Abstract
Topiramate is known to cause ocular side effects such as refractive changes and angle closure. We describe a patient with an electronegative electroretinogram (ERG) which may have been related to topiramate use. Electronegative ERG’s have been associated with other drugs in humans as well as topiramate use in rabbits. However, this would be the first suggestion of causality in humans.
Keywords: Electronegative, Electroretinogram, Topiramate, Topamax, Best disease, Vitelliform maculopathy
Introduction
Electrophysiology is an essential adjunct in distinguishing macular diseases and generalized retinal dysfunction [1]. For example, Best disease and adult onset vitelliform macular dystrophy, both characterized by a similar fundus appearance and normal electroretinogram (ERG), can be distinguished by the electro-oculogram (EOG) [2, 3]. We present a patient with vitelliform lesions in the macula and unexpected ERG results.
Case report
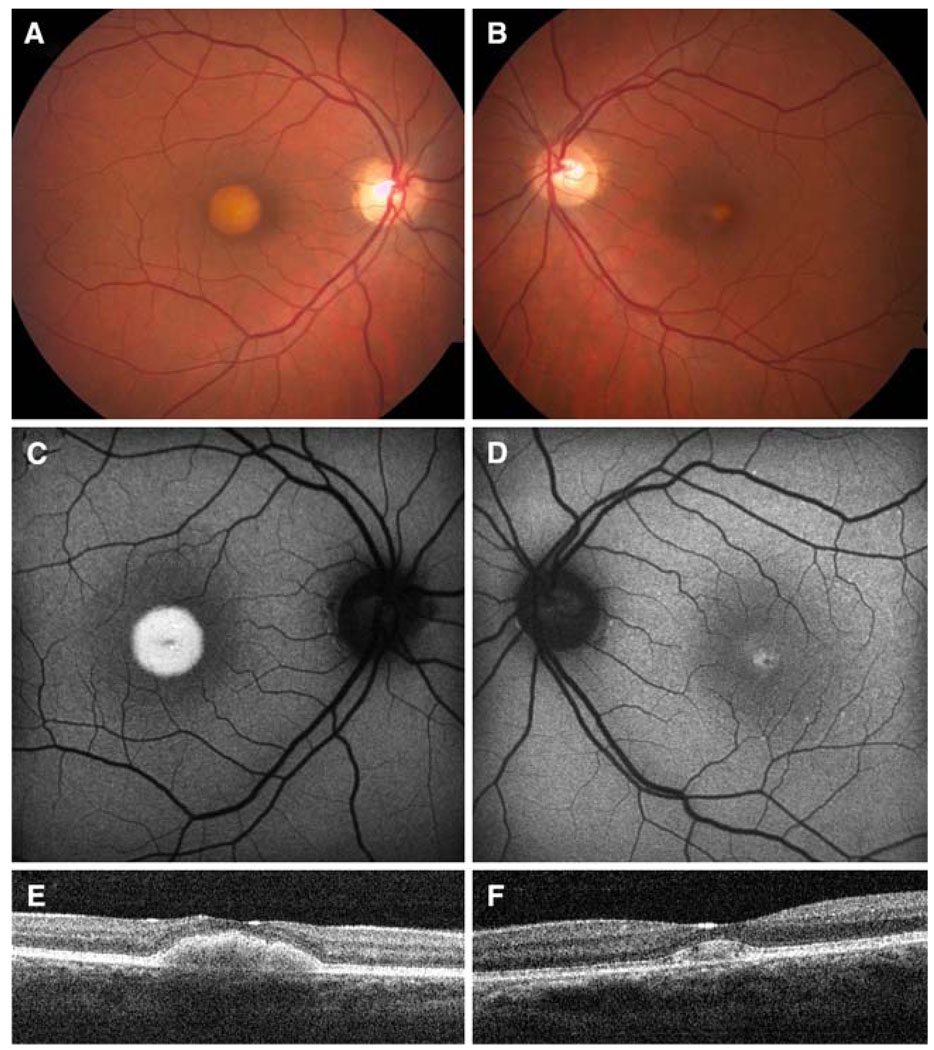
A 38 year old woman in her second trimester of pregnancy presented with complaints of decreased vision. Visual acuity was 20/60 in her right eye and 20/25 in her left eye. On fundoscopic exam, she had bilateral vitelliform maculopathy (Fig. 1 A, B). Scanning laser ophthalmoscopy showed hyperfluorescence of these central macular lesions (Fig. 1 C, D) and high resolution spectral OCT showed outer retinal accumulations without reflectivity (Fig. 1 E, F).
Fig. 1.
(A, C, E) right eye; (B, D, F) left eye (A, B) Color fundus photos had raised yellow lesions in the macula. (C, D) Autofluorescence showed increased hyperfluorescence with presumed lipofuscin accumulation. (E, F) Spectral OCT demonstrated accumulation of material in the subretinal space
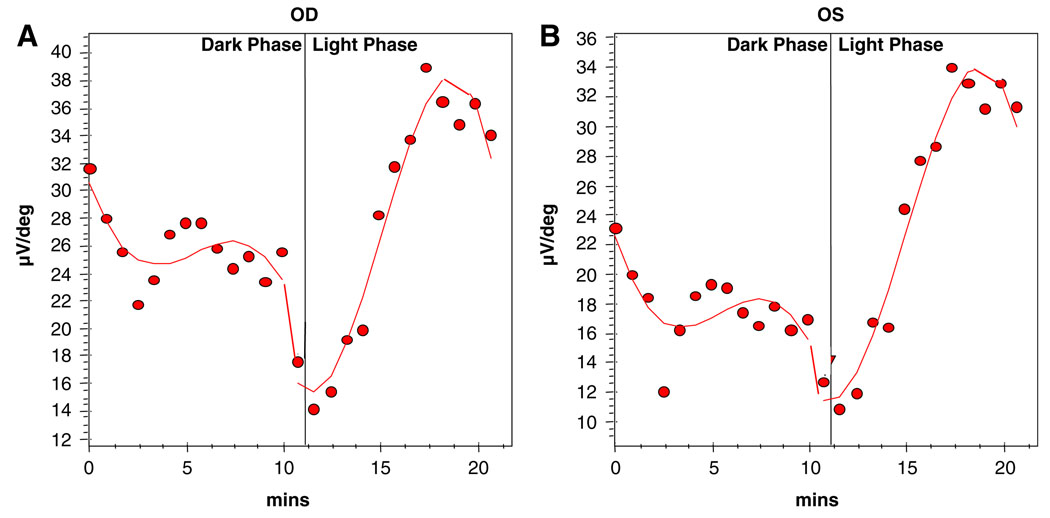
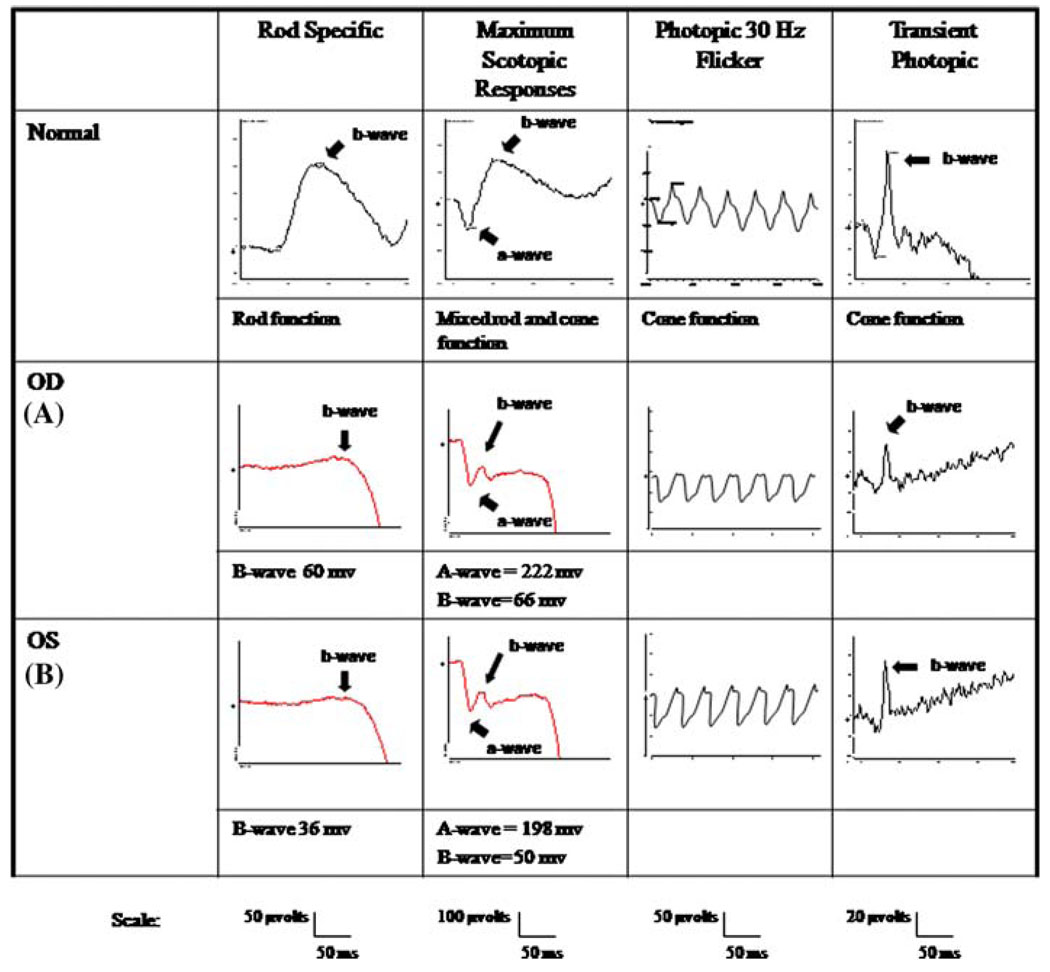
Electrophysiological testing showed normal EOGs in both eyes (Fig. 2) and she was diagnosed with adult vitelliform macular dystrophy. However, her maximal ERGs were electronegative in both eyes (Fig. 3); rods showed diminished responses in both eyes, and cone responses were borderline in the right eye.
Fig. 2.
Arden Light-Dark Ratio (A) Right eye 2.5 (nl > 1.8) (B) Left eye 3.1 (nl > 1.8)
Fig. 3.
(A) right eye; (B) left eye Maximal ERG showed electronegative responses. Full field ERG was performed with DTL recording electrodes according to International Society for Clinical Electrophysiology of Vision standards. Her scoptopic b-wave amplitudes were approximately 60 micro-volts right and 36 micro-volts left. Maximal responses a- and b-wave amplitudes were 222 micro-volts and 66 micro-volts on the right; 198 micro-volts and 50 micro-volts on the left
The patient’s only notable medical history was juvenile myoclonic epilepsy, diagnosed when she was 19 years old. She had taken topiramate 400 mg daily for 4 years until 9 months before presentation. She had recently been started on levetiracetam after a seizure relapse.
There was a documented eye exam from four years prior to presentation, at which time the patient had taken topiramate for 1.5 years. Fundus photos from this time showed smaller vitelliform lesions; EOG and full-field ERG were similar to our findings without significant progression. The patient had no family history of poor vision, night blindness, seizures, or muscular dystrophy.
Comment
Electronegative ERGs are most commonly associated with x-linked inherited disorders, such as congenital stationary night blindness and x-linked juvenile retinoschisis. Although these are unlikely in a female patient, it is possible that she had a rare autosomal form of congenital stationary night blindness.
Electronegative ERGs are also associated with acquired toxicities such as exposure to methanol, iron, vincristine, and quinine [4]. Recently, electronegative ERGs caused by topiramate have been reported in rabbits [5] but there have been no case reports in humans. It is well documented that topiramate causes ocular side effects such as refractive changes and angle closure [6]. We present this case to suggest that topiramate may also be the cause for our patient’s electronegative ERG, as previously reported in animal studies.
The abnormal b-wave and normal a-wave found in classically defined electronegative ERGs imply photoreceptor inner segment dysfunction with relative sparing of photoreceptor function [5]. The molecular mechanism of topiramate is unknown, but it has been demonstrated to modify several receptor-gated and voltage-sensitive ion channels [7] which may result in accumulation of GABA in the inner segment layer.
Another unusual aspect of this patient is the vitelliform maculopathy which is not associated with the ERG findings described. Although it is unlikely that the maculopathy was a result of topiramate, it is also unknown if the Best-like lesions were present prior to topiramate usage. In any case, we suggest that ophthalmologists be aware of this potential side effect of topiramate so that further cases may be collected and the etiology of this association be further elucidated.
Acknowledgements
We greatly appreciate the assistance of the Division of Medical Illustration and Neeco Palmer for sharing of advice, ideas, and equipment. We would also like to thank the patient for her cooperation. SHT is a Burroughs-Wellcome Program in Biomedical Sciences Fellow, and is also supported by the Charles E. Culpeper Scholarship, Foundation Fighting Blindness, Hirschl Trust, Schneeweiss Stem Cell Fund, Joel Hoffmann Scholarship, Jonas Family Fund, Jahnigen-Hartford-American Geriatrics Society, Eye Surgery Fund, Bernard Becker-Association of University Professors in Ophthalmology-Research to Prevent Blindness (RPB), and NIH grant EY004081.
Contributor Information
Irena Tsui, Email: irena.tsui@gmail.com, Brown Glaucoma Laboratory at Edward S. Harkness Eye Institute, Columbia University, New York, NY 10032, USA; Edward S. Harkness Eye Institute, Columbia University, New York, NY 10032, USA.
Daniel Casper, Edward S. Harkness Eye Institute, Columbia University, New York, NY 10032, USA.
Chai Lin Chou, Brown Glaucoma Laboratory at Edward S. Harkness Eye Institute, Columbia University, New York, NY 10032, USA; Department of Pathology & Cell Biology, College of Physicians and Surgeons, Columbia University, New York, NY 10032, USA.
Stephen H. Tsang, Brown Glaucoma Laboratory at Edward S. Harkness Eye Institute, Columbia University, New York, NY 10032, USA Department of Pathology & Cell Biology, College of Physicians and Surgeons, Columbia University, New York, NY 10032, USA.
References
- 1.Tsui I, Song B, Lin CS, Tsang S. A practical approach to retinal dystrophy. Retinal Physician. 2007;4:18–26. [Google Scholar]
- 2.Theischen M, Schilling H, Steinhorst UH. EOG in adult vitelliform macular degeneration, butterfly-shaped pattern dystrophy and Best disease. Ophthalmologe. 1997;9:230–233. doi: 10.1007/s003470050107. [DOI] [PubMed] [Google Scholar]
- 3.Glacet-Bernard A, Soubrane G, Coscas G. Macular vitelliform degeneration in adults. Retrospective study of a series of 85 patients. J Fr Ophtalmol. 1990;13:407–420. [PubMed] [Google Scholar]
- 4.Heckenlively JR, Arden GB. Principles and practice of clinical electrophysiology of vision. 2nd edn. Cambridge, Mass: MIT Press; 2006. pp. 809–822. [Google Scholar]
- 5.Kjellstrom S, Bruun A, Isaksson B, Eriksson T, Andreasson S, Ponjavic V. Retinal function and histopathology in rabbits treated with Topiramate. Doc Ophthalmol. 2006;113:179–186. doi: 10.1007/s10633-006-9027-8. [DOI] [PubMed] [Google Scholar]
- 6.Asensio-Sanchez VM, Torreblanca-Aguera B, Martinez-Calvo S, Calvo MJ, Rodriguez R. Severe ocular side effects with Topamax. Arch Soc Esp Oftalmol. 2006;81:345–348. doi: 10.4321/s0365-66912006000600010. [DOI] [PubMed] [Google Scholar]
- 7.White HS. Molecular pharmacology of topiramate: managing seizures and preventing migraine. Headache. 2005;45:S48–S56. doi: 10.1111/j.1526-4610.2005.4501006.x. [DOI] [PubMed] [Google Scholar]