Summary
Microtubule-binding proteins are conveniently divided into two large groups: MAPs (Microtubule-Associated Proteins), which can stabilize, anchor and/or nucleate microtubules, and motors, which use the energy of ATP hydrolysis for a variety of functions, including microtubule network organisation and cargo transportation along microtubules.
Here we describe the use of Taxol-stabilized microtubules for purification of MAPs, motors and their complexes from Xenopus egg extracts. Isolated proteins are analysed using SDS-gel electrophoresis and identified by various mass spectrometry and database mining technologies. Found proteins can be grouped into three classes: (i) known MAPs and motors; (ii) proteins previously reported as associated with the microtubule cytoskeleton, but without a clearly defined cytoskeletal function; (iii) proteins not yet described as having microtubule localization. Sequence-similarity methods employed for protein identification allow efficient identification of MAPs and motors from species with yet un-sequenced genomes.
Keywords: Animals; Brain; ultrastructure; Cattle; Electrophoresis, Polyacrylamide Gel; methods; Female; Mass Spectrometry; methods; Microtubule-Associated Proteins; chemistry; isolation & purification; Microtubules; chemistry; Oocytes; chemistry; Paclitaxel; chemistry; Tubulin; chemistry; Xenopus laevis
Keywords: tubulin, microtubule, microtubule-associated protein, MAP, motor, Xenopus, egg extracts, mass-spectrometry, proteomics.
1. Introduction
Microtubule cytoskeleton plays multiple roles both in interphase and in mitosis. Microtubules polymerize from α/β tubulin heterodimers (1, 2) and are organized in the cell by a number of accessory proteins, called motor proteins and MAPs (for “Microtubule-Associated Proteins”) (3, 4). Motor proteins, which are represented by the cytoplasmic dynein and the members of kinesin superfamily, use the energy of ATP hydrolysis for a variety of functions including to generate force to move along microtubules (5). The minimal definition of a MAP is a protein which can bind in vitro to microtubules, but more often by MAPs we understand proteins which also co-localize with microtubules in the cell (6), co-precipitate with microtubules (7) and/or affect microtubule polymerization dynamics (8, 9). Finally, many proteins which do not bind microtubules themselves, are tethered to them via MAPs (10) or motors, some of which are known to transport their cargos along microtubules (5). Both MAPs and motors can be purified on microtubules. Motors association with microtubules is ATP-sensitive, while MAPs can be usually eluted by salt. For simplicity, in this chapter we will call all the proteins eluted by ATP - “motors”, and those eluted by NaCl - “MAPs”.
Xenopus (Xenopus laevis) egg extracts are prepared from unfertilized eggs (11) and represent an abundant source of cytoskeletal proteins. Indeed, during the first 12 divisions after fertilization very little protein synthesis occurs and thus the egg has to supply most of the proteins needed for these rapid divisions. Freshly prepared egg extracts are in the M-phase of the cell cycle (“cytostatic factor-arrested”), but their status can be easily changed to interphase by addition of Ca2+ which triggers cyclin B destruction (12). This feature of egg extracts is extremely important for the studies of microtubule cytoskeleton as many accessory proteins are regulated by phosphorylation/dephosphorylation (13, 14) and/or through inhibition by importins during the interphase/M phase transition (15).
Here we describe methods to isolate and identify a number of proteins which bind to microtubules in Xenopus egg extracts. SDS-gel resolved proteins are identified using NanoLC MS/MS sequencing and database searching. Described methods can be applied to the isolation and identification of microtubule-binding proteins from other sources and model organisms. Of note, sequence-similarity searches make it possible to identify proteins from organisms from yet unsequenced genomes.
2. Materials and equipment
2.1. Xenopus egg extracts
Xenopus laevis females are from African Reptile Park, Tokai, South Africa. Pregnant mare serum gonadotropin (PMSG) and human chorionic gonadotropin (HCG) are from Sigma-Aldrich (corresponding Cat. No.: G4877 and CG-10, see Note 1).
Cytostatic-factor (CSF) - arrested Xenopus egg extracts are prepared as described in (16) with minor modifications. Extracts are snap-froze in liquid nitrogen in 200 μl aliquots in thin-walled PCR tubes followed by storage at −80°C. Prior to use, tubes with extracts are thawed under hot tap water and immediately put on ice.
Cytochalasin B is from Sigma-Aldrich (Cat. No. 30380)(see Note 2).
MMR buffer composition: NaCl 100 mM, KCl 2 mM, MgCl2 1 mM, CaCl2 2 mM, EDTA 0.1 mM, HEPES 5 mM, titrate to pH 7.8 with saturated solution of NaOH. Autoclave and store at room temperature (RT). This buffer can be also prepared as 20 × stock.
XB buffer composition: KCl 100 mM, MgCl2 1 mM, CaCl2 0.1 mM, HEPES 10 mM, Sucrose 50 mM, titrate to pH 7.7 with saturated solution of KOH. Autoclave and store at RT.
Dejelling buffer composition: 2% L-cystein (Fluka, Cat. No. 30089), EGTA 1 mM, titrate to pH 7.8 with saturated solution of NaOH.
“Proteases inhibitors cocktail” (PIs) contains leupeptine, aprotinine and pepstatine A (Euromedex, corresponding Cat. No.: SP-04-2217, A162-C and EI-9), make all together at 10 mg/ml in anhydrous DMSO and store at −20°C.
Xenopus sperm nuclei are prepared as described in Murray (17), frozen in liquid nitrogen in 10 μl aliquots and stored at −80°C.
Fix solution composition: 11% formaldehyde, 50% glycerol and Hoechst 33342 or 33258 at 10 μg/ml in MMR buffer.
Rhodamin-labelled tubulin is prepared as described in Hyman et al. (18).
2.2. MAPs and Motors purification
Cow brain tubulin is prepared as described in Castoldi and Popov (19) and stored at −80°C.
Taxol (Molecular Probes, Cat. No. P-3456) is dissolved in DMSO (Sigma-Aldrich, Cat. No. 41648) at 20 mM and stored at −20°C (see Note 3).
GTP (Roche, Cat. No. 106356) is prepared as 200 mM in water and stored at −20°C in 200 μl aliquots. ATP (Roche, Cat. No. 127531) is prepared as 300 mM in BRB80 (see below) and stored in 200 μl aliquots at −20°C. AMP-PNP (5′adenylylimidodiphosphate) is from Biochemika (Cat. No. 01910).
Brinkley Renaturing Buffer 80 (BRB80) (20), composition: Na-PIPES 80 mM, EGTA 1mM, MgCl2 1 mM, DTT 1mM, titrate to pH 7.8 with saturated solution of NaOH. BRB80 is prepared and stored until use as 5× stock solution.
BRB80 washing buffer composition: Na-PIPES 80 mM, EGTA 1mM, MgCl2 1 mM, DTT 1mM, 20 μM Taxol, 1mM GTP, titrated to pH 7.8 with saturated solution of NaOH.
All centrifugation procedures are carried out in the Optima TL100 tabletop centrifuge (Beckman).
2.3. SDS-PAGE
SDS-polyacrylamide gel electrophoresis (SDS-PAGE) is performed in the SE 400 apparatus (Hoefer Scientific Instruments; San Francisco, CA) according to manufacturer’s instructions or in an equivalent model. For more information on SDS-electrophoresis, see in Ausubel et al (21).
Isoelectrofocusing is performed using the Pharmacia system Multiphor II according to manufacturer’s instructions.
2D SDS-PAGE is performed using Bio-Rad Protean II xi Cell system according to manufacturer’s instructions.
2.4. Mass spectrometry
Cleland’s reagent (Dithiothreitol, DTT) is from Merck (Cat. No. 111474), iodoacetamide (Cat. No. I-6125), NH4HCO3 (Cat. No. A-6141) and acetonitrile are from Sigma-Aldrich.
Modified pig trypsin (Trypsin Gold) is from Promega (Cat. No. V5280).
HPLC solvents (Lichrosolv®) (H2O: cat. No. 1.15333, acetonitrile: cat. No. 1.00029), formic (Cat. No 1.00264) and trifluoroacetic (Cat. No 1.08262) acids are from Merck.
NanoLC set-up consisted of a FAMOS autosampler, a SWITCHOS column switching module and an ULTIMATE Plus pump (Dionex).
C18 PepMAP100 (1 mm × 300 μm ID, 5 μm) (Dionex) is used as a trap column and C18 PepMAP100 (15 cm × 75 μm ID, 3 μm) (Dionex) as an analytical column.
LTQ linear trap mass spectrometer (ThermoElectron Corp.) interfaced to the nanoLC system (2.4.5) via a dynamic nanospray probe with a silicatip™ uncoated needle (20 μm ID, 10 μm tip ID (Cat. No. FS360-20-10-N-20-C12) (New Objective).
3. Methods
3.1. Xenopus egg extract preparation
CSF-arrested Xenopus egg extracts are prepared according to Desai et al. (16). To induce egg maturation, three days before preparation eight frogs are injected subcutaneously with 100 Units of PMSG each. PMSG-”primed” animals can be used for laying eggs up to two weeks after PMSG injection. The day before extract preparation, frogs are injected with 500 Units of hCG each and are kept individually in 500 ml MMR in small plastic containers in a 16°C incubator. Under these conditions, frogs lay eggs 16–18 hours following hCG injection.
Collected eggs are washed with 800 ml of MMR to remove as much debris as possible (see Note 4). As much as 500 ml of dejelling buffer is added to eggs for a period of time between 5 to 7 minutes (see Note 5). Upon dejelling, eggs form a more compact mass. Dejelling buffer is then discarded and eggs are washed first with 200 ml of MMR, followed by four washes with XB buffer (prepare 500 ml). Finally eggs are washed four more times with CSF-XB buffer (prepare 250 ml). Last CSF-XB wash solution is supplemented with PIs at 0.01μg/ml (dilute 1:1000). After discarding the last wash solution, eggs are left in a small volume (~ 5 ml) of CSF-XB/PIs.
Dejelled and washed eggs are transferred into Ultra-clear centrifuges tubes (Beckman, Cat. No. 344057) using a wide bore polyethylene pipette (Sigma-Aldrich Cat. No. Z350796). Take care to remove as much buffer as possible from the top of the tube. Tubes with eggs are transferred into polypropylene tubes (Greiner, Cat. No. 187262, 18 × 95 mm) containing 0.5 ml of CSF-XB buffer and are then centrifuged at 800 rpm for 1 minute, followed by 30 seconds at 1500 rpm in a swinging bucket rotor centrifuge (type Eppendorf 5804 or Beckman SPINCHRON® Series). At this stage eggs should be densely packed in the tube but should not be lysed. Excess of buffer is removed from the top of the tube.
Eggs are crushed by centrifugation at 14000 g (12000 rpm) in a JS-13.1 rotor (Beckman) during 16 minutes at 4°C.
After centrifugation, tubes are transferred on ice. At this stage three distinct layers should be visible. The light yellow layer on top contains lipids and the dark layer on the bottom contains yolk and pigments. The cytoplasmic layer in the middle is called “CSF-arrested egg extract”. To collect this fraction the tube is punctured with an 18-gauge needle and the extract is aspired using a 2 ml syringe. Extract is then supplemented with PIs at 0.01μg/ml final concentration and stored on ice until use or is frozen for later use.
Upon addition of sperm nuclei, CSF-arrested egg extracts should be able to assemble half spindles and eventually bipolar spindles (see Note 6). To check the quality of extract, 20 μl is supplemented with 1 μl of sperm nuclei (1–5 × 107/ml) and 0.2–0.5 μl of Rhodamin-tubulin (the correct amount is determined empirically) (18). After 30 to 60 minutes incubation, 1 μl of the reaction is mixed with 2 μl of the formaldehyde Fix solution on a microscope slide, covered with a 18×18 mm coverslip and the presence of spindles is verified by fluorescence microscopy.
3.2. Preparation of Taxol-stabilized microtubules
Microtubules are polymerized in a 500 μl tubulin solution at 50 μM (5 mg/ml) in BRB80 supplemented with 1 mM GTP at 37°C during 30 minutes. Polymerized microtubules are supplemented with 10 μM Taxol and incubated for 10 minutes at 37°C (see Note 3 and 7).
Polymerized microtubules are then pelleted by centrifugation at 103000 g (50000 rpm) for 14 minutes at 20°C in the TLA100.3 rotor. Supernatant is discarded and microtubules are resuspended in 500 μl of BRB80 with 10 μM Taxol. Microtubule suspension is stored at RT and used on the same day.
3.3. Purification of motors and MAPs
As much as 4 ml of freshly prepared (or thawed) CSF-extract are used for purification. Extract is diluted in 2 volumes (8 ml) of BRB80 at 4°C and clarified by two successive 15 min centrifugations at 83000 g (45000 rpm) in a Beckman TLA100.3 rotor at 4°C through a 1 ml cushion of BRB80 buffer containing 40 % glycerol (see Note 8).
To bind MAPs and motors to microtubules, the clarified extract is pre-warmed in a water-bath at 20°C. Taxol-stabilized microtubules in suspension (500 μl, prepared as described above) is added to the clarified CSF-extract in the presence of 1 mM GTP and 1.5 mM AMP-PNP and the mixture is incubated at 20°C for 10 minutes (see Note 9 and 10).
The microtubules/extract solution is overlaid onto 1 ml cushion of BRB80 buffer containing 40 % glycerol and 10 μM Taxol and centrifuged for 10 minutes at 83000 g (45000 rpm) in a Beckman TLA100.3 rotor at 20°C.
Microtubule pellet containing MAPs and motors is resuspended in 3 ml of BRB80 washing buffer and centrifuged for 10 minutes at 83000 g (45000 rpm) in a Beckman TLA100.3 rotor at 20°C.
Repeat step 4 two more times.
The final pellet is resuspended in 1 ml of washing buffer containing 10 mM ATP and incubated for 10 minutes at 20°C. This step allows eluting motor proteins. After incubation, microtubules are pelleted for 10 min at 103000 g (50000 rpm) in a Beckman TLA100.3 rotor at 20°C and the supernatant containing eluted proteins (“motor proteins fraction”) is immediately transferred on ice.
Repeat step 6. Pool together both elution fractions from steps 6 and 7.
The remaining microtubule pellet is resuspended in 1 ml of washing buffer containing 0.5 M NaCl (add 1/10 v/v of 5M NaCl in H2O) and incubated for 10 minutes at 20°C. This step allows eluting MAPs and all other proteins sensitive to higher ionic strength (see Note 11). After incubation, microtubules are pelleted by centrifugation for 10 min at 103000 g (50000 rpm) in a Beckman TLA100.3 rotor at 20°C and the supernatant containing eluted proteins (“MAPs fraction”) is transferred on ice.
Both supernatants from steps 6/7 and 8 are then concentrated using a 0.5 ml concentrator with a 10.000 MWCO cut-off polyethersulfone membrane (Vivaspin, Cat. No. VS0101) to a volume of 50 μl. After this step, the motor protein fraction is ready for analysis by electrophoresis. The MAPs fraction at this stage contains 0.5 M NaCl that could perturb proteins migration on the acrylamide gel. MAPs fraction is thus diluted in water 10 times (by addition of 450 μl H2O) in order to reduce salt content to approximately 50 mM NaCl and concentrated one more time using Vivaspin 0.5 ml concentrator as described above. The MAPs fraction is now ready for analysis by electrophoresis. All steps of purification are schematically shown in Figure 1.
Figure 1.
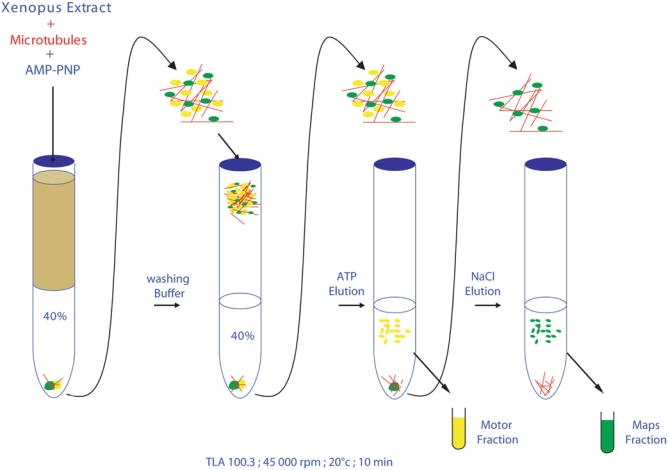
Schematic view of motors and MAPs purification
3.4. Proteins analysis on SDS-Polyacrylamide gel electrophoresis
3.4.1. D-SDS electrophoresis gel profile
Motors and MAPs fraction are loaded on a 6 to 18 % gradient electrophoresis gel on a vertical slab gel at 25mA/gel at 4°C.
After migration (until the front reached the bottom of the gel), the gels are stained with Coomassie Blue (see Note 12). Analysis of this gel is shown in Figure 2 (see Note 13).
Figure 2.
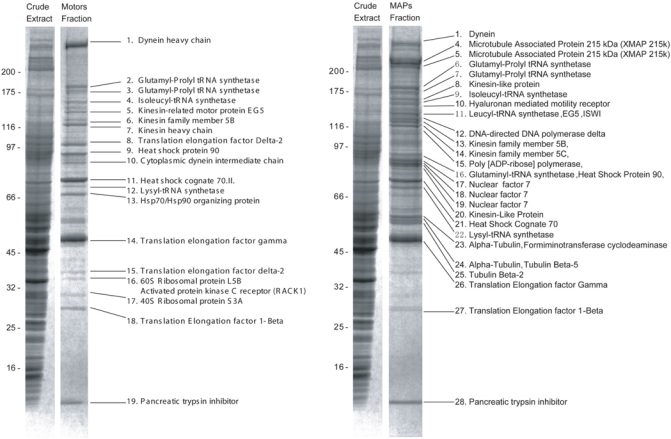
Analysis of proteins on a 1D SDS-electrophoresis gel (from Liska et al (32); courtesy of Proteomics).
3.4.2. 2D-SDS electrophoresis gel profile
Two-dimensional (2D) electrophoresis is performed with immobilised pH gradients for isoelectric focusing. Home-made linear 3–10.5 gradients are used (22) and prepared according to published procedures (23). IPG strips are cut with a paper cutter, and rehydrated in 7 M urea, 2 M thiourea, 4% CHAPS, 0.4% carrier ampholytes (3–10 range) and 5mM Tris cyanoethyl phosphine (Molecular Probes, Cat. No T6052) for 3–10.5 gradients (24). The protein sample is cup-loaded at the anode. Isoelectric focusing is carried out for a total of 60000 Vh (see Note 14).
After focusing, the strips are equilibrated for 2 × 10 minutes in 6 M urea, 2% SDS, 125 mM Tris-HCl pH 7.5 containing either 50mM DTT (first equilibration step) or 150mM iodoacetamide (second equilibration step). The equilibrated strip is loaded on the top of a 10% polyacrylamide gel, and submitted to SDS PAGE (10% gel) at 12W/gel (25).
After migration, the gels are stained with colloidal Coomassie Blue (26) (see Note 15). Analysis of this gel is shown in Figure 3.
Figure 3.
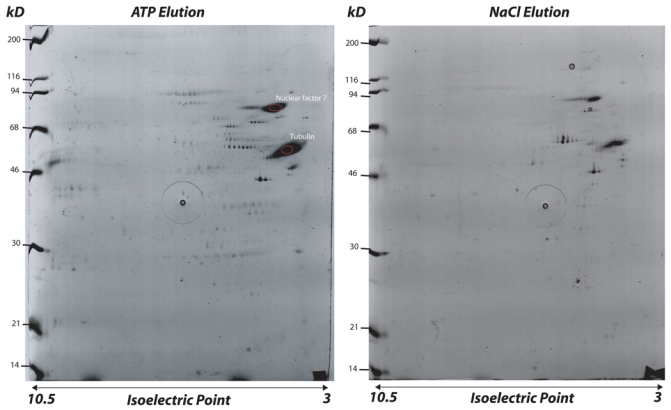
Analysis of proteins on a 2D SDS-electrophoresis gel: motors fraction (ATP elution) and MAPs fraction (NaCl elution).
3.5. Mass spectrometry analysis of proteins resolved on SDS-electrophoresis gels
3.5.1. In-gel digestion of proteins bands
Coomassie Blue-stained bands (spots) of interest are excised from 1D or 2D gels and digested in-gel as described in (27, 28) (see Note 16).
Briefly, gel pieces are cut in ca. 1 mm × 1mm cubes and dehydrated with acetonitrile. Proteins are reduced with 10 mM DTT in 100 mM ammonium bicarbonate at 56°C and alkylated with 55 mM iodoacetamide. After washing with 100 mM ammonium bicarbonate and dehydration with acetonitrile, a sufficient volume of digestion buffer (12.5 ng/μl of trypsin in 40 mM NH4HCO3/10 % acetonitrile) is added to cover the gel pieces. Samples are first incubated 2h at 4°C, and the digestion is then performed overnight at 37°C, after addition of more buffer if necessary (see Note 17).
After digestion, peptides are extracted, successively, with 50 μl (equal to 1–2 times the volume of gel particles) of acetonitrile and 100 μl of acetonitrile: 5% formic acid (50:50). The extracts are pooled together, dried down in a vacuum centrifuge and stored at −20°C (see Note 18).
3.5.2. NanoLC MS/MS sequencing
Dried samples are re-dissolved in 15–25 μL of 0.05 % trifluoroacetic acid (TFA) and 4 μL are loaded onto the trap column in 0.05 % TFA at the flow rate of 20 μL/min (see Note 19). After 4 min of loading and washing, peptides are eluted and separated on the analytical column at the flow rate of 200 nL/min with the following gradient: from 5 to 20 % of solvent B in 20 min, 20 to 50 % B in 16 min, 50 to 100 % B in 5 min, 100 % B during 10 min, and back to 5 % B in 5 min. Solvent A: 95:5 H2O:acetonitrile (v/v) with 0.1 % formic acid (v/v); solvent B: 20:80 H2O:acetonitrile (v/v) with 0.1 % formic acid (B).
The eluted peptides are introduced into the mass spectrometer via a nanospray needle at the voltage of 1.8 kV, and the capillary transfer temperature is set at 200°C. The analysis is performed in data-dependent acquisition mode powered by Xcalibur 1.4 software (ThermoElectron Corp.). The acquisition cycle consists of a survey scan covering the range of m/z 350–1500 followed by the consecutive acquisition of four MS/MS spectra from the most abundant precursor ions at the relative collision energy 35 %, isolation width 4.0, in 3 microscans with maximum ion injection time of 100 ms. The m/z of fragmented precursor ions are dynamically excluded for further 60 s, but otherwise no pre-defined exclusion lists is applied. Individual MS/MS spectra are exported into dta files by BioWorks 3.1 software from the same company
3.6. Bioinformatic tools for protein identification
Identification by Mascot software. For protein identification, dta files representing individual tandem mass spectra are converted into a single mgf- file and submitted to database searches using Mascot software version 2.1 (Matrix Science Ltd) installed on a local server. Typical database searching settings: mass tolerance for precursor and fragment ions: 2.0 and 0.5 Da respectively; instrument profile: ESI-Trap; database: MSDB; fixed modification: carbamidomethyl (cysteine); variable modification: oxidation (methionine). Protein identified with at least two peptides and a Mascot score > 100 are considered as significant hits.
Protein identification by MS BLAST. Selected dta files are interpreted de novo using appropriate software, such as DeNovoX (ThermoElectron Corporation) or PepNovo (29). The interpretation of each dta file results in a few peptide sequence proposals. The degenerate, redundant, and partially inaccurate and incomplete candidate sequences obtained by the interpretation of all selected dta files are assembled into a single query for MS BLAST (30) search as was described in great detail in (31),(28). The string can contain several thousands of peptide sequences assembled in arbitrary order. The string is then submitted to MS BLAST search at the servers at EMBL, Heidelberg URL http://dove.embl-heidelberg.de/Blast2/msblast.html or at Brigham and Women’s Hospital, Boston URL http://genetics.bwh.harvard.edu/msblast/. The statistical confidence of hits is evaluated and hits sorted according to MS BLAST scoring scheme (31). In this way it is possible to identify Xenopus proteins that are not present in a database by their similarity to available protein sequences from other species (32).
4. Notes
Pregnant mare serum gonadotropin (PMSG) and human chorionic gonadotropin (HCG) can be acquired from any other provider but has to be checked for efficiency.
Cytochalasin B (CB) is an inhibitor of actin polymerisation. Use of CB in Xenopus egg extract allows avoiding the contamination of microtubules with actin and actin-binding proteins (33).
Taxol is a potent microtubule-stabilizing agent (34). Taxol quality has to be tested. We observed that poor quality Taxol leads to partial microtubules depolymerization. This, in turn, decreases the yield of purified on microtubules proteins and results in the excessive contamination of the eluted proteins with tubulin dimers. We dilute Taxol in anhydrous DMSO, aliquot it in 10–50 μl and store at −20°C. Once thawed, aliquots of Taxol are either used up or discarded.
Eggs quality: egg quality is more important than egg quantity. Check and avoid lysed eggs or “activated” eggs (white eggs).
During and after dejelling, eggs become progressively more and more fragile and lyse easily if treated roughly. During and after this step, eggs must be manipulated carefully.
Extract: fresh or thawed? Before freezing extracts, we routinely test them for their competence to assemble spindles as described above. Only extracts that can assemble spindles are considered to be in the M-phase. Extracts that contain long microtubules not associated with sperm nuclei and/or decondensed DNA (round nuclei) are considered to be in “interphase” and are discarded. Freezing extracts considerably reduces their capacity to form bipolar spindles, but MAPs and motors can be purified from both freshly prepared and frozen extracts. We did not notice significant differences in the electrophoresis spectra of proteins isolated from fresh or thawed extracts (although we cannot exclude this for some proteins). Frozen extracts offer the advantage or knowing exactly the amount of extract available for purification, which is difficult to predict when starting with freshly laid eggs. Moreover, extract preparation and testing takes time, while thawing extracts allows starting the purification in the morning.
Tubulin quality is important as poor quality tubulin does not assemble well into microtubules. Usually about 70% of tubulin of freshly thawed tubulin should be able to assemble into microtubules.
Before centrifugation through glycerol cushion, mark the top of the cushion on the tube to visualize the border between cushion and extract after centrifugation. Extract is poured carefully along the tube wall on the top of the cushion to avoid mixing with 40% glycerol.
To scale up or down the purification procedure it is important to keep the amount of microtubules constant in respect to MAPs and motors which are to be purified on them. Generally speaking, microtubules must be in excess avoid competition between the proteins for binding sites on microtubules.
The non-hydrolysable analogue of ATP, AMP-PNP was previously shown to stabilize motors interaction with microtubules (35). The use of the reagent significantly increases the yield of proteins whose association with microtubules is ATP-sensitive.
At 0.5 M NaCl, there is a slight depolymerization of microtubules. This concentration is a compromise between the goal to elute all MAPs and keep microtubules intact.
For scanning, we use an UMAX Powerlook 1120 scanner. We suggest scanning the gel at a resolution of at least 600 dpi.
Analysis of identified proteins shows that many of them are already known motors (dynein, eg5, kinesin 5B, etc.) or MAPs (XMAP215, XNF7, RHAMM, etc.), other proteins like HSP90 or poly(ADP-ribose) polymerase (PARP) were previously shown to have a microtubule localization. Lastly, a number of identified proteins without a known association with microtubules should be handled with care because they could be genuine contaminants or yet unknown microtubule cytoskeleton-associated proteins.
Large proteins (with molecular mass over 120 kD) do not enter the isoelectric focusing gel. This represents a serious limitation of the 2-D gel analysis, especially evident for MAPs and motors, many of which are rather large proteins. Therefore, electrophoretic analysis of isolated proteins is a compromise between the high-resolution of the 2-D gels and the desire to have as many proteins as possible resolved on a single gel (1-D gel).
Handling of gels intended for mass-spectrometry analysis: plates for gels are washed using deionized water and stored in a clean, dust-free environment. Acrylamide solutions are filtered through 20 μm filter before pouring.
For cutting out protein spots and bands, we place the gels on a clean transluminator table and use a clean scalpel blade. It is not necessary to use a new blade for each band, but we wipe the blade clean after each band using an ethanol-wetted paper towel.
As plastic tubes accumulate static charges, they attract dust (a major source of keratin contamination!). To avoid contamination during sample preparation and digestion, we work in a laminar flow hood with gloves that are frequently rinsed with deionized water, and we use tubes stored in a clean, dust-free environment.
Centrifugation of pooled peptide extracts is recommended to eliminate eventual remaining gel particles.
Cross-contamination of samples: based on staining intensity, appropriate dilution and injection order should be carried out to avoid cross-contamination by column memory effect in LC-MS/MS analyses.
Acknowledgments
Research in the group of A.P. is funded by the “Avenir” grant of Inserm, ACI BCMS of the French Research Ministry, grant “Emergence” of the Department of Rhône-Alpes, “La Ligue contre le Cancer” (Comité de l’Isère) and « Association pour la Recherche sur le Cancer ».
References
- 1.Borisy GG, Taylor EW. The mechanism of action of colchicine. Colchicine binding to sea urchin eggs and the mitotic apparatus. J Cell Biol. 1967;34:535–48. doi: 10.1083/jcb.34.2.535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Borisy GG, Taylor EW. The mechanism of action of colchicine. Binding of colchincine-3H to cellular protein. J Cell Biol. 1967;34:525–33. doi: 10.1083/jcb.34.2.525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sloboda RD, Rudolph SA, Rosenbaum JL, Greengard P. Cyclic AMP-dependent endogenous phosphorylation of a microtubule-associated protein. Proc Natl Acad Sci U S A. 1975;72:177–81. doi: 10.1073/pnas.72.1.177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Weingarten MD, Lockwood AH, Hwo SY, Kirschner MW. A protein factor essential for microtubule assembly. Proc Natl Acad Sci U S A. 1975;72:1858–62. doi: 10.1073/pnas.72.5.1858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hirokawa N, Noda Y, Okada Y. Kinesin and dynein superfamily proteins in organelle transport and cell division. Curr Opin Cell Biol. 1998;10:60–73. doi: 10.1016/s0955-0674(98)80087-2. [DOI] [PubMed] [Google Scholar]
- 6.Morejohn LC. Microtubule Binding Proteins Are Not Necessarily Microtubule-Associated Proteins. Plant Cell. 1994;6:1696–99. doi: 10.1105/tpc.6.12.1696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dustin P. Microtubules. Sci Am. 1980;243:66–76. doi: 10.1038/scientificamerican0880-66. [DOI] [PubMed] [Google Scholar]
- 8.Hirokawa N. Microtubule organization and dynamics dependent on microtubule-associated proteins. Curr Opin Cell Biol. 1994;6:74–81. doi: 10.1016/0955-0674(94)90119-8. [DOI] [PubMed] [Google Scholar]
- 9.Cassimeris L, Spittle C. Regulation of microtubule-associated proteins. Int Rev Cytol. 2001;210:163–226. doi: 10.1016/s0074-7696(01)10006-9. [DOI] [PubMed] [Google Scholar]
- 10.Ookata K, Hisanaga S, Bulinski JC, Murofushi H, Aizawa H, Itoh TJ, et al. Cyclin B interaction with microtubule-associated protein 4 (MAP4) targets p34cdc2 kinase to microtubules and is a potential regulator of M-phase microtubule dynamics. J Cell Biol. 1995;128:849–62. doi: 10.1083/jcb.128.5.849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lohka MJ, Masui Y. Formation in vitro of sperm pronuclei and mitotic chromosomes induced by amphibian ooplasmic components. Science. 1983;220:719–21. doi: 10.1126/science.6601299. [DOI] [PubMed] [Google Scholar]
- 12.Murray AW, Kirschner MW. Cyclin synthesis drives the early embryonic cell cycle. Nature. 1989;339:275–80. doi: 10.1038/339275a0. [DOI] [PubMed] [Google Scholar]
- 13.Andersen SS. Xenopus interphase and mitotic microtubule-associated proteins differentially suppress microtubule dynamics in vitro. Cell Motil Cytoskeleton. 1998;41:202–13. doi: 10.1002/(SICI)1097-0169(1998)41:3<202::AID-CM2>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- 14.Andersen SSL. Balanced regulation of microtubule dynamics during the cell cycle: a contemporary view. BioEssays. 1999;21:53–60. doi: 10.1002/(SICI)1521-1878(199901)21:1<53::AID-BIES7>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- 15.Nachury MV, Maresca TJ, Salmon WC, Waterman-Storer CM, Heald R, Weis K. Importin beta is a mitotic target of the small GTPase Ran in spindle assembly. Cell. 2001;104:95–106. doi: 10.1016/s0092-8674(01)00194-5. [DOI] [PubMed] [Google Scholar]
- 16.Desai A, Murray A, Mitchison TJ, Walczak CE. The use of Xenopus egg extracts to study mitotic spindle assembly and function in vitro. Methods Cell Biol. 1999;61:385–412. doi: 10.1016/s0091-679x(08)61991-3. [DOI] [PubMed] [Google Scholar]
- 17.Murray AW. Cell cycle extracts. Methods Cell Biol. 1991;36:581–605. [PubMed] [Google Scholar]
- 18.Hyman A, Drechsel D, Kellogg D, Salser S, Sawin K, Steffen P, et al. Preparation of modified tubulins. Methods Enzymol. 1991;196:478–85. doi: 10.1016/0076-6879(91)96041-o. [DOI] [PubMed] [Google Scholar]
- 19.Castoldi M, Popov AV. Purification of brain tubulin through two cycles of polymerization-depolymerization in a high-molarity buffer. Protein Expr Purif. 2003;32:83–8. doi: 10.1016/S1046-5928(03)00218-3. [DOI] [PubMed] [Google Scholar]
- 20.Brinkley BR. Microtubule organizing centers. Annu Rev Cell Biol. 1985;1:145–72. doi: 10.1146/annurev.cb.01.110185.001045. [DOI] [PubMed] [Google Scholar]
- 21.Ausubel FM, Brent R, Kingston RE, Moore DD, Seidman JG, Smith JA, et al. Current Protocols In Molecular Biology 2005 [Google Scholar]
- 22.Gianazza E, Celentano F, Magenes S, Ettori C, Righetti PG. Formulations for immobilized pH gradients including pH extremes. Electrophoresis. 1989;10:806–8. doi: 10.1002/elps.1150101115. [DOI] [PubMed] [Google Scholar]
- 23.Rabilloud T, Valette C, Lawrence JJ. Sample application by in-gel rehydration improves the resolution of two-dimensional electrophoresis with immobilized pH gradients in the first dimension. Electrophoresis. 1994;15:1552–8. doi: 10.1002/elps.11501501223. [DOI] [PubMed] [Google Scholar]
- 24.Rabilloud T, Adessi C, Giraudel A, Lunardi J. Improvement of the solubilization of proteins in two-dimensional electrophoresis with immobilized pH gradients. Electrophoresis. 1997;18:307–16. doi: 10.1002/elps.1150180303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tastet C, Lescuyer P, Diemer H, Luche S, van Dorsselaer A, Rabilloud T. A versatile electrophoresis system for the analysis of high- and low-molecular-weight proteins. Electrophoresis. 2003;24:1787–94. doi: 10.1002/elps.200305400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Neuhoff V, Arold N, Taube D, Ehrhardt W. Improved staining of proteins in polyacrylamide gels including isoelectric focusing gels with clear background at nanogram sensitivity using Coomassie Brilliant Blue G-250 and R-250. Electrophoresis. 1988;9:255–62. doi: 10.1002/elps.1150090603. [DOI] [PubMed] [Google Scholar]
- 27.Shevchenko A, Wilm M, Vorm O, Mann M. Mass spectrometric sequencing of proteins silver-stained polyacrylamide gels. Anal Chem. 1996;68:850–8. doi: 10.1021/ac950914h. [DOI] [PubMed] [Google Scholar]
- 28.Shevchenko A, Sunyaev S, Liska A, Bork P, Shevchenko A. Nanoelectrospray tandem mass spectrometry and sequence similarity searching for identification of proteins from organisms with unknown genomes. Methods Mol Biol. 2003;211:221–34. doi: 10.1385/1-59259-342-9:221. [DOI] [PubMed] [Google Scholar]
- 29.Frank A, Pevzner P. PepNovo: de novo peptide sequencing via probabilistic network modeling. Anal Chem. 2005;77:964–73. doi: 10.1021/ac048788h. [DOI] [PubMed] [Google Scholar]
- 30.Shevchenko A, Sunyaev S, Loboda A, Shevchenko A, Bork P, Ens W, et al. Charting the proteomes of organisms with unsequenced genomes by MALDI-quadrupole time-of-flight mass spectrometry and BLAST homology searching. Anal Chem. 2001;73:1917–26. doi: 10.1021/ac0013709. [DOI] [PubMed] [Google Scholar]
- 31.Habermann B, Oegema J, Sunyaev S, Shevchenko A. The power and the limitations of cross-species protein identification by mass spectrometry-driven sequence similarity searches. Mol Cell Proteomics. 2004;3:238–49. doi: 10.1074/mcp.M300073-MCP200. [DOI] [PubMed] [Google Scholar]
- 32.Liska AJ, Popov AV, Sunyaev S, Coughlin P, Habermann B, Shevchenko A, et al. Homology-based functional proteomics by mass spectrometry: application to the Xenopus microtubule-associated proteome. Proteomics. 2004;4:2707–21. doi: 10.1002/pmic.200300813. [DOI] [PubMed] [Google Scholar]
- 33.Spudich JA, Lin S. Cytochalasin B, its interaction with actin and actomyosin from muscle (cell movement-microfilaments-rabbit striated muscle) Proc Natl Acad Sci U S A. 1972;69:442–6. doi: 10.1073/pnas.69.2.442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Schiff PB, Fant J, Horwitz SB. Promotion of microtubule assembly in vitro by taxol. Nature. 1979;277:665–7. doi: 10.1038/277665a0. [DOI] [PubMed] [Google Scholar]
- 35.Brady ST, Lasek RJ. Adenylyl imidodiphosphate (AMPPNP), a nonhydrolyzable analogue of ATP, produces a stable intermediate in the motility cycle of fast axonal transport. Biol Bull. 1984;167:503. [Google Scholar]


