Abstract
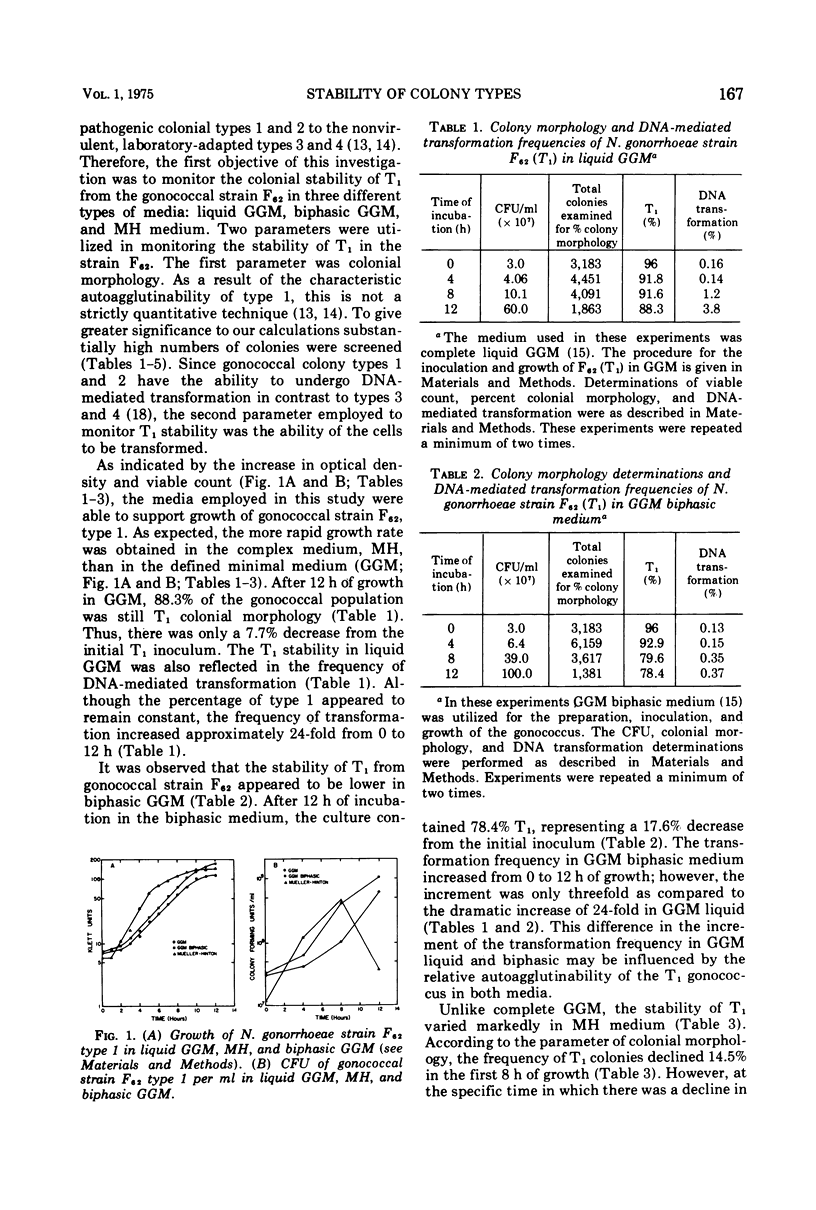
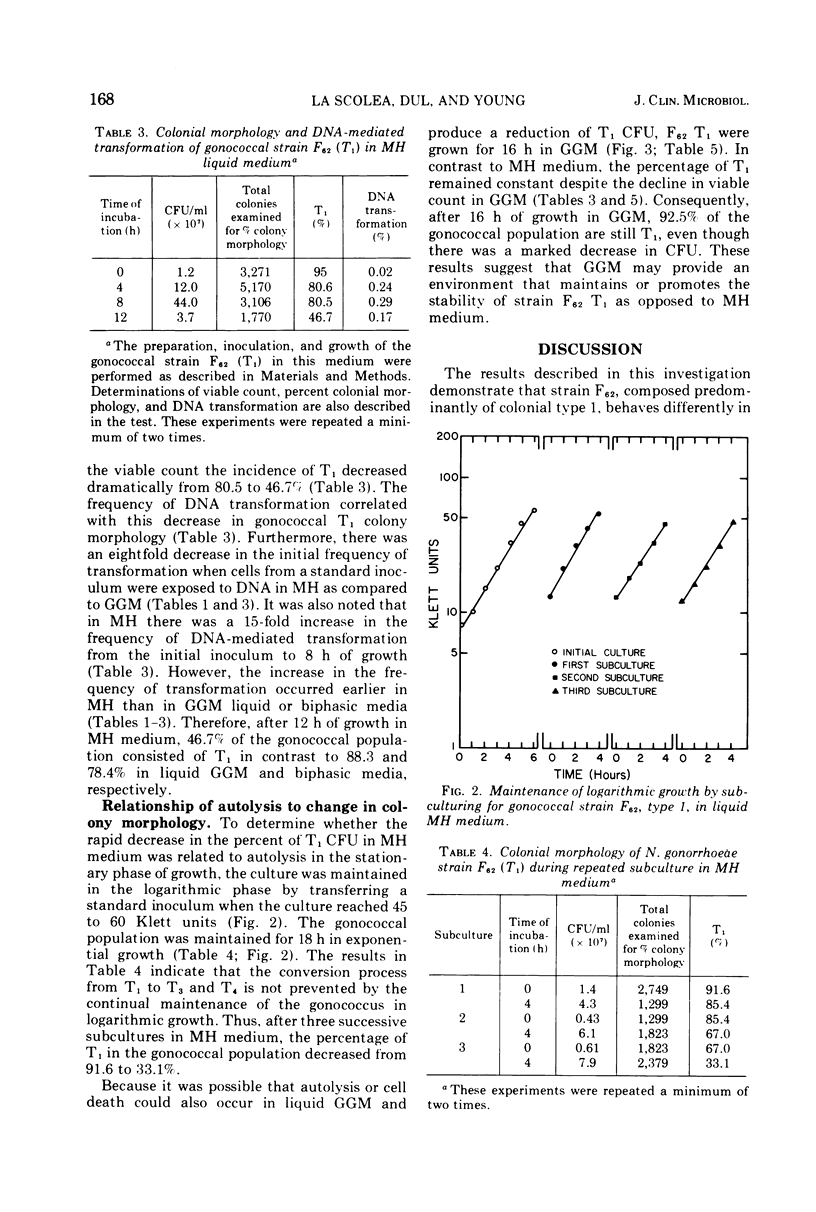
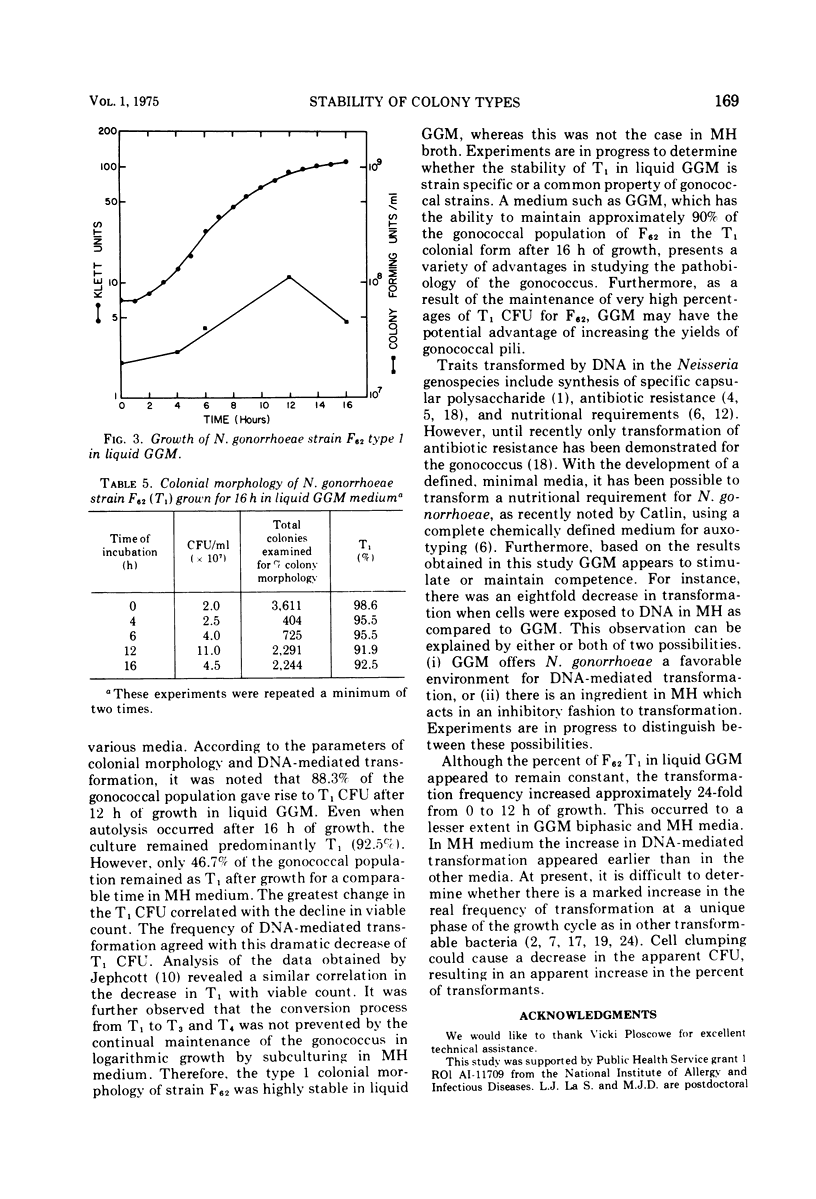
This investigation describes the surveillance of the colonial stability of the pathogenic type 1 from the gonococcal strain F62 to the nonvirulent types 3 and 4 in different liquid media. The maintenance of the colony types was monitored by the parameters of colonial morphology and deoxyribonucleic acid-mediated transformation. During growth in a complex medium, Mueller-Hinton broth, only 46.7% of the gonococcal population remained as type 1 after 12 h. The greatest change in the type 1 colony-forming units correlated with the decline in viable count. The conversion process could not be prevented by the continual maintenance of the gonococcus in logarithmic growth. The frequency of transformation from PRO(minus) (proline) to PRO(plus) was proportional to this decrease in type 1 colony-forming units. In contrast to Mueller-Hinton medium, the chemically defined minimal medium Gonococcal Genetic Medium (GGM) was capable of maintaining approximately 90% of the gonococcal population in the type 1 colonial form after 16 h of growth, despite a decrease in the viable count. Although the percentage of type 1 appeared to remain constant in GGM, the apparent transformation frequency increased approximately 24-fold from 0 to 12 h of growth. GGM appears to stimulate or maintain competence, as evidenced by an eightfold increase in transformation when cells are exposed to deoxyribonucleic acid in GGM as compared to Mueller-Hinton.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anagnostopoulos C., Spizizen J. REQUIREMENTS FOR TRANSFORMATION IN BACILLUS SUBTILIS. J Bacteriol. 1961 May;81(5):741–746. doi: 10.1128/jb.81.5.741-746.1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchanan T. M., Gotschlich E. C. Studies on gonococcus infection. 3. Correlation of gonococcal colony morphology with infectivity for the chick embryo. J Exp Med. 1973 Jan 1;137(1):196–200. doi: 10.1084/jem.137.1.196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CATLIN B. W. Transformation of Neisseria meningitidis by deoxyribonucleates from cells and from culture slime. J Bacteriol. 1960 Apr;79:579–590. doi: 10.1128/jb.79.4.579-590.1960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catlin B. W. Genetic studies of sulfadiazine-resistant and methionine-requiring Neisseria isolated from clinical material. J Bacteriol. 1967 Sep;94(3):719–733. doi: 10.1128/jb.94.3.719-733.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catlin B. W. Genetic transformation of biosynthetically defective Neisseria gonorrhoeae clinical isolates. J Bacteriol. 1974 Oct;120(1):203–209. doi: 10.1128/jb.120.1.203-209.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hotchkiss R. D. CYCLICAL BEHAVIOR IN PNEUMOCOCCAL GROWTH AND TRANSFORMABILITY OCCASIONED BY ENVIRONMENTAL CHANGES. Proc Natl Acad Sci U S A. 1954 Feb;40(2):49–55. doi: 10.1073/pnas.40.2.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunter K. M., McVeigh I. Development of a chemically defined medium for growth of Neisseria gonorrhoeae. Antonie Van Leeuwenhoek. 1970;36(2):305–316. doi: 10.1007/BF02069032. [DOI] [PubMed] [Google Scholar]
- JYSSUM K., LIE S. TRANSFORMATION OF AUXOTROPHS OF NEISSERIA MENINGITIDIS. Acta Pathol Microbiol Scand. 1965;63:445–455. doi: 10.1111/apm.1965.63.3.445. [DOI] [PubMed] [Google Scholar]
- James-Holmquest A. N., Swanson J., Buchanan T. M., Wende R. D., Williams R. P. Differential attachment by piliated and nonpiliated Neisseria gonorrhoeae to human sperm. Infect Immun. 1974 May;9(5):897–902. doi: 10.1128/iai.9.5.897-902.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jephcott A. E. Preliminary study of colony type stability of Neisseria gonorrhoeae in liquid culture. Br J Vener Dis. 1972 Oct;48(5):369–375. doi: 10.1136/sti.48.5.369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jephcott A. E., Reyn A., Birch-Andersen A. Neisseria gonorrhoeae 3. Demonstration of presumed appendages to cells from different colony types. Acta Pathol Microbiol Scand B Microbiol Immunol. 1971;79(3):437–439. doi: 10.1111/j.1699-0463.1971.tb00086.x. [DOI] [PubMed] [Google Scholar]
- KELLOGG D. S., Jr, PEACOCK W. L., Jr, DEACON W. E., BROWN L., PIRKLE D. I. NEISSERIA GONORRHOEAE. I. VIRULENCE GENETICALLY LINKED TO CLONAL VARIATION. J Bacteriol. 1963 Jun;85:1274–1279. doi: 10.1128/jb.85.6.1274-1279.1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kellogg D. S., Jr, Cohen I. R., Norins L. C., Schroeter A. L., Reising G. Neisseria gonorrhoeae. II. Colonial variation and pathogenicity during 35 months in vitro. J Bacteriol. 1968 Sep;96(3):596–605. doi: 10.1128/jb.96.3.596-605.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- La Scolea L. J., Jr, Young F. E. Development of a defined minimal medium for the growth of Neisseria gonorrhoeae. Appl Microbiol. 1974 Jul;28(1):70–76. doi: 10.1128/am.28.1.70-76.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pakula R. Factors regulating competence in transformation of streptococci. J Bacteriol. 1965 Nov;90(5):1320–1324. doi: 10.1128/jb.90.5.1320-1324.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sparling P. F. Genetic transformation of Neisseria gonorrhoeae to streptomycin resistance. J Bacteriol. 1966 Nov;92(5):1364–1371. doi: 10.1128/jb.92.5.1364-1371.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spencer H. T., Herriott R. M. Development of competence of Haemophilus influenzae. J Bacteriol. 1965 Oct;90(4):911–920. doi: 10.1128/jb.90.4.911-920.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swanson J., Kraus S. J., Gotschlich E. C. Studies on gonococcus infection. I. Pili and zones of adhesion: their relation to gonococcal growth patterns. J Exp Med. 1971 Oct 1;134(4):886–906. doi: 10.1084/jem.134.4.886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thongthai C., Sawyer W. D. Studies on the virulence of Neisseria gonorrhoeae. I. Relation of colonial morphology and resistance to phagocytosis by polymorphonuclear leukocytes. Infect Immun. 1973 Mar;7(3):373–379. doi: 10.1128/iai.7.3.373-379.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watt P. J., Glynn A. A., Ward M. E. Maintenance of virulent gonococci in laboratory culture. Nat New Biol. 1972 Apr 12;236(67):186–187. doi: 10.1038/newbio236186a0. [DOI] [PubMed] [Google Scholar]
- YOUNG F. E., SPIZIZEN J. Physiological and genetic factors affecting transformation of Bacillus subtilis. J Bacteriol. 1961 May;81:823–829. doi: 10.1128/jb.81.5.823-829.1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yasbin R. E., Young F. E. The influence of temperate bacteriophage phi105 on transformation and transfection in Bacillus subtilis. Biochem Biophys Res Commun. 1972 Apr 28;47(2):365–371. doi: 10.1016/0006-291x(72)90722-x. [DOI] [PubMed] [Google Scholar]


