Abstract
The JNK-interacting proteins, JIP3 and JIP4, are specific effectors of the small GTP-binding protein ARF6. The interaction of ARF6–GTP with the second leucine zipper (LZII) domains of JIP3/JIP4 regulates the binding of JIPs to kinesin-1 and dynactin. Here, we report the crystal structure of ARF6–GTP bound to the JIP4-LZII at 1.9 Å resolution. The complex is a heterotetramer with dyad symmetry arranged in an ARF6–(JIP4)2–ARF6 configuration. Comparison of the ARF6–JIP4 interface with the equivalent region of ARF1 shows the structural basis of JIP4's specificity for ARF6. Using site-directed mutagenesis and surface plasmon resonance, we further show that non-conserved residues at the switch region borders are the key structural determinants of JIP4 specificity. A structure-derived model of the association of the ARF6–JIP3/JIP4 complex with membranes shows that the JIP4-LZII coiled-coil should lie along the membrane to prevent steric hindrances, resulting in only one ARF6 molecule bound. Such a heterotrimeric complex gives insights to better understand the ARF6-mediated motor switch regulatory function.
Keywords: ARF6, effector specificity, heterotetramer, JIP4, leucine zipper
Introduction
ADP-ribosylation factors (Arfs) are small, ubiquitously expressed GTP-binding proteins that regulate membrane trafficking and the organization of the actin cytoskeleton (D'Souza-Schorey and Chavrier, 2006; Myers and Casanova, 2008). The Arf proteins comprise three different subfamilies: the Arfs, Arf-like proteins (Arls) and SARs (Kahn et al, 2006). In mammals, the Arf subfamily falls into three classes based on their sequence similarity: class I contains ARF1, ARF2 and ARF3; class II contains ARF4 and ARF5; and class III contains only ARF6. ARF6 and ARF1 are the least similar in amino-acid sequence, although they are 84% similar. The class I and class II Arfs are mainly involved in the secretory pathway, although they also function in endosomal compartments. By contrast, ARF6 is located in the plasma membrane and endosomal compartments where it regulates the traffic of plasma membrane receptors and lipids through the endocytic pathway.
To carry out their cellular functions, Arf proteins interact with various effectors that differ in nature and structure. As the effectors recognize the activated, GTP-bound forms of Arf proteins, we expect the primary binding site for the effectors to overlap the switch regions, that is, the switch I, interswitch and switch II domains of the Arf proteins that undergo major conformational changes on binding GDP/GTP. The crystal structures of ARF1 and ARF6 have shown that their switch regions have quite different conformations in the GDP-bound state, however, their GTP-bound structures are very similar (Ménétrey et al, 2000; Pasqualato et al, 2001). This raises the question about the structural basis of specificity of their interactions with downstream effectors. Although the majority of Arf effectors identified to date interact with more than one Arf protein in vitro (D'Souza-Schorey and Chavrier, 2006), some seem to distinguish between distinct Arf-subfamily members. For example, the FIP3/FIP4 proteins (Rab11 family-interacting proteins 3 and 4, also known as Arfophilin1 and Arfophilin2) interact specifically with ARF6 (Schonteich et al, 2007).
Recently, we identified a new family of ARF6-specific effectors: the mammalian c-Jun N-terminal kinase (JNK)-interacting proteins, JIP3 and JIP4, as well as their Drosophila orthologue, Sunday Driver (SYD) (Montagnac et al, 2009). These highly related proteins act as scaffolding factors for JNK/mitogen-activated protein kinase (MAPK) signalling modules, as well as binding partners of kinesin-1 and the dynein–dynactin complex, two molecular motors that migrate, respectively, toward the plus and minus ends of microtubules (Bowman et al, 2000; Cavalli et al, 2005). We have shown that ARF6–GTP binds to the second leucine zipper (LZII) domain of JIP3/4 and that this interaction interferes with its interaction with kinesin-1, whereas it favours its interaction with the dynactin complex. We have further shown that during cytokinesis, ARF6, JIP4, kinesin-1 and the dynactin complex control the trafficking of recycling endosomes in a mechanism necessary for abscission. Specific uncoupling of ARF6 from JIP4 has been observed using a mutant ARF6 (ARF6iSW) obtained by swapping the interswitch region between ARF6 and ARF1, showing that JIP4 makes critical interaction with the interswitch of ARF6. Expression of ARF6iSW leads to cytokinesis defects similar to those observed when ARF6 or JIP function was inhibited, showing that these proteins work together during abscission (Montagnac et al, 2009). Together, these findings showed a new function for ARF6 as a possible regulatory switch for microtubule-dependent molecular motors that work in opposing directions (Montagnac et al, 2009).
To investigate the structural basis of JIP3/4's specificity for ARF6, we determined the crystal structure of ARF6–GTP in a complex with the LZII domain of JIP4 at a resolution of 1.93 Å. The structure, we report here, shows that JIP4 LZII is a long and straight parallel coiled-coil structure that recruits two ARF6 molecules at its centre in dyad symmetry (2:2 stoichiometry). We also determined the stoichiometry of the ARF6–JIP4 complex in solution by analytical ultracentrifugation. We used these structural data to predict the key structural determinants that confer the specificity of JIP3/JIP4 for binding to ARF6-GTP, and tested our predictions by site-directed mutagenesis and surface plasmon resonance assays. Finally, by modelling the complex structure bound to a membrane, we propose that JIP4 interacts with only one membrane-anchored ARF6 molecule, showing an unexpected 1:2 stoichiometry that should impact binding of other JIP4 partners.
Results
Protein design and ARF6–JIP4 crystals
The ARF6-binding domain of human JIP4 was initially delimited to residues 347–480 (Montagnac et al, 2009). We further narrowed down this domain to residue 392–462, corresponding to the exact boundaries of the second leucine zipper motif of JIP4 (hereafter called JIP4-LZII). A truncated form of the GTP-locked ARF6-Q67L mutant comprising residues 13–175 (Δ12-ARF6-Q67L; hereafter called ARF6) and JIP4-LZII were independently expressed in Escherichia coli and purified. A complex was obtained by mixing the two purified proteins and subsequently concentrating the protein solution to 15–20 mg ml−1. The best crystals for structural analyses grew in the P6222 hexagonal space group and diffracted up to 1.93 Å. The structure was solved by the molecular replacement method using the crystal structure of ARF6GTPγS (PDB code 2J5X) as the search model. A large portion of the ARF6-binding domain of JIP4 was clearly visible in difference electron density maps, allowing unambiguous placement of the two helices of the leucine zipper and side-chains at the binding interface (see Materials and methods). The asymmetric unit contained three ARF6–GTP molecules and one JIP4-LZII dimer with one ARF6 molecule uncomplexed and one 2:2 stoichiometric complex of ARF6 and JIP4-LZII complex. The structure was refined to working and free R factors of 21.4 and 23.8%, respectively. Statistics for the structure determination and refinement are summarized in Table I.
Table 1.
Data collection and refinement statistics
| ARF6–JIP4-LZII | |
|---|---|
| Data collection | |
| Space group | P6222 |
| Unit Cell (Å) | a=137.3, b=137.3, c=165.0 |
| Resolution (Å) | 35–1.93 |
| Rmeas (%) | 5.9 (40.0) |
| I/σ(I) | 8.0 (2.1) |
| Completeness (%) | 99.9 (98.2) |
| Redundancy | 12.8 (11.8) |
| Refinement | |
| Resolution (Å) | 35–1.93 |
| Number of reflections | 62 165 |
| Rfactor/Rfree | 21.4/23.8 |
| Number of protein atoms | |
| Protein | 4944 |
| Water | 275 |
| B-factor (Å2) | |
| Protein | 30.4 |
| Water | 33.6 |
| r.m.s.d. | |
| Bond lengths (Å) | 0.009 |
| Bond angles (deg) | 1.170 |
| Values in parentheses are for highest resolution shell. | |
Overall description of the ARF6–JIP4 complex
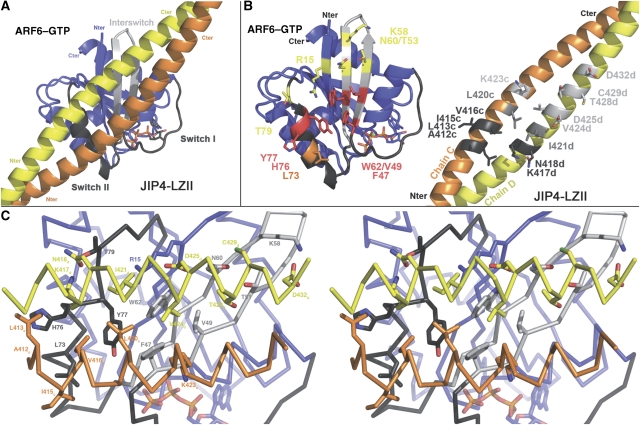
In the crystal, the overall complex is organized as an ARF6–(JIP4)2–ARF6 heterotetramer with the JIP4-LZII homodimer recruiting two ARF6 molecules at its centre in a dyad-symmetric manner (Figure 1). Each JIP4 helix bridges two opposing ARF6 molecules, and conversely, each ARF6 molecule interacts with both helices that comprise the leucine zipper. No interaction is observed between the two ARF6 molecules bound to JIP4-LZII in the crystal structure, suggesting that ARF6 molecules do not cooperate for binding to JIP4. The ARF6 molecules complexed to JIP4, as well as the uncomplexed ARF6, contain the G-domain fold typical of the Ras superfamily with a central six-stranded β sheet (β1–β6 strands) flanked by five α helices (α1–α5 helices) (Figure 1). The overall fold, the switch conformations and the Mg–GTP-binding site of all ARF6 molecules in the crystal are very similar and resemble closely those of the uncomplexed ARF6GTPγS solved previously (PDB code 2J5X; Pasqualato et al (2001)) with an average r.m.s.d. lower than 0.67 Å on 159 Cα atoms.
Figure 1.
The overall structure of the ARF6–JIP4-LZII complex. ARF6–GTP is drawn in blue with the switch regions in grey. The two monomers of JIP4-LZII are drawn in yellow and orange. Two orthogonal views of the complex are shown.
The second leucine zipper of JIP4 (396–458; Figure 2A) consists of nine heptad repeats with the characteristic leucine residue in position d except in heptad repeat 7, which shows a valine residue (alanine residue in JIP3) in place of the characteristic leucine (Figure 2B). The JIP4-LZII domain complexed to ARF6 consists of two unbroken α-helices that wind around each other in a 90 Å long and straight parallel coiled-coil structure (Figure 1 and 2C). The total surface area buried by both helices of the JIP4-LZII dimer is 3218 Å2. Note that although the ninth heptad repeat is present in our construct, it is not folded in the crystal.
Figure 2.
The second leucine zipper structure of JIP4. (A) Schematic representation of the JIP4 protein. (B) Sequence alignement of JIP3 and JIP4. The heptad repeats, the characteristic leucine residues at position ‘d' in each heptad, and their respective sequence numbers are indicated. On the JIP4 sequence, the ARF6-binding site (residues A412–D432) is highlighted in grey. (C) The JIP4-LZII structure is shown (with only one ARF6 molecule bound) aligned along the JIP3/JIP4 sequence alignment. It should be noted that the C-terminal part of the linker between the histidine tag and JIP4 has defined density at the N-terminus of the chain C and thus has been modelled (residues A386–F391), whereas the ninth heptad repeats of both monomers are not seen in the density and are not modelled.
To investigate the stoichiometry of the ARF6–JIP4 complex in solution, we carried out analytical ultracentrifugation experiments (AUC; Figure 3). In the presence of an excess of ARF6 over (JIP4)2, as in our crystallization conditions, two species were observed: one with a sedimentation coefficient of 2.0±0.1 S (corresponding to excess monomeric ARF6) and the other with a sedimentation coefficient of 3.4±0.1 S, corresponding to an (ARF6)2–(JIP4)2 heterotetramer (Figure 3; green curve). When calculated from the crystallographic structure, the complex had a hydrodynamic radius of 3.4 nm and a sedimentation coefficient of 3.6 S. The dimensions and shape of the ARF6–(JIP4)2–ARF6 heterotetramer in the crystal are, therefore, consistent with those of the (ARF6)2–(JIP4)2 heterotetramer in the solution. Furthermore, we carried out AUC experiments at a molar ratio of 2:1 (JIP4)2:ARF6. In this case, two species were observed with sedimentation coefficients of 1.5±0.1 S (corresponding to excess dimeric JIP4-LZII) and 2.7±0.1 S, compatible with an ARF6–(JIP4)2 heterotrimer (Figure 3; black curve). A heterotrimeric ARF6–(JIP4)2 model was created from the crystallographic structure by deleting one ARF6 molecule and this was used to calculate the hydrodynamic characteristics of such a complex, resulting in a hydrodynamic radius of 3.2 nm and a sedimentation coefficient of 2.6 S, in good agreement with the AUC data. The existence of an ARF6–(JIP4)2 heterotrimer in solution confirms the absence of cooperativity between the two ARF6 molecules bound to the dimer of JIP4.
Figure 3.
Stoichiometry of the ARF6–JIP4-LZII complexes in solution determined by analytical ultracentrifugation. Continuous sedimentation coefficient distribution analysis of ARF6 (100 μM; dashed blue line open triangles), JIP4 (100 μM; dashed red line filled triangles) and ARF6–JIP4 mixtures (12.5 μM ARF6, 50 μM JIP4; black line open squares and 50 μM ARF6, 12.5 μM JIP4; green line filled squares). The total integrated signal of each c(S) distribution was normalized. Sedimentation coefficients are expressed in Svedberg: 1 S=10−13 s.
To investigate the structure of JIP4-LZII in solution, we carried out AUC experiments on JIP4-LZII in the absence of ARF6. The data showed that JIP4-LZII is a stable dimer in solution with a sedimentation coefficient of 1.5±0.1 S (Figure 3; red curve), equal to that calculated by hydrodynamic modelling of (JIP4)2 from the crystallographic structure. These observations suggest that the overall structure of unbound JIP4-LZII is similar to that in the complex with ARF6.
Description of the ARF6–JIP4 interface
The ARF6–(JIP4)2–ARF6 heterotetramer structure has a two-fold axis of symmetry (which corresponds to the JIP4 homodimer axis) that generates two virtually identical interfaces between ARF6 and JIP4-LZII (Figure 1). This description of the ARF6–JIP4-LZII interface will address only one of the ARF6 molecules and its interface with the JIP4 homodimer (chains C and D) that will be distinguished by using the superscript suffixes ‘c' and ‘d' bearing in mind that the chains are related by a two-fold axis (Figure 4).
Figure 4.
The ARF6–JIP4-LZII interaction surface. (A) An overall view of the ARF6–JIP4-LZII interface. (B) An ‘open book' representation of the ARF6–JIP4 interface. Residues involved in the complex interface are labelled and shown as sticks. For ARF6, the residues that interact with chains C and D of JIP4 are shown, respectively, in orange and yellow; residues that interact with both chains are shown in red. For JIP4-LZII, residues that interact with the switch II and interswitch regions are shown, respectively, in light and dark grey; residues that interact with both are shown in mid grey. (C) Cross-eye stereo view of the interface. All the residues from ARF6 and JIP4-LZII that are involved in the interface are labelled and shown as sticks.
Our structure shows that ARF6 interacts with both the monomers of JIP4-LZII with the ARF6-binding site spanning three heptad repeats, namely repeats 3–5 consisting of 21 amino-acid residues (412–432; Figure 2B and C). The JIP4-LZII dimer interacts with the ARF6 switch interface, mainly at the interswitch (β2–β3 strands) and switch II regions (Figure 4A). This provides a rationale for the specificity of JIP4-LZII for the GTP-bound form of ARF6, as the switch II and interswitch regions differ markedly in conformation in the GDP-bound form (Ménétrey et al, 2000). ARF6 and JIP4-LZII have a standard Arf–effector interface with a buried accessible surface area of 1719 Å2 (743 and 976 Å2 for chains C and D, respectively), which involves both hydrophobic and hydrophilic interactions (Figure 4B).
The hydrophobic interface can be described as contacts between two flat surfaces rather than as a ‘lock and key' type of interaction as observed for other Arf–effector complexes (Hanzal-Bayer et al, 2002; Panic et al, 2003; Shiba et al, 2003; Wu et al, 2004; Ménétrey et al, 2007). The ARF6 residues at this hydrophobic interface include Phe 47, Val 49 and Trp 62 from the interswitch base, and Leu 73, His 76 and Tyr 77 from the switch II helix. Together, they form an elongated hydrophobic patch that includes the previously described hydrophobic pocket and triad patch (Phe 47, Trp 62 and Tyr 77 in ARF6) (Kawasaki et al, 2005; Ménétrey et al, 2007). On the other side of the interface, a larger hydrophobic patch is formed by the JIP4 dimer consisting of nine residues: Ala 412, Leu 413, Ile 415, Val 416, Leu 420 and Lys 423 from chain C, and Lys 417, Ile 421, Val 424 from chain D (Figure 4C).
Although both monomers of JIP4-LZII are involved in the hydrophobic patch interactions, only residues from one monomer participate in the hydrophilic interactions. These hydrophilic interactions consist of an elongated network of hydrogen bonds adjacent to the hydrophobic patch. First, the side chain of Thr 79 at the C-terminus of the ARF6 switch II region makes two hydrogen bonds with the Lys 417d and Asn 418d side chains from JIP4; these interactions are further stabilized by an additional hydrogen bond between the amine nitrogen (NZ) of the Lys 417d and the carbonyl main chain of ARF6-His 76. Second, the carboxylate group of Asp 425d from JIP4 makes hydrogen bonds with the Arg 15 (β1 strand), Trp 62 and Asn 60 (interswitch region) side chains, this latter interacting, in turn, with the thiol group of Cys 429d. Last, the side chains of two ARF6 interswitch residues (Thr 51 and Lys 58) make hydrogen bonds with, respectively, the hydroxyl group of Thr 428d and the carboxylate group of Asp 432d. Notably, modelling of the ARF6–JIP3 complex interface showed no significant difference with that of the ARF6–JIP4 complex, we thus predicted that the structure of the ARF6–JIP3 complex would be similar to that observed for ARF6–JIP4 complex. Finally and in contrast to what was described for other Arf–effector complexes (Hanzal-Bayer et al, 2002; Panic et al, 2003; Shiba et al, 2003; Wu et al, 2004; Ménétrey et al, 2007), this interface does not contain any obvious ‘hot spot' residue that would have a critical function in the interaction.
Affinity measurements and mutation experiments
Surface plasmon resonance (SPR) measurements found that the equilibrium dissociation constant (Kd) of the complex between JIP4-LZII (fused to GST) and Δ12-ARF6GTP-Q67L was 0.42 (±0.05) μM (Figure 5A and B and Supplementary Figure S1). Despite the lack of obvious hot-spot residues at the interface, we hypothesized that Val 416, Ile 421 and Asp 425 of JIP4 would be important for the ARF6–JIP4 interaction as they occupy central positions in both the hydrophobic and the hydrophilic patches (Figure 4C). Notably, Val 416 and Ile 421, which participate in the same ARF6-binding interface belong to different monomers of the JIP4-LZII dimer. To determine experimentally the role of these residues in complex formation, we generated point mutations in GST–JIP4-LZII and tested the interaction of these mutants with GTP-bound ARF6 in vitro. As these three residues are not involved in the interface between the two JIP4 monomers, we expected that their mutation would not affect the JIP4 dimer. SPR assays showed that V416A and I421A mutations reduced the affinity of GST–JIP4-LZII for Δ12-ARF6GTP-Q67L by 27-fold and 18-fold, respectively, when compared with the wild-type protein, whereas the D425A mutation resulted in a 5-fold decrease in affinity (Figure 5A and B). These data confirm that Val 416, Ile 421 and, to a lesser extent, Asp 425 of JIP4 have a significant function in the interaction with ARF6, and thus validate the ARF6–JIP4-LZII interface topology observed in our crystal structure. In addition, the similarity of the binding affinities of the V416A and I421A mutants supports the conclusion that both monomers of JIP4-LZII are equally important for the interaction with ARF6.
Figure 5.
Binding affinities of Arfs–JIP4-LZII complexes studied by surface plasmon resonance (SPR). (A) Real-time association and dissociation SPR profiles corresponding to the injection of ARF6 (3.9 μM) over immobilized wild-type and variant JIP4-LZII and (B) ARF6/(JIP4)2 molar ratio as a function of ARF6 concentration and corresponding fitted Kd values. (C) Real-time association and dissociation SPR profiles corresponding to the injection of the different Arf variants (1.8 μM) over immobilized JIP4-LZIIWT and (D) ARF/(JIP4)2 molar ratio as a function of Arf concentration and corresponding fitted Kd values.
Structure-based mutational analysis of the determinants of ARF6 specificity
We have shown previously that JIP4 interacts specifically with ARF6 as no interaction with ARF1 or ARF5 could be detected by pull-down assays (Montagnac et al, 2009). To quantify JIP4's specificity for ARF6, we studied its interaction with Δ17-ARF1-Q71L (hereafter called ARF1) by SPR and measured a Kd of 9.8 (±0.4) μM, which is 21-fold higher than that for Δ12-ARF6-Q67L (ARF6) (Figure 5C and D). These data confirm that JIP4 has a strong preference for ARF6 and can thus be considered as a specific ARF6 effector.
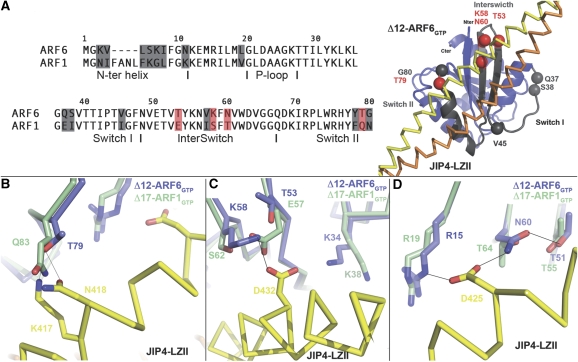
Although structural comparisons of ARF6 and ARF1 at the interface with JIP4 showed no major structural difference in the main chain conformation that might account for JIP4's specificity, discrete sequence differences between the two Arfs were observed that may underlie specificity (Figure 6). Overall, effectors interact mainly with the N-terminal part of Arf proteins that encompasses the critical switch region (G36–G80 in ARF6). There are 10 residue differences between ARF6 and ARF1 in this region (Figure 6A). Among these, two are buried in the core of the proteins (Val 57 and Tyr 78 in ARF6) and one conserved difference is located at the C-terminus of the switch I region (Val 45 in ARF6). The other seven residue differences are most likely to be essential for effectors to discriminate between ARF6 and ARF1: two are located at the N-terminus of switch I (Gln 37 and Ser 38 in ARF6 are replaced by Glu 41 and Ile 42 in ARF1); three at the tip of the interswitch (Thr 53, Lys 58 and Asn 60 in ARF6 are replaced by Glu 57, Ser 62 and Thr 64 in ARF1); and two at the extreme C-terminal end of switch II (Thr 79 and Gly 80 in ARF6 are replaced by Gln 83 and Asn 84 in ARF1). All these seven sequence differences are located at the edge of the switch region (Figure 6A).
Figure 6.
The structural basis of JIP4 specificity. (A) A sequence alignment of the ARF6 and ARF1 N-termini is shown (left). Sequence differences at the switch regions are indicated on the ARF6–JIP4-LZII structure by spheres (right). Residues in contact with JIP4-LZII or likely to affect the JIP4-LZII binding are indicated in red. (B–D) Detailed views of ARF6–JIP4-LZII interactions at positions not conserved in ARF1, namely Thr 79 (B), Thr 53–Lys 58 (C) and Asn 60 (D). The structure of ARF1–GTP (green) is superimposed on the structure of ARF6–GTP in the ARF6–JIP4-LZII complex (blue). Chain C of JIP4-LZII is shown in yellow.
A careful structural analysis suggested that four of these residues may have crucial functions in the specificity of the ARF6–JIP4 interaction. First, ARF6-Thr 79 at the C-terminus of switch II, which makes a double hydrogen bond with Lys 417 and Asn 418 of JIP4, is replaced by a glutamine residue in ARF1 (Gln 83). Owing to its longer side chain, ARF1-Gln 83 cannot make an hydrogen bond with Lys 417 and Asn 418 from JIP4 because of steric hindrance (Figure 6B). Second, ARF6-Thr 53 at the interswitch region does not make any direct contact with JIP4-LZII, but in ARF1 it is replaced by the longer and negatively charged Glu 57 residue that would face Asp 432 of JIP4, generating charge repulsion (Figure 6C). Note that this charge repulsion would be more important in the case of binding to JIP3 with a glutamate residue corresponding to Asp 432 of JIP4 (Figure 2B). Thus, ARF1-Glu 57 and ARF1-Gln 83 should induce charge repulsion and/or steric hindrance, providing an explanation for the lower affinity of JIP4 for ARF1 compared with ARF6. Third, Lys 58 in the interswitch region of ARF6 makes a hydrogen bond with Asp 432 of JIP4-LZII. In ARF1, this lysine residue is replaced by Ser 62, which cannot interact with Asp 432 of JIP4 because of its shorter side chain (Figure 6C). Last, ARF6-Asn 60, which is stabilized by an interaction with Thr 51 and makes a hydrogen bond with Asp 425 of JIP4, is replaced by a threonine residue in ARF1 (Thr 64). Owing to its shorter side chain, ARF1-Thr 64 cannot make a hydrogen bond with the carboxylate group of JIP4 Asp 425 (Figure 6D). Hence, both ARF1-Ser 62 and ARF1-Thr 64 are unlikely to interact with JIP4, in contrast to their ARF6 equivalents. The loss of these interactions should weaken binding of JIP4 to ARF1.
To explore experimentally the importance of these four sequence differences between ARF1 and ARF6 in binding to JIP4, we generated two variants of ARF1 and compared their binding affinity for JIP4-LZII with those of ARF1wt and ARF6wt (Figure 5C and D). First, we generated a variant of Δ17-ARF1-Q71L with Glu 57 and Gln 83 replaced by threonine, as in their ARF6 equivalents (E57T-Q83T; called hereafter ARF12mut). These changes to ARF1 should abolish the charge repulsion and steric hindrance allowing improved binding to JIP4. The second Δ17-ARF1-Q71L variant included, in addition to the ARF12mut substitutions, the replacement of residues Ser 62 and Thr 64 by their equivalents in ARF6 (E57T-S62K-T64N-Q83T; called hereafter ARF14mut). This variant is virtually identical to ARF6 at the level of the JIP4 interface in our crystal structure. Real-time SPR binding experiments showed that the affinity of ARF12mut for JIP4-LZII was 1.6-fold higher than that of ARF1wt, but still 13-fold lower than that of ARF6wt (Figure 5C and D), indicating that replacement of Glu 57 and Gln 83 by threonines did not improve significantly the affinity of ARF1 for JIP4-LZII. This shows that these two positions alone are not sufficient to determine the specificity for JIP4. By contrast, the affinity of ARF14mut for JIP4-LZII was enhanced by a factor of 54-fold compared with ARF1wt (Figure 5C and D). Surprisingly, this variant had a 2.6-fold higher affinity than ARF6wt for JIP4-LZII, and a 20-fold lower dissociation rate (Koff=0.025 s−1 for ARF14mut compared with 0.5 s−1 for ARF6wt; Figure 5C and D). As the residues at the interface were virtually identical in this ARF1 variant to those in ARF6, other residue(s) outside the interface probably change the dynamics of the binding interface and thus modulate the binding affinity for JIP4 measured in solution. For example, sequence differences at position Leu 19, Ile 106 and Met 112 in ARF6 (replaced by Val 23, Met 110 and Leu 116 in ARF1, respectively), which are hydrophobic buried residues that contact the C-terminal part of the switch II helix α2, might impact the flexibility of the switch helix.
Together, these data show that three sequence differences at the tip of the interswitch region (Thr 53, Lys 58 and Asn 60) in combination with one at the switch II extreme C-terminus (Thr 79), comprise the main structural determinants that allow JIP4 to discriminate between ARF6 and ARF1. In addition, they provide a rationale for the loss of interaction of JIP4 with the ARF6iSW mutant obtained by swapping the ARF6 interswitch region with that of ARF1 (Montagnac et al, 2009). Interestingly, these four sequence differences in ARF1 are also found in class II Arfs (ARF4 and ARF5) consistent with the observation that JIP4-LZII does not interact with ARF5–GTP in vitro (Montagnac et al, 2009). By contrast, residues at these positions are conserved in ARL1; we do not expect ARL1 to be a binding partner for JIP3/JIP4 because of sequence differences between ARL1 and ARF6 in the switch II region (ARL1-Tyr 77 and ARL1-Cys 80, equivalent to ARF6-Leu 73 and ARF6-His 76, respectively) that participate in JIP4 recognition (Supplementary Figure S2).
A model for the interaction of the JIP4–ARF6 complex at the membrane interface
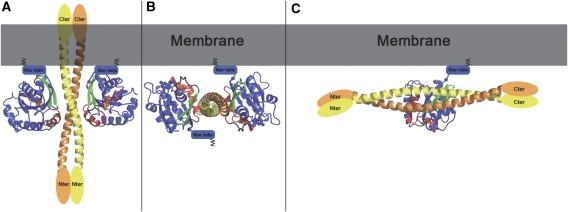
ARF6 interaction with the membrane is mainly mediated by its myristoylated N-terminal helix, which markedly restrains its position and orientation relative to the membrane. Modelling of the ARF6–(JIP4)2–ARF6 heterotetramer at the membrane interface according to the orientation of membrane-bound ARF6, showed that JIP4-LZII would stand perpendicular to the membrane, generating a severe clash between the membrane and the C-terminal part of the straight coiled-coil of JIP4-LZII (Figure 7A). We cannot exclude that, in the presence of membrane or in the context of the full-length protein, the leucine zipper coiled-coil of JIP4 could adopt a different structure in which its C-terminal part kinks to reposition toward the cytosol. However, sequence analysis of this and the flanking regions of JIP4 does not support the possibility of such a structural kink. Therefore, we conclude that the elongated leucine zipper of JIP4 should align tangentially to the membrane (Figure 7B and C). Then, modelling of the ARF6–JIP4-LZII heterotetramer bound to the membrane shows that only one ARF6–GTP molecule can be membrane anchored at a time. Indeed, the second ARF6–GTP molecule is oriented with its myristoylated N-terminal helix opposite to the membrane (Figure 7B). Thus, unlike the situation observed in the crystal in which a 2:2 ARF6:JIP4 stoichiometry was found, our model supports the idea that a dimer of JIP4 interacts with only one ARF6–GTP molecule in the presence of membrane (Figure 7C).
Figure 7.
ARF6–JIP4 interactions at the membrane. (A) Model of the ARF6–(JIP4)2–ARF6 heterotetramer at the membrane obtained when both ARF6 molecules are anchored to the membrane. ARF6 molecules are shown in blue with switch II in red and the interswitch in green. The myristoylated amphipatic N-terminal helix of ARF6 that is critical for interaction with membrane is indicated as a blue cylinder lying against the membrane. The orientation of ARF6 molecules is modelled such that both N-terminal parts are close to the membrane. (B) Model of the ARF6–(JIP4)2–ARF6 heterotetramer at the membrane considering that JIP4 is positioned tangentially with respect to the membrane. In this model, one ARF6 molecule is oriented with its N-terminal part close to the membrane, whereas that of the second ARF6 molecule is turned toward the cytosol, suggesting that only one ARF6 molecule interacts with JIP4 at the membrane. (C) Model of an ARF6–(JIP4)2 heterotrimer at the membrane.
Discussion
Structural determinants of the specificity of Arf binding to effectors
To date, six structures of complexes between Arf or Arl subfamily proteins and their effectors have been reported: ARF1–GGA1, ARF1–ARHGAP21, ARF6–CTA1 (cholera toxin A1 subunit), ARL1–Golgin245, ARL2–PDEδ and ARL2–BART (Hanzal-Bayer et al, 2002; Panic et al, 2003; Shiba et al, 2003; Wu et al, 2004; O'Neal et al, 2005; Ménétrey et al, 2007; Zhang et al, 2009). Together, these structures have provided a better understanding of how effectors recognize and interact with Arf proteins. All these effectors bind a common ‘hydrophobic area' at the junction of the switch I C-terminus and the switch II N-terminus at the base of the interswitch region. Within this area, two binding sites have been described: the critical hydrophobic pocket (Panic et al, 2003; Ménétrey et al, 2007) and the adjacent hydrophobic triad patch (Ménétrey et al, 2007). Owing to discrete sequence differences, both binding motifs allow the effectors to distinguish between Arf subfamilies and, thus, they seem to be structural determinants of specificity.
These structures do not explain, however, how effectors distinguish between two Arf proteins from the same subfamily, as close as ARF1 and ARF6. Indeed, the effectors GGA1, ARHGAP21 and CTA1 do not discriminate between ARF1–GTP and ARF6–GTP, at least in vitro (Price et al, 1992; Takatsu et al, 2002; Dubois et al, 2005). ARF1 and ARF6 share a virtually identical hydrophobic pocket and triad patch (except for one conserved sequence difference in the hydrophobic pocket: ARF1-Ile 49 versus ARF6-Val 46; Figure 6A), and they have no significant structural difference in these regions (Goldberg, 1998; Pasqualato et al, 2001). These observations suggest that the recognition of a specific effector may be due to interactions with other regions of ARF1 or ARF6. Seven residues that differ between ARF1 and ARF6, and are located at the border of the switch regions could contribute to the specificity of effector recognition. Our ARF6–JIP4 structure shows that these border positions, more precisely the extremities of the interswitch and switch II regions, participate in the interface with specific effectors. We carried out site-directed mutagenesis combined with SPR binding assays that showed four discrete sequence differences between ARF1 and ARF6 (at positions 53, 58, 60 and 79 of ARF6) that are responsible for the specific recognition of JIP4. It should be noted that earlier site-directed mutagenesis studies showed that replacement of the Gln 37–Ser 38 pair in the extreme N-terminus of the ARF6 switch I region by its ARF1 equivalent impaired ARF6-mediated induction of actin protrusions, suggesting that other unknown effector(s) recognize ARF6 at these positions (Al-Awar et al, 2000). Altogether, these observations suggest that non-conserved residues at the border of the switch regions are structural determinants for effector recognition of Arf-subfamily proteins.
Interestingly, another GTP-binding protein family, the Rab proteins that are involved in vesicular trafficking and share some effector-binding structural characteristics with Arf proteins (see below) (Kawasaki et al, 2005; Ménétrey et al, 2007), differs in their specific effector mode of recognition. Thus, although specific effector recognition of Rab proteins involves non-conserved residues outside the switch regions, probably combined with conformational variability in the conserved switch regions (Merithew et al, 2001), the Arf-subfamily mode of recognition involves non-conserved residues at the border of the switch regions.
ARF6–JIP4 association at the membrane
The structure described here resembles that of Rab5–Rabaptin5, Rab7–RILP, Rab11–FIP2/FIP3, Rab6–GCC185 and ARL1–Golgin245 in its overall quaternary organization as a dyad-symmetric heterotetramer, in which a homodimeric helical effector domain brings together two independent Rab or Arf molecules on either side (Wu et al, 2004, 2005; Zhu et al, 2004; Eathiraj et al, 2006; Jagoe et al, 2006; Shiba et al, 2006; Schweizer Burguete et al, 2008). (It should be noted that the Arl-binding domain of Golgin245 is a triple helix bundle homodimer, whereas the Rab/Arf-binding domain of RILP, Rabaptin5, FIP2/FIP3 and GCC185 are coiled-coils, as seen in JIP3 and JIP4.) The orientation and position of the effector helices relative to the GTPases switch region in these Rab–effector and ARL1–effector complexes differ substantially from those in the ARF6–JIP4 complex (Supplementary Figure S3).
The bivalent mode of binding of dimeric effectors by two Rab/Arf molecules is likely to increase the effector residence time at the membrane. Indeed, one of the Rab/Arf molecules may maintain the effector at the membrane whereas the other dissociates, to be inactivated and replaced by a new active one (Panic et al, 2003; Kawasaki et al, 2005). Prolonging the residence of the effectors at the membrane might be important for Arf/Rab to be able to trigger membrane traffic processes. Such a bivalent mode of recruitment, however, imposes a constraint on the orientation of these dimeric effectors relative to the membrane. So far, all dimeric effectors whose structures have been solved (those mentioned above) could probably dock on the membrane without steric hindrance between the effector and the membrane. The Rab/Arf-binding sites of Rabaptin5, Golgin245 and FIP2/FIP3 are situated at their extreme C-termini, whereas the medial Rab7-binding site of RILP adopts a helix-loop-helix hairpin structure. In all these cases, no steric hindrance constrains the effectors respective to the membrane, as they project into the cytosol allowing them to fulfill specific functions. One exception is the straight coiled-coil of the Rab6-binding domain of GCC185, which is not located at its extreme C-terminus. However, the hypervariable extended C-terminus of Rab6 may be long enough to accommodate the rest of GCC185 without steric clash with the membrane (Schweizer Burguete et al, 2008).
In the ARF6–JIP4 complex, the ARF6-binding site of JIP4 overlaps with the LZII region that is located in the middle of the molecule and adopts an elongated and fairly rigid structure. Our model predicts that this structural organization of the ARF6-binding site combined with a bivalent mode of binding would generate a severe clash with the membrane. Indeed, modelling showed that JIP4 stands perpendicular to the membrane and its C-terminal part thus penetrate into the membrane bilayer (Figure 7A). If JIP4 is assumed to lie tangentially to the membrane, modelling showed that binding could involve only one ARF6 molecule attached to the membrane (Figure 7B). Therefore, despite the fact that a 2:2 stoichiometry for the ARF6:JIP4 complex is found in the crystal, the complex is probably unable to attain such a 2:2 stoichiometry when ARF6 is bound to a membrane. This highlights that the plasma membrane may have a critical role in orienting the ARF6–JIP4 complex and determining its stoichiometry. In conclusion, our model suggests that at the plasma membrane, a dimer of JIP4 interacts with only one ARF6 molecule forming a heterotrimer (Figure 7C). By leaving the cytosolic face of the coiled-coil of JIP4 available for binding to other binding partners, like kinesin-1 or dynactin, this heterotrimeric complex model gives insights to understand the regulation of JIPs' interaction with motors by ARF6. Further functional and biophysical studies will be necessary to understand the detailed molecular mechanisms of the interaction of membrane-bound ARF6 with JIP3/JIP4, and the exact contribution of the membrane to the motor switch mechanism mediated by ARF6.
Materials and methods
Constructs, expression and purification
cDNA encoding the LZII of human JIP4 (JIP4-LZII, residues 392–462) was cloned into the pGST-1 plasmid. The V416A, I421A and D425A mutants of JIP4-LZII were generated using the QuickChange site-directed mutagenesis kit (Stratagene). The N-terminally truncated and GTP-locked human ARF6 variant, Δ12-ARF6-Q67L (residues 13–175) was cloned into the pET HTb plasmid. The double E57T-Q83T and quadruple E57T-S62K-T64N-Q83T variants of ARF1 were generated using the QuickChange site-directed mutagenesis kit using mouse Δ17-ARF1-Q71L cloned into pProEX HT plasmid as matrix (kind gift from Dr Soichi Wakatsuki). For biophysical experiments and crystallization, JIP4-LZII was expressed as a glutathione-S-transferase (GST)-fusion protein in E. coli BL21-DE3 cells. Cells were collected after induction with 0.3 mM IPTG for 4 h at 30°C. Frozen bacteria were resuspended in 50 mM Tris–HCl (pH 8) containing 250 mM NaCl, 1 mM EDTA, 10% glycerol, 1 mM DTT, 0.1% Triton X-100, 5 mM MgCl2, 1 mM PMSF, 2 μg/ml aprotinin, 2 μg/ml leupeptin, 0.7 mg ml−1 lysozyme and 0.02 mg/ml DNAse were disrupted by sonication. The lysate was ultracentrifuged at 40 000 g for 30 min at 4°C and the supernatant was incubated at 4°C with glutathione–Sepharose 4B beads (Amersham Biosciences) for 2 h. The protein, JIP4-LZII, domain was cleaved from the GST fusion protein using rTEV protease overnight at 4°C, then passed over a MonoQ 5/50 column (Amersham Biosciences) and eluted with a salt gradient. The JIP4-LZII domain was then dialysed and concentrated to 10–15 mg/ml, frozen in liquid nitrogen and stored at −80°C in 50 mM Tris–HCl (pH 8), 50 mM NaCl, 5 mM MgCl2 and 1 mM DTT. ARF6 and ARF1 variants were expressed and purified as described previously by Shiba et al (2003). Briefly, after purification by Ni2+–NTA affinity chromatography, the His6-tag was removed using rTEV protease, and the proteins were further purified by gel filtration chromatography, concentrated to 10–15 mg ml−1, frozen in liquid nitrogen and stored at −80°C in 25 mM Tris–HCl (pH 8), 100 mM NaCl, 5 mM MgCl2, 2 mM DTT and 0.1 mM GTP.
Analytical ultracentrifugation
Sedimentation experiments were carried out at 25°C in an XL-I analytical ultracentrifuge (Beckman–Coulter) equipped with Rayleigh Interference detection. ARF6 (300 μl, 100 μM), JIP4 (300 μl, 100 μM) or mixtures of the two (ARF6 12.5 μM+JIP4 50 μM or ARF6 50 μM+JIP4 12.5 μM) were centrifuged at 50 000 r.p.m. in an An60Ti rotor using 12 mm double-sector aluminium centrepieces. All samples were prepared in the interaction buffer (100 mM NaCl, 5 mM MgCl2 and 25 mM Tris (pH 8.0)). The partial specific volumes of ARF6, JIP4 and their complexes were estimated from their amino-acid sequences by using the software Sednterp 1.09 (available at http://www.rasmb.bbri.org) as 0.739, 0.744 and 0.740, respectively. The same software was used to estimate the buffer viscosity η (1.040 cP) and density ρ (1.00318 g ml−1). Interference profiles were recorded every 5 min. Sedimentation coefficient distributions, c(S), were determined by using the software Sedfit 11.7 (Brown and Schuck, 2006). Theoretical sedimentation coefficients were calculated from the crystal structure PDB file using Hydropro 7c (García De La Torre et al, 2000) with a hydrated radius of 3.1 Å for the atomic elements.
SPR measurements.
The assays were carried out at 25°C in the interaction buffer. To monitor protein–protein interactions, a goat anti-GST antibody (Biacore GST Capture Kit) was covalently coupled to a CM5 sensorchip, using a Biacore 2000 instrument and the Amine Coupling Kit (Biacore). Immobilization densities of 3500–6000 resonance units (RU; 1RU≈1 pg mm−2) were achieved. GST-fused JIP4-LZIIwt, JIP4-LZIIV416A, JIP4-LZIII421A and JIP4-LZIID425A were captured at densities (Rcapt) of 780 RU, 455 RU, 580 RU and 330 RU, respectively. A series of 11 concentrations of ARF6 (0.016–15 μM) was sequentially injected over captured GST–JIP4 at a flow rate of 50 μl min−1. After each injection, the interaction buffer was flowed on the surface until all the ARF6 molecules dissociated. At the end of the series, the surfaces were regenerated with a 2-min wash with 10 mM glycine–HCl (pH 1.5) and two 1-min washes with 0.1% SDS. Similarly, JIP4-LZIIwt was captured at a density of 550 RU and increasing concentrations of ARF6 (0.029–15 μM), ARF1 (0.095–24 μM), ARF12mut (0.098–25 μM) or ARF14mut (0.029–15 μM) were injected sequentially over captured JIP4-LZIIwt at a flow rate of 50 μl min−1.
The association and dissociation profiles were double referenced using the Scrubber 2.0 software (BioLogic Software), that is both the signals from a reference surface (with GST captured on the anti-GST antibody) and from blank experiments using interaction buffer instead of protein were subtracted. The steady-state SPR responses (Req) were plotted against the ARF concentration (C) and fitted according to the following equation:
where Kd is the equilibrium dissociation constant of the ARF–JIP4 interaction and Rmax the maximal binding capacity of GST–JIP4. The ARF:(JIP4)2 molar ratio was determined as follows:
Crystallization and structural determination
Crystallization conditions for the ARF6–JIP4-LZII complex were found using the stoichiometric variation screening approach (SVS; Stura et al (2001)). Drops were prepared by mixing equal volumes of protein solution (15–20 mg ml−1) and reservoir solution using the hanging drop vapour diffusion method at 17°C. Single crystals of ARF6–JIP4-LZII complex were obtained spontaneously using a reservoir containing 2.0 M ammonium sulphate, 0.1 M HEPES (pH 7.5), 0.2 M NaCl, 2 mM MgCl2, 6% 2-methyl-2,4-pentanediol using a stoichiometry ratio of 2 ARF6 for 1 JIP4-LZII dimer. Cryoprotection was carried out using crystallization conditions complemented with either 20% ethylene glycol or 25% glycerol in a two-step process, and crystals were flash frozen in liquid nitrogen. X-ray diffraction data sets were collected at −170°C on ID14-3/ID29 beamlines at the ESRF (Grenoble, France) and Proxima-1 beamline of Soleil (Saint-Aubin, France). Intensities were integrated with MOSFLM and scaled with SCALA (CCP4, 1994). The ARF6–JIP4-LZII crystals that diffracted up to 1.93 Å belonged to the primitive hexagonal space group P6222 with one ARF6–(JIP4)2–ARF6 complex and one uncomplexed ARF6 molecule in the asymmetric unit, and a=137.3 Å, b=137.3 Å, c=165.0 Å cell parameters.
Molecular replacement for ARF6 was carried out and solved using PHASER (McCoy et al, 2005) at 3.0 Å resolution using as search model the wild-type ARF6GTPγS ((Pasqualato et al, 2001); PDB code 2J5X). The Fo–Fc map calculated using phases from the ARF6 molecular replacement solution indicated two long and continuous regions of extra electron density in contact with the ARF6 switch that were easily interpreted and built as helices of the leucine zipper. Then, the N- and C-termini of both helices of the JIP4-LZII dimer were built manually as helix extensions. The structure was refined at 1.93 Å resolution by maximum likelihood refinement with Refmac (CCP4, 1994) and by graphical building using COOT (Emsley and Cowtan, 2004). The refined structure consists of one ARF6–(JIP4)2–ARF6 complex encompassing two ARF6 molecules (chain A, residues 12–172 and chain B, residues 12–172) complexed to one JIP4-LZII dimer (chain C, residues 386–452 and chain D, residues 391–452), and one uncomplexed ARF6 molecule (chain E, residues 11–172). It should be noted that in ARF6, three N-terminal residues (Gly, Ala and Met) belonging to the linker between the histidine tag and ARF6 were modelled in electron density and numbered as residues 10–12. Furthermore, in JIP4-LZII, six N-terminal residues (Ala, Met, Asp, Pro, Glu and Phe) belonging to the linker between the GST tag and ARF6 were modelled in the electron density and numbered as residues 386–391. The stereochemistry of the final refined model is excellent and there are no Φ–Ψ pairs outside allowed regions of the Ramachandran plot. The refined structure has a crystallographic R-value of 21.4% and a free R-value of 23.8%. Crystallographic statistics are summarized in Table I. Figures were produced using Pymol (DeLano, 2002).
Accession codes
The coordinates and structure factors of ARF6–JIP4-LZII have been deposited in the Protein Data Bank under accession numbers 2W83 and r2w83sf, respectively.
Supplementary Material
Supplementary data
Review Process File
Acknowledgments
We would like to thank Wolfgang Faigle for mass spectrometry measurements, and Lucien Cabanié and Ahmed El Marjou for help with protein purification. We are also grateful to the Institut de Biologie Physico-Chimique (IBPC, Paris, France) and Ines Gallay for providing access to the TECAN crystallization robot. X-ray data collection was carried out with the help of the staff of the ID14-3/ID29 beamlines at the European Synchrotron Radiation Facility (Grenoble, France) and of the Proxima-1 beamline at the Soleil synchrotron (Saint-Aubin, France). This study was supported by grants from La Ligue Nationale contre le Cancer (‘équipe labellisée 2008') and BNP-Paribas to PC and from the Association pour la Recherche contre le Cancer (Grant 4921) and ANR (ANR-08-JCJC-0110-01) to JM.
Footnotes
The authors declare that they have no conflict of interest.
References
- Al-Awar O, Radhakrishna H, Powell NN, Donaldson JG (2000) Separation of membrane trafficking and actin remodeling functions of ARF6 with an effector domain mutant. Mol Cell Biol 20: 5998–6007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowman AB, Kamal A, Ritchings BW, Philp AV, McGrail M, Gindhart JG, Goldstein LS (2000) Kinesin-dependent axonal transport is mediated by the sunday driver (SYD) protein. Cell 103: 583–594 [DOI] [PubMed] [Google Scholar]
- Brown PH, Schuck P (2006) Macromolecular size-and-shape distributions by sedimentation velocity analytical ultracentrifugation. Biophys J 90: 4651–4661 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavalli V, Kujala P, Klumperman J, Goldstein LSB (2005) Sunday Driver links axonal transport to damage signaling. J Cell Biol 168: 775–787 [DOI] [PMC free article] [PubMed] [Google Scholar]
- CCP4 (1994) The CCP4 suite: program for protein crystallography. Acta Crystallogr D Biol Crystallogr 50: 760–763 [DOI] [PubMed] [Google Scholar]
- D'Souza-Schorey C, Chavrier P (2006) ARF proteins: roles in membrane traffic and beyond. Nat Rev Mol Cell Biol 7: 347–358 [DOI] [PubMed] [Google Scholar]
- DeLano WL (2002) The PyMOL Molecular Graphics System. Delano Scientific, San Carlos, CA, USA. http://www.pymol.org
- Dubois T, Paleotti O, Mironov AA, Fraisier V, Stradal TE, De Matteis MA, Franco M, Chavrier P (2005) Golgi-localized GAP for Cdc42 functions downstream of ARF1 to control Arp2/3 complex and F-actin dynamics. Nat Cell Biol 7: 353–364 [DOI] [PubMed] [Google Scholar]
- Eathiraj S, Mishra A, Prekeris R, Lambright DG (2006) Structural basis for Rab11-mediated recruitment of FIP3 to recycling endosomes. J Mol Biol 364: 121–135 [DOI] [PubMed] [Google Scholar]
- Emsley P, Cowtan K (2004) Coot: model-building tools for molecular graphics. Acta Crystallogr D Biol Crystallogr 60: 2126–2132 [DOI] [PubMed] [Google Scholar]
- García De La Torre J, Huertas ML, Carrasco B (2000) Calculation of hydrodynamic properties of globular proteins from their atomic-level structure. Biophys J 78: 719–730 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldberg J (1998) Structural basis for activation of ARF GTPase: mechanisms of guanine nucleotide exchange and GTP-myristoyl switching. Cell 95: 237–248 [DOI] [PubMed] [Google Scholar]
- Hanzal-Bayer M, Renault L, Roversi P, Wittinghofer A, Hillig RC (2002) The complex of Arl2-GTP and PDE delta: from structure to function. EMBO J 21: 2095–2106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jagoe WN, Lindsay AJ, Read RJ, McCoy AJ, McCaffrey MW, Khan AR (2006) Crystal structure of rab11 in complex with rab11 family interacting protein 2. Structure 14: 1273–1283 [DOI] [PubMed] [Google Scholar]
- Kahn RA, Cherfils J, Elias M, Lovering RC, Munro S, Schurmann A (2006) Nomenclature for the human Arf family of GTP-binding proteins: ARF, ARL, and SAR proteins. J Cell Biol 172: 645–650 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawasaki M, Nakayama K, Wakatsuki S (2005) Membrane recruitment of effector proteins by Arf and Rab GTPases. Curr Opin Struct Biol 15: 681–689 [DOI] [PubMed] [Google Scholar]
- McCoy AJ, Grosse-Kunstleve RW, Storoni LC, Read RJ (2005) Likelihood-enhanced fast translation functions. Acta Crystallogr D Biol Crystallogr 61: 458–464 [DOI] [PubMed] [Google Scholar]
- Ménétrey J, Macia E, Pasqualato S, Franco M, Cherfils J (2000) Structure of Arf6-GDP suggests a basis for guanine nucleotide exchange factors specificity. Nat Struct Biol 7: 466–469 [DOI] [PubMed] [Google Scholar]
- Ménétrey J, Perderiset M, Cicolari J, Dubois T, Elkhatib N, El Khadali F, Franco M, Chavrier P, Houdusse A (2007) Structural basis for ARF1-mediated recruitment of ARHGAP21 to Golgi membranes. EMBO J 26: 1953–1962 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merithew E, Hatherly S, Dumas JJ, Lawe DC, Heller-Harrison R, Lambright DG (2001) Structural plasticity of an invariant hydrophobic triad in the switch regions of Rab GTPases is a determinant of effector recognition. J Biol Chem 276: 13982–13988 [DOI] [PubMed] [Google Scholar]
- Montagnac G, Sibarita J-B, Loubéry S, Daviet L, Romao M, Raposo G, Chavrier P (2009) ARF6 Interacts with JIP4 to control a motor switch mechanism regulating endosome traffic in cytokinesis. Curr Biol 19: 184–195 [DOI] [PubMed] [Google Scholar]
- Myers KR, Casanova JE (2008) Regulation of actin cytoskeleton dynamics by Arf-family GTPases. Trends Cell Biol 18: 184–192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Neal CJ, Jobling MG, Holmes RK, Hol WG (2005) Structural basis for the activation of cholera toxin by human ARF6-GTP. Science 309: 1093–1096 [DOI] [PubMed] [Google Scholar]
- Panic B, Perisic O, Veprintsev DB, Williams RL, Munro S (2003) Structural basis for Arl1-dependent targeting of homodimeric GRIP domains to the Golgi apparatus. Mol Cell 12: 863–874 [DOI] [PubMed] [Google Scholar]
- Pasqualato S, Ménétrey J, Franco M, Cherfils J (2001) The structural GDP/GTP cycle of human Arf6. EMBO Rep 2: 234–238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price SR, Welsh CF, Haun RS, Stanley SJ, Moss J, Vaughan M (1992) Effects of phospholipid and GTP on recombinant ADP-ribosylation factors (ARFs). Molecular basis for differences in requirements for activity of mammalian ARFs. J Biol Chem 267: 17766–17772 [PubMed] [Google Scholar]
- Schonteich E, Pilli M, Simon GC, Matern HT, Junutula JR, Sentz D, Holmes RK, Prekeris R (2007) Molecular characterization of Rab11-FIP3 binding to ARF GTPases. Eur J Cell Biol 86: 417–431 [DOI] [PubMed] [Google Scholar]
- Schweizer Burguete A, Fenn TD, Brunger AT, Pfeffer SR (2008) Rab and Arl GTPase family members cooperate in the localization of the golgin GCC185. Cell 132: 286–298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiba T, Kawasaki M, Takatsu H, Nogi T, Matsugaki N, Igarashi N, Suzuki M, Kato R, Nakayama K, Wakatsuki S (2003) Molecular mechanism of membrane recruitment of GGA by ARF in lysosomal protein transport. Nat Struct Biol 10: 386–393 [DOI] [PubMed] [Google Scholar]
- Shiba T, Koga H, Shin HW, Kawasaki M, Kato R, Nakayama K, Wakatsuki S (2006) Structural basis for Rab11-dependent membrane recruitment of a family of Rab11-interacting protein 3 (FIP3)/Arfophilin-1. Proc Natl Acad Sci USA 103: 15416–15421 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stura EA, Graille M, Taussig MJ, Sutton BJ, Gore MG, Silverman GJ, Charbonnier J-B (2001) Crystallization of macromolecular complexes: stoiciometric variation screening. J Cryst Growth 232: 580–590 [Google Scholar]
- Takatsu H, Yoshino K, Toda K, Nakayama K (2002) GGA proteins associate with Golgi membranes through interaction between their GGAH domains and ADP-ribosylation factors. Biochem J 365: 369–378 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu M, Lu L, Hong W, Song H (2004) Structural basis for recruitment of GRIP domain golgin-245 by small GTPase Arl1. Nat Struct Mol Biol 11: 86–94 [DOI] [PubMed] [Google Scholar]
- Wu M, Wang T, Loh E, Hong W, Song H (2005) Structural basis for recruitment of RILP by small GTPase Rab7. EMBO J 24: 1491–1501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang T, Li S, Zhang Y, Zhong C, Lai Z, Ding J (2009) Crystal structure of the ARL2-GTP-BART complex reveals a novel recognition and binding mode of small GTPase with effector. Structure 17: 602–610 [DOI] [PubMed] [Google Scholar]
- Zhu G, Zhai P, Liu J, Terzyan S, Li G, Zhang XC (2004) Structural basis of Rab5–Rabaptin5 interaction in endocytosis. Nat Struct Mol Biol 11: 975–983 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary data
Review Process File