P < 0.0001; Supplementary Fig. 6) than either IdU- or CldU-labeled cells in both brain regions, suggesting that these thymidine analogs are not labeling S-phase cells with equal probability. Moreover, the detection of IdU and CldU was not specific to the antibodies we tested for the dentate gyrus (F(2,26) = 34.10, P < 0.0001) and SVZ (F(2,26) = 11.64, P < 0.0005). Although BD detected more IdU-labeled cells than Accurate (P < 0.0001), some IdU-labeled cells were detected with the Accurate antibody. Also, although Accurate detected more CldU-labeled cells than BD (P < 0.05), a substantial number of CldU-labeled cells were detected with the BD antibody.
Figure 5.
The thymidine analogs, IdU and CldU, are not as sensitive as BrdU. Overall, BrdU-labeled cells in the dentate gyrus were more abundant than either IdU- or CldU-labeled cells (** P < 0.05) suggesting that these thymidine analogs are not labeling cells undergoing S-phase with equal probability. Also, BrdU antibodies did not label IdU or CldU with specificity. Although BD detected more IdU-labeled cells than Accurate, some IdU-positive cells were found with the Accurate antibody. In addition, both BD and Accurate detected a substantial number of CldU-labeled cells. Bars represent mean ± SEM, * P < 0.05.
DISCUSSION
These results demonstrate that commonly used BrdU antibodies do not label an equivalent number of cells. BrdU antibodies from Vector and Novocastra stained fewer cells in both the dentate gyrus and SVZ than BD, Roche, Dako, and Accurate antibodies. Antibody penetration was similar for low (Vector) and high (BD) staining antibodies suggesting that variations in the number of BrdU-labeled cells are likely due to differences in antibody sensitivity rather than antibody permeability. The differences in the number of BrdU-labeled cells across antibodies were not eliminated by increasing the concentration of the less sensitive antibodies. Moreover, a high staining antibody (BD) did not label nonspecifically since all BrdU-labeled cells expressed Ki67, an endogenous marker of proliferating cells. Additional variability in BrdU labeling occurred with different denaturation pretreatments – HCl alone and HCl + formamide stained more cells in the dentate gyrus than Steam heating. Pretreatments also differentially affected immunofluorescent staining for the mature neuronal marker, NeuN – greater staining was observed with HCl alone pretreatment. Finally, labeling with the thymidine analogs IdU and CldU resulted in fewer stained cells compared to BrdU. While both IdU and CldU labeled a comparable number of cells in the dentate gyrus and SVZ, each stained fewer cells than BrdU using sensitive antibodies. Moreover, labeling with IdU and CldU at molarities comparable to those used for staining the maximal number of BrdU-labeled cells yielded antibody cross-reactivity. Given that the present work utilized peroxidase staining and brightfield microscopy to evaluate the number of newly generated cells, additional work is needed to assess the extent to which these findings apply to fluorescence methods. Nonetheless, these data suggest that current methodologies for studying adult neurogenesis are not equally sensitive and in some cases can lack specificity.
BrdU antibodies
Antibody selection is an important issue (Saper and Sawchenko, 2003; Rhodes and Trimmer, 2007), which until now has not been examined with regard to adult neurogenesis. In this study, differences in the number of BrdU-labeled cells were detected in the dentate gyrus and SVZ depending on the antibody used. Since antibody permeability did not differ between a high (BD) and low (Vector) staining antibody, the differences we observed are likely a result of varied antibody sensitivity.
Vector and Novocastra were the least sensitive and stained the fewest cells. These antibodies, although from different suppliers, are generated from the same clone (85-2C8). Despite a similar origin, Novocastra generally stained more cells than Vector. Increasing the concentration from 1:200 to 1:100 rendered Novocastra, but not Vector, equivalently sensitive to the high staining antibodies at the 2h post-BrdU survival time. At the 3 week survival time, Novocastra stained more cells than Vector at the 1:100 concentration, however, Novocastra still labeled fewer cells than the more sensitive BrdU antibodies. One possible explanation for staining differences between the 2h and 3 week survival times for Novocastra at 1:100 is that after a 2h survival, BrdU-labeled cells are easier to detect because cells that incorporated BrdU have not divided and thus diluted the label.
DNA denaturation pretreatments
BrdU antibodies recognize BrdU in single-stranded DNA (Gratzner, 1982; Vanderlaan and Thomas, 1985) thus necessitating denaturation pretreatments before primary antibody incubation. To investigate whether pretreatments alter the number of BrdU-labeled cells, we compared three BrdU staining protocols. Two used HCl for denaturation (Cameron and McKay, 2001) but one also included high temperature incubation in the solvent formamide (Kuhn et al., 1996). The third protocol used steam heating alone for denaturation (Tang et al., 2007). Using a sensitive and specific BrdU antibody (BD), we found that protocols using HCl yielded the highest number of BrdU-labeled cells in the dentate gyrus. Thus, combined use of less sensitive antibodies and suboptimal pretreatments would likely further degrade staining, altering data qualitatively and quantitatively.
In addition to differences in staining, cell counting methods are also critical (Saper, 1996) and may influence the number of BrdU-labeled cells reported. Here, we used a modified optical fractionator method that is common in studies of adult neurogenesis (Cameron and McKay, 2001; Drapeau et al., 2003; Malberg et al., 2003; Ngwenya et al., 2005; Siwak-Tapp et al., 2007). Although some have argued that such modified stereological methods simply count profiles (Coggeshall and Lekan, 1996), it remains to be tested whether modified and strict stereological approaches produce similar outcomes. One way in which these different counting methods may yield inconsistent results in the numbers of BrdU-labeled cells is under conditions, such as harsh pretreatments, that lead to differential cell loss from the surface of the tissue section (i.e., lost caps). However, this explanation is unlikely to account for the data presented here since we avoided counting cells in the outermost focal plane where lost caps are likely to be located.
In contrast to the dentate gyrus, the number of labeled cells in the SVZ was unaffected by different pretreatments. This regional difference in sensitivity may be related to variations in BrdU availability for uptake. Because of its location in the ventricles, as opposed to in the parenchyma, proliferating cells in the SVZ may have greater access to BrdU than those in the dentate gyrus. Even with histological procedures that reduce staining intensity, cells heavily labeled with BrdU are likely to remain detectable in the SVZ. However, these findings should not be used as evidence that multiple BrdU injections be employed to heavily label cells throughout the brain. Indeed, the choice of injection protocol must be thoughtfully considered within the context of the questions addressed in a particular experiment (Christie and Cameron, 2006).
Pretreatments also affected NeuN immunofluorescent staining in the dentate gyrus. The signal:noise ratio of NeuN staining was highest for HCl alone and substantially reduced with the other methods suggesting that the assessment of new cell phenotype is sensitive to pretreatment methods. It is likely, however, that methods diminishing NeuN staining reduce the probability that cells containing relatively less NeuN (i.e., immature cells) will be detectable. The extent to which this variable contributes to inconsistencies in reports of adult neurogenesis in regions outside of the dentate gyrus and SVZ remains unknown (Gould, 2007), but it may be relevant that many negative reports used the HCl + formamide method (Kornack and Rakic, 2001; Ehninger et al., 2003; Bhardwaj et al., 2006).
IdU and CldU as alternatives to BrdU
The thymidine analogs IdU and CldU have an advantage over BrdU labeling alone; both can be injected into the same animal so cells produced at different timepoints can be simultaneously assessed (Vega and Peterson, 2005; Thomas et al., 2007; Dupret et al., 2007). For this technique to produce interpretable results, antibodies for one analog must not cross react with the other. Previous work identified antibodies that recognize IdU or CldU exclusively (Burns and Kuan, 2005; Vega and Peterson, 2005). However, these studies did not use IdU and CldU at molarities comparable to those of BrdU which labels the maximal number of cells (i.e., 200–300 mg/kg; Cameron and McKay, 2001). We demonstrate that when doses of IdU and CldU are increased to an equivalently saturating dose of BrdU, detection specificity is lost resulting in antibody cross-reactivity. Another difficulty with this approach is that IdU and CldU are less soluble than BrdU, necessitating larger injection volumes. This problem is most evident with IdU, which required a concentration of 6mg/ml to achieve solubility. Large injection volumes are stressful, making this an unsuitable treatment for adult neurogenesis studies (Mirescu and Gould, 2006).
At doses similar to those of a saturating BrdU dose, neither IdU nor CldU labeled as many cells in the dentate gyrus or SVZ as labeled by BrdU. It is unlikely that these differences are due to a stimulatory effect of BrdU itself on cell proliferation since the numbers of Ki67-labeled cells in the dentate gyrus is similar in BrdU-injected and non-injected animals, even when high saturating doses of BrdU are used (Kee et al., 2002; Hancock et al., 2009). Rather, the data presented here suggest that studies using IdU and CldU (even at high doses) underestimate the number of newborn cells. This effect is likely due to differences in the sensitivity of the analogs themselves, but could also be related to the sensitivity of the antibodies used to detect them.
Prior work examining lower doses of BrdU, IdU, and CldU found similar numbers of labeled cells in the SVZ across markers using fluorescence methods (Bauer and Patterson, 2005). It is possible that at high doses, like those used here, IdU and CldU are not completely available for uptake by cells in S-phase, either because they remain somewhat insoluble or have saturated uptake mechanisms. Making relative comparisons may require lower doses to prevent cross-reactivity that can also be eliminated by using BrdU antibodies at greater dilutions. Indeed, a study using lower doses of IdU and CldU, as well as less concentrated BrdU antibodies, reported no such cross-reactivity (Vega and Peterson, 2005). Future studies should include controls injected with a single analog to examine nonspecific staining with BrdU antibodies and should assess whether each analog labels similar numbers of cells given identical experimental conditions.
Potential contribution of histology to variability in adult neurogenesis studies
Adult neurogenesis in the dentate gyrus was first reported in the 1960s but was not accepted for decades. Earlier evidence may have been discounted because it was reported to be a quantitatively minor process (Gross, 2000). The original studies used 3H-thymidine autoradiography on relatively thick tissue sections (Altman and Das 1965; 1966; 1967), a method which underestimates the number of labeled cells (Gross, 2000). The application of BrdU labeling provided further evidence that new cells in the dentate gyrus were neurons and that adult neurogenesis is robust, supporting the idea that the production of new neurons in the adult brain may be functionally relevant (Cameron and McKay, 2001).
The present report suggests that commonly used BrdU methods likely underestimate the number of new neurons, and furthermore emphasize the need to standardize BrdU labeling procedures. It also raises the question of whether studies which use less sensitive antibodies, thymidine analogs, or suboptimal pretreatments remain useful when making relative comparisons across groups. Since histological procedures are usually standardized across studies within a laboratory and similarly applied among groups within an experiment, it is likely that degradation of staining would affect all groups equally. But, it is theoretically possible that a population of cells containing only a small amount of BrdU exist in a certain experimental group, perhaps because the manipulation slows entry into S-phase or enhances the survival of cells that underwent multiple divisions after labeling. In these instances, the most sensitive conditions may be necessary to stain this lightly labeled population and thus detect group differences. Although such scenarios may be uncommon, they are perhaps relevant when studies of similar design but varying histological methods produce inconsistent results. It is noteworthy that in many instances, relative group differences are preserved across a range of antibodies. For example, Vector, Roche, and BD have all been used to demonstrate enhancements in adult neurogenesis following learning (Epp et al., 2007; Leuner et al., 2006a) and physical activity (Stranahan et al., 2006; Pereira et al., 2007) as well as stress-induced decrements in cell proliferation (Malberg et al., 2003; Mirescu et al., 2004). Still, conflicting reports exist regarding the regulation of adult neurogenesis by these factors (Leuner et al., 2006b; Mirescu and Gould, 2006; Thomas et al., 2007). While some differences are likely due to variables including BrdU dose and regimen, cell counting methods, animal age, strain, species and sex, as well as duration and specifics of the experimental manipulation (Mitra et al., 2006; Mohapel et al., 2006; Engesser-Cesar et al., 2007), the possibility that differences in histological procedures play some role should be considered.
Supplementary Material
Acknowledgments
Funded by: Grants from the National Institutes of Health to B.L. (F32MH76471, K99MH084148) and E.G. (MH54970), National Alliance for Research on Schizophrenia and Depression Young Investigator Award to B.L., United Negro College Fund·Merck Postdoctoral Science Research Fellowship to E.R.G.
REFERENCES
- Abrous DN, Koehl M, Le Moal M. Adult neurogenesis: from precursors to network and physiology. Physiol Rev. 2005;85:523–569. doi: 10.1152/physrev.00055.2003. [DOI] [PubMed] [Google Scholar]
- Altman J, Das GD. Autoradiographic and histological evidence of postnatal hippocampal neurogenesis in rats. J Comp Neurol. 1965;124:319–335. doi: 10.1002/cne.901240303. [DOI] [PubMed] [Google Scholar]
- Altman J, Das GD. Autoradiographic and histological studies of postnatal neurogenesis. I. A longitudinal investigation of the kinetics, migration and transformation of cells incorporating tritiated thymidine in neonate rats, with special reference to postnatal neurogenesis in some brain regions. J Comp Neurol. 1966;126:337–389. doi: 10.1002/cne.901260302. [DOI] [PubMed] [Google Scholar]
- Altman J, Das GD. Postnatal neurogenesis in the guinea-pig. Nature. 1967;214:1098–1101. doi: 10.1038/2141098a0. [DOI] [PubMed] [Google Scholar]
- Bauer S, Patterson PH. The cell cycle-apoptosis connection revisited in the adult brain. J Cell Biol. 2005;171:641–650. doi: 10.1083/jcb.200505072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhardwaj RD, Curtis MA, Spalding KL, Buchholz BA, Fink D, Björk-Eriksson T, Nordborg C, Gage FH, Druid H, Eriksson PS, Frisén J. Neocortical neurogenesis in humans is restricted to development. Proc Natl Acad Sci USA. 2006;103:12564–12568. doi: 10.1073/pnas.0605177103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burns KA, Kuan CY. Low doses of bromo-and iododeoxyuridine produce near-saturation labeling of adult proliferative populations in the dentate gyrus. Eur J Neurosci. 2005;21:803–807. doi: 10.1111/j.1460-9568.2005.03907.x. [DOI] [PubMed] [Google Scholar]
- Cameron HA, Gould E. Distinct populations of cells in the adult dentate gyrus undergo mitosis or apoptosis in response to adrenalectomy. J Comp Neurol. 1996;369:56–63. doi: 10.1002/(SICI)1096-9861(19960520)369:1<56::AID-CNE4>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- Cameron HA, McKay RD. Restoring production of hippocampal neurons in old age. Nat Neurosci. 1999;2:894–897. doi: 10.1038/13197. [DOI] [PubMed] [Google Scholar]
- Cameron HA, McKay RD. Adult neurogenesis produces a large pool of new granule cells in the dentate gyrus. J Comp Neurol. 2001;435:406–417. doi: 10.1002/cne.1040. [DOI] [PubMed] [Google Scholar]
- Christie BR, Cameron HA. Neurogenesis in the adult hippocampus. Hippocampus. 2006;16:199–207. doi: 10.1002/hipo.20151. [DOI] [PubMed] [Google Scholar]
- Coggeshall RE, Lekan HA. Methods for determining the numbers of cells and synapses: a case for more uniform standards of review. J Comp Neurol. 1996;364:6–15. doi: 10.1002/(SICI)1096-9861(19960101)364:1<6::AID-CNE2>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- Dayer AG, Ford AA, Cleaver KM, Yassaee M, Cameron HA. Short-term and long-term survival of new neurons in the rat dentate gyrus. J Comp Neurol. 2003;460:563–572. doi: 10.1002/cne.10675. [DOI] [PubMed] [Google Scholar]
- Drapeau E, Mayo W, Aurousseau C, Le Moal M, Piazza PV, Abrous DN. Spatial memory performances of aged rats in the water maze predict levels of hippocampal neurogenesis. Proc Natl Acad Sci USA. 2003;100:14385–143890. doi: 10.1073/pnas.2334169100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dupret D, Fabre A, Döbrössy MD, Panatier A, Rodríguez JJ, Lamarque S, Lemaire V, Oliet SH, Piazza PV, Abrous DN. Spatial learning depends on both the addition and removal of new hippocampal neurons. PLoS Biol. 2007;5:e214. doi: 10.1371/journal.pbio.0050214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehninger D, Kempermann G. Regional effects of wheel running and environmental enrichment on cell genesis and microglia proliferation in the adult murine neocortex. Cereb Cortex. 2003;13:845–851. doi: 10.1093/cercor/13.8.845. [DOI] [PubMed] [Google Scholar]
- Eisch AJ, Mandyam CD. Adult neurogenesis: can analysis of cell cycle proteins move us “beyond BrdU”? Curr Pharm Biotechnol. 2007;8:147–165. doi: 10.2174/138920107780906540. [DOI] [PubMed] [Google Scholar]
- Engesser-Cesar C, Anderson AJ, Cotman CW. Wheel running and fluoxetine antidepressant treatment have differential effects in the hippocampus and the spinal cord. Neuroscience. 2007;144:1033–1044. doi: 10.1016/j.neuroscience.2006.10.016. [DOI] [PubMed] [Google Scholar]
- Epp JR, Spritzer MD, Galea LA. Hippocampus-dependent learning promotes survival of new neurons in the dentate gyrus at a specific time during cell maturation. Neuroscience. 2007;149:273–285. doi: 10.1016/j.neuroscience.2007.07.046. [DOI] [PubMed] [Google Scholar]
- Eriksson PS, Perfilieva E, Björk-Eriksson T, Alborn AM, Nordborg C, Peterson DA, Gage FH. Neurogenesis in the adult human hippocampus. Nat Med. 1998;4:1313–1317. doi: 10.1038/3305. [DOI] [PubMed] [Google Scholar]
- Fornal CA, Stevens J, Barson JR, Blakley GG, Patterson-Buckendahl P, Jacobs BL. Delayed suppression of hippocampal cell proliferation in rats following inescapable shocks. Brain Res. 2007;1130:48–53. doi: 10.1016/j.brainres.2006.10.081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gage FH. Neurogenesis in the adult brain. J Neurosci. 2002;22:612–613. doi: 10.1523/JNEUROSCI.22-03-00612.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gould E, Reeves AJ, Fallah M, Tanapat P, Gross CG, Fuchs E. Hippocampal neurogenesis in adult Old World primates. Proc Natl Acad Sci USA. 1999;96:5263–5267. doi: 10.1073/pnas.96.9.5263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gould E. How widespread is adult neurogenesis in mammals? Nat Rev Neurosci. 2007;8:481–488. doi: 10.1038/nrn2147. [DOI] [PubMed] [Google Scholar]
- Gratzner HG. Monoclonal antibody to 5-bromo- and 5-iododeoxyuridine: A new reagent for detection of DNA replication. Science. 1982;218:474–475. doi: 10.1126/science.7123245. [DOI] [PubMed] [Google Scholar]
- Gross CG. Neurogenesis in the adult brain: death of a dogma. Nat Rev Neurosci. 2000;1:67–73. doi: 10.1038/35036235. [DOI] [PubMed] [Google Scholar]
- Gundersens HJ, Jensen EB, Kiêu K, Nielsen J. The efficiency of systematic sampling in stereology--reconsidered. J Microsc. 1999;193:199–211. doi: 10.1046/j.1365-2818.1999.00457.x. [DOI] [PubMed] [Google Scholar]
- Hancock A, Priester C, Kidder E, Keith JR. Does 5-bromo-2'-deoxyuridine (BrdU) disrupt cell proliferation and neuronal maturation in the adult rat hippocampus in vivo? Behav Brain Res. 2009;199:218–221. doi: 10.1016/j.bbr.2008.11.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kee N, Sivalingam S, Boonstra R, Wojtowicz JM. The utility of Ki-67 and BrdU as proliferative markers of adult neurogenesis. J Neurosci Methods. 2002;115:97–105. doi: 10.1016/s0165-0270(02)00007-9. [DOI] [PubMed] [Google Scholar]
- Kempermann G, Kuhn HG, Gage FH. Genetic influence on neurogenesis in the dentate gyrus of adult mice. Proc Natl Acad Sci USA. 1997;94:10409–10414. doi: 10.1073/pnas.94.19.10409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Key G, Petersen JL, Becker MH, Duchrow M, Schluter C, Askaa J, Gerdes J. New antiserum against Ki-67 antigen suitable for double immunostaining of paraffin wax sections. J Clin Pathol. 1993;46:1080–1084. doi: 10.1136/jcp.46.12.1080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kornack DR, Rakic P. Cell proliferation without neurogenesis in adult primate neocortex. Science. 2001;294:2127–2130. doi: 10.1126/science.1065467. [DOI] [PubMed] [Google Scholar]
- Kuhn HG, Dickinson-Anson H, Gage FH. Neurogenesis in the dentate gyrus of the adult rat: age-related decrease of neuronal progenitor proliferation. J Neurosci. 1996;16:2027–2033. doi: 10.1523/JNEUROSCI.16-06-02027.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhn HG, Cooper-Kuhn CM. Bromodeoxyuridine and the detection of neurogenesis. Curr Pharm Biotechnol. 2007;8:127–131. doi: 10.2174/138920107780906531. [DOI] [PubMed] [Google Scholar]
- Leuner B, Waddell J, Gould E, Shors TJ. Temporal discontiguity is neither necessary nor sufficient for learning-induced effects on adult neurogenesis. J Neurosci. 2006a;26:13437–13442. doi: 10.1523/JNEUROSCI.2781-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leuner B, Gould E, Shors TJ. Is there a link between adult neurogenesis and learning? Hippocampus. 2006b;16:216–224. doi: 10.1002/hipo.20153. [DOI] [PubMed] [Google Scholar]
- Leuner B, Kozorovitskiy Y, Gross CG, Gould E. Diminished adult neurogenesis in the marmoset brain precedes old age. Proc Natl Acad Sci USA. 2007;104:17169–17173. doi: 10.1073/pnas.0708228104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lind D, Franken S, Kappler J, Jankowski J, Schilling K. Characterization of the neuronal marker NeuN as a multiply phosphorylated antigen with discrete subcellular localization. J Neurosci Res. 2005;79:295–302. doi: 10.1002/jnr.20354. [DOI] [PubMed] [Google Scholar]
- Lindsey BW, Tropepe V. A comparative framework for understanding the biological principles of adult neurogenesis. Prog Neurobiol. 2006;80:281–307. doi: 10.1016/j.pneurobio.2006.11.007. [DOI] [PubMed] [Google Scholar]
- Malberg JE, Duman RS. Cell proliferation in adult hippocampus is decreased by inescapable stress: reversal by fluoxetine treatment. Neuropsychopharmacology. 2003;28:1562–1571. doi: 10.1038/sj.npp.1300234. [DOI] [PubMed] [Google Scholar]
- Mirescu C, Peters JD, Gould E. Early life experience alters response of adult neurogenesis to stress. Nat Neurosci. 2004;7:841–846. doi: 10.1038/nn1290. [DOI] [PubMed] [Google Scholar]
- Mirescu C, Gould E. Stress and adult neurogenesis. Hippocampus. 2006;16:233–238. doi: 10.1002/hipo.20155. [DOI] [PubMed] [Google Scholar]
- Mitra R, Sundlass K, Parker KJ, Schatzberg AF, Lyons DM. Social stress-related behavior affects hippocampal cell proliferation in mice. Physiol Behav. 2006;89:123–127. doi: 10.1016/j.physbeh.2006.05.047. [DOI] [PubMed] [Google Scholar]
- Mohapel P, Mundt-Petersen K, Brundin P, Frielingsdorf H. Working memory training decreases hippocampal neurogenesis. Neuroscience. 2006;142:609–613. doi: 10.1016/j.neuroscience.2006.07.033. [DOI] [PubMed] [Google Scholar]
- Ngwenya LB, Peters A, Rosene DL. Light and electron microscopic immunohistochemical detection of bromodeoxyuridine-labeled cells in the brain: different fixation and processing protocols. J Histochem Cytochem. 2005;53:821–832. doi: 10.1369/jhc.4A6605.2005. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. New York: Academic Press; 1998. [Google Scholar]
- Pereira AC, Huddleston DE, Brickman AM, Sosunov AA, Hen R, McKhann GM, Sloan R, Gage FH, Brown TR, Small SA. An in vivo correlate of exercise-induced neurogenesis in the adult dentate gyrus. Proc Natl Acad Sci USA. 2007;104:5638–5643. doi: 10.1073/pnas.0611721104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhodes KJ, Trimmer JS. Antibodies as valuable neuroscience research tools versus reagents of mass distraction. J Neurosci. 2006;26:8017–8020. doi: 10.1523/JNEUROSCI.2728-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saper CB. Any way you cut it: a new journal policy for the use of unbiased counting methods. J Comp Neurol. 1996;364:5. doi: 10.1002/(SICI)1096-9861(19960101)364:1<5::AID-CNE1>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- Saper CB, Sawchenko PE. Magic peptides, magic antibodies: guidelines for appropriate controls for immunohistochemistry. J Comp Neurol. 2003;465:161–163. doi: 10.1002/cne.10858. [DOI] [PubMed] [Google Scholar]
- Siwak-Tapp CT, Head E, Muggenburg BA, Milgram NW, Cotman CW. Neurogenesis decreases with age in the canine hippocampus and correlates with cognitive function. Neurobiol Learn Mem. 2007;88:249–259. doi: 10.1016/j.nlm.2007.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stranahan AM, Khalil D, Gould E. Social isolation delays the positive effects of running on adult neurogenesis. Nat Neurosci. 2006;9:526–533. doi: 10.1038/nn1668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang X, Falls DL, Li X, Lane T, Luskin MB. Antigen-retrieval procedure for bromodeoxyuridine immunolabeling with concurrent labeling of nuclear DNA and antigens damaged by HCl pretreatment. J Neurosci. 2007;27:5837–5844. doi: 10.1523/JNEUROSCI.5048-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas RM, Hotsenpiller G, Peterson DA. Acute psychosocial stress reduces cell survival in adult hippocampal neurogenesis without altering proliferation. J Neurosci. 2007;27:2734–2743. doi: 10.1523/JNEUROSCI.3849-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanderlaan M, Thomas CB. Characterization of monoclonal antibodies to bromodeoxyuridine. Cytometry. 1985;6:501–505. doi: 10.1002/cyto.990060603. [DOI] [PubMed] [Google Scholar]
- Vega CJ, Peterson DA. Stem cell proliferative history in tissue revealed by temporal halogenated thymidine analog discrimination. Nat Methods. 2005;2:167–169. doi: 10.1038/nmeth741. [DOI] [PubMed] [Google Scholar]
- West MJ, Slomianka L, Gundersen HJ. Unbiased stereological estimation of the total number of neurons in the subdivisions of the rat hippocampus using the optical fractionator. Anat Rec. 1991;231:482–497. doi: 10.1002/ar.1092310411. [DOI] [PubMed] [Google Scholar]
- Wojtowicz JM, Kee N. BrdU assay for neurogenesis in rodents. Nature Protoc. 2006;1:1399–1405. doi: 10.1038/nprot.2006.224. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.