Abstract
The physiological function of Sentrin/SUMO-specific proteases (SENPs) remains largely unexplored, and little is known about the regulation of SENPs themselves. Here, we show that a modest increase of reactive oxygen species (ROS) regulates SENP3 stability and localization. We found that SENP3 is continuously degraded through the ubiquitin-proteasome pathway under basal condition and that ROS inhibit this degradation. Furthermore, ROS causes SENP3 to redistribute from the nucleoli to the nucleoplasm, allowing it to regulate nuclear events. The stabilization and redistribution of SENP3 correlate with an increase in the transcriptional activity of the hypoxia-inducing factor-1 (HIF-1) under mild oxidative stress. ROS-enhanced HIF-1 transactivation is blocked by SENP3 knockdown. The de-SUMOylating activity of SENP3 is required for ROS-induced increase of HIF-1 transactivation, but the true substrate of SENP3 is the co-activator of HIF-1α, p300, rather than HIF-1α itself. Removing SUMO2/3 from p300 enhances its binding to HIF-1α. In vivo nude mouse xenografts overexpressing SENP3 are more angiogenic. Taken together, our results identify SENP3 as a redox sensor that regulates HIF-1 transcriptional activity under oxidative stress through the de-SUMOylation of p300.
Keywords: p300, ROS, SENP3, SUMO2/3, ubiquitin–proteasome system
Introduction
Small ubiquitin-like modifier (SUMO) modification is an important post-translational protein modification that has gained much prominence due to the large number of SUMO substrates (Hay, 2005). SUMOylation is catalysed by SUMO-specific E1, E2 and E3 ligases, and is reversed by a family of Sentrin/SUMO-specific proteases (SENPs) (Yeh et al, 2000). SUMOylation can regulate a broad spectrum of cellular processes, including DNA replication/repair, cell division, cell signalling and nuclear transport. De-SUMOylation mediated by SENPs has been shown to have an important function in many of these processes as well (Cheng et al, 2004, 2005, 2007; Degerny et al, 2005; Di Bacco et al, 2006; Deyrieux et al, 2007; Dorval and Fraser, 2007; Halliwell, 2007; Haindl et al, 2008). For example, SENP1 deconjugates SUMO1 from hypoxia-inducible factor-1α (HIF-1α) to control its stability and has a critical function in the regulation of hypoxic response (Cheng et al, 2007). SENP3 is required for rRNA processing through deconjugation of SUMO2/3 from nucleophosmin (Haindl et al, 2008). Importantly, the conjugation and deconjugation of SUMO modification is a highly dynamic event, and only a small fraction of a substrate is SUMOylated at a given time (Hay, 2005). Apparently, the SUMOylation/de-SUMOylation balance for a specific substrate is delicately regulated. What triggers de-SUMOylation and how SENPs are regulated under various physiological and pathological conditions are, therefore, intriguing questions.
Oxidative stress, a common challenge to cellular homoeostasis, is mediated predominantly through the production of reactive oxygen species (ROS). Many extracellular insults, such as changes in temperature, pH, osmotic pressure, oxygen tension and sugar concentration, also lead to an increase in the production of intracellular ROS (Halliwell, 2003). Hypoxia itself paradoxically causes an increase in ROS (Chandel et al, 1998). The extent and duration of ROS increase usually determine the consequences of the cellular adaptive response to oxidative stress. Although severe oxidative stress causes cell senescence and even cell death, mild oxidative stress paradoxically promotes cell survival, during which global alterations of gene expression and protein post-translational modification occur (Halliwell, 2007). It has been reported that SUMO conjugation, in particular SUMO2/3 conjugation, is a major response to oxidative stress (Saitoh and Hinchey, 2000; Manza et al, 2004; Li et al, 2006; Dorval and Fraser, 2007; Cimarosti et al, 2008; Yang et al, 2008). Thus, it is highly likely that the balance between SUMOylation and de-SUMOylation may have a critical function in the cellular adaptive response to ROS production. It is unknown, however, whether SUMO2/3-specific protease, SENP3, is involved in the cellular response to oxidative stress (Tempe et al, 2008).
HIF-1, consisting of an oxygen-regulated α-subunit and a constitutively expressed β-subunit, is a master transcriptional regulator of gene expression in response to hypoxia. HIF-1 activation is a multistep process involving the stabilization of HIF-1α protein, dimerization of the HIF-α and HIF-β subunits, translocation to the nucleus, binding to the HIF-1 responsive elements (HRE), and the formation of active transcriptional complexes with the accessory proteins p300/CBP (Arany et al, 1996; Ebert and Bunn, 1998; Kallio et al, 1998; Yamashita et al, 2001; Freedman et al, 2002; Lando et al, 2002). Recent studies show that the ROS generated in mitochondria under hypoxia are both necessary and sufficient for HIF-1 activation (Ebert and Bunn, 1998; Chandel et al, 2000; Brunelle et al, 2005; Guzy et al, 2005; Mansfield et al, 2005; Guzy and Schumacker, 2006). In addition, HIF-1 activation occurs in response to a variety of environmental stimuli other than hypoxia (Zelzer et al, 1998; Chandel et al, 2000; Haddad and Land, 2001; Harris, 2002; Lu et al, 2002; Jung et al, 2003; Semenza, 2003; Wang et al, 2004; Kamat et al, 2005; Thomas and Kim, 2005; De Ponti et al, 2007), for instance, during inflammation (Cramer et al, 2003; Jung et al, 2003; Melillo, 2004; Walmsley et al, 2005) and insulin administration (Zelzer et al, 1998; Roth et al, 2004; Carroll and Ashcroft, 2006; Treins et al, 2006; Glassford et al, 2007; Zhou et al, 2007), in which ROS generated by NADPH oxidase are required (Shiose et al, 2001; Biswas et al, 2007; Xia et al, 2007). An immediate stabilization of HIF-1α is often observed on exposure to exogenous hydrogen peroxide (H2O2) or accompanying endogenous ROS generation (Chandel et al, 2000; Guzy et al, 2005). Moreover, HIF-1α accumulation induced by normoxic ROS generation is responsible for initiating the expression of an HRE-controlled luciferase reporter, as well as HIF-1 target genes that may relate to tumourigenesis and malignant phenotypes of cancer cells (Biswas et al, 2007; Gao et al, 2007; Xia et al, 2007; Guzy et al, 2008). Therefore, how ROS regulate HIF-1 activation under both hypoxia and normoxia is an important question. To date, the stabilization of HIF-1α is suggested as a mechanism by which ROS activate HIF-1 (Brunelle et al, 2005; Guzy et al, 2005; Mansfield et al, 2005). Whether other steps in HIF-1 activation may be promoted by ROS has not been addressed. We herein propose a new mechanism in which a mild oxidative stress induced by low doses of H2O2 can rapidly stabilize the SUMO2/3-specific protease, SENP3, and SENP3 in turn promotes the transcriptional activity of HIF-1 through deconjugation of SUMO2/3 from p300, the co-activator of HIF-1α. This mechanism has a critical function in HIF-1 activation under both normoxia and hypoxia, working independently of HIF-1α stabilization.
Results
Mild oxidative stress induces a rapid stabilization of SENP3 protein
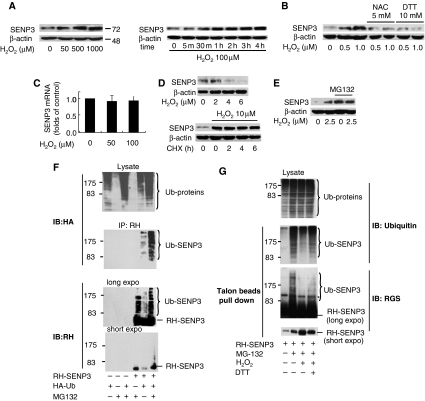
HeLa cells were exposed to various concentrations of H2O2 and the expression of SUMO2/3-specific protease, SENP3, was evaluated. The increase in SENP3 protein was seen after treatment with 50 μM H2O2, and this increase occurred in a dose-dependent manner (Figure 1A, left). A similar SENP3 increase could be observed in some non-tumour cells, for instance, human umbilical vein endothelial cells (HUVEC), but the required doses of H2O2 were much lower (2.5 μM) than in HeLa cells (data not shown). The obvious increase in SENP3 protein on H2O2 exposure was seen at 30 min, reached a plateau at 60 min, and remained stable over time (Figure 1A, right). Interestingly, other stresses, such as UV radiation, low pH, hypotonic and hypertonic, or hypothermal and hyper-thermal stimuli that increase ROS, and even hypoxia, also increased SENP3 protein levels (data not shown). As ROS generation is common to these stress inducers, we tested whether the increase in SENP3 protein level could be blocked by anti-oxidants such as N-acetyl cysteine (NAC), or the thiol-reducing agent dithiothreitol (DTT). Indeed, increase of SENP3 was blocked by NAC and DTT (Figure 1B), suggesting that the level of SENP3 protein is regulated by changes in redox state.
Figure 1.
Mild oxidative stress induces a rapid stabilization of SENP3 protein. (A) HeLa cells were treated with the indicated concentrations of hydrogen peroxide (H2O2) for 1 h (left panel) or 100 μM H2O2 for the indicated time (right panel). SENP3 protein level was evaluated by immunoblotting (IB) using SENP3 antibody. β-actin was used as a loading control. (B) HeLa cells were pre-treated with 5 mM NAC for 4 h or 10 mM DTT for 1 h, before H2O2 was added to the medium, for an additional 1 h. SENP3 protein levels were evaluated by IB. (C) HeLa cells were treated with the indicated concentrations of H2O2 for 1 h. SENP3 mRNA levels were evaluated by real-time PCR and shown as folds of control. (D) SENP3 protein levels were evaluated by IB after HUVEC cells were treated with cycloheximide (CHX) for different times (upper panel), or pre-treated with H2O2 for 2 h before CHX was added and co-incubated for the indicated time (lower panel). β-actin was used as a loading control. (E) SENP3 protein levels were evaluated by IB after HUVEC cells were treated with H2O2 as indicated for 1 h with or without 30 μM MG132. (F) HEK293T cells were transfected with RH-SENP3 or/and HA-Ubiquitin (HA-Ub) for 36 h, and MG132 was then added for additional 10 h as indicated. Co-IP was carried out with RH antibody and IB was carried out as indicated. The blots with long time of exposure (long expo) and short time of exposure (short expo) are displayed to show the differences in quantity between ubiquitin-conjugated and non-conjugated SENP3, respectively. (G) HEK293T cells were transfected with RH-SENP3 for 36 h. MG132, H2O2 and DTT were added as indicated for an additional 1 h. RH-SENP3 was pulled down with Talon beads and IB was carried out using anti-ubiquitin and anti-RGS antibodies, respectively.
The rapid increase in the SENP3 protein level by H2O2 could not be due to an increase in transcription, as the SENP3 messenger RNA remained unchanged following exposure to H2O2 (Figure 1C). We then tested whether SENP3 was regulated through a post-transcriptional mechanism. After exposure to the protein synthesis inhibitor, cycloheximide (CHX), SENP3 protein rapidly decreased and became undetectable after 6 h in HUVEC (Figure 1D, upper panel). However, if H2O2 was pre-incubated with cells for 2 h before CHX addition, the decrease in SENP3 protein level was blocked (Figure 1D, bottom panel), suggesting that the SENP3 protein was stabilized by H2O2. To test whether turnover of SENP3 protein was regulated by the ubiquitin–proteasome pathway, the proteasome inhibitor, MG132, was added to medium. The SENP3 protein level was increased when HUVEC cells were treated with MG132 alone and remained stable when exposed to a low concentration of H2O2 (Figure 1E). Immunoprecipitation (IP) was then carried out on HEK293T cells co-transfected with RGS-SENP3 and HA-ubiquitin, in the presence or absence of MG132. As shown in Figure 1F, addition of MG132 led to an accumulation of ubiquitin-conjugated SENP3 and an increase in total SENP3. These results indicate that SENP3 protein was being constantly ubiquitin-conjugated and degraded by the proteasome. Furthermore, endogenous ubiquitin conjugation of SENP3 was attenuated by H2O2 and partially reversed by DTT (Figure 1G). Taken together, these results indicate that the H2O2-mediated increase in SENP3 protein stability is because of the inhibition of the ubiquitin–proteasome pathway.
SENP3 redistributes between the nucleolus and the nucleoplasm on H2O2 exposure
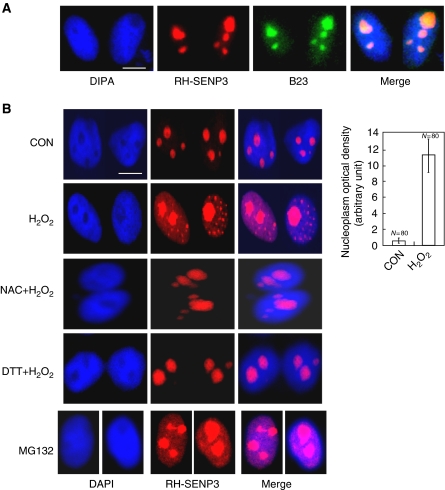
SENP3 is reported to be preferentially localized in the nucleoli (Nishida et al, 2000). We, therefore, check whether the localization of SENP3 can be altered when cells are exposed to H2O2. RGS-His (RH)-tagged SENP3 was overexpressed and detected using antibody against RH. We showed that most of the RH-tagged SENP3 co-localized with the nucleolus marker B23, in untreated cells as expected, and was almost invisible in the nucleoplasm (Figure 2A). However, SENP3 became visible in the nucleoplasm when cells were exposed to H2O2, seen as a dim dispersion with numerous bright foci, whereas SENP3 staining in the nucleoli did not decrease (Figure 2B). A similar redistribution of endogenous SENP3 was also observed by immunofluorescence in cells exposed to H2O2 (Supplementary Figure S1). Furthermore, SENP3 redistribution from the nucleoli to the nucleoplasm was reversed by pre-treating cells with NAC or DTT (Figure 2B), indicating that the localization of SENP3 was regulated by ROS. To determine the association between SENP3 localization and the ubiquitin–proteasome pathway, we then used MG132 to inhibit SENP3 degradation by the proteasome. Strikingly, MG132 led to SENP3 accumulation in the nucleoplasm in a pattern similar to that caused by H2O2 (Figure 2B, bottom panel). This implies that in resting cells, SENP3 is frequently transported from the nucleoli to the nucleoplasm where it is degraded. On oxidative stress, this degradation is blocked by inhibition of the ubiquitination of SENP3, which stabilizes SENP3 as it accumulates in the nucleoplasm.
Figure 2.
SENP3 redistributes between the nucleolus and the nucleoplasm on H2O2 exposure. (A) HeLa cells were transfected with RH-SENP3 for 36 h. Immunofluorescence was carried out with RH and B23 antibodies. B23 is a nucleolar marker. DAPI was used for counterstaining the nuclei. Scale bar=9 μm. (B) HeLa cells were transfected with RH-SENP3 for 36 h, and the cells were treated with H2O2 (100 μM) or MG132 (10 mM), respectively, for 1 h, or pretreated with 5 mM NAC for 4 h or 10 mM DTT for 1 h before H2O2 exposure for another 1 h. Immunofluorescence was carried out using RH antibody. DAPI was used for counterstaining the nuclei. Scale bar=9 μm. Bar charts showed the difference in the rhodamine intensity that reflected SENP3 quantity in the nucleoplasm in eighty cells with or without H2O2 treatment.
SENP3 participates in H2O2-induced global changes in SUMO2/3 modification and can regulate the SUMOylation status of HIF-1α
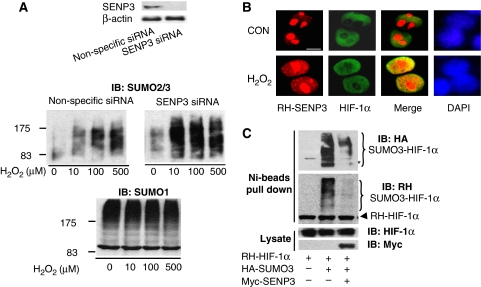
As SENP3 is a de-SUMOylating enzyme, its increase and redistribution following H2O2 exposure may affect the balance of protein SUMOylation/de-SUMOylation. Global SUMOylation patterns were examined in cells with intact or knocked-down SENP3 on exposure to H2O2 at a very low dose range. Knock down of SENP3 by siRNA was validated (Figure 3A, upper panel). In cells exposed to H2O2, SUMO2/3-conjugated proteins were gradually increased (Figure 3A, middle panel, left). However, in samples with SENP3 knocked down, SUMO2/3 conjugation was markedly increased following treatment with escalating doses of H2O2 (Figure 3A, middle panel, right). This suggests that in SENP3-intact cells, SENP3 normally antagonizes SUMO2/3 conjugation induced by ROS. In contrast, SUMO-1 conjugation is not changed by H2O2 in the same dose range (Figure 3A, bottom panel).
Figure 3.
SENP3 participates in H2O2-induced changes in SUMO2/3 modification and can interact with HIF-1. (A) HeLa cells were transfected with non-specific siRNA and SENP3 siRNA for 72 h, and SENP3 expression was markedly knocked down (upper panel). The cells were then treated with various concentrations of H2O2 for 1 h before global protein SUMOylation was evaluated using the SUMO2/3 antibody (middle panel) and the SUMO1 antibody (bottom panel). (B) After being transfected with HA-HIF-1α and RH-SENP3 for 48 h, HeLa cells were exposed to 100 μM H2O2 for 1 h. Cell monolayers were fixed and immunofluorescence was carried out using anti-HIF-1α and anti-RH antibodies. Cell nuclei were stained with DAPI. Scale bar=9 μm. (C) After HeLa cells were transfected with RH-HIF-1α with or without HA-SUMO3 and Myc-SENP3 as indicated for 48 h, cells were lysed and RH-HIF-1α was pulled down using Ni beads. The pulled-down HIF-1α and cell lysates were analysed by IB as indicated.
As our data (Figure 2 and Supplementary Figure 1) show that SENP3 accumulates in the nucleoplasm on H2O2 exposure, allowing for SENP3 de-SUMOylation activity in the milieu outside the nucleoli, and also because our previous studies have indicated the association of ROS with HIF-1, we checked whether HIF-1, an important nuclear protein, could be affected by SENP3. Immunoprecipitation showed that SENP3 could have physical interaction with HIF-1α (Supplementary Figure S2). Immunofluorescence staining showed that, although in resting cells SENP3 was mainly localized in the nucleolus and only slightly dispersed in the nucleoplasm, and HIF-1α was localized in the nucleoplasm, the co-existence of the two proteins in nucleoplasm was greatly enhanced by treatment with H2O2 (Figure 3B). Indeed, SENP3 could remove SUMO3 (Figure 3C), but not SUMO1 (data not shown), from HIF-1α. Collectively, these data indicate that SENP3 participates in the removal of SUMO2/3 from a number of proteins in the nucleoplasm under mild oxidative stress.
Mild oxidative stress enhances the transcriptional activity of HIF-1 through SENP3, but this action is not attributed to the de-SUMOylation of HIF-1α by SENP3.
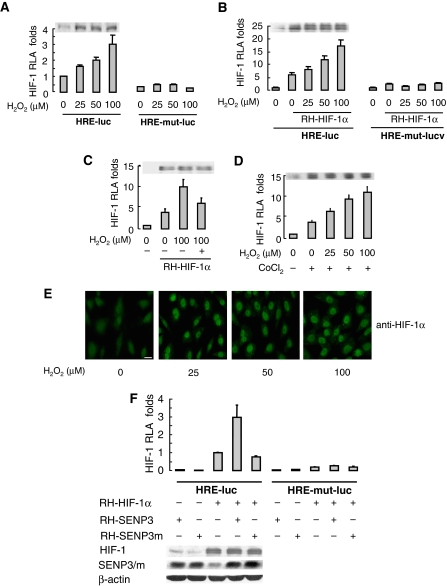
We then examined the association of SENP3 with HIF-1 activation under oxidative stress. On the basis of the above data, low concentrations of H2O2 that stabilize SENP3 (i.e. 50–100 μM in HeLa cells) were selected to mimic mild oxidative stress, and HIF-1 transactivation was assessed by luciferase reporter assay. When cells were exposed to 50 μM H2O2, the endogenous and ectopically expressed HIF-1α was immediately stabilized and the transcriptional activity of HIF-1 was induced. However, the transcriptional activity of HIF-1 continuously increased with increasing doses of H2O2, whereas the quantities of HIF-1α remained unchanged (Figure 4A and B). H2O2-enhanced HIF-1 transactivation could be blocked by NAC (Figure 4C). Moreover, HIF-1 transactivation induced by cobalt chloride (CoCl2) could also be enhanced by H2O2 exposure (Figure 4D). Immunofluorescent staining confirmed that when H2O2 was added, HIF-1α accumulated in the nucleus, but the quantity in the nucleus remained the same when H2O2 was further increased (Figure 4E). These results indicate that the transcriptional activity of HIF-1 can be enhanced by a modest ROS increase and this action is independent of HIF-1α protein level.
Figure 4a.
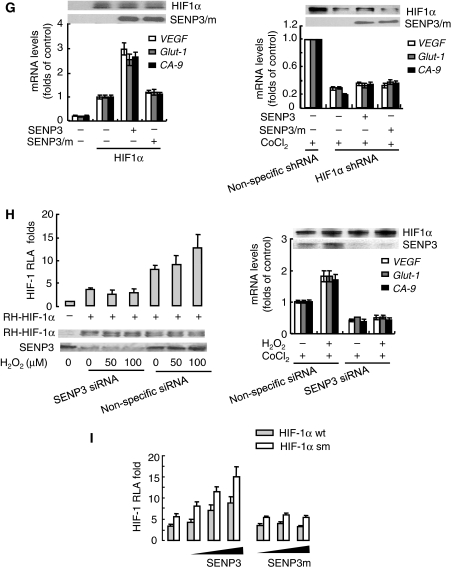
Mild oxidative stress enhances the transcriptional activity of HIF-1 through SENP3, but this action is not attributed to the de-SUMOylation of HIF-1α by SENP3. (A–D) HeLa cells were co-transfected with the constructs of luciferase reporters for HRE or HRE mutant, and Renilla, with or without RH-HIF-1α. At 40 h post-transfection, cells were exposed to the indicated concentrations of H2O2 and CoCl2, as described in Materials and methods section, before cells were lysed, and the relative luciferase activity (RLA) for HIF-1 was assayed. NAC, when used, was pre-incubated with cells for 4 h before H2O2 was added to the medium. The cell lysates of one of the representative experiments were subjected to IB with anti-HIF-1α antibody. The results of the RLA are given as the mean±s.d. of three independent experiments, and samples were duplicated in each experiment. (E) After HeLa cells were exposed to the indicated concentration of H2O2 for 1 h, cell monolayers were fixed and immunofluorescence was carried out using anti-HIF-1α antibody. Scale bar=10 μm. (F) HeLa cells were co-transfected with the constructs of luciferase reporters for HRE or HRE mutant, and Renilla, along with RH-HIF-1α, RH-SENP3 or RH-SENP3 mutant were additionally co-transfected as indicated. At 48 h post-transfection, cells were lysed and HIF-1 RLA was assayed.
As the same concentrations of H2O2 can enhance HIF-1 transactivation and concomitantly stabilize SENP3, the causal relationship between SENP3 and HIF-1 transactivation needs to be determined. As shown in Figure 4F, the HIF-1 transactivation was enhanced by overexpression of wild-type SENP3, but not by the SENP3 mutant lacking de-SUMOylation activity, indicating that the enhancement of HIF-1 transcriptional activity required SENP3 de-SUMOylation activity. The quantity of HIF-1α was not changed by the overexpression of SENP3. Furthermore, expression of HIF-1 target genes, including vascular endothelial growth factor (VEGF), Glut-1 and CA-9 was also upregulated by the overexpression of SENP3, and this upregulation could be reversed by ablation of endogenous HIF-1α using shRNA (Figure 4G). Strikingly, when endogenous SENP3 was depleted by siRNA, H2O2 could no longer boost the transcriptional activities and the target gene expression of HIF-1 at all, even if the accumulation of HIF-1α remained unchanged (Figure 4H). These results suggest that H2O2-enhanced HIF-1 transcriptional activity is mediated by SENP3, and this led us to hypothesize that SENP3 may de-SUMOylate HIF-1α to achieve this effect. However, to our surprise, enhancement of HIF-1 transcriptional activity by overexpression of SENP3 did not disappear in cells co-expressing mutant HIF-1α that had mutated SUMOylation sites; the increase in transcriptional activity seemed to be as effective as in cells expressing the wild-type HIF-1α and was even more robust (Figure 4I). This indicates that effect of SENP3 is not dependent on de-SUMOylation of HIF-1α.
Figure 4b.
(G) HeLa cells were co-transfected with the constructs for RH-HIF-1α, with or without RH-SENP3 or RH-SENP3 mutant as indicated (left panel), or with shRNA to knock down endogenous HIF-1α (right panel). At 48 h after transfection of SENP3 constructs, or 42 h post-transfection of HIF-1α shRNA, plus treatment with 150 μM CoCl2 for 6 h, cells were collected for real-time PCR to determine the expression of three major HIF-1 target genes as indicated. The levels of mRNA were shown as folds of control. (H) HeLa cells were co-transfected with the constructs of luciferase reporters for HRE and Renilla, and RH-HIF-1α, together with SENP3 siRNA or non-specific siRNA as indicated. At 72 h post-transfection, cells were treated with the indicated concentrations of H2O2 before they were lysed and HIF-1 RLA was assayed (left panel). HeLa cells were transfected with siRNA to knock down endogenous SENP3 (right panel). At 66 h after transfection of siRNA, plus treatment with 150 μM CoCl2 for 6 h, cells were collected for real-time PCR to determine the expression of the HIF-1 target genes as indicated. (I) HeLa cells were transfected with the constructs of luciferase reporters for HRE and Renilla, along with wild type (wt) HIF-1α, or SUMOylation sites-mutated (sm) HIF-1α (K391, 477R) with or without increasing amounts of RH-SENP3 or RH-SENP3 mutant as indicated. At 48 h post-transfection, the cells were lysed and RLA was assayed.
The effect of SENP3 on the enhancement of HIF-1 transcriptional activity depends on p300 and its de-SUMOylation
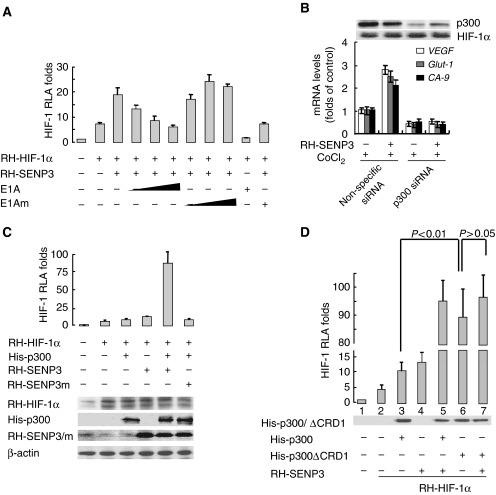
We then searched for the target of SENP3 among the proteins related to HIF-1. As p300 is one of the master co-activators of HIF-1 (Arany et al, 1996; Lando et al, 2002) and its activity can be inhibited by the conjugation of SUMO (Girdwood et al, 2003), we tested whether p300 mediates the effect of SENP3 on HIF-1 transactivation. A luciferase reporter assay showed that the enhancement of HIF-1 transactivation by SENP3 was blocked by E1A protein, a specific inhibitor for p300, in a dose-dependent manner, whereas the E1A mutant had no such inhibitory effect (Figure 5A). More specifically, depletion of endogenous p300 by siRNA abolished the SENP3 caused-enhancement of HIF-1 target gene expression (Figure 5B). Furthermore, co-transfection of SENP3 and p300 dramatically increased HIF-1 transactivation, which showed a synergistic effect (Figure 5C). As the effect of SENP3 on HIF-1 transcriptional activities may be mediated by p300, a SUMO-less, truncated p300 with a His tag, His-p300ΔCRD1, was used to determine the association of SUMOylation status of p300 with HIF-1 transcriptional activities. Interestingly, p300ΔCRD1 could boost HIF-1 transcriptional activity dramatically compared with the wild-type, full-length p300, and p300ΔCRD1 co-transfection with SENP3 was no longer able to increase HIF-1 transcriptional activity further (Figure 5D). Taken together, these data indicate that either non-SUMOylated or de-SUMOylated forms of p300 help to increase HIF-1 transcriptional activity.
Figure 5.
The effect of SENP3 on the enhancement of HIF-1 transcriptional activity depends on p300 and its de-SUMOylation. (A) HeLa cells were co-transfected with the constructs of luciferase reporters for HRE and Renilla, RH-HIF-1α and RH-SENP3, and increasing amounts of E1A or E1A mutant as indicated. At 48 h post-transfection, the cells were lysed and HIF-1 RLA was assayed. (B) HeLa cells were transfected with siRNA to knock down endogenous p300. At 66 h post-transfection of siRNA, cells were incubated with 150 μM CoCl2 for 6 h and then collected for real-time PCR. (C) HeLa cells were co-transfected with the constructs of luciferase reporters for HRE and Renilla, RH-HIF-1α and RH-SENP3 or its mutant. His-p300 was additionally co-transfected as indicated. At 48 h post-transfection, the cells were lysed and HIF-1 RLA was assayed. (D) HeLa cells were co-transfected with the constructs of luciferase reporters for HRE and Renilla, RH-HIF-1α and RH-SENP3. His-p300 or His-p300ΔCRD1 were additionally co-transfected as indicated. p300ΔCRD1 is a p300 truncate that lacks the domain containing two sites for SUMOylation and is thus unable to be SUMOylated. At 48 h post-transfection, the cells were lysed and HIF-1 RLA was assayed. The results showed that overexpression of p300ΔCRD1 (the sixth bar, left to right) alone could promote the HIF-1 transactivation to an extent much higher than what its full-length counterpart did (compare the sixth bar with the third, with statistically significant difference), and comparable to the synergistic effect of SENP and full-length p300 (compare the sixth bar with the fifth). SENP3 could not further promote the enhancing effect of p300ΔCRD1 (compare the sixth bar with the seventh, with statistically insignificant difference).
p300 is a direct substrate for SENP3 and de-SUMOylation of p300 potentiates its interaction with HIF-1
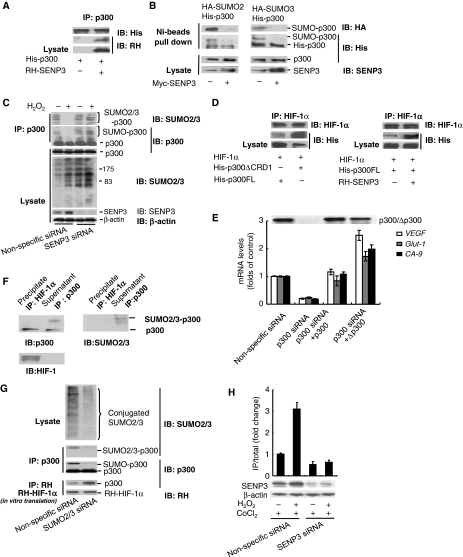
p300 is reported to have a SUMO1, SUMO2 and SUMO3 modification (Girdwood et al, 2003), but whether SENP3 can affect SUMO2/3 modification of p300 is not clear. As shown in Figure 6A, p300 and SENP3 interacted physically. SENP3 could not deconjugate SUMO1 from p300 (data not shown), whereas SUMO2/3-conjugated p300 was a direct substrate for SENP3 (Figure 6B). After cells were exposed to a low concentration of H2O2, endogenous SUMO2/3 modification of endogenous p300, that was immunoprecipitated, was obviously decreased. In contrast, SUMO2/3 modification of proteins in cell lysates was enhanced. It is clear that deconjugation of SUMO2/3 from p300 caused by H2O2 was mediated by SENP3, because when endogenous SENP3 was silenced, H2O2 could no longer trigger de-SUMOylation on p300, although SUMO2/3 modification of p300 under basal condition seemed to change modestly (Figure 6C). These imply that deconjugation of SUMO2/3 from p300 occurs specifically under a mild oxidative stress. Meanwhile, the specificity of SUMO isopeptidases was verified through comparing SUMO2/3 modification of endogenous p300 in SENP1−/−/SENP2−/− mouse embryonic fibroblasts (MEF) (Supplementary Figure S3) and SENP3-knocked-down HEK293 cells (Figure 6C), respectively. The results indicate that SENP1 and SENP2 can affect global SUMO2/3 conjugations (see the lysates), but SENP1 is not responsible for deconjugation of SUMO2/3 from p300, and SENP2 might deconjugate to some extent under basal condition (see SUMO2/3 conjugation on p300 by IP) (Supplementary Figure S3). Deconjugation of SUMO2/3 from p300 in response to oxidative stress is uniquely executed by SENP3 (Figure 6C).
Figure 6.
p300 is a direct substrate of SENP3 and de-SUMOylation of p300 potentiates its interaction with HIF-1. (A) HeLa cells were transfected with the constructs for His-p300 alone or RH-SENP3 as indicated. At 48 h post-transfection, the cells were lysed and co-IP was carried out with anti-p300 antibody. Bound proteins and cell lysates were analysed by IB as indicated. (B) HeLa cells were co-transfected with His-p300, and HA-SUMO2 or HA-SUMO3, with or without Myc-SENP3 as indicated. At 48 h post-transfection, the cells were lysed and a pull-down assay was performed using Ni-beads. Bound proteins and cell lysates were analysed by IB as indicated. (C) HEK293T cells were transfected with non-specific siRNA or SENP3 siRNA for 72 h to deplete endogenous SENP3. The cells were treated with 100 μM H2O2 for 1 h before they were lysed and p300 was pulled down using p300 antibody. Bound proteins and cell lysates were analysed by IB as indicated. (D) HeLa cells were transfected with HIF-1α and His-p300, and His-p300ΔCRD1 (left panel) or RH-SENP3 (right panel) as indicated. At 48 h post-transfection, the cells were lysed and IP was carried out using anti-HIF-1α and IB was carried out as indicated. (E) HeLa cells were co-transfected with siRNA specific to endogenous p300 and/or wild-type or truncated p300 constructs as indicated for 72 h. The expressions of three major HIF-1 target genes were determined by real-time PCR. (F) HeLa cells were transfected with HA-SUMO3 for 48 h. Endogenous p300 that bound with HIF-1α in HeLa cells was immunoprecipitated using anti-HIF-1α antibody, whereas p300 in the rest portion, that is, in the supernatant was immunoprecipitated using anti-p300 antibody. Samples were then detected by anti-p300, anti-SUMO2/3 and anti-HIF-1α antibodies, respectively. (G) HeLa cells were transfected with non-specific siRNA or siRNAs for SUMO2 and SUMO3. At 72 h post-transfection, the cells were lysed and p300 was immunoprecipitated using anti-p300 antibody. The efficiency of SUMO2/3 silencing was evaluated in whole-cell lysates with anti-SUMO2/3 antibody. The SUMOylation status of p300 was determined using anti-SUMO2/3 and anti-p300 antibodies. The whole-cell lysates were then incubated with in vitro-translated RH-tagged HIF-1α for 1 h. IP was carried out using anti-RH antibody, and IB was carried out using anti-p300 and anti-RH antibodies. (H) Endogenous SENP3 was knocked down by siRNA and cells were exposed to H2O2 for 1 h. p300 that was recruited to HRE DNA was immunoprecipitated using anti-p300 antibody. The bound HRE in the precipitates were then quantitatively analysed by real-time PCR.
We then further examined how the SUMOylation state of p300 affected its interaction with HIF-1α. After overexpression of HIF-1α with the truncated p300ΔCRD1 or the full-length p300, the amount of p300ΔCRD1 bound to HIF-1α was more abundant than full-length p300 (Figure 6D, left). Likewise, after overexpression of HIF-1α and p300 with or without SENP3, the amount of p300 bound to HIF-1α was more abundant in samples with SENP3 than in samples without SENP3 (Figure 6D, right). To further confirm whether a SUMO-less p300 would enhance the expression of HIF-1 target genes, endogenous p300 was knocked down by siRNA, and siRNA-resistant full-length p300 or p300ΔCRD were used to rescue. As predicted, p300ΔCRD promoted HIF-1 target gene expression more strongly than its wild-type counterpart (Figure 6E). Furthermore, we immunoprecipitated endogenous HIF-1α, and then examined the SUMO2/3 modifications on endogenous p300 bound with HIF-1α in the precipitates and on the rest of p300 in the supernatant, respectively. Immunoblots showed that p300 bound with HIF-1α was non-SUMOylated, whereas that in the rest portion was SUMOylated (Figure 6F). These data indicate that p300 that cannot be SUMOylated or that is de-SUMOylated by SENP3 has preferential binding to HIF-1α. Next, in vitro-expressed HIF-1α was immunoprecipitated and incubated with the whole-cell lysates derived from cells with intact or silenced SUMO2/3 expression. After interaction in vitro, HIF-1α and p300 were co-immunoprecipitated, and HIF-1α, p300 and SUMO2/3 were evaluated. The results indicate that the non-SUMOylated, rather than the SUMOylated, p300 binds favourably with HIF-1α (Figure 6G). In these experiments it was notable that, for unknown reasons, immunoblotting for SUMO2/3 modification conjugated to p300 appeared as a single band in HeLa cells, regardless of whether p300 was exogenously expressed (Figure 6B) or endogenous (Figure 6F), but it appeared as multiple bands in HEK293T cells (Figure 6C). Fortunately, the antibodies against SUMO2/3 or the specific tags verified the nature of these bands. Finally, chromatin IP (ChIP) was carried out to determine whether SENP3 affected the recruitment of p300 to HIF-1-dependent target genes. The result showed that an increased recruitment of p300 induced by H2O2 was blocked in SENP3-depleted cells (Figure 6H).
Overexpression of SENP3 promotes in vivo angiogenesis in tumour xenografts
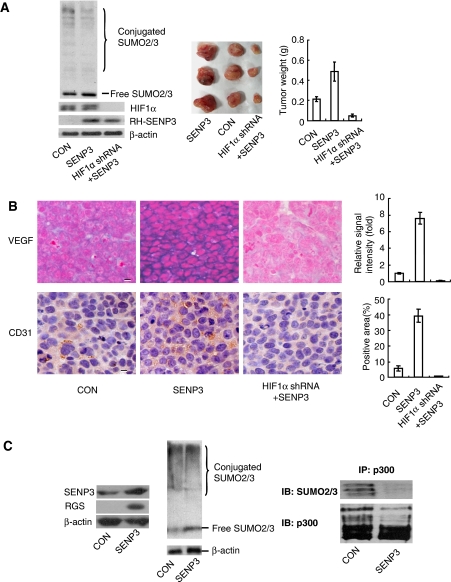
The above data indicate that it is SENP3 that mediates H2O2-induced enhancement of HIF-1 transcriptional activity. We, therefore, intended to see whether overexpressing SENP3 could promote in vivo angiogenesis, a phenotype controlled predominantly by the HIF-1 target gene VEGF, in nude mice. Before cells were inoculated into nude mice, overexpression of SENP3, SUMO2/3 modification pattern or depletion of HIF-1α in these cells was verified (Figure 7A, left). Tumour derived from cells stably overexpressing SENP3 grew larger and showed more potent angiogenesis, as compared with controls. However, in cells overexpressing SENP3 but depleting HIF-1α these characteristics were completely ablated, and even basal tumour growth was blocked (Figure 7A, middle and right). In SENP3 overexpressing tumours, in situ hybridization indicated stronger expression of VEGF mRNA in tumour cells, and CD31 immunohistochemistry indicated a more abundant volume of mouse capillary endothelial cells (Figure 7B). In accord with these alterations in phenotypes, SENP3 expression level and SUMO2/3 modification pattern (Figure 7C, left and middle), the general expression of HIF-1 target genes was promoted in SENP3-overexpressing tumours (Supplementary Figure S4), and the endogenous p300 that was immunoprecipitated from the SENP3-overexpressing tumour tissues bore a remarkably attenuated SUMO2/3 conjugation (Figure 7C, right), suggesting that the enhanced malignant phenotypes driven by HIF-1 might be correlated with the de-SUMOylation of p300 by SENP3.
Figure 7.
Overexpression of SENP3 promotes in vivo angiogenesis in tumour xenografts. (A) HeLa cells were stably transfected with empty vector+non-specific shRNA (as a control group), SENP3+non-specific shRNA (as SENP3 group) or SENP3+HIF-1α shRNA (as SENP3+HIF-1α shRNA group). Cell colonies were examined for specificity and efficiency of protein expressions and SUMO2/3 modification pattern (left panel). The cells were injected subcutaneously into the left flank of 4-week-old BALB/c-nu-nu mice. After 3 weeks, the mice were sacrificed and the tumour xenografts were dissected (middle panel). The bar chart showed mean±s.d. of the tumour weights (right panel). (B) In situ hybridization for human VEGF mRNA (left, upper panel, blue-purple signal), and immunohistochemistry for mouse CD31 (left, lower panel, yellow-brown signal) in tumor tissues. The bar charts showed the relative hybridization signal intensity (right, upper panel) and the areas positive for CD31 (right, lower panel) in tumour tissues. Scale bar=10 μM. (C) The mixed tumour tissues were homogenized. The enforced-expressed SENP3 (left panel) and SUMO2/3 modification pattern (middle panel) were examined by IB. P300 was immunoprecipitated using anti-p300 antibody, and examined for SUMOylation status by IB as indicated (right panel).
Discussion
ROS, SUMOylation and SENP3
Redox regulation of protein SUMOylation has been implicated recently in several investigations. Bossis and Melchior (2006) show that ROS, at low concentrations, result in the rapid disappearance of most SUMO1 conjugates, which is due to direct and reversible inhibition of SUMO conjugating enzymes. Their study has attributed a decrease in SUMOylation under oxidative stress to a decrease in SUMO1 conjugation. Xu et al (2008) have described that SUMO proteases, including human SENP1 and SENP2, are inhibited under oxidizing conditions. In this study, we have identified a new mechanism in which ROS can also regulate SUMOylation/de-SUMOylation by controlling the stability and localization of SENP3. It is interesting to note that the concentrations that induce SENP3 stabilization in HeLa cells in our study (50 or 100 μM) are 10–20-fold lower than the levels that cause inactivation of SUMO-1 conjugation ligase (1 mM) (Bossis and Melchior, 2006). The effective concentrations in non-tumor cells are even lower in this study (10 μM), and doses 1000-fold lower than that cause inactivation of SENP1 (10 mM) (Xu et al, 2008). This suggests that SENP3 is more sensitive to mild oxidative stress than SENP1, SENP2 or SUMO1-conjugating enzyme, and is more likely to function under physiological conditions.
Although SUMO2/3 modification patterns are specifically responsive to stresses induced by H2O2, mild heat stress, UV radiation and genotoxic agents (Saitoh and Hinchey, 2000; Sramko et al, 2006), it is not clear whether deconjugation of SUMO2/3 occurs upon oxidative stress. This study shows that the SUMO2/3-specific protease, SENP3, is rapidly stabilized under a mild oxidative stress induced by very low concentrations of H2O2 or other stimuli. Our findings are in agreement with the concept that SENPs may act as redox sensors (Xu et al, 2008), but they suggest an alternative way of ‘sensing'.
We find that SENP3 is endogenously ubiquitinated and subjected to proteasome degradation under basal conditions. During the preparation of this paper, (Kuo et al, 2008) had reported that in an enforced expression 293T cell system, SENP3 can be degraded by the ubiquitin–proteasome system. These findings explain why SENP3 is expressed at low levels in the basal state. As SUMO2/3 conjugation is rapidly induced by various stress responses (Saitoh and Hinchey, 2000; Manza et al, 2004; Li et al, 2006; Dorval and Fraser, 2007; Cimarosti et al, 2008; Yang et al, 2008), SUMO2/3 deconjugation by SENP3 requires a prompt means to regulate this process. ROS can quickly stabilize SENP3, which allows for a new SUMOylation/de-SUMOylation balance in response to oxidative stress. Indeed, the de-SUMOylating activity of SENP3 has a function in forming a SUMO2/3 modification pattern for the global profile, as well as for the specific target proteins on H2O2 exposure.
We also show that SENP3 redistributes in the subnuclear compartments on H2O2 treatment. It is likely that SENP3, although preferentially residing in the nucleolus, is continuously degraded in the nucleoplasm in the basal state. ROS inhibit SENP3 ubiquitination and block ubiquitin–proteasome-mediated degradation, thus causing SENP3 accumulation outside of the nucleolus. Redistribution of SENP3 may enable SENP3 to de-SUMOylate its extra-nucleolar substrates and to regulate related nuclear events, one of which is the activation of HIF-1 under oxidative stress.
ROS, HIF-1 and SENP3
It has been shown that ROS, generated by mitochondria or NADPH oxidase, are required for HIF-1α accumulation in response to hypoxia or growth-factor stimulation (Ebert and Bunn, 1998; Chandel et al, 2000; Shiose et al, 2001; Park et al, 2003; Biswas et al, 2007), despite controversial documentation (Liu et al, 2004; Tuttle et al, 2007). A recent in vivo study by Gao et al (2007) has shown that antioxidants diminish tumourigenesis through inhibition of HIF-1, strongly implying that increased ROS generation is responsible for HIF-1 activation and the tumourigenic phenotype. HIF-1α stability is regulated through its hydroxylation by the HIF prolyl hydroxylases (PHDs). Although how ROS stabilize HIF-1α is not clear, suppression of PHDs by ROS is apparently relevant. Gerald et al (2004) have reported that in junD-null cells, the accumulation of H2O2 reduces the activity of HIF PHDs that target HIF-1α for degradation, and subsequently, HIF-1α proteins accumulate and enhance the transcription of HIF-1 target genes. Stabilization of HIF-1α through inhibition of PHDs, thus, would be the only mechanism suggested to date by which ROS activate HIF-1 transactivation (Brunelle et al, 2005; Guzy et al, 2005; Mansfield et al, 2005; Gao et al, 2007). However, HIF-1 activation is regulated by multiple processes in addition to HIF-1α stabilization by PHDs. SUMO1 conjugation has been indicated to increase HIF-1α stabilization and transcriptional activity (Bae et al, 2004). Conversely, HIF-1α proteins require de-SUMOylation by SENP1 to ensure their hypoxic accumulation within the nucleus (Cheng et al, 2007). Moreover, HIF-1α–HIF-1β dimers do not show transcriptional activity unless they bind with accessory proteins p300/CBP to form active transcriptional complexes (Arany et al, 1996; Ebert and Bunn, 1998; Kallio et al, 1998). Whether ROS can regulate HIF-1 activation at these steps has not been addressed. In this study, we find that HIF-1α becomes stable on exposure to exogenous H2O2. Furthermore, HIF-1 transcriptional activity is augmented up to 3–5-fold by ROS in a dose-dependent manner, regardless of the unchanging quantity of HIF-1α protein after the initial stabilization (see Figure 4A–E). We, therefore, try to discriminate the role of ROS in promoting HIF-1 transactivation from the stabilization of HIF-1α.
One of the most conspicuous findings of this study is that after endogenous SENP3 is silenced, the H2O2-enhanced transcriptional activities of HIF-1 are entirely abolished, even if the HIF-1α protein level remains stable (Figure 4H). This previously undescribed phenomenon suggests that removal of SUMO2/3 by SENP3 is essential for ROS-induced HIF-1 activation, and it also implies that stabilization of HIF-1α is required but not sufficient for ROS-induced HIF-1 activation.
Although we find that HIF-1α can be modified by SUMO2/3, and SENP3 can deconjugate SUMO2/3 from HIF-1α, this de-SUMOylation turns out not to account for HIF-1 transactivation enhanced by SENP3. Overexpression of SENP3 can enhance HIF-1 transcriptional activity in cells co-expressing mutant HIF-1α that lack the sites for SUMOylation as well, even more obviously than in wild type (Figure 4I). This may be explained by previous findings that imply that when HIF-1α lose SUMO modification, it becomes more stable in the nucleus and thus, more potent in transcriptional activity (Cheng et al, 2007). Regardless, the effect of SENP3 of enhancing HIF-1 transcriptional activity is confirmed by the upregulation of a series of HIF-1 target genes in vitro, and finally by a potentiated in vivo angiogenesis in tumours stably overexpressing SENP3. It is, therefore, necessary to examine the association of ROS and SENP3 with the co-activator of HIF-1, p300.
ROS, de-SUMOylation and p300
Oxidative stress causes redistribution of SENP3 to the nucleoplasm where SENP3 can regulate SUMOylation status of p300. p300 functions as general transcriptional co-activator and is involved in multiple signal-dependent transcription events (Chan and La Thangue, 2001). p300 has been known for some time to be phosphorylated (Chan and La Thangue, 2001) and has recently been reported to be modified by three types of SUMO (Girdwood et al, 2003). However, little is known about how these post-translational modifications are regulated. We show in this study that, upon exposure to low doses of H2O2, SUMO2/3 are deconjugated from p300 in a process mediated by SENP3, in spite of an increase in global cellular SUMO2/3 conjugation.
To coordinate HIF-1 transactivation, complex interplays of other transcriptional factors, for instance, p53 or FOXO3, with p300 may be needed (Chan and La Thangue, 2001; Emerling et al, 2008). However, whether the post-translational modifications of p300 are associated with its function as a co-activator for HIF-1 has never been reported. We find that p300 de-SUMOylation is beneficial for its binding to HIF-1. Thus, we report here that p300 deconjugation of SUMO2/3 by SENP3 represents a new mechanism underlying the increase of HIF-1 transcriptional activity in response to a mild oxidative stress.
As p300 serves as co-activator of multiple transcriptional factors (TFs), based on the literature (Freedman et al, 2002), we chose several TFs and used available luciferase reporters to investigate whether SENP3 affected their transcriptional activity. p53, NF-κB and Stat3, but not AP-1, were affected by SENP3, but the effect varies (Supplementary Figure S5). This indicates that de-SUMOylation of p300 by SENP3 is not necessarily beneficial for all TFs. In addition, some of these TFs themselves may be direct substrates of SENP3, which makes the regulation more complex.
It has been shown that modifications at two sites in the HIF-1α transactivation domain may affect HIF-1α binding with p300: one is the hydroxylation of asp 803, and another is the S-nitrosation of cys 800 (Lando et al, 2002; Yasinska and Sumbayev, 2003). In addition, a conserved cysteine in the C-terminal activation domain of HIF-1α must be kept in the form of a free thiol, allowing for its interaction with p300, which is redox sensitive (Ema et al, 1999). Whether these regulations have a function in ROS-enhanced p300/HIF-1 interaction is worth further study. However, given that the ROS-enhanced transcriptional activities of HIF-1 are entirely abolished after silencing of endogenous SENP3, the mechanism of 300 de-SUMOylation, that we report in this study, is critical in HIF-1 activation regulated by ROS.
ROS are usually increased in cancer cells due to oncogene activation, relative lack of blood supply or other variances, and may be increased under various physiological or pathological conditions in non-cancer cells, including growth-factor stimulation, inflammation, ischaemia and ischaemia/reperfusion. HIF-1 activation serves as a significant cellular adaptive response to a modest, non-fatal ROS increase, which may result in angiogenesis, cell proliferation and other behaviours somehow related to transformation in non-cancerous cells and malignant phenotypes in cancer cells. We agree that HIF-1α stabilization is an essential basis for HIF-1 activation in these contexts, but the transcriptional activity of HIF-1 will not be guaranteed if another regulation is absent, such as the ROS-triggered stabilization of SENP3 to execute de-SUMOylation of p300. In addition, as soon as HIF-1α accumulates to saturation, this mechanism provides a fine-tuning modulation in response to further subtle increases of ROS. Therefore, our findings may show a fundamental regulation of HIF-1 by ROS under diverse conditions, regardless of hypoxia or normoxia, and especially in cancers.
Conclusions
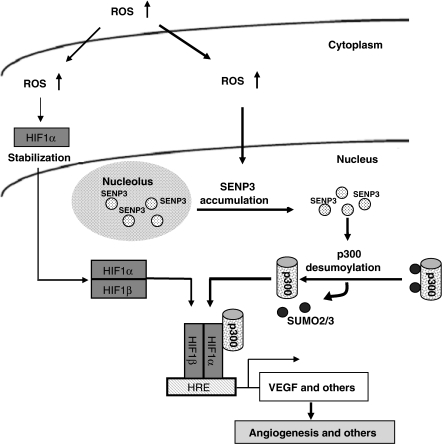
SUMO2/3-specific protease, SENP3, is rapidly stabilized and redistributed from the nucleolus to the nucleoplasm in response to mild oxidative stress, thus serving as a redox sensor. The increased nucleoplasmic SENP3, in turn, acts as an effector to enhance HIF-1 transcriptional activity by de-SUMOylating p300. This, in addition to HIF-1α stabilization, constitutes a second mechanism for HIF-1 transactivation. Meanwhile, this seems to be a fine-tuning regulation in HIF-1 activation in response to a mild oxidative stress (illustrated in Figure 8).
Figure 8.
The hypothetical illustration for a role of SENP3 and de-SUMO2/3 of p300 in ROS-enhanced HIF-1 transactivation.
Materials and methods
Cell culture and treatments
HeLa and HEK293T cells were cultured in Dulbecco's modified Eagle's medium (GibcoBRL, Gaithersburg, MD) supplemented with 10% newborn calf serum (Biochrom AG, Germany). HUVEC were cultured in M200 medium containing low-serum growth supplement (Cascade, Portland, OR). All media were supplemented with 100 U/ml penicillin and 100 mg/l streptomycin. Cells were maintained at 37 °C in a humidified atmosphere with 5% CO2 at 37°C.
SENP1−/− MEF were obtained and cultured as described previously (Cheng et al, 2007). SENP2−/− MEF were obtained and cultured similarly.
To examine the association with ROS, cells were treated with anti-oxidant NAC and DTT. When used, NAC was administered 4 h and DTT was administered 2 h before other treatments.
Constructs and transient transfections
The constructs are described in Supplementary data. The construct were transiently transfected or co-transfected into cells using Lipofectamine 2000 (Invitrogen, Carlsbad, CA) according to the manufacturer's instructions.
Immunoblotting (IB)
Cells were lysed in sample solution. Proteins were separated on 7.5, 10 or 12% SDS–PAGE gels, transferred to nitrocellulose membranes, and bands were detected using various antibodies as indicated. The antiserum against SENP3 was prepared using a method similar to that for SENP5 (Gong and Yeh, 2006). Other antibodies used for IB are described in Supplementary data. The membranes were incubated with the primary antibodies at 4 °C overnight and horseradish peroxidase-conjugated secondary antibodies (Santa Cruz Biotechnology, Santa Cruz, CA) for 2 h at the room temperature (RT) before detection using an enhanced chemiluminescence (ECL) system (Pierce Biotechnology, Rockford, IL).
Reverse transcription and real-time PCRs
Reverse transcription was carried out; quantitative real-time PCR was carried out on the ABI Prism 7300 system (Applied Biosystems, Foster City, CA) using SYBR Green and following the manufacturer's instructions. The primers and the procedures are described in Supplementary data.
Luciferase reporter assay
Cells were transfected with a luciferase reporter specific for HRE. Relative luciferase activity was assayed as described previously (Yang et al, 2004; Yi et al, 2004; Huang et al, 2008).
Under normoxia condition, in the absence of ectopically expressed HIF-1α, cells were treated with H2O2 as indicated, every 30 min for 6 h to ensure the accumulation of HIF-1α, according to the method described by Chandel et al (2000). Under chemical hypoxia caused by CoCl2 treatment, cells were pre-treated with 150 μM CoCl2 for 6 h and then treated with H2O2 only once for 1 h at various concentrations.
Immunoprecipitation, co-immunoprecipitation, Talon beads and Ni-beads pull down
IP, co-IP, Talon beads and Ni-beads pull down were carried out as described in Supplementary data. Briefly, transfected cells were lysed in IP or denaturing buffers, and immunoprecipitated using specific antibodies or beads as indicated. The immunoprecipitated or pulled-down extracts were washed three times, mixed with loading buffer and subjected to SDS–PAGE analysis. Proteins were detected using various antibodies as indicated.
Immunofluorescence
Immunofluorescence procedures were described in Supplementary data. Rhodamine intensity, that reflected SENP3 protein quantity in the nucleoplasm, was estimated in eighty cells by a Zeiss KS400 Version 2.2 software.
siRNA
siRNA specific for SENP3, HIF-1α and p300, and control non-specific siRNA oligonucleotides were synthesized (RIBOBIO, China). The sequences of siRNA oligonucleotides are described in Supplementary data. Cells were transfected with siRNA oligonucleotides using Lipofectamine 2000.
In vitro expression of HIF-1α and assay for HIF-1/p300 interaction
RH-HIF-1α was in vitro translated, and it's binding with p300, and the SUMOylation status of p300 were analysed as described in Supplementary data.
ChIP assay
To evaluate whether SENP3 affected p300 recruitment to HRE of HIF-1 target genes, ChIP assay was carried out on SENP3 intact or depleted cells with or without H2O2 treatment using a method described in Supplementary data.
SENP3 stable-expressing cells with intact or depleted HIF-1α and xenografts in nude mice
To obtain cells overexpressing SENP3 with intact or depleted HIF-1α, the SENP3 expression construct and HIF-1α shRNA or empty constructs (Song et al, 2008) were co-transfected stably. Cells were examined before inoculation into mice. The mice bearing the tumour xenografts were killed. All procedures are indicated in Supplementary data.
Tumour examinations
Tumours were dissected and their masses determined. Histochemical examinations were done on the tumour masses to evaluate angiogenesis, and biochemical analysis to evaluate SENP3 expression, global SUMO2/3 modification pattern and SUMO2/3 modification of p300. In situ hybridization for VEGF and immunohistochemistry for CD31 were carried out on paraformaldehyde-fixed and paraffin-embedded tumour sections, using previous methods (Yang et al, 2004; Huang et al, 2007). The procedures and reagents are described in Supplementary data.
Supplementary Material
Supplementary Figure S1
Supplementary Figure S2
Supplementary Figure S3
Supplementary Figure S4
Supplementary Figure S5
Supplementary data
Review Process File
Acknowledgments
We thank Dr RD Ye of the University of Illinois at Chicago for the HRE reporter construct, Dr ND Perkins of the University of Dundee, United Kingdom, for the tagged p300 constructs and Dr GQ Chen of Shanghai Jiao Tong University School of Medicine for the HIF-1α shRNA constructs. Dr Dangsheng Li is appreciated for discussion on the paper. This study was supported by grants from the National Natural Science Foundation of China (30570965 to JY), the Ministry of Science and Technology of China (2006CB910104 to JY), Shanghai Municipal Science and Technology Commission (08JC1413800 to JY, 08ZR1412300 to YW), Shanghai Municipal Education Commission (J50201 to Shanghai Jiao Tong University School of Medicine, The Faculty of Basic Medicine) and NIH grants (CA-239520 to ETHY and CA-16672 to MDACC). ETHY is the McNair Scholar of the Texas Heart Institute at St Luke's Episcopal Hospital.
References
- Arany Z, Huang LE, Eckner R, Bhattacharya S, Jiang C, Goldberg MA, Bunn HF, Livingston DM (1996) An essential role for p300/CBP in the cellular response to hypoxia. Proc Natl Acad Sci USA 93: 12969–12973 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bae SH, Jeong JW, Park JA, Kim SH, Bae MK, Choi SJ, Kim KW (2004) Sumoylation increases HIF-1alpha stability and its transcriptional activity. Biochem Biophys Res Commun 324: 394–400 [DOI] [PubMed] [Google Scholar]
- Biswas S, Gupta MK, Chattopadhyay D, Mukhopadhyay CK (2007) Insulin-induced activation of hypoxia-inducible factor-1 requires generation of reactive oxygen species by NADPH oxidase. Am J Physiol Heart Circ Physiol 292: H758–H766 [DOI] [PubMed] [Google Scholar]
- Bossis G, Melchior F (2006) Regulation of SUMOylation by reversible oxidation of SUMO conjugating enzymes. Mol Cell 21: 349–357 [DOI] [PubMed] [Google Scholar]
- Brunelle JK, Bell EL, Quesada NM, Vercauteren K, Tiranti V, Zeviani M, Scarpulla RC, Chandel NS (2005) Oxygen sensing requires mitochondrial ROS but not oxidative phosphorylation. Cell Metab 1: 409–414 [DOI] [PubMed] [Google Scholar]
- Carroll VA, Ashcroft M (2006) Role of hypoxia-inducible factor (HIF)-1alpha versus HIF-2alpha in the regulation of HIF target genes in response to hypoxia, insulin-like growth factor-I, or loss of von Hippel-Lindau function: implications for targeting the HIF pathway. Cancer Res 66: 6264–6270 [DOI] [PubMed] [Google Scholar]
- Chan HM, La Thangue NB (2001) p300/CBP proteins: HATs for transcriptional bridges and scaffolds. J Cell Sci 114: 2363–2373 [DOI] [PubMed] [Google Scholar]
- Chandel NS, Maltepe E, Goldwasser E, Mathieu CE, Simon MC, Schumacker PT (1998) Mitochondrial reactive oxygen species trigger hypoxia-induced transcription. Proc Natl Acad Sci USA 95: 11715–11720 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandel NS, McClintock DS, Feliciano CE, Wood TM, Melendez JA, Rodriguez AM, Schumacker PT (2000) Reactive oxygen species generated at mitochondrial complex III stabilize hypoxia-inducible factor-1alpha during hypoxia: a mechanism of O2 sensing. J Biol Chem 275: 25130–25138 [DOI] [PubMed] [Google Scholar]
- Cheng J, Kang X, Zhang S, Yeh ET (2007) SUMO-specific protease 1 is essential for stabilization of HIF1alpha during hypoxia. Cell 131: 584–595 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng J, Perkins ND, Yeh ET (2005) Differential regulation of c-Jun-dependent transcription by SUMO-specific proteases. J Biol Chem 280: 14492–14498 [DOI] [PubMed] [Google Scholar]
- Cheng J, Wang D, Wang Z, Yeh ET (2004) SENP1 enhances androgen receptor-dependent transcription through desumoylation of histone deacetylase 1. Mol Cell Biol 24: 6021–6028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cimarosti H, Lindberg C, Bomholt SF, Ronn LC, Henley JM (2008) Increased protein SUMOylation following focal cerebral ischemia. Neuropharmacology 54: 280–289 [DOI] [PubMed] [Google Scholar]
- Cramer T, Yamanishi Y, Clausen BE, Forster I, Pawlinski R, Mackman N, Haase VH, Jaenisch R, Corr M, Nizet V, Firestein GS, Gerber HP, Ferrara N, Johnson RS (2003) HIF-1alpha is essential for myeloid cell-mediated inflammation. Cell 112: 645–657 [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Ponti C, Carini R, Alchera E, Nitti MP, Locati M, Albano E, Cairo G, Tacchini L (2007) Adenosine A2a receptor-mediated, normoxic induction of HIF-1 through PKC and PI-3K-dependent pathways in macrophages. J Leukoc Biol 82: 392–402 [DOI] [PubMed] [Google Scholar]
- Degerny C, Monte D, Beaudoin C, Jaffray E, Portois L, Hay RT, de Launoit Y, Baert JL (2005) SUMO modification of the Ets-related transcription factor ERM inhibits its transcriptional activity. J Biol Chem 280: 24330–24338 [DOI] [PubMed] [Google Scholar]
- Deyrieux AF, Rosas-Acosta G, Ozbun MA, Wilson VG (2007) Sumoylation dynamics during keratinocyte differentiation. J Cell Sci 120: 125–136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Bacco A, Ouyang J, Lee HY, Catic A, Ploegh H, Gill G (2006) The SUMO-specific protease SENP5 is required for cell division. Mol Cell Biol 26: 4489–4498 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorval V, Fraser PE (2007) SUMO on the road to neurodegeneration. Biochim Biophys Acta 1773: 694–706 [DOI] [PubMed] [Google Scholar]
- Ebert BL, Bunn HF (1998) Regulation of transcription by hypoxia requires a multiprotein complex that includes hypoxia-inducible factor 1, an adjacent transcription factor, and p300/CREB binding protein. Mol Cell Biol 18: 4089–4096 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ema M, Hirota K, Mimura J, Abe H, Yodoi J, Sogawa K, Poellinger L, Fujii-Kuriyama Y (1999) Molecular mechanisms of transcription activation by HLF and HIF1alpha in response to hypoxia: their stabilization and redox signal-induced interaction with CBP/p300. EMBO J 18: 1905–1914 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emerling BM, Weinberg F, Liu JL, Mak TW, Chandel NS (2008) PTEN regulates p300-dependent hypoxia-inducible factor 1 transcriptional activity through Forkhead transcription factor 3a (FOXO3a). Proc Natl Acad Sci USA 105: 2622–2627 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freedman SJ, Sun ZY, Poy F, Kung AL, Livingston DM, Wagner G, Eck MJ (2002) Structural basis for recruitment of CBP/p300 by hypoxia-inducible factor-1 alpha. Proc Natl Acad Sci USA 99: 5367–5372 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao P, Zhang H, Dinavahi R, Li F, Xiang Y, Raman V, Bhujwalla ZM, Felsher DW, Cheng L, Pevsner J, Lee LA, Semenza GL, Dang CV (2007) HIF-dependent antitumorigenic effect of antioxidants in vivo. Cancer Cell 12: 230–238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerald D, Berra E, Frapart YM, Chan DA, Giaccia AJ, Mansuy D, Pouyssegur J, Yaniv M, Mechta-Grigoriou F (2004) JunD reduces tumor angiogenesis by protecting cells from oxidative stress. Cell 118: 781–794 [DOI] [PubMed] [Google Scholar]
- Girdwood D, Bumpass D, Vaughan OA, Thain A, Anderson LA, Snowden AW, Garcia-Wilson E, Perkins ND, Hay RT (2003) P300 transcriptional repression is mediated by SUMO modification. Mol Cell 11: 1043–1054 [DOI] [PubMed] [Google Scholar]
- Glassford AJ, Yue P, Sheikh AY, Chun HJ, Zarafshar S, Chan DA, Reaven GM, Quertermous T, Tsao PS (2007) HIF-1 regulates hypoxia- and insulin-induced expression of apelin in adipocytes. Am J Physiol Endocrinol Metab 293: E1590–E1596 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gong L, Yeh ET (2006) Characterization of a family of nucleolar SUMO-specific proteases with preference for SUMO-2 or SUMO-3. J Biol Chem 281: 15869–15877 [DOI] [PubMed] [Google Scholar]
- Guzy RD, Hoyos B, Robin E, Chen H, Liu L, Mansfield KD, Simon MC, Hammerling U, Schumacker PT (2005) Mitochondrial complex III is required for hypoxia-induced ROS production and cellular oxygen sensing. Cell Metab 1: 401–408 [DOI] [PubMed] [Google Scholar]
- Guzy RD, Schumacker PT (2006) Oxygen sensing by mitochondria at complex III: the paradox of increased reactive oxygen species during hypoxia. Exp Physiol 91: 807–819 [DOI] [PubMed] [Google Scholar]
- Guzy RD, Sharma B, Bell E, Chandel NS, Schumacker PT (2008) Loss of the SdhB, but Not the SdhA, subunit of complex II triggers reactive oxygen species-dependent hypoxia-inducible factor activation and tumorigenesis. Mol Cell Biol 28: 718–731 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haddad JJ, Land SC (2001) A non-hypoxic, ROS-sensitive pathway mediates TNF-alpha-dependent regulation of HIF-1alpha. FEBS Lett 505: 269–274 [DOI] [PubMed] [Google Scholar]
- Haindl M, Harasim T, Eick D, Muller S (2008) The nucleolar SUMO-specific protease SENP3 reverses SUMO modification of nucleophosmin and is required for rRNA processing. EMBO Rep 9: 273–279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halliwell B (2003) Oxidative stress in cell culture: an under-appreciated problem? FEBS Lett 540: 3–6 [DOI] [PubMed] [Google Scholar]
- Halliwell B (2007) Oxidative stress and cancer: have we moved forward? Biochem J 401: 1–11 [DOI] [PubMed] [Google Scholar]
- Harris AL (2002) Hypoxia—a key regulatory factor in tumour growth. Nat Rev Cancer 2: 38–47 [DOI] [PubMed] [Google Scholar]
- Hay RT (2005) SUMO: a history of modification. Mol Cell 18: 1–12 [DOI] [PubMed] [Google Scholar]
- Huang XZ, Wang J, Huang C, Chen YY, Shi GY, Hu QS, Yi J (2008) Emodin enhances cytotoxicity of chemotherapeutic drugs in prostate cancer cells: the mechanisms involve ROS-mediated suppression of multidrug resistance and hypoxia inducible factor-1. Cancer Biol Ther 7: 468–475 [DOI] [PubMed] [Google Scholar]
- Jung YJ, Isaacs JS, Lee S, Trepel J, Neckers L (2003) IL-1beta-mediated up-regulation of HIF-1alpha via an NFkappaB/COX-2 pathway identifies HIF-1 as a critical link between inflammation and oncogenesis. FASEB J 17: 2115–2117 [DOI] [PubMed] [Google Scholar]
- Kallio PJ, Okamoto K, O'Brien S, Carrero P, Makino Y, Tanaka H, Poellinger L (1998) Signal transduction in hypoxic cells: inducible nuclear translocation and recruitment of the CBP/p300 coactivator by the hypoxia-inducible factor-1alpha. EMBO J 17: 6573–6586 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamat CD, Green DE, Curilla S, Warnke L, Hamilton JW, Sturup S, Clark C, Ihnat MA (2005) Role of HIF signaling on tumorigenesis in response to chronic low-dose arsenic administration. Toxicol Sci 86: 248–257 [DOI] [PubMed] [Google Scholar]
- Kuo ML, den Besten W, Thomas MC, Sherr CJ (2008) Arf-induced turnover of the nucleolar nucleophosmin-associated SUMO-2/3 protease Senp3. Cell Cycle 7: 3378–3387 [DOI] [PubMed] [Google Scholar]
- Lando D, Peet DJ, Whelan DA, Gorman JJ, Whitelaw ML (2002) Asparagine hydroxylation of the HIF transactivation domain a hypoxic switch. Science 295: 858–861 [DOI] [PubMed] [Google Scholar]
- Li T, Santockyte R, Shen RF, Tekle E, Wang G, Yang DC, Chock PB (2006) Expression of SUMO-2/3 induced senescence through p53- and pRB-mediated pathways. J Biol Chem 281: 36221–36227 [DOI] [PubMed] [Google Scholar]
- Liu Q, Berchner-Pfannschmidt U, Moller U, Brecht M, Wotzlaw C, Acker H, Jungermann K, Kietzmann T (2004) A Fenton reaction at the endoplasmic reticulum is involved in the redox control of hypoxia-inducible gene expression. Proc Natl Acad Sci USA 101: 4302–4307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu H, Forbes RA, Verma A (2002) Hypoxia-inducible factor 1 activation by aerobic glycolysis implicates the Warburg effect in carcinogenesis. J Biol Chem 277: 23111–23115 [DOI] [PubMed] [Google Scholar]
- Mansfield KD, Guzy RD, Pan Y, Young RM, Cash TP, Schumacker PT, Simon MC (2005) Mitochondrial dysfunction resulting from loss of cytochrome c impairs cellular oxygen sensing and hypoxic HIF-alpha activation. Cell Metab 1: 393–399 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manza LL, Codreanu SG, Stamer SL, Smith DL, Wells KS, Roberts RL, Liebler DC (2004) Global shifts in protein sumoylation in response to electrophile and oxidative stress. Chem Res Toxicol 17: 1706–1715 [DOI] [PubMed] [Google Scholar]
- Melillo G (2004) HIF-1: a target for cancer, ischemia and inflammation—too good to be true? Cell Cycle 3: 154–155 [PubMed] [Google Scholar]
- Nishida T, Tanaka H, Yasuda H (2000) A novel mammalian Smt3-specific isopeptidase 1 (SMT3IP1) localized in the nucleolus at interphase. Eur J Biochem 267: 6423–6427 [DOI] [PubMed] [Google Scholar]
- Park JH, Kim TY, Jong HS, Kim TY, Chun YS, Park JW, Lee CT, Jung HC, Kim NK, Bang YJ (2003) Gastric epithelial reactive oxygen species prevent normoxic degradation of hypoxia-inducible factor-1alpha in gastric cancer cells. Clin Cancer Res 9: 433–440 [PubMed] [Google Scholar]
- Roth U, Curth K, Unterman TG, Kietzmann T (2004) The transcription factors HIF-1 and HNF-4 and the coactivator p300 are involved in insulin-regulated glucokinase gene expression via the phosphatidylinositol 3-kinase/protein kinase B pathway. J Biol Chem 279: 2623–2631 [DOI] [PubMed] [Google Scholar]
- Saitoh H, Hinchey J (2000) Functional heterogeneity of small ubiquitin-related protein modifiers SUMO-1 versus SUMO-2/3. J Biol Chem 275: 6252–6258 [DOI] [PubMed] [Google Scholar]
- Semenza GL (2003) Targeting HIF-1 for cancer therapy. Nat Rev Cancer 3: 721–732 [DOI] [PubMed] [Google Scholar]
- Shiose A, Kuroda J, Tsuruya K, Hirai M, Hirakata H, Naito S, Hattori M, Sakaki Y, Sumimoto H (2001) A novel superoxide-producing NAD(P)H oxidase in kidney. J Biol Chem 276: 1417–1423 [DOI] [PubMed] [Google Scholar]
- Song LP, Zhang J, Wu SF, Huang Y, Zhao Q, Cao JP, Wu YL, Wang LS, Chen GQ (2008) Hypoxia-inducible factor-1alpha-induced differentiation of myeloid leukemic cells is its transcriptional activity independent. Oncogene 27: 519–527 [DOI] [PubMed] [Google Scholar]
- Sramko M, Markus J, Kabat J, Wolff L, Bies J (2006) Stress-induced inactivation of the c-Myb transcription factor through conjugation of SUMO-2/3 proteins. J Biol Chem 281: 40065–40075 [DOI] [PubMed] [Google Scholar]
- Tempe D, Piechaczyk M, Bossis G (2008) SUMO under stress. Biochem Soc Trans 36: 874–878 [DOI] [PubMed] [Google Scholar]
- Thomas R, Kim MH (2005) Epigallocatechin gallate inhibits HIF-1alpha degradation in prostate cancer cells. Biochem Biophys Res Commun 334: 543–548 [DOI] [PubMed] [Google Scholar]
- Treins C, Murdaca J, Van Obberghen E, Giorgetti-Peraldi S (2006) AMPK activation inhibits the expression of HIF-1alpha induced by insulin and IGF-1. Biochem Biophys Res Commun 342: 1197–1202 [DOI] [PubMed] [Google Scholar]
- Tuttle SW, Maity A, Oprysko PR, Kachur AV, Ayene IS, Biaglow JE, Koch CJ (2007) Detection of reactive oxygen species via endogenous oxidative pentose phosphate cycle activity in response to oxygen concentration: implications for the mechanism of HIF-1alpha stabilization under moderate hypoxia. J Biol Chem 282: 36790–36796 [DOI] [PubMed] [Google Scholar]
- Walmsley SR, Cadwallader KA, Chilvers ER (2005) The role of HIF-1alpha in myeloid cell inflammation. Trends Immunol 26: 434–439 [DOI] [PubMed] [Google Scholar]
- Wang FS, Wang CJ, Chen YJ, Chang PR, Huang YT, Sun YC, Huang HC, Yang YJ, Yang KD (2004) Ras induction of superoxide activates ERK-dependent angiogenic transcription factor HIF-1alpha and VEGF-A expression in shock wave-stimulated osteoblasts. J Biol Chem 279: 10331–10337 [DOI] [PubMed] [Google Scholar]
- Xia C, Meng Q, Liu LZ, Rojanasakul Y, Wang XR, Jiang BH (2007) Reactive oxygen species regulate angiogenesis and tumor growth through vascular endothelial growth factor. Cancer Res 67: 10823–10830 [DOI] [PubMed] [Google Scholar]
- Xu Z, Lam LS, Lam LH, Chau SF, Ng TB, Au SW (2008) Molecular basis of the redox regulation of SUMO proteases: a protective mechanism of intermolecular disulfide linkage against irreversible sulfhydryl oxidation. FASEB J 22: 127–137 [DOI] [PubMed] [Google Scholar]
- Yamashita K, Discher DJ, Hu J, Bishopric NH, Webster KA (2001) Molecular regulation of the endothelin-1 gene by hypoxia. Contributions of hypoxia-inducible factor-1, activator protein-1, GATA-2, AND p300/CBP. J Biol Chem 276: 12645–12653 [DOI] [PubMed] [Google Scholar]
- Yang J, Li H, Chen YY, Wang XJ, Shi GY, Hu QS, Kang XL, Lu Y, Tang XM, Guo QS, Yi J (2004) Anthraquinones sensitize tumor cells to arsenic cytotoxicity in vitro and in vivo via reactive oxygen species-mediated dual regulation of apoptosis. Free Radic Biol Med 37: 2027–2041 [DOI] [PubMed] [Google Scholar]
- Yang W, Sheng H, Warner DS, Paschen W (2008) Transient global cerebral ischemia induces a massive increase in protein sumoylation. J Cereb Blood Flow Metab 28: 269–279 [DOI] [PubMed] [Google Scholar]
- Yasinska IM, Sumbayev VV (2003) S-nitrosation of Cys-800 of HIF-1alpha protein activates its interaction with p300 and stimulates its transcriptional activity. FEBS Lett 549: 105–109 [DOI] [PubMed] [Google Scholar]
- Yeh ET, Gong L, Kamitani T (2000) Ubiquitin-like proteins: new wines in new bottles. Gene 248: 1–14 [DOI] [PubMed] [Google Scholar]
- Yi J, Yang J, He R, Gao F, Sang H, Tang X, Ye RD (2004) Emodin enhances arsenic trioxide-induced apoptosis via generation of reactive oxygen species and inhibition of survival signaling. Cancer Res 64: 108–116 [DOI] [PubMed] [Google Scholar]
- Zelzer E, Levy Y, Kahana C, Shilo BZ, Rubinstein M, Cohen B (1998) Insulin induces transcription of target genes through the hypoxia-inducible factor HIF-1alpha/ARNT. EMBO J 17: 5085–5094 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Q, Liu LZ, Fu B, Hu X, Shi X, Fang J, Jiang BH (2007) Reactive oxygen species regulate insulin-induced VEGF and HIF-1alpha expression through the activation of p70S6K1 in human prostate cancer cells. Carcinogenesis 28: 28–37 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figure S1
Supplementary Figure S2
Supplementary Figure S3
Supplementary Figure S4
Supplementary Figure S5
Supplementary data
Review Process File