Abstract
To fulfil their function, nuclear pore complexes (NPCs) must discriminate between inert proteins and nuclear transport receptors (NTRs), admitting only the latter. This specific permeation is thought to depend on interactions between hydrophobic patches on NTRs and phenylalanine-glycine (FG) or related repeats that line the NPC. Here, we tested this premise directly by conjugating different hydrophobic amino-acid analogues to the surface of an inert protein and examining its ability to cross NPCs unassisted by NTRs. Conjugation of as few as four hydrophobic moieties was sufficient to enable passage of the protein through NPCs. Transport of the modified protein proceeded with rates comparable to those measured for the innate protein when bound to an NTR and was relatively insensitive both to the nature and density of the amino acids used to confer hydrophobicity. The latter observation suggests a non-specific, small, and pliant interaction network between cargo and FG repeats.
Keywords: hydrophobicity, nuclear pore complex, transport, selectivity
Introduction
Eukaryotic cell nuclei are separated from the cytoplasm by a double lipid bilayer system known as the nuclear envelope (NE). Exchange of material between the two compartments proceeds solely through nuclear pore complexes (NPCs), large protein assemblies that span the NE and provide a medium for the exchange. NPCs support two modes of transport: small molecules, such as ions, metabolites, and other small solutes can pass by diffusion, which becomes increasingly restricted as the particles approach a size limit of ∼5 nm (30–40 kDa for globular proteins). On the other hand, most proteins and their complexes cannot cross NPCs unless they are bound to soluble nuclear transport receptors (NTRs) that recognize specific nuclear import signal (NLS) or nuclear export signal (NES) peptides displayed by the cargo (Paine et al, 1975; Corbett and Silver, 1997; Mattaj and Englmeier, 1998; Gorlich and Kutay, 1999; Keminer and Peters, 1999; Yoneda, 2000; Fried and Kutay, 2003; Suntharalingam and Wente, 2003; Weis, 2003; Fahrenkrog et al, 2004; Pemberton and Paschal, 2005; Lim et al, 2008).
How do NPCs allow the passage of small molecules, suppress that of large molecules, yet permit transport of the same large molecules when bound to NTRs has been the focus of intense study. Fulfilling the above functions necessitates that a device exists, which not only discriminates between small and large molecules, but is also able to selectively admit NTRs and their bound cargoes over a background of free cargoes. The likely constituents of this device are arrays of hydrophobic peptide repeats, most commonly in the form of FG, FXFG, or GLFG, present in natively disordered domains carried by one-third of the proteins that make up the pores (called nucleoporins or nups) (Rout and Wente, 1994; Rout et al, 2000; Bayliss et al, 2002; Denning et al, 2003; Tran and Wente, 2006; Patel et al, 2007; Stewart, 2007; Lim et al, 2007b). How the presence of these repeats provides NPCs their discriminatory capability is under debate (Rout et al, 2000, 2003; Ben-Efraim and Gerace, 2001; Macara, 2001; Ribbeck and Gorlich, 2001, 2002; Pyhtila and Rexach, 2003; Peters, 2005; Burke, 2006; Elbaum, 2006; Frey and Gorlich, 2007; Patel et al, 2007; Weis, 2007; Lim et al, 2007b). Two major views have dominated the field. The first (Rout et al, 2000) invokes the idea of entropic exclusion. In this model, the FG domains form an unlinked polymer brush, which excludes the passage of large molecules through thermal undulations. Passage of NTRs is afforded, as they are able to bind to sites present in FG repeats, therefore, overcoming this exclusion. The second view suggests that the unfolded domains of nups form a mesh within NPCs that is mediated by FG motif interactions. The size of the mesh dictates the maximum size for passive diffusion and NTRs are incorporated or ‘dissolved' into the meshwork by binding to the FG repeats, transiently replacing contacts between the FG domains (Ribbeck and Gorlich, 2001, 2002). More recently, a two-gated model using a central (hydrophobic) diffusion barrier, formed by a meshwork of cohesive FG domains, and peripheral (entropic) barriers, formed by repulsive FG domains, has been proposed (Patel et al, 2007).
Regardless of differences in modus operandi, all models assume that selective permeation of NPCs to NTRs depends on interactions between the latter and FG repeats in the pores (Radu et al, 1995; Bayliss et al, 2000; Ribbeck and Gorlich, 2001, 2002; Strawn et al, 2004; Frey et al, 2006; Frey and Gorlich, 2007; Stewart, 2007; Yazicioglu et al, 2007; Isgro and Schulten, 2007b). This is supported by several lines of investigations including crystallographic (Bayliss et al, 2000, 2002; Liu and Stewart, 2005), NMR (Morrison et al, 2003), solution and single-molecule mechanical studies of NTR-FG repeat interactions (Lim et al, 2007b; Otsuka et al, 2008; Patel and Rexach, 2008) and studies involving deletions or swaps of FG-carrying nucleoporins (Strawn et al, 2004; Zeitler and Weis, 2004). Furthermore, NTR mutants impaired in FG binding (Bayliss et al, 1999; Quimby et al, 2001; Ribbeck and Gorlich, 2001; Yazicioglu et al, 2007) were shown to have attenuated transport rates, clearly pointing to the capacity to interact with FG repeats as a requisite for nucleocytoplasmic transport. Two experimental studies have made use of synthetic media containing a subset of nups to reproduce some of the selectivity properties of NPCs towards NTRs. In one study, a saturated FG-repeat hydrogel (made from the N-terminal of the yeast nucleoporin Nsp1p) (Frey and Gorlich, 2007) was shown to preferentially support the passage of NTRs over comparably sized inert proteins that cannot interact with FG repeats. More recently, NTRs were shown to have a greater capacity than an inert protein to cross a synthetic filter, one side of which was modified with the FG-repeat domains of the yeast nucleoporins Nsp1p and Nup100 (Jovanovic-Talisman et al, 2008).
NTRs are distinguished from other cytoplasmic proteins by having a greater surface hydrophobicity (Ribbeck and Gorlich, 2002). In addition, substitutions that conserve hydrophobicity in FG domains of several nups were shown neither to affect the interactions between the nups themselves, nor did they interrupt their binding to NTRs (Patel et al, 2007). In contrast, substitution with non-hydrophobic residues impaired both binding activities (Frey et al, 2006; Patel and Rexach, 2008) and inhibited the transport of NTRs through in vitro assembled FG-repeat gels (Frey et al, 2006). The results obtained from all of the above studies indicate that NTRs are able to interact with FG repeats present in some nucleoporins and suggest that the interactions have a strong hydrophobic component. However, the exact nature of the interactions, as well as their specificity, valence, and architecture are still unknown.
To address these issues in a systematic manner, we used a protein that is normally barred from passage through NPCs and modified its surface hydrophobicity by conjugating different hydrophobic amino-acid analogues, each to different degrees. We then examined the ability of the various protein derivates to cross NPCs and characterized their ensuing transport dynamics. Using this approach, we could discern the role of surface hydrophobicity in a molecule's capacity to cross the permeability barrier formed by NPCs from other potential contributions and to gain insight into the nature of the interaction network formed between FG repeats and transporting cargoes in intact NPCs within cells.
Results
Modifying the surface hydrophobicity of bovine serum albumin
For the hydrophobicity-conferring moieties to exert their effect in full, their exposure to the solvent should be maximized and their interactions with and masking by neighbouring side chains minimized. We, therefore, took a synthetic approach in which we covalently attached hydrophobic amino-acid side-chain analogues to the target protein through distal functional groups of polar side-chains abundant on protein surfaces, primarily lysine and serine (Ser) residues. As a model protein, we used bovine serum albumin (BSA), which is cross-linked by 17 disulfide bonds that render its globular structure extremely stable. Such high stability was required, as the conjugation procedure involved rather harsh conditions (see Materials and methods) and we wished to be able to modify the protein surface even to large extents, both of which may induce partial or complete unfolding. Quite surprisingly, given its copious use in various studies, the three-dimensional structure of BSA is unknown. Therefore, to locate residues of BSA that could be used for modification and follow their distribution over the protein surface, we used a model based on the structure of human serum albumin (Supplementary Figure 1). K-means clustering analysis of all probable conjugation sites on the surface of BSA revealed that they are not arranged into clusters, with the average calculated silhouette value being not significantly different from that derived for randomly distributed sites on a surface of a similarly sized ellipsoid (see Materials and methods).
Three hydrophobic amino-acid side-chain analogues were used to modify the protein: 3-phenylpropionic acid (C9H6O2), 3-indolepropionic acid (C11H11NO2), and 4-methyl valeric acid (C6H12O2). Given the composition of FG repeats, the choice of the first, a phenylalanine (Phe) analogue, was natural. The second modifier, an analogue of tryptophan (Trp), is also aromatic, but is larger and less hydrophobic than Phe. The choice of this analogue was motivated by the findings that substitution of Phe residues in FG repeats by Trp does not alter domain–domain interactions between FG nups and their binding to NTRs (Patel et al, 2007; Patel and Rexach, 2008), and the observation that mutation of a tryptophyl residue (Trp7) in the NTR NTF2 weakens the interaction of the receptor with FG repeats and slows down its passage through the pores (Bayliss et al, 1999; Ribbeck and Gorlich, 2001). The third substance, an analogue of leucine (Leu), which is hydrophobic, but not aromatic, was chosen to test the importance of π−π interactions to passage through the NPC. As a control, we used glycolic acid (C2H4O3) that, on conjugation, adds a hydrophilic moiety, analogous to that of a Ser residue, to the protein's surface. The modifying substances were conjugated to FITC-labelled BSA, using different modifier/protein stoichiometries, and the degree of modification was determined, post facto, by mass spectrometry (Supplementary Figure 2). The derivatized proteins were then subjected to far-UV circular dichroism and gel-filtration analyses to verify that no massive structural rearrangements had occurred and that the increase in surface hydrophobicity did not lead to aggregation of the proteins. The results (Supplementary Figure 3) indicated that the modifications of the protein neither lead to significant alterations in secondary structure or size nor have they led to aggregation.
Modified BSA is able to pass through NPCs unassisted by NTRs
We first examined whether the increase in surface hydrophobicity enabled BSA to cross the NPC. Experiments were conducted on HeLa cells whose plasma membrane was permeabilized by digitonin and were, therefore, largely devoid of soluble nuclear transport factors. As the derivatized BSA molecules had a propensity to bind to cytoplasmic components, presumably cytoskeletal elements, which complicated kinetic analysis (see below), most studies were performed on cells treated with the microtubule-disrupting agent nocodazole. The results obtained from untreated HeLa cells, as well as from isolated Xenopus oocyte nuclei (Supplementary Figure 4), were qualitatively similar.
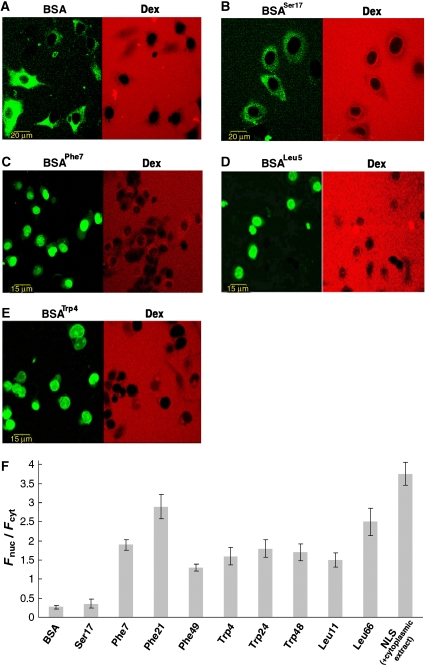
On account of its size (∼70 kDa) and lack of nuclear localization signals (NLSs), innate BSA is virtually excluded from NPCs (Ribbeck and Gorlich, 2001; Kramer et al, 2007; Naim et al, 2007) and indeed failed to enter the cell nuclei to a significant extent within the time scale of our experiments (Figure 1A and F). However, when modified by any of the hydrophobic amino-acid analogues described above, considerable nuclear entry of the protein was observed (Figure 1, panels C–E and F). This apparent promiscuity indicates that the essential characteristic for permeating NPCs is indeed surface hydrophobicity. This is consistent with experimental observations (Bayliss et al, 2000, 2002; Liu and Stewart, 2005) and binding simulations (Isgro and Schulten, 2005, 2007a, 2007b; Frey et al, 2006) showing that FG repeats can interact with the three amino acids used, as well as with other hydrophobic moieties, on NTRs. To verify that entry into the nucleus was not promoted by the modification process itself, we tested the ability of BSA conjugated to a Ser analogue to cross NPCs. As shown in Figure 1B and F, this derivative failed to enter cell nuclei. Likewise, BSA molecules modified by NLS peptides (CTPPKKKRKV) failed to enter the nucleus in the absence of cytosolic extracts (data not shown, see below). Thus, the ability of BSA to cross NPCs is not conferred by the mere modification of its surface, but rather requires that the modifiers be hydrophobic in nature.
Figure 1.
Nuclear entry of BSA molecules modified by amino-acid side-chain analogues on their surface. The modifier used in each panel is denoted in superscript by the three-letter code of the corresponding amino-acid side chain it carries and the number indicates the degree of modification. (A–E) Confocal images of digitonin-permeabilized, nocodazole-treated HeLa cells 30 min after the introduction of a transport mixture containing innate or modified BSA (green) and a 66-kDa dextran (red) probe serving as an NE integrity marker. (F) Quantitative analysis of nuclear entry. Shown is the ratio between (background-corrected) nuclear and cytoplasmic mean fluorescence intensity (Fnuc/Fcyt) measured 30 min after introduction of innate BSA, BSA molecules modified by different amino-acid analogues, and BSA linked to SV40 large T-antigen NLS peptides (in the presence of cytosolic extract and an energy regenerating system). Data are presented as the mean±2 s.e.m. of determinations involving 20–30 cells for each column.
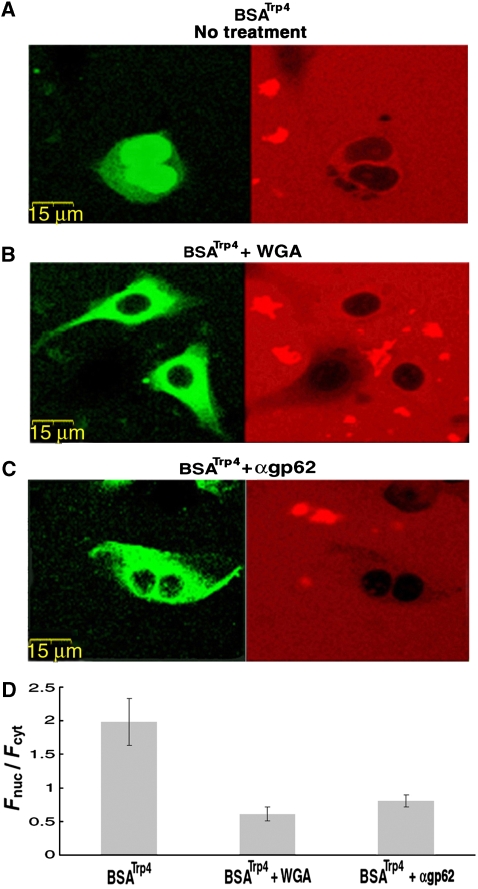
In all the cases we tested (Figure 1F), the BSA molecules derivatized by hydrophobic moieties accumulated in the nuclei (i.e. Fnuc/Fcyt>1). Given the rather non-specific nature of hydrophobic interactions and the highly crowded environment in the cell nucleus, such an accumulation is not unexpected. To verify that nuclear import of the modified BSA proceeded through NPCs, we repeated the experiments in the presence of the lectin wheat germ agglutinin (WGA) or an antibody raised against the central pore protein gp62, both known to block macromolecular transport through NPCs. As shown in Figure 2, these treatments effectively inhibited passage of the BSA NTR mimics through the pores.
Figure 2.
Inhibition of modified BSA transport by NPC blockers. (A–C) Confocal images of HeLa cells 30 min after the introduction of BSATrp4 either alone (A) or together with WGA (B) or αgp62 (C). The latter two are known blockers of NPC-mediated transport. Cells were permeabilized twice with the pore forming protein SLO, which is used for reversible permeabilization of the plasma membrane (Walev et al, 2001) (see Materials and methods) with the transport substrate introduced during the second permeabilization. (D) Quantitative analysis of nuclear entry. Shown is the ratio between (background-corrected) nuclear and cytoplasmic mean fluorescence intensity (Fnuc/Fcyt) measured 30 min after the second SLO permeabilization in which BSATrp4 was introduced to the cells. Data are presented as the mean±2 s.e.m. of determinations involving 6–12 cells for each column. Note that nuclear entry of the modified BSA in the absence of the blockers was as effective as that observed in digitonin-permeabilized cells.
Transport rate is insensitive to the nature and density of the moieties used to confer hydrophobicity
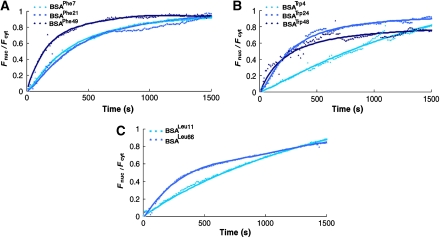
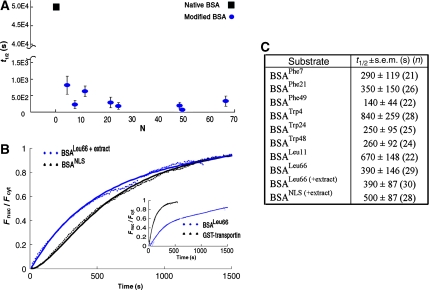
Crystallographic analyses reveal a few, well-defined sites on NTR surfaces that accommodate different FG-containing segments, albeit with different affinities (Stewart, 2007 and references therein). Molecular dynamics simulations suggest the presence of 8–14 binding sites (Isgro and Schulten, 2007a, 2007b). However, the number of sites simultaneously engaged during translocation is unknown. To gain insight into the valence and architecture of the interaction network between NTRs and the NPC, we followed the transport dynamics of BSA molecules carrying an increasing number of any of the three amino acids used to confer hydrophobicity. As can be expected from the results shown in Figure 1, the type of amino acid used for conjugation had a relatively small effect on the rate of nuclear import of BSA, the difference in t1/2 values not exceeding three folds for cargoes modified to a similar extent, but with different conjugates (Figures 3 and 4C). This apparent indifference to conjugate type given, we plotted the t1/2 values measured for all derivatives, regardless of their type, as a function of the number of conjugates present on the cargo (Figure 4A). Notably, at our resolution, the plot shows characteristics of a step function with a very sharp decrease in t1/2 with the addition of just four hydrophobic amino acids to the BSA surface. This latter number is very close to that derived from in vitro binding assays, which indicated that a minimum of six FG motifs are needed to detect binding between the yeast transport receptor Kap95 and GST–FG domain fusions (Patel and Rexach, 2008). Thus, the presence of a small number of hydrophobic spots on the cargo surface is sufficient to allow passage through the pores. Increasing the number of conjugates further had little effect on the overall transport rate, neither increasing nor decreasing it by more than two to three folds.
Figure 3.
Nuclear accumulation versus time traces of modified BSA. Shown are traces of BSA modified by (A) phenylalanine, (B) tryptophan, or (C) leucine analogues to varying degrees. Each data point represents the average obtained from multiple traces (see Figure 4C, for exact numbers) that were normalized before averaging. For convenience of presentation, error bars were not included (but see Figures 4A and 4C). The fits shown (solid lines) are provided only as guidance to the eyes.
Figure 4.
Nuclear import kinetics of BSA modified by either hydrophobic amino acids or by NLSs and of an NTR. (A) A plot of t1/2 values measured for all BSA derivatives used in this study (blue circles) versus N—the estimated number of hydrophobic moieties attached to the protein. With our experimental setup and timeframe of the measurements, we could not accurately determine the nuclear entry rate of unmodified BSA because it was too low. It has been estimated that BSA traffics to the nucleus at least 600 times slower than transportin (Ribbeck and Gorlich, 2001). Combining this with our data, we estimate that native BSA enters the nucleus with a t1/2 of about 50 000 s, under the experimental conditions we used. This latter value is marked in the graph by a black square (note the break in the y axis). (B) Comparison between nuclear import kinetics of BSALeu66 and BSA derivatized by NLSs, which serve as substrates for the transport receptor complex importin α/β. In these experiments, the cells were supplemented by cytoplasmic extract and an energy regenerating system. (Inset) Nuclear import kinetics of BSALeu66 as compared with that of the (unloaded) nuclear import receptor transportin (fused to GST), both measured in unsupplemented cells. Data describing the nuclear import of the transport receptor were taken from Naim et al (2007). (C) t1/2 values derived for nuclear transport of BSA derivatives used in this study. The numbers in parenthesis denote the number of cells used in the analysis.
Transport of modified BSA proceeds at rates comparable to those exhibited by NTRs under the same experimental conditions
Next, we compared the nuclear import kinetics of BSA modified by 66 Leu moieties (BSALeu66) with that of BSA covalently linked to 10 SV40 large T-antigen NLSs, which serve as substrates for the NTR complex importin α/β1. Receptor-mediated traffic through NPCs typically requires, in addition to NTRs, the participation of an effector system centring on the small GTPase Ran. This system controls the association and dissociation of cargoes with or from NTRs in the appropriate cellular compartment and provides loci for metabolic energy input in the form of hydrolysis of Ran-bound GTP (Tran and Wente, 2006; Stewart, 2007). As mentioned above, NLS-BSA indeed failed to enter nuclei of permeabilized cells, which are largely devoid of soluble cytoplasmic factors. Thus, for these experiments, the cells were supplemented by cytosolic extract and an energy regenerating system that, together, readily support receptor-mediated transport through NPCs in permeabilized cells (Adam et al, 1990). Under these conditions, BSALeu66 imported to the nucleus with a t1/2 of ∼400 s (Figure 4B and C). Expectedly, NLS-BSA accumulated in the nucleus to a larger extent than BSALeu66 (Figure 1F). However, its transport into the nucleus was in fact slightly slower, t1/2 being 500 s. This might be partially because of the fact that the transport complex formed by NLS-BSA is at least three times larger than BSALeu66 (depending on the number of bound importin α/β complexes) . In addition, its translocation through the pores involves binding to an NTR in the cytoplasm and its subsequent release in the nucleus and, therefore, depends on auxiliary activity of the Ran system. To eliminate the dependence on the two latter processes, we compared nuclear import kinetics of BSALeu66 with that of the free NTR, transportin, which can effectively import into the nucleus in a Ran-independent manner (Ribbeck and Gorlich, 2002). In an earlier study (Naim et al, 2007), we found that unloaded transportin enters the nuclei of permeabilized HeLa cells (not supplemented by cytosolic extract) with a t1/2 of ∼90 s. This value is only four times smaller than the one we measured for BSALeu66 under the same experimental conditions (Figure 4, panels B (inset) and C).
Modified BSA does not impair the permeability barrier of the NPC
Certain alcohols have been shown to compromise the permeability barrier of the NPC (Ribbeck and Gorlich, 2002; Patel et al, 2007), potentially by specifically disrupting interactions between FG repeats in the pores. The BSA derivates used in this study could act similarly by forming stable interactions with FG repeats, obliterating the ability of the latter to exclude inert molecules (i.e. other BSA molecules) from the pores. This is, however, not the case. Even the most extensively modified BSA molecules we used did not permeate the pores to a 66-kDa-dextran probe that was introduced as a marker for NE integrity in all transport assays (Figure 1). Entry of the probe into the nucleus was likewise not observed when di-phenylalanine methyl ester was added at high concentrations (data not shown).
Discussion
Deciphering the nature of the selectivity barrier of the NPC has been the subject of various experimental and theoretical studies, which collectively point to interactions between hydrophobic patches on the surface of NTRs and FG nups (Radu et al, 1995; Bayliss et al, 2000; Ribbeck and Gorlich, 2001, 2002; Strawn et al, 2004; Frey et al, 2006; Frey and Gorlich, 2007; Stewart, 2007; Yazicioglu et al, 2007; Zilman et al, 2007; Isgro and Schulten, 2007b; Jovanovic-Talisman et al, 2008). This study differs from most earlier works in two main aspects: first, the experiments were conducted on intact NPCs within cells rather than on reconstituted sieves or grafted surfaces or pores (Frey et al, 2006; Lim et al, 2006, 2007a, 2007b; Frey and Gorlich, 2007; Jovanovic-Talisman et al, 2008; Patel and Rexach, 2008), in which specific subsets of nups were used, or on nup-deficient cells (Strawn et al, 2004; Zeitler and Weis, 2004). Second, we made use of specially designed synthetic cargoes, which allowed us to single out the role of surface hydrophobicity in granting NTRs their ability to cross NPCs.
The main conclusions from our results can briefly be summarized in the following way: cargo surface hydrophobicity is sufficient to provide access to the NPC, the interactions between cargo and FG repeats in the pores are non-specific, and their number is limited.
The first two conclusions above are drawn from the fact that the ability of our NTR mimics to transport through NPCs was independent of amino-acid identity. We used three different amino acids to confer surface hydrophobicity to our otherwise inert cargo. The three amino acids used are all hydrophobic, but have a different structure and vary in the details of their chemical nature, being aromatic (Phe), polar-aromatic (Trp), and non-aromatic (Leu). Yet, all three were able to facilitate transport in a cargo that, by itself, could not cross the NPC, and there were no major differences in the transport dynamics of BSA cargoes modified by either of them. These results are in line with earlier experimental evidence showing that transport receptors are distinguished from other cytoplasmic proteins by a greater surface hydrophobicity (Ribbeck and Gorlich, 2002). The apparent promiscuity with respect to amino-acid identity is consistent with experimental observations (Patel et al, 2007; Patel and Rexach, 2008) that substitution of Phe with Trp in FG domains of several nups does not affect the interactions between FG-carrying nup domains and themselves or transport receptors. It is also supported by X-ray crystallography studies (Bayliss et al, 2000, 2002; Liu and Stewart, 2005), which reveal that the hydrophobic cavities on the surface of NTRs, to which Phe side chains of FG-repeat cores bind, are populated by a fairly large repertoire of hydrophobic and non-polar amino acids including Trp, Phe, Leu, Ile, and Val, as well as Met, Tyr, and Pro. In addition, it was shown that besides the aromatic ring of Phe in the FG-repeat region, there are secondary contributions from adjacent hydrophobic residues, which lie in the linker between FG cores (Liu and Stewart, 2005). Molecular dynamics simulations of binding of FG-carrying peptides to NTRs (Isgro and Schulten, 2005, 2007a, 2007b) likewise indicated that the binding spots on the latter include different hydrophobic amino acids. Thus, we conclude that surface hydrophobicity per se and not specific interactions is the primary determinant of the recognition of NTRs by the NPC.
Let us now turn to the issue of the number of interactions formed between transporting cargoes and FG repeats. Crystallographic (Bayliss et al, 2000, 2002; Liu and Stewart, 2005), NMR (Morrison et al, 2003), mutational analyses (Chi and Adam, 1997; Kose et al, 1997; Kutay et al, 1997; Bednenko et al, 2003), as well as molecular dynamics simulations (Isgro and Schulten, 2005, 2007a, 2007b), and in vitro binding assays (Patel and Rexach, 2008) have been used to study the details of the interactions between FG repeats of different nups and NTRs. These studies have yielded a broad range of numbers for the sites used for interactions on NTRs, varying between 2 and 14. Likewise, the number of FG repeats and the distance and chemical nature of the linker regions between them varies between different nups (5–30 FG-repeat units) (Rout and Wente, 1994; Rout et al, 2000; Denning et al, 2003; Tran and Wente, 2006; Patel et al, 2007; Stewart, 2007; Lim et al, 2007b). Thus, the interaction network formed between FG repeats and NTRs during translocation represents a complex system whose valance and strength is difficult to quantify and may vary for different cargoes and for different regions of the pore.
The ability of cargo to interact with the NPC can lead to opposite effects, depending on the strength of the interaction. Theoretical studies have showed that, under certain conditions, attractive interactions between a molecule and cognate binding sites inside a channel (or a pore) may facilitate transport of the former through the channel because of an increase in occupation probability (Lu et al, 2003; Berezhkovskii and Bezrukov, 2005; Bauer and Nadler, 2006; Bezrukov et al, 2007; Zilman et al, 2007; Zilman, 2009). On the other hand, the residence time in the channel scales roughly exponentially with overall interaction strength (Zilman et al, 2007), which could lead to blockage of traffic with too strong an interaction. Thus, the number of interactions that ultimately leads to efficient transport depends on their strength as well as on the distribution of binding spots in the channel reflecting a balance between maximization of partitioning of particles into the channel and minimization of their residence therein. The concentration of FG repeats in the NPC is estimated to be at the millimolar range (Bayliss et al, 1999; Rout et al, 2000; Denning et al, 2003; Strawn et al, 2004; Tran and Wente, 2006; Stewart, 2007), amounting to over 3000 repeats that, if evenly distributed in the central pore channel, would be spaced a few nanometers from each other (consistent with the unit size in saturated FG-repeat hydrogel; Frey and Gorlich, 2007). Given this very high effective concentration, if all FG repeats were available for NTR binding, one would expect to find the aforementioned optimum when scanning the number of binding sites present on a cargo over a broad range. We did not find such an optimum using BSA molecules carrying from 4 to 66 hydrophobic moieties on their surface. Even the cargoes that were modified with the least number of moieties (i.e. 4) were able to efficiently cross the NPCs into the nucleus. Increasing the number of moieties did not significantly accelerate passage. Importantly, it did not lead to significant attenuation either. This is surprising as even if we consider only the lower binding affinities reported for the interactions between FG repeats and NTRs (micromolar range; Pyhtila and Rexach, 2003; Patel and Rexach, 2008), simultaneously breaking all bonds formed between FG repeats and hydrophobic residues present on the more extensively modified BSA molecules is highly unlikely. For such heavily modified cargoes, one would expect transport to slow down dramatically or halt altogether. We thus conclude that the average number of interactions formed simultaneously between the BSA cargoes and FG repeats is limited, with the optimum (if exists) lying somewhere between one and four. This number lies within the range of FG binding spots found on NTRs by crystallographic analyses (Bayliss et al, 2000, 2002; Liu and Stewart, 2005) and likely does not differ significantly for them. The limited number of interactions that seem to be formed between NTRs and FG repeats implies that only a small fraction of the latter is available for binding at a given time. This may be due to steric occlusion, the spatial distribution of FG repeats within the pores (Peters, 2005), or other reasons.
Taken together, our observations suggest that the predominant interaction network formed between cargo and NPC is based on hydrophobic interaction and is small, flexible, and highly adaptive. A flexible, opportunistic interaction module would render transport robust against random occlusions or redistributions of FG binding sites in the pores. This is consistent with experiments showing that massive deletion or swap of FG nups does not abolish transport selectivity through NPCs (Strawn et al, 2004; Zeitler and Weis, 2004). A dynamic, relatively unstructured arrangement is also in line with the unique physicochemical characteristics of FG-carrying domains (Denning et al, 2003; Lim et al, 2007a, 2007b) and is supported by modelling studies showing that transport is relatively insensitive to the number and distribution of FG repeats in the pore (Zilman et al, 2007). Keeping the (average) number of contacts between an NTR and the NPC small, further increases the adaptability of the system. Importantly, it ensures that the gain in partition of NTRs into the NPC, facilitated by binding to FG sites, is not beset by too slow dissociation from the pores brought about by the need to simultaneously disengage numerous interactions to proceed. We note that the characteristics revealed here for the interactions between cargo and the NPC cannot be used to discriminate between existing models proposed for NPC selectivity, as these models differ mainly in the way the interaction among the FG repeats themselves is treated rather than in the way the interaction between cargo and FG repeats is perceived. Our data can be useful in placing constraints on future models in which the interaction network between FG repeats and NTRs is more detailed. Finally, the use of NTR mimics, as used in this work, allows systematic exploration of the effects of size, shape, and hydrophobicity on macromolecular transport through NPCs and may provide a new means for nuclear delivery of macromolecules independent of NTR-mediated transport.
Materials and methods
Preparation of conjugates
Surface-exposed Nɛ-amino (Lys) and hydroxyl (Ser and Thr) groups, present on BSA, served as primary and secondary sites for conjugation, respectively. In cases in which extensive modification was performed, binding to unexposed lysil and seryl residues or to accessible but less active functional groups (e.g. free cysteins) could also take place. The 3-indolepropionic acid, 3-phenylpropionic acid, 4-methyl valeric acid, and glycolic acid were attached to FITC-labelled BSA (all purchased from Sigma) through an N,N′-dicyclohexyl carbodiimide cross-linker, following Fuchs and Fuchs (1969). The degree of modification was varied by using different protein/modifier stoichiometries, ranging from 1:20 to 1:300. The conjugates were dissolved in 100 μl of dry dioxane. The cross-linker, also dissolved in dioxane, was then added and the solution was stirred for 30 min at room temperature. The supernatant, which contained the active ester derivative of the conjugates, was added drop wise to a solution of 10 mg/ml FITC-BSA in 0.1 M borate buffer (pH 8.5). The solution was stirred overnight at 4°C, centrifuged, and the supernatant was desalted against PBS (pH 7.2). The degree of modification was determined post facto by mass spectrometry (Supplementary Figure 2). The modified molecules were subjected to gel-filtration analysis to verify that no major changes in size have occurred consequent to conjugation. The molecules were further analysed for structural alterations by far-UV circular dichroism (Supplementary Figure 3).
Assessment of conjugation sites distribution on BSA surface
An assessment of the spatial clustering of surface-exposed Nɛ-amino (Lys) and hydroxyl (Ser) groups, present in BSA, was performed by K-means clustering analysis (Kaufman and Rousseeuw, 1990) using an algorithm implemented in MATLAB. The association of a point with a cluster is tested by calculating its average silhouette value, s, which reflects the ratio between the proximity of a point to other points in its own cluster versus its proximity to points in the nearest cluster. s approaches 1 for a perfectly clustered point, 0 for a point, which could be clustered with another cluster equally well, and −1 for a point, which is clustered inappropriately. For our analysis, we calculated the average s, which results when the conjugation sites on the surface of BSA is divided into clusters containing four or more points. s was found to have a value between 0.4 and 0.5, similar to the value obtained when the same procedure was applied to an ellipsoid with dimensions similar to those of BSA (ra=80 Å, rb=80 Å, rc=40 Å) and carrying the same number of residues distributed randomly on its surface.
Cell permeabilization
HeLa cells (ATCC CCL-2; up to five passages) were cultured in DMEM-10% fetal calf serum (FCS)/antibiotics (all purchased from Life Technologies) and were synchronized by serum starvation (0.15% FCS, for 14 h). For permeabilization with digitonin, cells were washed with cold transport buffer (TB; 30 mM sodium chloride, 90 mM potassium acetate, 5 mM magnesium acetate, 1 mM EDTA, 2 mM DTT, 250 mM sucrose, and 20 mM Tris–HCl pH 7.4) and incubated for 5 min with 8 μg/ml digitonin (Sigma) on ice, after which they were rinsed for five times with cold TB. In cases in which disrupting the microtubles was necessary, nocodazole at 5 μg/ml was added to the cells 1 h before the experiments. Permeabilization with streptolysin O (SLO) was performed by subjecting the cells to 50 μl of TB containing 0.3 mg/ml SLO (Sigma) and 0.1 M DTT for 30 min at 37°C. The cells were washed with TB and then were subjected to a mixture containing transport inhibitors (WGA or α-gp62) and an NE integrity marker. After 30 min, a second permeabilization was performed (also with SLO), which was used to introduce the transport probes.
Transport assays
Measurements were performed on permeamibilized HeLa cells pre-treated with nocodazol applied 1 h before the recordings. Experiments were also made on non-treated cells, yielding qualitatively similar results. In some of the experiments, the cells were supplemented with cytoplasmic extract (kindly provided by Ronen Kopito and Michael Elbaum, Weizmann Institute of Science) and an energy regeneration system as described (Kopito and Elbaum, 2007). The isolation of Xenopus oocyte nuclei, also used in this study, is described in Nevo et al (2000). With the exception of the latter (for details, see legend to Supplementary Figure 4), transport assays were conducted in TB at 37°C in an open perfusion micro-incubator (PDMI II, Harvard Apparatus) mounted on the stage of the microscope used for the recordings. Cells were viewed with an IX70-based Olympus FluoView 500 confocal laser-scanning microscope, using a 0.85-numerical-aperture × 40 (UplanApo) objective. The focal plane was set such that it traversed through the mid-section of the nuclei. FITC-BSA, Alexa Fluor Dextran, and their hydrophobic derivatives were visualized using an argon laser (λex=488 nm) and a 505–525-nm band-pass filter. TRITC-dextran and TRITC-BSA-NLS were excited at 543 nm, using a He–Ne laser, and the emitted fluorescence was collected between 560 and 600 nm. Images were acquired every 10 s after addition of the transport mixture, in a sequential mode, to prevent spectral bleed-through of the fluorescence emitted from the tracers and the 66-kDa dextran that was co-added as a marker for NE integrity and were processed using the ImagePro Plus (v4.5) software.
Supplementary Material
Supplementary Information
Review Process File
Acknowledgments
We thank Meir Wilchek (Weizmann Institute of Science) for advice regarding chemical conjugation of amino-acid analogues to BSA, members of the laboratories of Eitan Reuveny and Haim Garty (Weizmann Institute of Science) for the preparation of Xenopus laevis oocytes, Vladimir Kiss for assistance with the confocal microscope measurements, and Alla Shainskaya (Mass Spectrometry Unit, Weizmann Institute of Science) for performing the mass spectrometry analyses. We also thank Michael Elbaum, Michael Fainzilber, Ronen Kopito, Abraham Minsky, and Itay Rousso (Weizmann Institute of Science) for helpful discussions and comments. This work was supported by a grant from the Human Frontiers Science Program (ZR).
Footnotes
The authors declare that they have no conflict of interest.
References
- Adam SA, Marr RS, Gerace L (1990) Nuclear protein import in permeabilized mammalian cells requires soluble cytoplasmic factors. J Cell Biol 111: 807–816 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauer WR, Nadler W (2006) Molecular transport through channels and pores: effects of in-channel interactions and blocking. Proc Natl Acad Sci USA 103: 11446–11451 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bayliss R, Littlewood T, Stewart M (2000) Structural basis for the interaction between FxFG nucleoporin repeats and importin-beta in nuclear trafficking. Cell 102: 99–108 [DOI] [PubMed] [Google Scholar]
- Bayliss R, Littlewood T, Strawn LA, Wente SR, Stewart M (2002) GLFG and FxFG nucleoporins bind to overlapping sites on importin-beta. J Biol Chem 277: 50597–50606 [DOI] [PubMed] [Google Scholar]
- Bayliss R, Ribbeck K, Akin D, Kent HM, Feldherr CM, Gorlich D, Stewart M (1999) Interaction between NTF2 and xFxFG-containing nucleoporins is required to mediate nuclear import of RanGDP. J Mol Biol 293: 579–593 [DOI] [PubMed] [Google Scholar]
- Bednenko J, Cingolani G, Gerace L (2003) Importin beta contains a COOH-terminal nucleoporin binding region important for nuclear transport. J Cell Biol 162: 391–401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben-Efraim I, Gerace L (2001) Gradient of increasing affinity of importin beta for nucleoporins along the pathway of nuclear import. J Cell Biol 152: 411–417 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berezhkovskii AM, Bezrukov SM (2005) Optimizing transport of metabolites through large channels: molecular sieves with and without binding. Biophys J 88: L17–L19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bezrukov SM, Berezhkovskii AM, Szabo A (2007) Diffusion model of solute dynamics in a membrane channel: mapping onto the two-site model and optimizing the flux. J Chem Phys 127: 115101. [DOI] [PubMed] [Google Scholar]
- Burke B (2006) Cell biology. Nuclear pore complex models gel. Science 314: 766–767 [DOI] [PubMed] [Google Scholar]
- Chi NC, Adam SA (1997) Functional domains in nuclear import factor p97 for binding the nuclear localization sequence receptor and the nuclear pore. Mol Biol Cell 8: 945–956 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corbett AH, Silver PA (1997) Nucleocytoplasmic transport of macromolecules. Microbiol Mol Biol Rev 61: 193–211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denning DP, Patel SS, Uversky V, Fink AL, Rexach M (2003) Disorder in the nuclear pore complex: the FG repeat regions of nucleoporins are natively unfolded. Proc Natl Acad Sci USA 100: 2450–2455 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elbaum M (2006) Materials science. Polymers in the pore. Science 314: 766–767 [DOI] [PubMed] [Google Scholar]
- Fahrenkrog B, Koser J, Aebi U (2004) The nuclear pore complex: a jack of all trades? Trends Biochem Sci 29: 175–182 [DOI] [PubMed] [Google Scholar]
- Frey S, Gorlich D (2007) A saturated FG-repeat hydrogel can reproduce the permeability properties of nuclear pore complexes. Cell 130: 512–523 [DOI] [PubMed] [Google Scholar]
- Frey S, Richter RP, Gorlich D (2006) FG-rich repeats of nuclear pore proteins form a three-dimensional meshwork with hydrogel-like properties. Science 314: 815–817 [DOI] [PubMed] [Google Scholar]
- Fried H, Kutay U (2003) Nucleocytoplasmic transport: taking an inventory. Cell Mol Life Sci 60: 1659–1688 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuchs S, Fuchs Y (1969) Immunological assay for plant hormones using specific antibodies to indoleacetic acid and gibberellic acid. Biochim Biophys Acta 192: 528–530 [DOI] [PubMed] [Google Scholar]
- Gorlich D, Kutay U (1999) Transport between the cell nucleus and the cytoplasm. Annu Rev Cell Dev Biol 15: 607–660 [DOI] [PubMed] [Google Scholar]
- Isgro TA, Schulten K (2005) Binding dynamics of isolated nucleoporin repeat regions to importin-beta. Structure 13: 1869–1879 [DOI] [PubMed] [Google Scholar]
- Isgro TA, Schulten K (2007a) Association of nuclear pore FG-repeat domains to NTF2 import and export complexes. J Mol Biol 366: 330–345 [DOI] [PubMed] [Google Scholar]
- Isgro TA, Schulten K (2007b) Cse1p-binding dynamics reveal a binding pattern for FG-repeat nucleoporins on transport receptors. Structure 15: 977–991 [DOI] [PubMed] [Google Scholar]
- Jovanovic-Talisman T, Tetenbaum-Novatt J, McKenney AS, Zilman A, Peters R, Rout MP, Chait BT (2008) Artificial nanopores that mimic the transport selectivity of the nuclear pore complex. Nature 457: 1023–1027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufman L, Rousseeuw P (1990) Finding groups in data. An introduction to cluster analysis. In Applied Probability and Statistics, Kaufman L, Rousseeuw P (eds), pp 342. New York: Wiley [Google Scholar]
- Keminer O, Peters R (1999) Permeability of single nuclear pores. Biophys J 77: 217–228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kopito RB, Elbaum M (2007) Reversibility in nucleocytoplasmic transport. Proc Natl Acad Sci USA 104: 12743–12748 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kose S, Imamoto N, Tachibana T, Shimamoto T, Yoneda Y (1997) Ran-unassisted nuclear migration of a 97-kD component of nuclear pore-targeting complex. J Cell Biol 139: 841–849 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kramer A, Ludwig Y, Shahin V, Oberleithner H (2007) A pathway separate from the central channel through the nuclear pore complex for inorganic ions and small macromolecules. J Biol Chem 282: 31437–31443 [DOI] [PubMed] [Google Scholar]
- Kutay U, Izaurralde E, Bischoff FR, Mattaj IW, Gorlich D (1997) Dominant-negative mutants of importin-beta block multiple pathways of import and export through the nuclear pore complex. EMBO J 16: 1153–1163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim RY, Fahrenkrog B, Koser J, Schwarz-Herion K, Deng J, Aebi U (2007a) Nanomechanical basis of selective gating by the nuclear pore complex. Science 318: 640–643 [DOI] [PubMed] [Google Scholar]
- Lim RY, Huang NP, Koser J, Deng J, Lau KH, Schwarz-Herion K, Fahrenkrog B, Aebi U (2006) Flexible phenylalanine-glycine nucleoporins as entropic barriers to nucleocytoplasmic transport. Proc Natl Acad Sci USA 103: 9512–9517 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim RY, Koser J, Huang NP, Schwarz-Herion K, Aebi U (2007b) Nanomechanical interactions of phenylalanine-glycine nucleoporins studied by single molecule force-volume spectroscopy. J Struct Biol 159: 277–289 [DOI] [PubMed] [Google Scholar]
- Lim RY, Ullman KS, Fahrenkrog B (2008) Biology and biophysics of the nuclear pore complex and its components. Int Rev Cell Mol Biol 267: 299–342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu SM, Stewart M (2005) Structural basis for the high-affinity binding of nucleoporin Nup1p to the Saccharomyces cerevisiae importin-beta homologue, Kap95p. J Mol Biol 349: 515–525 [DOI] [PubMed] [Google Scholar]
- Lu D, Grayson P, Schulten K (2003) Glycerol conductance and physical asymmetry of the Escherichia coli glycerol facilitator GlpF. Biophys J 85: 2977–2987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macara IG (2001) Transport into and out of the nucleus. Microbiol Mol Biol Rev 65: 570–594 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattaj IW, Englmeier L (1998) Nucleocytoplasmic transport: the soluble phase. Annu Rev Biochem 67: 265–306 [DOI] [PubMed] [Google Scholar]
- Morrison J, Yang JC, Stewart M, Neuhaus D (2003) Solution NMR study of the interaction between NTF2 and nucleoporin FxFG repeats. J Mol Biol 333: 587–603 [DOI] [PubMed] [Google Scholar]
- Naim B, Brumfeld V, Kapon R, Kiss V, Nevo R, Reich Z (2007) Passive and facilitated transport in nuclear pore complexes is largely uncoupled. J Biol Chem 282: 3881–3888 [DOI] [PubMed] [Google Scholar]
- Nevo R, Markiewicz P, Kapon R, Elbaum M, Reich Z (2000) High-resolution imaging of the nuclear pore complex by AC scanning force microscopy. Single Mol 1: 109–114 [Google Scholar]
- Otsuka S, Iwasaka S, Yoneda Y, Takeyasu K, Yoshimura SH (2008) Individual binding pockets of importin-beta for FG-nucleoporins have different binding properties and different sensitivities to RanGTP. Proc Natl Acad Sci USA 105: 16101–16106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paine PL, Moore LC, Horowitz SB (1975) Nuclear envelope permeability. Nature 254: 109–114 [DOI] [PubMed] [Google Scholar]
- Patel SS, Belmont BJ, Sante JM, Rexach MF (2007) Natively unfolded nucleoporins gate protein diffusion across the nuclear pore complex. Cell 129: 83–96 [DOI] [PubMed] [Google Scholar]
- Patel SS, Rexach MF (2008) Discovering novel interactions at the nuclear pore complex using bead halo: a rapid method for detecting molecular interactions of high and low affinity at equilibrium. Mol Cell Proteomics 7: 121–131 [DOI] [PubMed] [Google Scholar]
- Pemberton LF, Paschal BM (2005) Mechanisms of receptor-mediated nuclear import and nuclear export. Traffic 6: 187–198 [DOI] [PubMed] [Google Scholar]
- Peters R (2005) Translocation through the nuclear pore complex: selectivity and speed by reduction-of-dimensionality. Traffic 6: 421–427 [DOI] [PubMed] [Google Scholar]
- Pyhtila B, Rexach M (2003) A gradient of affinity for the karyopherin Kap95p along the yeast nuclear pore complex. J Biol Chem 278: 42699–42709 [DOI] [PubMed] [Google Scholar]
- Quimby BB, Leung SW, Bayliss R, Harreman MT, Thirumala G, Stewart M, Corbett AH (2001) Functional analysis of the hydrophobic patch on nuclear transport factor 2 involved in interactions with the nuclear pore in vivo. J Biol Chem 276: 38820–38829 [DOI] [PubMed] [Google Scholar]
- Radu A, Moore MS, Blobel G (1995) The peptide repeat domain of nucleoporin Nup98 functions as a docking site in transport across the nuclear pore complex. Cell 81: 215–222 [DOI] [PubMed] [Google Scholar]
- Ribbeck K, Gorlich D (2001) Kinetic analysis of translocation through nuclear pore complexes. EMBO J 20: 1320–1330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ribbeck K, Gorlich D (2002) The permeability barrier of nuclear pore complexes appears to operate via hydrophobic exclusion. EMBO J 21: 2664–2671 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rout MP, Aitchison JD, Magnasco MO, Chait BT (2003) Virtual gating and nuclear transport: the hole picture. Trends Cell Biol 13: 622–628 [DOI] [PubMed] [Google Scholar]
- Rout MP, Aitchison JD, Suprapto A, Hjertaas K, Zhao Y, Chait BT (2000) The yeast nuclear pore complex: composition, architecture, and transport mechanism. J Cell Biol 148: 635–651 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rout MP, Wente SR (1994) Pores for thought: nuclear pore complex proteins. Trends Cell Biol 4: 357–365 [DOI] [PubMed] [Google Scholar]
- Stewart M (2007) Molecular mechanism of the nuclear protein import cycle. Nat Rev Mol Cell Biol 8: 195–208 [DOI] [PubMed] [Google Scholar]
- Strawn LA, Shen T, Shulga N, Goldfarb DS, Wente SR (2004) Minimal nuclear pore complexes define FG repeat domains essential for transport. Nat Cell Biol 6: 197–206 [DOI] [PubMed] [Google Scholar]
- Suntharalingam M, Wente SR (2003) Peering through the pore: nuclear pore complex structure, assembly, and function. Dev Cell 4: 775–789 [DOI] [PubMed] [Google Scholar]
- Tran EJ, Wente SR (2006) Dynamic nuclear pore complexes: life on the edge. Cell 125: 1041–1053 [DOI] [PubMed] [Google Scholar]
- Walev I, Bhakdi SC, Hofmann F, Djonder N, Valeva A, Aktories K, Bhakdi S (2001) Delivery of proteins into living cells by reversible membrane permeabilization with streptolysin-O. Proc Natl Acad Sci USA 98: 3185–3190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weis K (2003) Regulating access to the genome: nucleocytoplasmic transport throughout the cell cycle. Cell 112: 441–451 [DOI] [PubMed] [Google Scholar]
- Weis K (2007) The nuclear pore complex: oily spaghetti or gummy bear? Cell 130: 405–407 [DOI] [PubMed] [Google Scholar]
- Yazicioglu MN, Goad DL, Ranganathan A, Whitehurst AW, Goldsmith EJ, Cobb MH (2007) Mutations in ERK2 binding sites affect nuclear entry. J Biol Chem 282: 28759–28767 [DOI] [PubMed] [Google Scholar]
- Yoneda Y (2000) Nucleocytoplasmic protein traffic and its significance to cell function. Genes Cells 5: 777–787 [DOI] [PubMed] [Google Scholar]
- Zeitler B, Weis K (2004) The FG-repeat asymmetry of the nuclear pore complex is dispensable for bulk nucleocytoplasmic transport in vivo. J Cell Biol 167: 583–590 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zilman A (2009) Effects of multiple occupancy and interparticle interactions on selective transport through narrow channels: theory versus experiment. Biophys J 96: 1235–1248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zilman A, Di Talia S, Chait BT, Rout MP, Magnasco MO (2007) Efficiency, selectivity, and robustness of nucleocytoplasmic transport. PLoS Comput Biol 3: e125. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Information
Review Process File