Abstract
Greatwall (GW) is a new kinase that has an important function in the activation and the maintenance of cyclin B–Cdc2 activity. Although the mechanism by which it induces this effect is unknown, it has been suggested that GW could maintain cyclin B–Cdc2 activity by regulating its activation loop. Using Xenopus egg extracts, we show that GW depletion promotes mitotic exit, even in the presence of a high cyclin B–Cdc2 activity by inducing dephosphorylation of mitotic substrates. These results indicate that GW does not maintain the mitotic state by regulating the cyclin B–Cdc2 activation loop but by regulating a phosphatase. This phosphatase is PP2A; we show that (1) PP2A binds GW, (2) the inhibition or the specific depletion of this phosphatase from mitotic extracts rescues the phenotype induced by GW inactivation and (3) the PP2A-dependent dephosphorylation of cyclin B–Cdc2 substrates is increased in GW-depleted Xenopus egg extracts. These results suggest that mitotic entry and maintenance is not only mediated by the activation of cyclin B–Cdc2 but also by the regulation of PP2A by GW.
Keywords: cyclin B–Cdc2, greatwall, mitosis, PP2A
Introduction
The entry into mitosis is driven by the activation of the cell-cycle kinase cyclin B–Cdc2 or MPF. MPF activity oscillates through the cell cycle, peaking at mitosis and dropping during interphase. The primary event controlling MPF activation is the binding of Cdc2 to cyclin B. The expression of cyclin B is restricted to late S and G2 phases and thus, the formation of the complex can only take place during this phase of the cell cycle (Pines and Hunter, 1989; Nurse, 1990). After cyclin B–Cdc2 association, which only yields a partially active complex, the CAK kinase phosphorylates Cdc2 at thr 161. This phosphorylation induces a change in the T loop of Cdc2, making the catalytic cleft fully accessible to ATP (Russo et al, 1996; Draetta, 1997; Fesquet et al, 1997). Finally, Cdc2 is regulated by phosphorylation at thr 14 and tyr 15, which involves a balance of the inhibitory kinases Myt1/Wee1 and the activatory phosphatase Cdc25. Myt1/Wee1 phosphorylate Cdc2 at residues thr 14 and tyr 15 during G2, whereas Cdc25 reverses these inhibitory phosphorylations at mitotic entry (Morgan, 1997). This model proposes that after thr 161 phosphorylation, cyclin B–Cdc2 complexes are held in an inactive state by phosphorylation at Thr 14 and Tyr 15 by Myt1 and Wee1. At the end of the G2 phase, the MPF feedback loop is activated by the abrupt dephosphorylation of these residues by Cdc25. This dephosphorylation promotes an initial activation of cyclin B–Cdc2, which in turns activates Cdc25 and inactivates Wee1 and Myt1 by phosphorylation, resulting in full activation of the cyclin B–Cdc2 complex (Perdiguero and Nebreda, 2004; Perry and Kornbluth, 2007).
Apart from CAK, Cdc25, Myt1 and Wee1, a new MPF regulator, GW, has been described. The depletion of GW from metaphase II-arrested Xenopus egg extracts (CSF extracts) induces mitotic exit, whereas the same depletion prevents mitotic entry in cycling extracts. GW kinase has an important function in both, the activation and the maintenance of cyclin B–Cdc2 activity, however, the mechanism by which it regulates this complex is completely unknown (Yu et al, 2006; Zhao et al, 2008).
Results
Greatwall maintains the mitotic state independently of MPF activity by inhibiting dephosphorylation
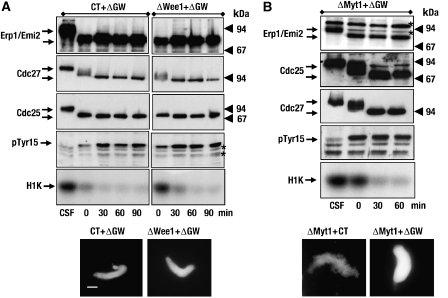
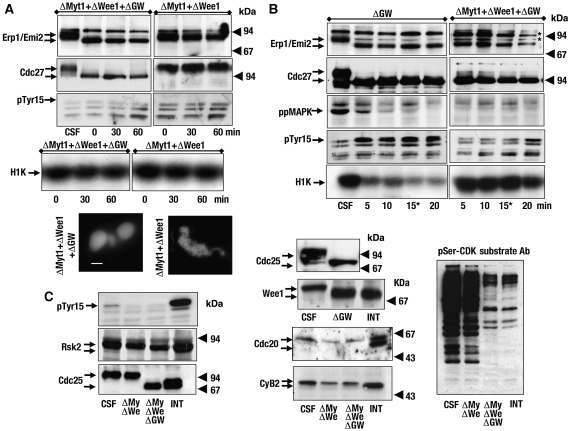
The removal of GW in mitosis induces the inactivation of MPF concomitantly with phosphorylation of Cdc2 at tyr 15, indicating that it could regulate the MPF feedback loop. To characterize the mechanism by which GW regulates cyclin B–Cdc2 kinase activity, we used CSF extracts depleted of GW alone or co-depleted of Wee1 or Myt1 and GW, and analysed the state of DNA condensation, the phosphorylation of Erp1/Emi2, Cdc27, Cdc25, and tyr 15 of Cdc2 and the activity of cyclin B–Cdc2. Our antibodies efficiently depleted the corresponding proteins from the extracts (Supplementary Figure S1). Moreover, as previously described, GW depletion induced the MPF inactivation, as reflected by dephosphorylation of Erp1/Emi2, Cdc27 and Cdc25; rephosphorylation on tyr 15 of Cdc2 and a decondensation of the DNA (Figure 1A, left panels). This corresponds to a specific effect of GW removal, as the observed phenotype is clearly rescued by the addition of a recombinant wild-type form of GW, but not by a kinase-dead version (Supplementary data, Figure S2A). As tyr 5 of Cdc2 was phosphorylated in the absence of GW, we expected that the co-depletion of Wee1 or Myt1 and GW would reverse this phenotype. However, Wee1/GW co-depletion neither reversed the phosphorylation of tyr 15 nor prevented the MPF inactivation (Figure 1A, right panels), although, a slight delay of this inactivation was observed (compare Figure 1A, H1K, times 0 of CT+ΔGW and ΔWee1+ΔGW). Moreover, all the analysed MPF substrates were dephosphorylated and DNA decondensed under these conditions. From these results, we conclude that Wee1 is not the main target of GW. We next investigated whether GW could maintain MPF activity by inhibiting Myt1 kinase. To test this hypothesis, we co-depleted Myt1 kinase and GW from CSF extracts. Similar results were observed (Figure 1B), therefore, Myt1 is also not the main target of GW. Next, we asked whether this kinase could regulate phosphorylation of tyr 15 of Cdc2 by inhibiting both kinases, Wee1 and Myt1, or by activating Cdc25 phosphatase. To analyse this hypothesis, we co-depleted Wee1 and Myt1 in CSF extracts before GW depletion. When Wee1 and Myt1 co-depletion was followed by the depletion with control antibodies, CSF extracts remained in mitosis, but the triple depletion of Myt1, Wee1 and GW still induced mitotic exit (Figure 2A). However, interestingly, due to the double removal of Myt1 and Wee1, we no longer observed any inhibitory phosphorylation of Cdc2 on tyr 15 and the cyclin B–Cdc2 kinase activity remained high (Figure 2A, left panels). We obtained the same results when Myt1, Wee1, Cdc25 and GW were depleted from the CSF extracts (data not shown). Thus, surprisingly, extracts still exited mitosis in the presence of a high cyclin B–Cdc2 activity. We conclude that GW preserves the mitotic state by a new unknown mechanism that is independent of cyclin B–Cdc2 activity. Moreover, this new mechanism seems to be very rapid, as Cdc27 and Erp1/Emi2 are dephosphorylated immediately after GW depletion. To measure the kinetics of this dephosphorylation, we developed a time-course analysis in which anti-GW antibodies bound to Dynabeads were added to the extract after control or after Myt1–Wee1 co-depletions. Samples were removed at the indicated time points after addition of anti-GW antibodies. The depletion of GW induced a rephosphorylation of Cdc2 on tyr 15 and a decrease of cyclin B–Cdc2 activity (Figure 2B), due to Cdc25 and Wee1 dephosphorylation 5 min after antibody addition (Figure 2C). As expected, prior co-depletion of Myt1 and Wee1 prevented the rephosphorylation of Cdc2 on tyr 15 as well as the decrease of MPF activity. However, dephosphorylation of the different MPF-dependent substrates (Erp1/Emi2, Cdc27 and MAPK) was observed as early as 5 min after antibody addition in both conditions.
Figure 1.
Co-depletion of GW with Wee1 or Myt1 does not prevent mitotic exit. (A) CSF extracts were co-depleted with control (CT) or anti-Wee1 (ΔWee1) and anti-GW (ΔGW) antibodies. Phosphorylation of the indicated proteins was analysed by western blot. Cyclin B–Cdc2 activity was measured by H1 histone phosphorylation assay (H1K). Finally, chromatin condensation was visualized by light microscopy. Asterisks denote non-specific bands of anti-pTyr 15 antibody. (B) Similar to (A) except that the depletion of Myt1 (ΔMyt1) was studied instead that of Wee1 before GW immunoprecipitation. Bar, 5 μm.
Figure 2.
GW depletion induces mitotic exit in CSF extracts in the presence of high cyclin B–Cdc2 activity. (A) A triple depletion with Myt1–Wee1–GW antibodies or Myt1–Wee1–Control antibodies were carried out in CSF extracts and the phosphorylation of the indicated proteins, as well as the cyclin B–Cdc2 activity and chromatin condensation were analysed. (B) CSF extracts were immunoprecipitated twice with control antibodies or with anti-Myt1 and anti-Wee1 antibodies. Subsequently, anti-GW antibody-bound Dynabeads were added to the supernatants and samples were removed at the indicated times. * Time-point 0 min in GW immunodepletions of Figure 1 corresponds to time-point 15 min of this figure. (C) Supernatants of GW, Myt1–Wee1 or Myt1–Wee1–GW immunoprecipitates were used to analyse the phosphorylation of the indicated proteins using western blot. Bar 5 μm.
These results show that GW maintains phosphorylation of, at least, four different MPF-dependent substrates, that is, Cdc25, Cdc27, MAPK and Erp1/Emi2. To investigate if this protection against phosphorylation is a general response, we analysed the phosphorylation state of four different proteins (Rsk2, Wee1, Cdc25 and Cdc20) whose phosphorylation depends directly or indirectly on cyclin B–Cdc2 activity during mitosis. Most of the analysed proteins (Rsk2, Wee1 and Cdc25 shown in Figure 2C and Cdc27, MAPK and Erp1/Emi2 in Figure 2B) were dephosphorylated after GW depletion. However, this is not the result of a non-specific dephosphorylation, as cyclin B2, Cdc20 (Figure 2C) and Cdc2 (see phospho tyr 15, Figure 2B) conserved their phosphorylation states under these conditions. Finally, we analysed the general cyclin B–Cdc2-dependent phosphorylation state in these extracts by using an antibody directed against the phosphorylated serine of the Cdk consensus motif. As shown in Figure 2C (right panel), a strong signal, corresponding to phosphorylated MPF substrates, was present in CSF as well as Myt1–Wee1 co-depleted extracts, however, this signal decreased markedly in both Myt1–Wee1–GW co-depleted extracts and in interphase extracts. Thus, GW keeps the mitotic state by maintaining phosphorylation of the majority of MPF substrates, although some of them are not subjected to this regulation.
The PP1/PP2A inhibitors, microcystin and okadaic acid, rescue the phenotype induced by Greatwall inactivation in CSF extracts
The general and rapid dephosphorylation induced by GW removal in CSF extracts suggests that this kinase could act as a phosphatase inhibitor. It has recently been shown that the phosphatase, calcineurin, is required to release CSF extracts from meiotic M phase (Mochida and Hunt, 2007; Nishiyama et al, 2007). In addition, our results show that the overexpression of GW in CSF extracts delays mitotic exit induced by calcium (Supplementary Figure S2B). Thus, one putative target of GW could be calcineurin. We tested the role of calcineurin in this pathway by using the specific inhibitor cyclosporin. However, the inhibition of this phosphatase did not affect the exit of mitosis induced by GW removal, although, as described, it clearly delayed the dephosphorylation of Cdc27 and cyclin B degradation after Ca2+ addition (Supplementary Figure S3B). Thus, GW does not regulate mitosis through calcineurin inhibition.
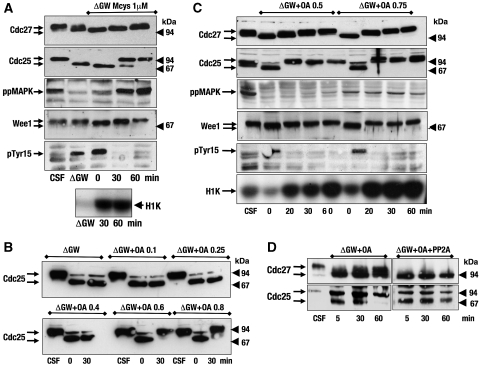
We next questioned whether GW could inhibit PP1 and/or PP2A, the major phosphatases present in Xenopus egg extracts. To test this hypothesis, we used the potent PP1/PP2A phosphatase inhibitor, microcystin, and we investigated whether it could rescue mitotic exit in GW-depleted CSF extracts. To this end, we first depleted GW from CSF extracts and we subsequently added microcystin. Samples were taken just after GW depletion and 0, 30 and 60 min after microcystin addition. The results are shown in Figure 3A. We observed the first dephosphorylation of the different analysed proteins just after GW depletion, followed by a rephosphorylation of these proteins at 30 min after microcystin addition. Moreover, after GW depletion, we observed a phosphorylation on tyr 15 of Cdc2 that was concomitant with Cdc25 and Wee1 dephosphorylation and with a clear decrease in cyclin B–Cdc2 activity. However, 30 min later, tyr 15 was dephosphorylated again, MPF substrates were re-phosphorylated and cyclin B–Cdc2 complex was reactivated. Thus, microcystin rescues the phenotype induced by GW inactivation.
Figure 3.
Phosphatase inhibitors, microcystin and okadaic acid (OA), rescue the phenotype induced by GW inactivation in CSF extracts. (A) GW-depleted CSF extracts were supplemented with microcystin (1 μM) and the phosphorylation of the indicated proteins as well as the cyclin B–Cdc2 activity were analysed. (B) CSF extracts were devoid of GW and supplemented with increasing doses of OA (from 0.1 to 0.8 μM). (C) GW-depleted CSF extracts were supplemented with 0.5 or 0.75 μM OA. (D) GW-depleted CSF extracts were supplemented with 0.75 μM OA and subsequently supplemented or not with purified PP2A (Upstate).
Microcystin is a potent inhibitor of both PP1 and PP2A (MacKintosh et al, 1990; Rivas et al, 2000). To elucidate which of these two phosphatases could be involved in mitotic exit, we used the phosphatase inhibitor, okadaic acid (OA), specificity of which for PP1 and PP2A, at different doses, has been described (Felix et al, 1990). We tested the dose–response of Cdc25 dephosphorylation on OA in GW-depleted CSF extracts, to analyse at what dose this inhibitor was capable to reverse Cdc25 dephosphorylation. As shown in Figure 3B, we observed a complete rephosphorylation of Cdc25, 30 min after GW depletion due to a 600-nM dose of OA. We next tried to determine, more accurately, the minimal dose that was capable of rescuing the GW phenotype. A total of 500 nM OA was sufficient to reverse the dephosphorylation of Cdc27, Cdc25, Wee1 and MAPK, although phosphorylation of the latter was only observed at 60 min probably due to the fact that it is induced indirectly by MPF-dependent phosphorylation of c-Mos (Figure 3C). At this dose, we also observed a dephosphorylation of Cdc2 at tyr 15 and an increase in cyclin B–Cdc2 activity at 20 min after the addition of the drug. As 500 nM of OA has been reported to inhibit 70% of PP2A activity and only 20% of PP1, it is likely that PP2A, rather than PP1, could be involved in the reversion of mitotic exit induced by GW depletion. In agreement with this hypothesis, the addition of the PP1 inhibitor, Inhibitor 2, was not able to reverse GW phenotype in CSF extracts (Supplementary Figure S4). Moreover, we observed a reversion of the effect of OA in GW-depleted CSF extracts when an active form of PP2A phosphatase was added after this phosphatase inhibitor (Figure 3D).
GW binds PP2A in human cells and in CSF extracts
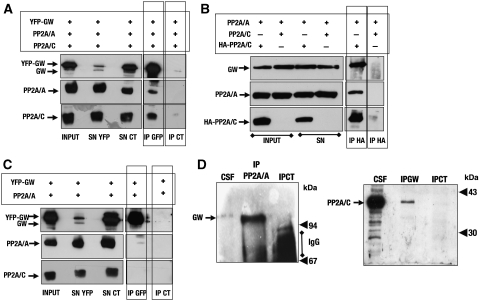
The results presented above suggest that GW maintains the mitotic state by regulating PP2A activity, suggesting that GW could bind PP2A. To investigate whether GW could associate with PP2A, we co-transfected YFP-tagged GW, and non-tagged PP2A/A and /C subunits in HEK293 cells and we subsequently immunoprecipitated cell lysate with either an anti-YFP or a control antibody. As shown in Figure 4A, both PP2A/A and C subunits were present in the immunoprecipitate when anti-YFP antibody, but not a control antibody, was used. We next co-transfected HEK293 cells with a non-tagged PP2A/A subunit and with either HA-tagged or non-tagged PP2A/C subunit, and the cell lysates were then immunoprecipitated with anti-HA antibodies. The results show that endogenous GW was present in the immunoprecipitate when HA-tagged PP2A/C, but not non-tagged PP2A/C, was used in co-transfection (Figure 4B). Finally, we analysed whether GW and PP2A/A could associate when co-transfected in the absence of PP2A/C overexpression. Under these conditions, we did not observed any association of these two proteins, indicating that PP2A/C subunit is required to mediate GW binding with PP2A (Figure 4C). Thus, GW, PP2A/A and PP2A/C interact in HEK293 cells and this interaction is dependent on the PP2A/C subunit.
Figure 4.
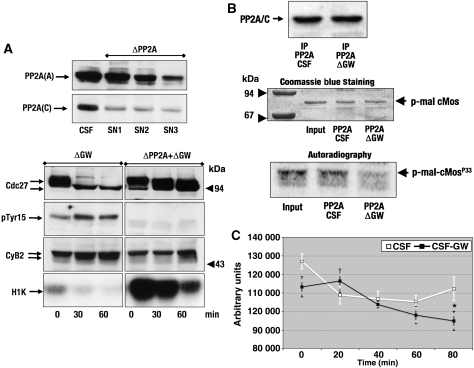
PP2A binds GW in human cells and CSF extracts. (A) HEK293 cells were co-transfected with YFP-GW, PP2A/A subunit and PP2A/C subunit. Cells were then lysed and immunoprecipitated with anti-GFP antibodies or with control antibodies. The presence of GW, PP2A/A and PP2A/C in 40 ng of total protein of the input and the supernatant, as well as the IP corresponding to 500 μg of total protein were analysed by SDS–PAGE and western blot. (B) HEK293 cells were co-transfected with PP2A/A and either PP2A/C or HA-PP2A/C and immunoprecipitated with anti-HA antibodies. The presence of GW, PP2A/A and PP2A/C was then analysed in the inputs, the supernatants and the Ips. (C) HEK293 cells were co-transfected with YFP-GW and PP2A/A subunit, lysed and immunoprecipitated with anti-GFP or control antibodies as described in Material and methods section. The presence of GW, PP2A/A and PP2A/C was then analysed in the inputs and supernatants by SDS–PAGE and western blot. (D) A total of 50 μl CSF extracts were immunoprecipitated with anti-PP2A/A monoclonal antibodies (6F9) or control antibodies, and the immunoprecipitates as well as a 1.5-μl CSF sample were used to analyse the presence of GW by immunoblotting. The smeared bands present in control IP between 94 and 67 kDa correspond to immunoglobulins in which the heavy and light chains have not been correctly dissociated after boiling. The same amount of CSF extracts were used to immunoprecipitate GW with anti-GW or control antibodies, and the immunoprecipitates as well as a 1.5-μl CSF sample were treated as described above to analyse the presence of PP2A/C.
We next analysed whether endogenous GW and PP2A could bind in CSF extracts. To this end, we immunoprecipitated PP2A from CSF extracts by using a monoclonal antibody against the PP2A/A subunit (Kremmer et al, 1997). The results show that GW is clearly present in this immunoprecipitate (Figure 4D, left panel). We next carried out the reverse immunoprecipitation by using anti-GW antibodies. As depicted in Figure 4D (right panel), PP2A was present in the GW IP and completely absent when control antibodies were used, however, unlike the high amount of GW observed in PP2A IP, only a small quantity of PP2A was detected in the GW IP, suggesting that GW does not bind to all PP2A complexes, but probably to one particular sub-complex of this phosphatase.
GW maintains the mitotic state by promoting PP2A inhibition
The results above show that GW binds PP2A and that the inhibition of this phosphatase rescues the phenotype induced by GW inactivation. To further investigate whether PP2A is the target of GW, we removed this phosphatase from CSF extracts before GW depletion by using a monoclonal antibody directed against PP2A/A subunit (Kremmer et al, 1997). Samples of the PP2A-depleted extracts were taken at the indicated times and used to analyse the phosphorylation of Cdc27, cyclin B2 and tyr 15 of Cdc2 and to measure cyclin B–Cdc2 kinase activity. As shown in Figure 5A (upper panel), 61% of PP2A/A and 83% of PP2A/C were depleted from these extracts. Moreover, this removal clearly prevented the dephosphorylation of Cdc27, as well as phosphorylation of tyr 15 of Cdc2 and cyclin B–Cdc2 inactivation although a small decrease in cyclin B–Cdc2 activity was observed 1 h after GW depletion, probably due to the PP2A left in these extracts (lower panel). Similar results were obtained when PP2A was removed from the extracts by using microcystin–agarose beads (Supplementary Figure S5A). Thus, PP2A depletion rescues the phenotype induced by GW inactivation in CSF extracts.
Figure 5.
GW maintains the mitotic state by promoting PP2A inhibition. (A) CSF extract was incubated with anti-PP2A/A monoclonal antibodies bound to protein G–Sepharose beads. Three runs of immunodepletion were carried out to remove PP2A. The last supernatant was then depleted of GW by a subsequent immunoprecipitation and used to analyse the phophorylation of Cdc27, Cdc2 and cyclin B2 and to measure cyclin B–Cdc2 activity. The levels of PP2A/A and C were also examined in the three supernatants recovered after PP2A/A immunoprecipitation. (B) Radiolabelled p-mal-cMos was incubated with a PP2A complex obtained from CSF (PP2A CSF) or GW-depleted CSF extracts (PP2A Δ GW). After 1-h incubation, the supernatants were submitted to SDS–PAGE, stained with Coomassie Blue and the phosphorylation of p-mal-cMos revealed by autoradiography. One-tenth of the PP2A immunoprecipitates from CSF (IP PP2A CSF) and GW-depleted CSF extracts (IP PP2A ΔGW) were used to measure the amount of PP2A/C immunoprecipitated in each condition by western blotting. Coomassie Blue staining showing the levels of phosphorylated p-mal-cMos, as well as a scan of this gel using Typhoon Scanner, from the input (10 μl p-mal-cMosp33) and the supernatant of the dephosphorylation reactions with PP2A from CSF (PP2A CSF) and GW-depleted CSF extracts (PP2A ΔGW) are shown. (C) A procedure similar to that followed in (B) except that supernatants were taken at 0, 20, 40, 60 and 80 min of incubation. The gels were scanned using a Typhoon Scanner and quantified by using ImageQuant TL software. Statistical analysis of the results, obtained from three different independent experiments, was performed using unpaired Student's t test. The amounts of phosphorylated p-mal-cMos present at each time were expressed as mean±s.e.m. Statistical difference in the last time point is indicated by an asterisk (*) P<0.0212.
Finally, we analysed whether GW modulates the PP2A-dependent dephosphorylation of cyclin B–Cdc2 substrates during mitosis. With this aim, a p-mal-tagged form of the cyclin B–Cdc2 substrate, c-Mos (Castro et al, 2001b), was purified and used as a substrate for PP2A.
p-mal-tagged cMos protein was first phosphorylated in the presence of ATPγ33 by a cyclin B–Cdc2 complex immunoprecipitated from CSF extracts. A sample of radiolabelled p-mal-cMosp33 was then incubated with a PP2A complex obtained by immunoprecipitation from either CT or GW-depleted CSF extracts. After 1-h incubation, the level of p-mal-cMos phosphorylation was analysed. The results show that despite the fact that similar amounts of PP2A/C were present in immunoprecipitates from control and GW-depleted CSF extracts (Figure 5B, upper panel) and that the same quantity of p-mal-cMosP33 was incubated with both PP2A IPs (Figure 5B, Coomassie blue staining), a higher decrease of the phosphorylation levels of p-mal-cMosp33 was observed when PP2A was obtained from GW-depleted extracts. The quantification of the autoradiography indicates a threefold decrease in the radiolabelled p-mal-cMosP33 signal when PP2A from GW-depleted extracts was used compared with CT (Supplementary Figure S5B). We next repeated this assay activity as a time course by triplicate and we measured c-Mos phosphorylation at 0, 20, 40, 60 and 80 min. The results of these experiments are shown as the mean value plus error bars in Figure 5C. Confirming the results shown above, dephosphorylation of c-Mos was higher when PP2A was obtained from GW-depleted CSF extracts. This difference was observed after 40 min and was statistically significant at 80 min (*P<0.0212). Thus, these results clearly show that dephosphorylation of cyclin B–Cdc2 substrates by PP2A is regulated by GW in mitotic egg extracts.
Discussion
It is established that, at mitosis entry, cyclin B–Cdc2 is irreversibly activated and that this irreversibility is directly induced by this complex through a feedback loop. Our results clearly show that the irreversibility of cyclin B–Cdc2 activation is not exclusively induced by the MPF feedback loop. We characterize a pathway controlled by the recently identified GW kinase that acts in parallel to MPF feedback loop and is essential for the irreversibility of cyclin B–Cdc2 activation and for the maintaining of the mitotic state.
The GW kinase was first identified at Goldberg's laboratory where it was shown that depletion of this protein from CSF extracts induces mitotic exit concomitantly with rephosphorylation of tyr 15 of Cdc2 and cyclin B–Cdc2 inactivation (Yu et al, 2004, 2006; Zhao et al, 2008). From their results, the authors suggested that GW could maintain the mitotic state by controlling cyclin B–Cdc2 feedback loop. Surprisingly, we found that GW inactivation induces mitotic exit by promoting a rapid dephosphorylation of different mitotic substrates independently of cyclin B–Cdc2 activity. Moreover, although we cannot exclude a direct control of GW on the Myt1–Wee1–Cdc25 pathway, we show that even in the absence of this pathway, GW is still required to maintain the mitotic state. In this light, it is likely that cyclin B–Cdc2 inactivation after GW depletion is not the cause of mitotic exit, but the consequence of Cdc25, Wee1 and Myt1 dephosphorylation. Moreover, we show that this phenotype is reversed by the addition of the phosphatase inhibitors, microcystin and OA, and that this reversion is not observed if additional active PP2A phosphatase is further supplemented. We also present data showing that GW binds PP2A in vivo through its PP2A–C subunit although this association is likely restricted to a specific sub-complex of this phosphatase. Finally, we show that the depletion of PP2A completely rescues the phenotype induced by GW inactivation in CSF extracts and that GW depletion results in an increase of the capacity of PP2A to dephosphorylate cyclin B–Cdc2 substrates. Thus, all these results clearly indicate that GW maintains the mitotic state by regulating PP2A.
Until now, mitotic entry and exit was equated to cyclin B–Cdc2 activation and inactivation, respectively, and once this kinase was activated, mitosis was thought to be irreversible. It now seems that mitotic control is not only under the control of cyclin B–Cdc2 kinase activity, but also under the control of phosphatases that counterbalances the kinase activity of cyclin B–Cdc2. A role of the inhibition of PP1 in the maintenance of the mitotic state has already been shown, however, in this case the regulation of this phosphatase is directly controlled by the mitotic kinase cyclin B–Cdc2 itself (Wu et al, 2009). However, our results show a new PP2A pathway regulated by GW that is required for the maintenance of mitosis and that is independent of cyclin B–Cdc2. This might have important consequences for the regulation of mitosis by checkpoints, as this GW-dependent regulation may well be a target of the G2/M and M checkpoints. One might imagine a situation in which the DNA-damage checkpoint induces G2 arrest by preventing the activation of GW. Under these conditions, PP2A phosphatase would be active and would dephosphorylate mitotic substrates. As Cdc25, Wee1 and Myt1 are also mitotic substrates, GW inhibition would also results in the inactivation of cyclin B–Cdc2 and G2 arrest. The opposite situation might be true for the spindle assembly checkpoint. In this case, this surveillance mechanism could maintain an active GW, favouring the stability of phosphorylation of mitotic substrates, thereby, maintaining the mitotic state. Finally, we also hypothesize that GW might be the target of the newly described G2–prophase checkpoint (Matsusaka and Pines, 2004). This checkpoint induces a G2 delay or a prophase–G2 reversion if there is a depolymerization of microtubules, either at G2 or at prophase. Interestingly, cells in which microtubule poisons are added at prophase, with a high cyclin A–Cdk activity and with condensed DNA, are capable of decondensing chromatin and reversing to G2 (Matsusaka and Pines, 2004), a situation that is reminiscent to the one we observed in interphase and CSF extracts immunodepleted of GW.
In summary, we show that two different kinase activities, regarding cyclin B–Cdc2 and GW, are essential to maintain the mitotic state, the former is required to phosphorylate mitotic substrates and the latter to prevent the dephosphorylation of these substrates. These data provide a completely new view of the regulation of mitosis. Until now, mitotic entry and exit were equated to cyclin B–Cdc2 activation and inactivation, respectively, and once this kinase was activated, mitosis was thought to be irreversible. It now seems that mitosis is not only under the control of cyclin B–Cdc2 kinase activity, but is also modulated by PP2A that counterbalances cyclin B–Cdc2, preventing a premature mitotic entry and assuring a correct mitotic progression.
Materials and methods
c-DNA cloning, immunization procedures, protein purification and antibodies
For the immunization protocol, Xenopus GW cDNA was amplified from pGEM-GW (a generous gift from Dr M Goldberg) by PCR. The PCR product was subcloned into the EcoR1–Sal1 site of pGEX5X1. pCMVsport6–Xenopus Wee1 was obtained from RZPD Deutsches Ressourcenzentrum für Genomforschung GmbH, amplified by PCR and subcloned at the BamHI and XhoI site of pGEX4T2. The fusion proteins of both kinases were expressed in Escherichia coli. Inclusion bodies were prepared and subjected to SDS–PAGE and electroeluted according to standard procedures. These proteins were dialysed against 500 mM NaCl, 100 mM NaHCO3 buffer and used to immunize rabbits. Immune sera were first pre-cleared of the anti-GST antibodies in a GST-immobilized column and were subsequently affinity purified on immobilized GST–GW and GST–Wee1 columns, respectively. Anti-Myt1 antibodies were generated against a peptide (H2N-CRNLLGMFDDATEQ-COOH) corresponding to the C-terminal sequence of Xenopus Myt1 protein. Peptides were coupled to thyroglobulin for immunization and to immobilized bovine serum albumin for affinity purification as previously described by Abrieu et al (2001).
Monoclonal p44/42 MAPK and PP2A/A (6G3) antibodies as well as polyclonal phospho-tyr 15 Cdc2 and anti-phospho-Ser Cdk substrates were obtained from Cell Signaling Technology. Anti-PP2A C subunit and anti-human PP1 alpha antibodies were obtained from Upstate/Millipore. Monoclonal anti-Rsk2 was provided by Santa Cruz Biotechnology, CA. Anti-GFP polyclonal antibody and anti-HA monoclonal antibody were obtained from Torrey Pines and Roche, respectively. Affinity purified antibodies against Cdc20, Cdc27, cyclin B2, Cdc25, Plx1 and Erp1/Emi2 were obtained as previously described (Abrieu et al, 1998; Lorca et al, 1998; Castro et al, 2001a; Bernis et al, 2007). Anti-Xenopus PP2A subunit C antibodies were a generous gift from Dr D Fesquet. 6F9 anti-PP2A/A monoclonal antibodies were kindly provided by Dr G Walter and Dr T Hunt.
Preparation of Xenopus egg extract and sperm nuclei and immunoprecipitation
CSF egg extracts were prepared from unfertilized Xenopus egg that were arrested at metaphase stage of the second meiotic division as previously described (Murray, 1991). Interphase egg extracts were prepared from de-jellied unfertilized eggs transferred in MMR/4 (25 mM NaCl, 0.5 mM KCl, 0.25 mM MgCl2, 0.025 mM Na EGTA, 1.25 mM HEPES–NaOH (pH 7.7)) Extracts were prepared 15 or 40 min after ionophore addition by the same procedure as described for CSF extracts. De-membraned sperm nuclei were prepared as described (Murray, 1991). Immunoprecipitations/immunodepletions were carried out using 10 μl of extracts, 10 μl of magnetic Protein A-Dynabeads (Dynal) and 2 μg of each antibody. Beads were washed twice with RIPA (10 mM NaH2PO4, 100 mM NaCl, 5 mM EDTA, 1% Triton X-100, 0.5% deoxycholate, 80 μM β-glycerophosphate, 50 mM NaF, 1 mM DTT), followed by a washing twice with 50 mM TRIS (pH 7.5) and incubated for 15 min at RT with 10 μl Xenopus egg extracts. For immunodepletion, the supernatant was recovered and used for subsequent experiments. When two subsequent immunodepletions were carried out, the supernatant from the first immunodepletion was recovered and used for the second. Two and three consecutive immunoprecipitations were made to completely remove the endogenous Cdc25 and Myt1 proteins, respectively, whereas one immunoprecipitation was enough to completely deplete endogenous GW, Wee1 and Plx1.
Immunodepletion of PP2A/A was carried by using 55 μl CSF extracts and 40 μl 6F9 anti-PP2A/A monoclonal antibodies bound to protein G–Sepharose beads. After 15-min incubation, extracts were centrifuged and supernatant was used for a subsequent immunodepletion. Three consecutive runs of immunodepletion were required to remove 90% of PP2A/A and PP2A/C.
H1 kinase and p-mal-cMos dephosphorylation assays
A total of 1 μl extract was frozen in liquid nitrogen at the indicated times. Extract samples were then thawed by the addition of 19 μl H1 buffer including [γ32P]ATP (Chen and Murray, 1997) and incubated for 10 min at room temperature. Reactions were stopped by adding Laemmli sample buffer and analysed by SDS–PAGE.
Purified p-mal-cMos protein was phosphorylated using cyclin B–Cdc2 complex immunoprecipitated from 60 μl CSF extracts with anti-cyclin B2 antibodies. Briefly, cyclin B2–Cdc2 immunoprecipitates were washed twice with RIPA buffer and twice with 50 mM Tris (pH 7.5) and were subsequently incubated for 20 min at room temperature with 20 μl purified p-mal-cMos protein (1 μg/μl) in the presence of 40 μl phosphorylation buffer (100 μM ATP, 50 mM Tris (pH 7.5) 100 mM MgCl2 and 4 μl [γ33P]ATP 10 μCi/μl). Free [γ33P]ATP was eliminated from the supernatant by using micro bio-spin chromatography columns (Bio-Rad) and used to analyse the dephosphorylation activity of PP2A.
PP2A was immunoprecipitated from 25 μl CSF or GW-depleted CSF extracts by using monoclonal anti-PP2A/C antibody (1D6, Upstate), washed twice with 50 mM Tris (pH 7.5) and incubated for 1 h at 30°C in the presence of pre-phosphorylated p-mal-cMos (10 μl) and 20 μl of dephosphorylation buffer (50 mM Tris (pH 7.5), 0.1 mM CaCl2). Supernatant was submitted to PAGE and Coomassie Blue staining, and the phosphorylation of p-mal-cMos was measured by autoradiography.
GW and PP2A/A-C overexpression and immunoprecipitation
HEK293 cells were transfected with pCS2-GW, pCS2-YFP-GW, pCS2-PP2A/C, pCS2-HA-PP2A/C subunit or pCMV-PP2A/A constructs by using the transfection reagent JetPei (PolyPlus transfection). After 36 h, cells were lysed using a lysis buffer containing 20 mM Tris (pH 8), 1 mM EDTA, 150 mM NaCl, 0.5% IGEPAL, 100 mM Na3VO4, 100 mM NaF and a complete EDTA-free protease inhibitor cocktail tablet. Total protein (500 μg) was used for immunoprecipitation with 50 μl Dynabeads+; 2 or 5 μl anti-GFP; or anti-HA antibodies. As anti-GFP antibodies cross-react with YFP, they can successfully immunoprecipitate this protein (see Figure 4).
Light microscopy
A DMR A Leica microscope DM 4500B with a × 63 immersion oil objective (HCX PL APO), tube factor 1 was used for epifluorescence imaging. Images were captured with a CoolSnap HQ camera (Roger Scientific) and the whole set was driven by MetaMorph (Universal Imaging, Downingtown, PA).
Supplementary Material
Supplementary Figure S1
Supplementary Figure S2
Supplementary Figure S3
Supplementary Figure S4
Supplementary Figure S5
Review Process File
Acknowledgments
We thank Drs S Galas and M Goldberg for the generous gift of anti-Wee1 and anti-GW antibodies. We also thank Drs T Hunt and G Walter for kindly providing 6F9 anti-PP2A/A monoclonal antibody. We are also indebted to Drs D Fisher and C Jessus for helpful discussion. We acknowledge the technical support provided by J Casanova and MRI. This study was supported by the Ligue Nationale Contre le Cancer (Equipe Labellisée). AB and EB are fellows of the Fondation pour la Recherche Medicale and Ligue Nationale Contre le Cancer.
Footnotes
The authors declare that they have no conflict of interest.
References
- Abrieu A, Brassac T, Galas S, Fisher D, Labbe JC, Doree M (1998) The polo-like kinase plx1 is a component of the MPF amplification loop at the G2/M-phase transition of the cell cycle in Xenopus eggs. J Cell Sci 111: 1751–1757 [DOI] [PubMed] [Google Scholar]
- Abrieu A, Magnaghi-Jaulin L, Kahana JA, Peter M, Castro A, Vigneron S, Lorca T, Cleveland DW, Labbe JC (2001) Mps1 is a kinetochore-associated kinase essential for the vertebrate mitotic checkpoint. Cell 106: 83–93 [DOI] [PubMed] [Google Scholar]
- Bernis C, Vigneron S, Burgess A, Labbe JC, Fesquet D, Castro A, Lorca T (2007) Pin1 stabilizes Emi1 during G2 phase by preventing its association with SCF (betatrcp). EMBO Rep 8: 91–98 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castro A, Peter M, Lorca T, Mandart E (2001a) c-Mos and cyclin B/cdc2 connections during Xenopus oocyte maturation. Biol Cell 93: 15–25 [DOI] [PubMed] [Google Scholar]
- Castro A, Peter M, Magnaghi-Jaulin L, Vigneron S, Galas S, Lorca T, Labbe JC (2001b) Cyclin B/cdc2 induces c-Mos stability by direct phosphorylation in Xenopus oocytes. Mol Biol Cell 12: 2660–2671 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen RH, Murray A (1997) Characterization of spindle assembly checkpoint in Xenopus egg extracts. Methods Enzymol 283: 572–584 [DOI] [PubMed] [Google Scholar]
- Draetta GF (1997) Cell cycle: will the real Cdk-activating kinase please stand up. Curr Biol 7: R50–R52 [DOI] [PubMed] [Google Scholar]
- Felix MA, Cohen P, Karsenti E (1990) Cdc2 H1 kinase is negatively regulated by a type 2A phosphatase in the Xenopus early embryonic cell cycle: evidence from the effects of okadaic acid. EMBO J 9: 675–683 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fesquet D, Morin N, Doree M, Devault A (1997) Is Cdk7/cyclin H/MAT1 the genuine cdk activating kinase in cycling Xenopus egg extracts? Oncogene 15: 1303–1307 [DOI] [PubMed] [Google Scholar]
- Kremmer E, Ohst K, Kiefer J, Brewis N, Walter G (1997) Separation of PP2A core enzyme and holoenzyme with monoclonal antibodies against the regulatory A subunit: abundant expression of both forms in cells. Mol Cell Biol 17: 1692–1701 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorca T, Castro A, Martinez AM, Vigneron S, Morin N, Sigrist S, Lehner C, Doree M, Labbe JC (1998) Fizzy is required for activation of the APC/cyclosome in Xenopus egg extracts. EMBO J 17: 3565–3575 [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacKintosh C, Beattie KA, Klumpp S, Cohen P, Codd GA (1990) Cyanobacterial microcystin-LR is a potent and specific inhibitor of protein phosphatases 1 and 2A from both mammals and higher plants. FEBS Lett 264: 187–192 [DOI] [PubMed] [Google Scholar]
- Matsusaka T, Pines J (2004) Chfr acts with the p38 stress kinases to block entry to mitosis in mammalian cells. J Cell Biol 166: 507–516 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mochida S, Hunt T (2007) Calcineurin is required to release Xenopus egg extracts from meiotic M phase. Nature 449: 336–340 [DOI] [PubMed] [Google Scholar]
- Morgan DO (1997) Cyclin-dependent kinases: engines, clocks, and microprocessors. Annu Rev Cell Dev Biol 13: 261–291 [DOI] [PubMed] [Google Scholar]
- Murray A (1991) Cell cycle extracts. Methods Cell Biol 36: 581–605 [PubMed] [Google Scholar]
- Nishiyama T, Yoshizaki N, Kishimoto T, Ohsumi K (2007) Transient activation of calcineurin is essential to initiate embryonic development in Xenopus laevis. Nature 449: 341–345 [DOI] [PubMed] [Google Scholar]
- Nurse P (1990) Universal control mechanism regulating onset of M-phase. Nature 344: 503–508 [DOI] [PubMed] [Google Scholar]
- Perdiguero E, Nebreda AR (2004) Regulation of Cdc25C activity during the meiotic G2/M transition. Cell cycle 3: 733–737 [PubMed] [Google Scholar]
- Perry JA, Kornbluth S (2007) Cdc25 and Wee1: analogous opposites? Cell division 2: 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pines J, Hunter T (1989) Isolation of a human cyclin cDNA: evidence for cyclin mRNA and protein regulation in the cell cycle and for interaction with p34cdc2. Cell 58: 833–846 [DOI] [PubMed] [Google Scholar]
- Rivas M, Garcia C, Liberona JL, Lagos N (2000) Biochemical characterization and inhibitory effects of dinophysistoxin-1, okadaic acid and microcystine 1-r on protein phosphatase 2a purified from the mussel Mytilus chilensis. Biol Res 33: 197–206 [DOI] [PubMed] [Google Scholar]
- Russo AA, Jeffrey PD, Pavletich NP (1996) Structural basis of cyclin-dependent kinase activation by phosphorylation. Nat Struct Biol 3: 696–700 [DOI] [PubMed] [Google Scholar]
- Wu JQ, Guo JY, Tang W, Yang CS, Freel CD, Chen C, Nairn AC, Kornbluth S (2009) PP1-mediated dephosphorylation of phosphoproteins at mitotic exit is controlled by inhibitor-1 and PP1 phosphorylation. Nat Cell Biol 11: 644–651 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu J, Fleming SL, Williams B, Williams EV, Li Z, Somma P, Rieder CL, Goldberg ML (2004) Greatwall kinase: a nuclear protein required for proper chromosome condensation and mitotic progression in Drosophila. J Cell Biol 164: 487–492 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu J, Zhao Y, Li Z, Galas S, Goldberg ML (2006) Greatwall kinase participates in the Cdc2 autoregulatory loop in Xenopus egg extracts. Mol Cell 22: 83–91 [DOI] [PubMed] [Google Scholar]
- Zhao Y, Haccard O, Wang R, Yu J, Kuang J, Jessus C, Goldberg ML (2008) Roles of Greatwall kinase in the regulation of Cdc25 phosphatase. Mol Biol Cell 19: 1317–1327 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figure S1
Supplementary Figure S2
Supplementary Figure S3
Supplementary Figure S4
Supplementary Figure S5
Review Process File