Abstract
The decatenation activity of topoisomerase II (Top2), which is widely conserved within the eukaryotic domain, is essential for chromosomal segregation in mitosis. It is less clear, however, whether Top2 performs the same function uniformly across the whole genome, and whether all its functions rely on decatenation. In the fission yeast, Schizosaccharomyces pombe, telomeres are bound by Taz1, which promotes smooth replication fork progression through the repetitive telomeric sequences. Hence, replication forks stall at taz1Δ telomeres. This leads to telomeric entanglements at low temperatures (⩽20°C) that cause chromosomal segregation defects and loss of viability. Here, we show that the appearance of entanglements, and the resulting cold sensitivity of taz1Δ cells, is suppressed by mutated alleles of Top2 that confer slower catalytic turnover. This suppression does not rely on the decatenation activity of Top2. Rather, the enhanced presence of reaction intermediates in which Top2 is clamped around DNA, promotes the removal of telomeric entanglements in vivo, independently of catalytic cycle completion. We propose a model for how the clamped enzyme–DNA complex promotes proper chromosomal segregation.
Keywords: DNA replication, Schizosaccharomyces pombe, telomeres, topoisomerase II
Introduction
In proliferating eukaryotic cells, linear chromosomes face two important problems. First, as the semi-conservative DNA replication machinery is unable to completely replicate the terminal DNA sequences, chromosome ends erode with each cell cycle. Second, free DNA ends can be recognized as double-strand breaks (DSBs) by the specialized DNA repair machineries whose activities can be deleterious at natural chromosome ends. These problems are solved by various specialized proteins that bind the G-rich repetitive sequences situated at the end of chromosomes and form the telomere (Ferreira et al, 2004; Rog and Cooper, 2008). Telomeres recruit and regulate telomerase, a reverse transcriptase that re-synthesizes the DNA lost due to the end replication problem (Bianchi and Shore, 2008). Telomeres also suppress their own non-homologous end joining (NHEJ), homologous recombination (HR) and checkpoint activation, promoting chromosome stability.
In fission yeast, telomeric sequences are bound by Taz1, which recruits Rap1. Together, Taz1 and Rap1 negatively regulate telomerase activity at telomeres, conferring telomere length homoeostasis (Cooper et al, 1997; Kanoh and Ishikawa, 2001), and inhibit NHEJ, protecting chromosomes from lethal fusions (Ferreira and Cooper, 2001; Miller et al, 2005). Fortunately for taz1Δ and rap1Δ cells, growing fission yeast spend little time in G1 and, therefore NHEJ frequencies are virtually zero in growing cultures, allowing taz1Δ and rap1Δ cells to be viable (Ferreira and Cooper, 2001, 2004).
Taz1 also has a positive effect on telomeric sequence maintenance, as it favours replication fork (RF) progression through the telomere region. Hence, taz1+ deletion results in stalling of the RF at telomeres (Miller et al, 2006). This stalling is thought to lead to at least two different outcomes: rapid telomere loss in the absence of telomerase, and a high rate of telomere rearrangement (Miller et al, 2006; Rog et al, 2009). Fission yeast cells lacking Taz1 also show chromosome entanglements, distinct from NHEJ-mediated fusions, at cold growing temperatures (⩽20°C). These entanglements cannot be resolved at mitosis and therefore induce lethality; hence, taz1Δ cells are cold sensitive (Miller and Cooper, 2003). Such mitotic defects are only observed in taz1Δ cells that have undergone the preceding S-phase at ⩽20°C (Miller and Cooper, 2003). Furthermore, although Rap1 shares many functions with Taz1, it is dispensable for the prevention of both entanglements and stalled telomeric RFs. Hence, the entanglements seem to arise as a by-product of stalled RF processing.
Interestingly, chromosome entanglements and the cold sensitivity of taz1Δ cells are suppressed by a mutation of the Top2 gene (top2+), top2-191 (Miller and Cooper, 2003). Top2 is a homodimeric enzyme, widely conserved within the eukaryotic domain, able to create a transient DSB in a DNA duplex and to promote the passage of another duplex through this ‘DNA gate'. This strand passage activity confers the ability of Top2 to modify the topology of DNA molecules in vitro and in vivo. It should be noted that Top2 is able to relax supercoiled DNA, and to resolve or promote catenation between two DNA circles (Baldi et al, 1980; Hsieh and Brutlag, 1980; Liu et al, 1980; Goto and Wang, 1982) (Figure 1A).
Figure 1.
Mutant top2 alleles used in this study. (A) Diagram of Top2 catalytic cycle. The Top2 dimer (light blue semi-circles) binds a ‘gate' DNA duplex, and changes conformation on ATP binding, undergoing ‘closure' and simultaneously inducing a transient DSB in the gate duplex, to which Top2 remains bound at either end as it transports a second duplex through the DSB. The purple squares represent the covalent bonds formed transiently between the 5′ phosphates flanking the DSB and tyr 835 of each Top2 subunit. Clamp re-opening requires ATP hydrolysis and release. (B) Simplified scheme of the Top2 polypeptide chain, pointing out the target sites mutagenized during the course of this study. Ala 802 is mutagenized to valine in the top2-191 allele. The Y835F mutant is designed to kill the cleavage activity of Top2, and the K423Q mutant is designed to inhibit ATPase activity and clamp re-opening.
Top2 is essential in eukaryotes for chromosomal condensation and segregation (Holm et al, 1985; Uemura et al, 1987; Adachi et al, 1991; Shamu and Murray, 1992). It is generally accepted that its strand passage activity is required during mitosis to decatenate residual intertwining that persists between sister chromatids after the completion of semi-conservative DNA replication. As a consequence, yeast cells bearing catalytically dead Top2 mutations fail to properly segregate chromosomes during mitosis and die. In addition, cells harbouring extra-chromosomal circular DNA molecules accumulate catenated circles following replication (Sundin et al, 1980; DiNardo et al, 1984). Top2 is also able to relieve the topological constraints induced by RF and transcriptional progression, but this activity is not essential, as topoisomerase I also can perform the same (Brill et al, 1987).
Studies in higher eukaryotes have led to the generation of an additional idea that Top2 is the main component of a ‘chromosomal scaffold' (Adolphs et al, 1977; Paulson and Laemmli, 1977). Top2 has been suggested to anchor DNA to this scaffold by linking it to specific regions of the genome (Mirkovitch et al, 1984; Adachi et al, 1989), and to have a function in re-setting the spatial organization of replication origins during mitosis (Lemaitre et al, 2005). However, the existence and precise function of this scaffold, and the role of Top2 in chromosomal organization, are yet to be definitively understood.
The observation that taz1Δ cells harbouring the top2-191 allele are rescued from cold sensitivity suggested that Top2 has a crucial function in preventing the accumulation of telomeric entanglements. Here we explore this role by examining the in vitro activities of Top2-191 and developing additional top2+ alleles that confer cold-resistance to taz1Δ cells. Surprisingly, suppression of telomeric entanglement is not dependent on the DNA cleavage activity of Top2. Hence, the in vivo functions of Top2 are not confined to its catenation–decatenation activity. Rather, our data suggest that slowing down the Top2 catalytic cycle suppresses telomeric entanglement by enhancing the lifetime of the Top2–DNA clamp.
Results
The top2-191 allele acts in a dominant manner to suppress taz1Δ cold sensitivity
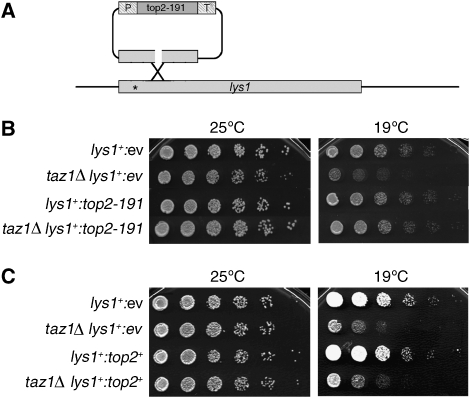
Top2 can either catenate or decatenate DNA circles in vitro (Hsieh and Brutlag, 1980; Goto and Wang, 1982). It is therefore conceivable that Top2 could either generate or remove chromosome entanglements in vivo. If Top2 generated the entanglement of taz1Δ telomeres, a mutation in top2+ that suppressed this defect would be expected to be a loss-of-function, recessive allele. Conversely, if Top2 removed or prevented entanglement, a suppressing allele would be expected to be a gain-of-function and dominant allele. We assessed whether top2-191 is recessive by integrating it under control of the inducible nmt81 promoter (an attenuated allele of the strong inducible nmt1 promoter) at the lys1 locus (Figure 2A), in a strain that also expresses wild-type (wt) endogenous Top2. Surprisingly, deletion of taz1+ fails to induce cold sensitivity in the top2+lys1:top2-191 background (Figure 2B). In contrast, deletion of taz1+ induces cold sensitivity in a strain bearing an empty nmt81 cassette integrated at the lys1 locus. Like taz1Δ strains containing single alleles of top2 (either wt or top2-191), taz1Δ top2+lys1:top2-191 cells harbour markedly elongated telomeres (data not shown). Thus, although the presence of a copy of top2-191 suppresses taz1Δ cold sensitivity (and the telomeric entanglements that arise in taz1Δ cells at cold temperatures (Miller and Cooper, 2003), it does not suppress the telomere elongation sustained by taz1Δ cells. Hence, top2-191 suppresses cold sensitivity in a dominant manner, indicating that Top2-191 has gained an activity that prevents or removes entanglement, rather than lost an activity that generates entanglement.
Figure 2.
top2-191 suppresses the cold sensitivity of taz1Δ cells in a gain-of-function manner. (A) Scheme of the integration strategy. The vector (top of diagram) bears the wt N-terminal region of lys1+ encompassing the site mutated in the chromosomal lys1-131 allele (asterisk), flanking various top2 alleles. Integration events produce genomes bearing one wild-type copy of lys1+ and a mutated copy of its N-terminal region. P and T represent the nmt81 promoter and terminator, respectively. (B) top2-191 is dominant for cold sensitivity suppression. Strains harbouring the indicated insertions at lys1 were spotted on minimal medium lacking thiamine (to induce transcription from nmt81). ‘ev' denotes ‘empty vector'. (C) Overexpression of wt top2+ fails to suppress taz1Δ cold sensitivity. Strains harbouring the indicated insertions at lys1 were spotted on minimal medium lacking thiamine (to induce transcription from nmt81).
To check the general functionality of the integrated top2-191 allele, we introduced it into a strain harbouring a cold-sensitive allele of top2 (top2-250) at the endogenous top2+ locus. The Top2-250 protein is fully functional at 32°C but loses its decatenation activity at 19°C, conferring cold-associated lethality (Uemura et al, 1987). We found that the integrated top2-191 allele was able to fully restore the growth of top2-250 strains at 19°C (data not shown). Hence, the integrated copy of top2-191 is fully capable of decatenating chromosomes at mitosis at 19°C.
As top2-191 suppresses taz1Δ cold sensitivity in a gain-of-function capacity, we investigated whether the presence of an extra functional copy of wt Top2 could also confer suppression. A strain bearing both endogenous top2+ and nmt81-top2+ shows cold sensitivity on taz1+ deletion to the same extent as strains bearing only endogenous Top2 (Figure 2C). Although we cannot rule out the possibility that nmt81-driven top2+ is simply not expressed at high enough levels, it is notable for its failure to suppress given that nmt81-driven top2-191 confers complete suppression of taz1Δ cold sensitivity. Nonetheless, the integrated top2+ copy is functional, as it fully rescues both the thermosensitivity of top2-191 cells and a full deletion of top2+ (data not shown). Hence, Top2-191 can both provide wt Top2 function at 19°C and a function not provided by comparable levels of wt Top2.
Purified Top2-191 shows a slower catalytic cycle than wt Top2 at permissive temperature
The top2-191 allele loses its decatenation activity at 36°C, causing chromosomal segregation defects. However, at 19°C, it gains a function that promotes chromosomal segregation. Hence, our initial hypothesis was that the Top2-191 protein has a higher decatenation rate at permissive temperature, despite its loss of activity at non-permissive temperature. To test this hypothesis, we purified both the versions of the enzyme, using Saccharomyces cerevisiae as an expression system (Worland and Wang, 1989). The purification was successful, achieving >95% purity for both alleles (data not shown). The purified Top2 shows a plasmid relaxation activity that depends strictly on the presence of ATP (data not shown), as is characteristic of type II topoisomerases. We measured the relaxation rate of both alleles using supercoiled plasmid pBR322 as substrate, at 25°C (permissive temperature for Top2-191) and 37°C (restrictive temperature for Top2-191). As expected, relaxation by the Top2-191 enzyme is almost completely inactivated at restrictive temperature, whereas the relaxation carried out by wt Top2 is unaffected (Figure 3A). Notably, however, Top2-191 also shows a significantly slower relaxation rate than the wt enzyme at permissive temperature (Figure 3A). Hence, the suppression activity of Top2-191 does not reflect an enhanced strand passage rate.
Figure 3.
Top2-191 shows a slower catalytic cycle than wt Top2 at all temperatures. Top2 and Top2-191 were expressed in Saccharomyces cerevisiae and purified according to the procedure described in Material and methods section. (A) The plasmid relaxation activity of Top2-191 is slower than wt. Equal concentrations (50 ng) of Top2 or Top2-191 were mixed with ATP in relaxation buffer and pre-incubated for 10 min at the indicated temperature. The relaxation reactions were started by adding 250 ng of supercoiled pBR322 DNA and stopped at the indicated time after DNA addition (for details, see Materials and methods section). Samples were phenol extracted, ethanol precipitated, resolved by 0.7% agarose gel electrophoresis and stained with gelRed. (B) The cleavable complex intermediate accumulates in reactions containing Top2-191. A gel showing plasmid cleavage products is shown. The triangles above the gels indicate increasing quantities of enzyme (0, 40 and 67 ng), which were mixed with ATP in cleavage buffer and pre-incubated for 10 min at 19°C. Supercoiled pBR322 DNA was added and incubated for 30 min before trapping by SDS (for details, see Materials and methods section). The different forms of the plasmid (linear, closed circle and nicked) were separated on an agarose gel. (C) Quantification of the cleavage assay. DNA was transferred from the gel to a nylon membrane and hybridized with a random primed radio-labelled pBR322. The signals from each band were quantified and cleavage ratios calculated as the ratio of signal for ‘linear' to the total signal over the lane. Cleavage ratio was plotted as a function of enzyme concentration for both wt Top2 and Top2-191. (D) Schematic of Top2 catalytic cycle pointing out (in yellow shaded oval) the cleavable complex intermediate that we detected at higher levels in reactions containing Top2-191.
The slower plasmid relaxation rate showed by Top2-191 might reflect slower catalytic turnover, and in turn, the accumulation of some catalytic intermediate. Hence, we sought to assess the steady state levels of catalytic cycle intermediates. During the Top2 catalytic cycle, ATP binding is required for the enzyme to form a clamp around DNA. Once the clamp is formed, Top2 cleaves the DNA and the enzyme is transiently covalently bound to its cleaved DNA substrate (forming the ‘cleavable complex'; Figure 1A and 3D). This intermediate can be trapped by the addition of SDS to the reaction, which disrupts the ternary structure of the enzyme and converts the reversible DSB within the cleavable complex into an irreversible DSB (Liu et al, 1983). We performed this SDS-trapping procedure on a reaction containing pBR322 DNA as a substrate, ATP and increasing amounts of either wt or mutant Top2 at 19°C. We were able to trap DSB-containing DNA substrate (‘linear', Figure 3B) only in the presence of both the enzyme and the ATP (Figure 3B and data not shown). We reproducibly observed twofold higher levels of this intermediate in reactions with Top2-191 than in reactions with equivalent concentrations of wt Top2 (Figure 3C). Along with our observation that the plasmid relaxation rate of Top2-191 is slower than that of wt Top2 at permissive temperature, this accumulation of cleavable complex suggests that the Top2–DNA clamp is stabilized by the top2-191 mutation.
Suppression of entanglement by Top2-191 is independent of its DNA strand-passage activity
The accumulation of cleavable complex seen upon the incubation of DNA with Top2-191 in vitro led us to question whether the mutant enzyme's cleavage activity is involved in the suppression of telomeric entanglement. In other words, might suppression be afforded by the cleaved DNA within the cleavable complex, or by the presence of a Top2–DNA ‘closed clamp' within this complex? Topoisomerases catalyse transient DNA strand breakage through transesterification of a phosphate from the DNA backbone to a tyrosine residue in the enzyme. Sequence alignments with S. cerevisiae Top2 predict that the catalytic tyrosine of fission yeast Top2 is at position 835 (Figure 1). We mutated this tyrosine to phenylalanine by site-directed mutagenesis of an integration vector bearing the top2-191 allele, and integrated the resulting double mutant allele (encoding ‘Top2-191-Y835F') at the fission yeast lys1 locus. It should be noted that although clamp closure, cleavage and strand passage are thought to occur simultaneously (Figure 1A), it is known that closure can occur without cleavage and passage (Oestergaard et al, 2004). As expected, Top2-191-Y835F was unable to suppress the cold lethality conferred by top2-250, confirming that the double mutant allele is indeed catalytically dead (Figure 4B). Surprisingly, however, we found that Top2-191-Y835F completely suppresses taz1Δ cold sensitivity (Figure 4A). Therefore, we conclude that the cleavage activity of Top2-191 is dispensable for its ability to suppress telomeric entanglement in taz1Δ cells.
Figure 4.
Top2-191 molecules lacking cleavage activity can relieve taz1Δ telomeric entanglement. (A) Cleavage-dead Top2-191 suppresses taz1Δ cold sensitivity. Strains bearing the indicated insertion at the lys1 locus were spotted on minimal medium lacking thiamine. (B) Cleavage-dead Top2-191 fails to provide the essential decatenation function of Top2. The indicated strains were streaked on YES medium. All clones were obtained from the same parental diploid and four clones of each genotype are shown. The survivors seen at 19°C in the top2-250 nmt81-top2-191-Y835F patches are likely to arise from gene conversion between the two top2 copies.
As the active form of Top2 is a homodimer, the foregoing results leave open the possibility that Top2-191 suppresses cold sensitivity by heterodimerizing with wt Top2 and inhibiting its decatenation activity. If this were true, insertion of any catalytically dead Top2 allele would suppress cold sensitivity. However, in striking contrast to Top2-191-Y835F, Top2-Y835F fails to suppress the cold sensitivity of taz1Δ cells (Figure 4A). Hence, the ‘191' mutation counteracts the appearance of telomeric entanglements by conferring an extra activity rather than by decreasing wt Top2 activity through a dominant-negative effect; that is, top2-191 is a genuine gain-of-function allele. Furthermore, this extra activity does not rely on the classical decatenation activity of Top2.
Wild-type Top2 limits entanglement in taz1Δ cells
The observation that Top2-191 has a slower catalytic cycle than wt Top2 suggests that suppression of taz1Δ cold sensitivity is achieved by an intermediate in the catalytic cycle. Any such intermediate should also be produced during the wt Top2 catalytic cycle, albeit with a reduced lifetime. If this were true, wt Top2 should also be able to counteract taz1Δ telomeric entanglement, although with a reduced efficiency compared with Top2-191. We have observed that the cold sensitivity phenotype of taz1Δ cells is not absolute; residual growth is always observed. We therefore questioned whether telomeric entanglements are limited by wt Top2 activity.
To test this idea, we generated a strain in which endogenous Top2 is C-terminally tagged with nine copies of the Simian Virus 5 antigen (9PK). Top2-9PK seems to be functional, as cells harbouring this tagged protein are healthy. Interestingly, however, deletion of taz1+ induces a much more severe cold sensitivity in cells harbouring Top2-9PK than in cells harbouring untagged Top2 (Figure 5). The integration of untagged top2+ at the lys1 locus suppresses this exacerbated cold sensitivity, rescuing the growth at 19°C to the levels seen in top2+ taz1Δ strains (Figure 5). This observation suggests that C-terminal tagging of Top2 inhibits an inherent ability to prevent or remove entanglement, and implies that wt Top2 indeed limits entanglement.
Figure 5.
Top2-9PK exacerbates the cold sensitivity of taz1Δ cells in a recessive manner. Equal numbers of cells of the indicated genotype (status of top2 and lys1 loci are indicated on the left, status of taz1 at the bottom) were plated in minimal medium lacking thiamine. A representative area of each plate is shown. Colonies were scored after 7 days of incubation and the percentage viability at 19°C (to the right of each panel) was calculated as the ratio of number of colonies formed at 19°C to 32°C.
A top2 point mutation impairing clamp re-opening imparts the ability to suppress entanglements
The foregoing results prompt the hypothesis that the ‘closed clamp' form of Top2 has an activity that promotes the removal of taz1Δ telomeric entanglements, and that Top2-191 stabilizes this closed clamp more effectively than wt Top2. If this hypothesis is correct, a mutation that impairs clamp re-opening should also confer the ability to suppress entanglements. As clamp re-opening is dependent on the ATPase activity of Top2, we generated a mutation in a highly conserved region of the ATP binding domain of Top2 (K in position 423 is mutated to Q, Figure 1B). In Drosophila melanogaster Top2, the corresponding mutation has been shown to reduce the efficiency of ATP hydrolysis in vitro, in turn favouring the persistence of the Top2 clamp around DNA (Hu et al, 1998) (Figure 6A). To determine whether the K423Q mutation imparts the same properties to the fission yeast enzyme, we expressed and purified Top2-K423Q and carried out relaxation and cleavage assays using the wt enzyme as a control. We found more pronounced accumulation of cleavable complex upon SDS trapping of plasmid DNA with Top2-K423Q than with wt Top2; the relevant cleavage activity is strictly dependent on ATP (Figure 6B,D and data not shown). Furthermore, the K423Q mutation almost completely abolishes the ability of Top2 to accomplish plasmid relaxation at all temperatures (Figure 6C and data not shown). These data indicate that Top2-K423Q is able to clamp around DNA but unable to complete the catalytic cycle and ‘re-set' the enzyme.
Figure 6.
A Top2 mutant that impairs clamp re-opening suppresses taz1Δ telomeric entanglement. (A) Schematic of the likely effect of K423Q mutation on the Top2 catalytic cycle. The last step, clamp opening, requires ATP hydrolysis, which is blocked by the mutation. As a result, the clamped conformation of the homodimer persists; cleavage of the gate duplex within the protein–DNA clamp should also be favoured. (B) Cleavable complex accumulates in reactions containing Top2-K423Q. As shown in Figure 4, increasing quantities of enzyme (0, 20 and 33 ng) were mixed with ATP in cleavage buffer and pre-incubated for 10 min at 19°C. Supercoiled pBR322 DNA was added and incubated for 30 min before trapping by SDS (as described in Materials and methods section). Different forms of the plasmid (linear, closed circle and nicked) were separated on an agarose gel. (C) Top2-K423Q lacks relaxation activity. The relaxation assay was carried out at 25°C essentially as described in Figure 4. Identical results were obtained at 36°C (data not shown). (D) Quantification of the cleavage assay. DNA was transferred from the gel to a nylon membrane and hybridized with a random primed radio-labelled pBR322. The signals from each band were quantified and cleavage ratios calculated as the ratio of signal for ‘linear' to the total signal over the lane. Cleavage ratio was plotted as a function of enzyme concentration for both wt Top2 and Top2-K423Q. (E) Top2-K423Q suppresses taz1Δ cold sensitivity. Fivefold serial dilutions of the indicated strains were spotted on minimal medium lacking thiamine. (F) Top2-K423Q fails to provide the essential function of Top2. Four independent colonies of the indicated genotypes were streaked on YES medium. As in shown in Figure 2, survivors in the top2-191 nmt81-top2-K423Q patches are likely to arise from gene conversion between the two top2 copies.
Strikingly, integration of DNA encoding Top2-K423Q at the lys1 locus allows near-complete suppression of taz1Δ cold sensitivity (Figure 6E), whereas it fails to suppress the telomere elongation phenotype in taz1Δ cells. This integrated allele was not able to suppress the thermosensitivity of top2-191 (Figure 6F), consistent with the requirement of ATP hydrolysis for the essential decatenation reaction and with our in vitro data showing that Top2-K423Q is essentially dead for multiple strand passage events (Figure 6C). These data confirm that an intermediate in the Top2 catalytic cycle, most probably the closed clamp, suppresses telomeric entanglements in vivo.
Discussion
In this study, we show that top2-191 acts in a dominant manner to suppress the telomeric entanglements induced by taz1+ deletion. We find that the ‘191' mutation slows down the catalytic cycle of Top2, thereby prolonging the lifetime of catalytic intermediates. These intermediates could comprise either the cleavable complex or the closed clamped in which the enzyme encircles DNA; our observation that a cleavage-dead allele (Y835F) of Top2-191 also suppresses taz1Δ cold sensitivity favours the closed clamp as the relevant suppressing intermediate. Furthermore, we find that a separate allele engineered to stabilize the closed clamp intermediate recapitulates the suppression of taz1Δ telomeric entanglement conferred by top2-191. Our observation that Top2-Y835F fails to suppress cold sensitivity rules out the possibility that Top2-191 acts as a ‘dominant negative' allele that blocks wt Top2 activity. Thus, our data imply that Top2 has a ‘non-canonical' function, independent of decatenation, in limiting telomere entanglements.
An important question is whether the non-canonical activity carried out by Top2-191 is relevant for understanding the role of wild-type Top2 in telomere physiology, or whether it reflects a unique property of a particular mutant. For several reasons, we think that this non-canonical activity is also carried out by wt Top2, albeit less effectively. First, the Top2–DNA clamp is a normal intermediate in the catalytic cycle; wt Top2 is, therefore, capable of forming these clamps transiently. Hence, wt Top2 would counteract the appearance of entanglements with a reduced efficiency because of the volatility of the relevant intermediate. Indeed, we found that fusing endogenous wt Top2 with a 9PK tag exacerbates the cold sensitivity induced by the loss of Taz1. This enhanced lethality is suppressed by the integration of an extra copy of Top2, indicating that Top2-9PK is recessive, lacking an activity that suppresses entanglement in taz1Δ cells. We suggest that this activity is the persistent formation of enzyme–DNA clamps. However, we failed to suppress entanglements through limited overexpression of wild-type Top2. Although we cannot exclude that an insufficient level of expression accounts for this lack of effect, we speculate that it reflects the non-catalytic nature of the activity involved. If the number of sites (i.e. entanglements) where Top2 must act does not exceed the number of available Top2 molecules, and if the relevant activity does not involve catalytic turnover (and would be ‘structural'), then the overexpression of the less effective allele (in this case, the wt allele) would not be expected to suppress. In this scenario, the ability of Top2 to adopt the ‘suppressing' conformation would be the only relevant parameter governing the efficiency of suppression.
Although the suppression conferred by Top2-191 is independent of its ability to cleave DNA, it remains possible that suppression requires the presence of separate Top2 molecules harbouring DNA cleavage activity. The Top2-K423Q allele most probably retains DNA cleavage activity, and Top2-191-Y835F could heterodimerize with endogenous Top2, forming a dimer that would be able to nick one DNA strand. Furthermore, it is important to note that our experiments did not rule out the possibility that decatenation activity is required for the removal of entanglements, as this activity is essential for viability and was always present in our experiments. As the decatenation activity of Top2 relies on the same reaction cycle measured in our relaxation and cleavable complex formation assays, we suspect that the decatenation activity of Top2-191 is also slower than that of wt Top2. However, we cannot exclude the possibility of a difference in relaxation and decatenation activity. Furthermore, the Top2-191–DNA clamp could favour disentanglement by enhancing the decatenation activity of a second, wt Top2 dimer. This could also be achieved through the recruitment of additional proteins. An interaction between Top2 and a subunit of the condensin complex has been reported in Drosophila (Bhat et al, 1996). Therefore, one interesting possibility is that the clamped form recruits condensin more efficiently, in turn favouring decatenation. In a related scenario, the clamped form might directly influence chromosome organization (e.g. chromosome looping and/or cohesion) to provide a better substrate for decatenation.
We have found that Top2-191 does not suppress RF stalling at taz1Δ telomeres (data not shown). Hence, an attractive hypothesis is that Top2 favours the denaturation of the unreplicated region though transient stabilization of catenanes behind the RF. In support of this view, it has been shown in vitro that the Top2 clamp can bind points at which two DNA duplexes cross and can stabilize knotted plasmid molecules (Roca et al, 1993). The prolonged binding at such a site behind a stalled RF may allow the chromosome to accommodate the superhelical stress produced by the denaturation of the unreplicated region. The stabilized catenanes would subsequently be removed through wt Top2 activity. In this view, clamped Top2 would prevent the appearance of entanglement rather than promoting its removal. ChIP analysis has shown that Top2 localizes near moving RFs in S. cerevisiae (Bermejo et al, 2007). We can envision that clamped Top2 near stalled RFs might have a general role in the resolution of collapsed forks, through this non-canonical activity.
The ReqQ helicase, Rqh1, has been implicated in processing stalled taz1Δ telomeric RFs (Rog et al, 2009). Indeed, inactivation of Rqh1 helicase activity (or diminution of Rqh1 sumoylation) suppresses all of the phenotypes associated with stalled telomeric RFs—entanglement at cold growing temperatures, telomeric hyper-recombination and the immediate loss of telomeres on telomerase inhibition. Top2-191 presents an intriguing contrast to Rqh1 in being involved in only a subset of the phenotypes associated with stalled telomeric RFs, as top2-191 fails to suppress the rapid telomere loss seen upon deletion of trt1+ in a taz1Δ background. Hence, we surmise that Rqh1 acts upstream of Top2 in the processing of stalled RFs. Rqh1 seems to promote the collapse of the stalled taz1Δ telomeric RFs, preventing the replication re-start and producing abortive structures that can elicit entanglement. In contrast, the Top2 clamp either promotes the resolution of a collapsed fork to a structure that remains ‘disentangled' but nonetheless fails to resume replication, or removes entanglements that are downstream of collapsed telomeric RFs.
In summary, we have found that Top2 can influence chromosomal organization and/or topology at telomeric regions independently of its decatenation activity. We propose that Top2 acts as a ‘structural switch' through inter-conversion between the clamped and the open form, influencing the topology of telomeric DNA and favouring the resolution of abortive structure stemming from stalled RFs. Widely used anti-tumoral agents target Top2 and convert the cleavable complex intermediate into an irreversible DNA lesion. Hence, our observation that Top2 reaction intermediates can profoundly influence the fate of stalled telomeric RFs may turn out to have broad implications regarding the cytotoxicity of these compounds.
Materials and methods
Yeast strains and media
S. pombe strains (Table I) were constructed and maintained according to standard procedures. For the integration of various mutant forms of top2+ at the lys1 locus, the relevant fragments were inserted between the BamHI sites of a vector (pINT81) lacking a replication origin, and bearing the nmt81 promoter and terminator. pINT81 also bears the N-terminal region of the lys1+ gene, encompassing the site that is mutated in lys1-131 strains as well as two BlpI sites. For integration, the vector was partially digested with BlpI. Successful integration events were selected on minimal media lacking lysine and verified by PCR.
Table 1.
Schizosaccharomyces pombe strains used in this study
| JCF 542 | h− lys1+:nmt81top2+ taz1∷hyg |
| JCF 543 | h− lys1+:nmt81top2+ taz1∷hyg top2-9PK:kanR |
| JCF 544 | h− lys1+:nmt81empty vector taz1∷hyg top2-9PK:kanR |
| JCF 547 | h− lys1+:nmt81top2-K423Q |
| JCF 548 | h− lys1+:nmt81top2-K423Q taz1∷hyg |
| JCF 552 | h− lys1+:nmt81top2-Y835F |
| JCF 554 | h− lys1+:nmt81top2-Y835F taz1∷hyg |
| JCF 1876 | h− lys1+:nmt81empty vector |
| JCF 1877 | h− lys1+:nmt81top2-191 |
| JCF 1880 | h− lys1+:nmt81empty vector taz1∷hyg |
| JCF 1881 | h− lys1+:nmt81top2-191 taz1∷hyg |
| JCF 1882 | h− lys1+:nmt81top2-191-Y835F |
| JCF 1883 | h+ leu1-32 lys1-131 top2-191 |
| JCF 1887 | h− lys1+:nmt81top2+ top2-9PK:kanR |
| JCF 1888 | h− lys1+:nmt81top2+ |
| JCF 1889 | h− lys1+:nmt81empty vector top2-9PK:kanR |
| JCF 1890 | h− leu1-32 lys1-131 top2-250 |
| JCF 1892 | h+ ade6-M210 lys1+:nmt81top2-191 |
| JCF 1894 | h− lys1+:nmt81top2-191 leu1-32 top2-250 |
| JCF 1896 | h− lys1+:nmt81top2-191-Y835F top2-9PK:kanR |
| JCF 1898 | h+ lys1+:nmt81top2-191-Y835F |
Viability assay
S. pombe cells were grown to log phase, pelleted and adjusted to OD=1. Fivefold serial dilutions were then spotted on solid medium and incubated at the indicated temperatures.
Expression and purification of S. pombe Top2
A S. cerevisiae–Escherichia coli shuttle vector bearing the S. pombe top2+ sequence (without the intron) under GAL control was transformed into a S. cerevisiae strain deleted for TOP1. The expression was initiated by the addition of galactose to the medium, and extraction and purification were carried out as described previously (Worland and Wang, 1989). The purification of Top2-191 was carried out in parallel using a vector obtained by site-directed mutagenesis (Stratagene) of the wild-type vector.
Relaxation assay and trapping of cleavable complexes
To assess plasmid relaxation rates, 40 ng of purified enzyme was added to 50 μl of a reaction buffer (150 mM potassium acetate, 6 mM magnesium acetate, 50 mM Tris (pH 7.8), 5 mM β-mercaptoethanol, 250 μg/ml BSA and 1 mM ATP) and pre-incubated for 10 min at the indicated temperature. Relaxation was then initiated by the addition of 300 ng of supercoiled pBr322 (TopoGEN). Aliquots were collected and the reaction stopped at the indicated times by mixing with 40 μl of ‘stop' buffer (1% SDS, 40 mM EDTA, 300 mM NaCl). DNA was phenol extracted, ethanol precipitated, electrophoresed in 0.7 % agarose and stained with gelRed reagent.
To trap cleavable complexes, indicated amounts of wt or mutant enzyme were added to 50 μl of reaction buffer (50 mM potassium acetate, 6 mM magnesium acetate, 50 mM Tris (pH 7.8), 5 mM β-mercaptoethanol, 250 μg/ml BSA and 1 mM ATP). The samples were pre-incubated at 19°C for 12 min, followed by the addition of 300 ng of supercoiled pBr322 and incubation continued for 30 min. The trapping was achieved by adding 100 μl of 1.5% SDS and mixing rapidly. A total volume of 19 μl of NaCl (5 M) and 26 μl of EDTA (0.5 M) were added along with proteinase K. The digestion was carried out overnight at 37°C before phenol extraction and ethanol precipitation.
Supplementary Material
Review Process File
Acknowledgments
We thank our lab members, Sonia Trigueros and Frank Uhlmann for discussion and critical reading of the paper. We also thank John Nitiss (St. Jude Children′s Research Hospital, Memphis, TN, USA) and the Yeast Genetic Resource Center (YGRC, Osaka, Japan) for sending strains and plasmids. We are especially grateful to Sonia Trigueros for her tremendous help with Top2 purification. This study was supported by the EMBO long-term fellowship program and Cancer Research UK (TG).
References
- Adachi Y, Kas E, Laemmli UK (1989) Preferential, cooperative binding of DNA topoisomerase II to scaffold-associated regions. EMBO J 8: 3997–4006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adachi Y, Luke M, Laemmli UK (1991) Chromosome assembly in vitro: topoisomerase II is required for condensation. Cell 64: 137–148 [DOI] [PubMed] [Google Scholar]
- Adolphs KW, Cheng SM, Paulson JR, Laemmli UK (1977) Isolation of a protein scaffold from mitotic HeLa cell chromosomes. Proc Natl Acad Sci USA 74: 4937–4941 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baldi MI, Benedetti P, Mattoccia E, Tocchini-Valentini GP (1980) In vitro catenation and decatenation of DNA and a novel eucaryotic ATP-dependent topoisomerase. Cell 20: 461–467 [DOI] [PubMed] [Google Scholar]
- Bermejo R, Doksani Y, Capra T, Katou YM, Tanaka H, Shirahige K, Foiani M (2007) Top1- and Top2-mediated topological transitions at replication forks ensure fork progression and stability and prevent DNA damage checkpoint activation. Genes Dev 21: 1921–1936 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhat MA, Philp AV, Glover DM, Bellen HJ (1996) Chromatid segregation at anaphase requires the barren product, a novel chromosome-associated protein that interacts with topoisomerase II. Cell 87: 1103–1114 [DOI] [PubMed] [Google Scholar]
- Bianchi A, Shore D (2008) How telomerase reaches its end: mechanism of telomerase regulation by the telomeric complex. Mol Cell 31: 153–165 [DOI] [PubMed] [Google Scholar]
- Brill SJ, DiNardo S, Voelkel-Meiman K, Sternglanz R (1987) Need for DNA topoisomerase activity as a swivel for DNA replication for transcription of ribosomal RNA. Nature 326: 414–416 [DOI] [PubMed] [Google Scholar]
- Cooper JP, Nimmo ER, Allshire RC, Cech TR (1997) Regulation of telomere length and function by a Myb-domain protein in fission yeast. Nature 385: 744–747 [DOI] [PubMed] [Google Scholar]
- DiNardo S, Voelkel K, Sternglanz R (1984) DNA topoisomerase II mutant of Saccharomyces cerevisiae: topoisomerase II is required for segregation of daughter molecules at the termination of DNA replication. Proc Natl Acad Sci USA 81: 2616–2620 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferreira MG, Cooper JP (2001) The fission yeast Taz1 protein protects chromosomes from Ku-dependent end-to-end fusions. Mol Cell 7: 55–63 [DOI] [PubMed] [Google Scholar]
- Ferreira MG, Cooper JP (2004) Two modes of DNA double-strand break repair are reciprocally regulated through the fission yeast cell cycle. Genes Dev 18: 2249–2254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferreira MG, Miller KM, Cooper JP (2004) Indecent exposure: when telomeres become uncapped. Mol Cell 13: 7–18 [DOI] [PubMed] [Google Scholar]
- Goto T, Wang JC (1982) Yeast DNA topoisomerase II. An ATP-dependent type II topoisomerase that catalyzes the catenation, decatenation, unknotting, and relaxation of double-stranded DNA rings. J Biol Chem 257: 5866–5872 [PubMed] [Google Scholar]
- Holm C, Goto T, Wang JC, Botstein D, Sundin O, Varshavsky A, de la Barre AE, Gerson V, Gout S, Creaven M, Allis CD, Dimitrov S (1985) DNA topoisomerase II is required at the time of mitosis in yeast. Cell 41: 553–563 [DOI] [PubMed] [Google Scholar]
- Hsieh T, Brutlag D (1980) ATP-dependent DNA topoisonmerase from D. melanogaster reversibly catenates duplex DNA rings. Cell 21: 115–125 [DOI] [PubMed] [Google Scholar]
- Hu T, Chang S, Hsieh T (1998) Identifying Lys359 as a critical residue for the ATP-dependent reactions of Drosophila DNA topoisomerase II. J Biol Chem 273: 9586–9592 [DOI] [PubMed] [Google Scholar]
- Kanoh J, Ishikawa F (2001) spRap1 and spRif1, recruited to telomeres by Taz1, are essential for telomere function in fission yeast. Curr Biol 11: 1624–1630 [DOI] [PubMed] [Google Scholar]
- Lemaitre JM, Danis E, Pasero P, Vassetzky Y, Mechali M (2005) Mitotic remodeling of the replicon and chromosome structure. Cell 123: 787–801 [DOI] [PubMed] [Google Scholar]
- Liu LF, Liu CC, Alberts BM (1980) Type II DNA topoisomerases: enzymes that can unknot a topologically knotted DNA molecule via a reversible double-strand break. Cell 19: 697–707 [DOI] [PubMed] [Google Scholar]
- Liu LF, Rowe TC, Yang L, Tewey KM, Chen GL (1983) Cleavage of DNA by mammalian DNA topoisomerase II. J Biol Chem 258: 15365–15370 [PubMed] [Google Scholar]
- Miller KM, Cooper JP (2003) The telomere protein Taz1 is required to prevent and repair genomic DNA breaks. Mol Cell 11: 303–313 [DOI] [PubMed] [Google Scholar]
- Miller KM, Ferreira MG, Cooper JP (2005) Taz1, Rap1 and Rif1 act both interdependently and independently to maintain telomeres. EMBO J 24: 3128–3135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller KM, Rog O, Cooper JP (2006) Semi-conservative DNA replication through telomeres requires Taz1. Nature 440: 824–828 [DOI] [PubMed] [Google Scholar]
- Mirkovitch J, Mirault ME, Laemmli UK (1984) Organization of the higher-order chromatin loop: specific DNA attachment sites on nuclear scaffold. Cell 39: 223–232 [DOI] [PubMed] [Google Scholar]
- Oestergaard VH, Knudsen BR, Andersen AH (2004) Dissecting the cell-killing mechanism of the topoisomerase ii-targeting drug ICRF-193. J Biol Chem 279: 28100–28105 [DOI] [PubMed] [Google Scholar]
- Paulson JR, Laemmli UK (1977) The structure of histone-depleted metaphase chromosomes. Cell 12: 817–828 [DOI] [PubMed] [Google Scholar]
- Roca J, Berger JM, Wang JC (1993) On the simultaneous binding of eukaryotic DNA topoisomerase II to a pair of double-stranded DNA helices. J Biol Chem 268: 14250–14255 [PubMed] [Google Scholar]
- Rog O, Cooper JP (2008) Telomeres in drag: Dressing as DNA damage to engage telomerase. Curr Opin Genet Dev 18: 212–220 [DOI] [PubMed] [Google Scholar]
- Rog O, Miller KM, Ferreira MG, Cooper JP (2009) Sumoylation of RecQ helicase controls the fate of dysfunctional telomeres. Mol Cell 33: 559–569 [DOI] [PubMed] [Google Scholar]
- Shamu CE, Murray AW (1992) Sister chromatid separation in frog egg extracts requires DNA topoisomerase II activity during anaphase. J Cell Biol 117: 921–934 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sundin O, Varshavsky A, de la Barre AE, Gerson V, Gout S, Creaven M, Allis CD, Dimitrov S (1980) Terminal stages of SV40 DNA replication proceed via multiply intertwined catenated dimers. Cell 21: 103–114 [DOI] [PubMed] [Google Scholar]
- Uemura T, Ohkura H, Adachi Y, Morino K, Shiozaki K, Yanagida M (1987) DNA topoisomerase II is required for condensation and separation of mitotic chromosomes in S. pombe. Cell 50: 917–925 [DOI] [PubMed] [Google Scholar]
- Worland ST, Wang JC (1989) Inducible overexpression, purification, and active site mapping of DNA topoisomerase II from the yeast Saccharomyces cerevisiae. J Biol Chem 264: 4412–4416 [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Review Process File