Abstract
The actin nucleation-promoting factors SCAR/WAVE and WASp, together with associated elements, mediate the formation of muscle fibres through myoblast fusion during Drosophila embryogenesis. Our phenotypic analysis, following the disruption of these two pathways, suggests that they function in a sequential manner. Suppressor of cyclic AMP receptor (SCAR) activity is required before the formation of pores in the membranes of fusing cells, whereas Wiskott–Aldrich syndrome protein (WASp) promotes the expansion of nascent pores and completion of the fusion process. Genetic epistasis experiments are consistent with this step-wise temporal progression. Our observations further imply a separate, Rac-dependent role for the SCAR complex in promoting myoblast migration. In keeping with the sequential utilization of the two systems, we observe abnormal accumulations of filamentous actin at the fusion sites when both pathways are disrupted, resembling those present when only SCAR-complex function is impaired. This observation further suggests that actin-filament accumulation at the fusion sites might not depend on Arp2/3 activity altogether.
Keywords: actin, fusion, myoblasts, SCAR, WASp
Introduction
The evolutionarily conserved Arp2/3 complex acts as a crucial mediator of actin polymerization in living cells, and the forces generated by actin-related protein (Arp)2/3-based branched polymerization provide a mechanistic basis for a diverse array of fundamental cellular processes (Goley & Welch, 2006; Kaksonen et al, 2006). Nucleation of actin polymerization by the Arp2/3 complex is commonly stimulated by nucleation-promoting factors (NPFs), which act as crucial links between signal-transduction pathways and remodelling of the actin-based cytoskeleton. Members of the Wiskott–Aldrich syndrome protein (WASp) and suppressor of cyclic AMP receptor (SCAR)/WASp family, verprolin homology domain-containing protein (WAVE) protein families are considered to be the two main NPFs mediating the function of Arp2/3 (Takenawa & Suetsugu, 2007). While the molecular basis for Arp2/3 activation is shared by both NPFs, these modular proteins are subject to distinct modes of regulation by components of the signalling pathways. Thus, although Arp2/3-based actin polymerization constitutes a common outcome, the two NPFs are often utilized in distinct settings (Bompard & Caron, 2004).
The fruitfly Drosophila melanogaster provides a system for assessing the physiological roles of the Arp2/3 machinery and its associated NPFs, as it has single WASp (Wsp; Ben-Yaacov et al, 2001) and SCAR/WAVE (SCAR; Zallen et al, 2002) homologues. Although genetic analyses have commonly identified distinct, non-overlapping roles for SCAR and Wsp during development, a series of recent studies has revealed requirements for both NPFs in the process of embryonic myoblast fusion.
The formation of functional muscle fibres in Drosophila embryos is based on successive rounds of fusion between founder-cell myoblasts and the more numerous class of fusion-competent myoblasts. Recognition and attachment between founder-cell myoblasts and fusion-competent myoblasts, mediated by cell-type-specific adhesion molecules, sets in motion a series of events that culminates in the opening of fusion pores and eventual breakdown of the aligned myoblast membranes. A role for SCAR in this process was suggested both by the study of Kette (nucleosome assembly protein 1), a conserved member of the SCAR-regulatory complex (Schroter et al, 2004), and by direct assessment of SCAR function (Richardson et al, 2007; Berger et al, 2008). Complementing studies of Wsp, and of the Wsp-regulatory element D-WIP/Sltr/Vrp-1 (Kim et al, 2007; Massarwa et al, 2007; Schafer et al, 2007), have put forward strong evidence that both types of Arp2/3 NPFs provide essential contributions to myoblast fusion in Drosophila embryos.
The shared involvement of both SCAR and Wsp in myoblast fusion allowed us to assess the functional contributions of Arp2/3 NPFs within a common physiological framework. We found that SCAR is required for myoblast migration as well as myoblast fusion, in keeping with the pleiotropic nature of its function throughout Drosophila development (Zallen et al, 2002). Furthermore, we have shown that SCAR and Wsp act in a sequential manner to promote myoblast fusion. Our findings therefore imply that the two NPF systems are responsible for distinct mechanistic functions, even when operating in close spatial and temporal correspondence.
Results And Discussion
Multiple roles for SCAR during embryonic myogenesis
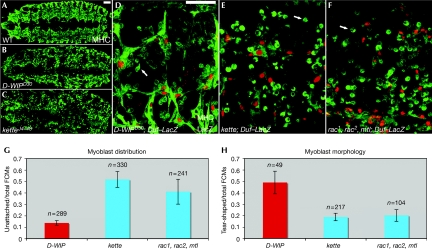
To assess and compare the defects in embryonic myogenesis resulting from the disruption of SCAR and Wsp function, we examined embryos having strong loss-of-function alleles of kette and D-WIP, respectively (Fig 1). Genetic disruption of these regulatory elements provides the most severe myogenic phenotypes associated with zygotic Arp2/3 NPF loss-of-function (see supplementary information online for further discussion and for detailed analysis of SCAR-complex phenotypes). As previously reported, both D-WIP (Fig 1B) and kette (Fig 1C) stage-16-mutant embryos show characteristic abnormalities in muscle development associated with defects in the myoblast fusion process, including markedly thin muscle fibres and numerous unfused myoblasts, which are almost absent in wild-type embryos at this stage.
Figure 1.
Distinct myogenic phenotypes result from the disruption of the Wsp and SCAR pathways. (A–C) Full dorsal views of stage-16 wild-type (A), D-WIPD30 (B) and ketteJ4–48 (C) embryos stained with anti-MHC (green) to show the embryonic musculature. (D–F) Higher magnification images of D-WIPD30 (D), ketteJ4–48 (E) and rac1, rac2, mtl triple-mutant (F) embryos, stained with anti-MHC (green) and for the rp298/Duf–LacZ reporter (Nose et al, 1998), expressed in myotube nuclei (red). Although the muscle pattern of both NPF pathway mutants is severely disrupted, unfused myoblasts in the D-WIP embryos (B,D) are mostly clustered and attached to small myotubes, whereas those in the kette (C,E) and rac (F) embryos are far more dispersed. Arrows in (D–F) point to representative unattached myoblasts, which are commonly ‘tear'-shaped in the D-WIP embryo, but retain a rounded, non-migratory morphology in the kette and rac embryos. Scale bars (in A,D), 50 μm. (G,H) Quantitative assessment of myogenic phenotypes. The proportion of unattached myoblasts (G) and of those showing a migratory morphology (H) was determined in various mutant backgrounds. n represents the total number of myoblasts counted in at least three different embryos of each genotype. D-WIP, D-WASp-interacting protein; FCM, fusion-competent myoblast; MHC, myosin heavy chain; NPF, nucleation-promoting factor; SCAR, suppressor of cyclic AMP receptor; Wsp, Wiskott–Aldrich syndrome protein; WT, wild type.
Closer examination reveals clear phenotypic distinctions between the D-WIP- and kette-mutant embryos (Fig 1D,E,G,H). These are most readily apparent in both distribution and morphology of the unfused myoblasts. In D-WIP embryos, unfused myoblasts are typically clustered and attached to myotubes, and approximately half of those that remain unattached show a migratory, ‘tear-shaped' morphology, in which a broad lamellipodium is extended in the direction of cell movement. In kette mutants, however, unattached myoblasts are much more abundant and commonly retain a rounded shape, suggesting a requirement for the SCAR complex in the acquisition of a cell morphology appropriate for migration.
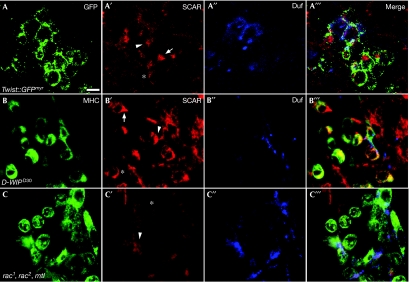
To investigate this issue further, we used SCAR antibodies to localize the endogenous SCAR protein in the myogenic mesoderm (Fig 2). We carried out this analysis in wild-type (Fig 2A–A′″) and D-WIP-mutant embryos (Fig 2B–B′″), in which the fusion arrest enriches for myoblasts that are at various stages of migration and fusion. The SCAR protein is found to be expressed in both myotubes and individual fusion-competent myoblasts, where it shows a dynamic pattern of localization. SCAR is uniformly distributed in the cytoplasm of round, pre-migratory myoblasts; however, as myoblasts acquire a tear-shaped morphology, SCAR becomes asymmetrically distributed and is conspicuously enriched in the lamellipodial extension of nearly all of the tear-shaped cells. SCAR remains concentrated within this structure as the migrating myoblasts orient and move towards the myotubes. Myotube–myoblast attachment leads to the establishment of fusion sites, which can be visualized by the accumulation of the myotube-specific fusion receptor Dumbfounded (Duf; Fig 2A″,B″); SCAR is enriched at these sites. The localization pattern of the SCAR protein during these phases of myogenesis is therefore consistent with functional roles in both migration and fusion.
Figure 2.
Localization pattern of SCAR in migrating and fusing myoblasts. (A–A′″) A stage 13 wild-type twist-GAL4∷UAS–GFPmyr embryo stained with anti-GFP (A, green) to visualize the contours of myoblasts, anti-SCAR (A′, red) and anti-Duf (A′′, blue), which mark myotube surfaces. The asterisk marks a rounded (pre-migratory) myoblast in which SCAR is uniformly localized in the membrane; an arrow points to a ‘tear'-shaped, migrating myoblast, in which SCAR localizes to the lamellipodium; and the arrowhead to a myoblast–myotube fusion interface. (B–B′″) A stage 14-D-WIPD30 embryo. Anti-MHC (B, green) was used as the general myoblast marker. SCAR localization within the enriched population of unfused myoblasts resembles that seen in wild-type embryos. Cells are marked as in A′,A′″. (C–C′″) A rac triple-mutant embryo stained as in (B–B′″). SCAR is uniformly distributed within the unfused myoblasts, all of which show a rounded morphology. Scale bar, 10 μm. GFP, green fluorescent protein; MHC, myosin heavy chain; SCAR, suppressor of cyclic AMP receptor; UAS, upstream activation sequence.
Signalling through the small GTPase Rac provides a primary pathway for stimulation of SCAR/WAVE complexes and their associated NPF activity. Therefore, we examined embryos having strong loss-of-function alleles in all three Drosophila rac orthologues—rac1, rac2 and mtl (rac ‘triple' mutants). As reported previously (Hakeda-Suzuki et al, 2002; Richardson et al, 2007), compromising rac function in this manner results in strong impairment of myoblast fusion (Fig 1F). Furthermore, the abundance and rounded morphology of unattached myoblasts are reminiscent of the defects associated with the disruption of SCAR-complex activity (Fig 1E,G,H).
Further examination of rac triple-mutant embryos revealed significant abnormalities in the pattern of SCAR-protein localization during myoblast fusion and migration (Fig 2C–C′″). SCAR becomes uniformly distributed in myoblasts and myotubes, and fails to show any specific pattern of subcellular localization. This holds true both for the asymmetric distribution within unattached myoblasts, regardless of their morphology, and for the enrichment at fusion sites. The rac function thus seems to be essential for correct localization of the SCAR protein in both migrating and fusing myoblasts.
Sequential roles for SCAR and Wsp in myoblast fusion
Phenotypic distinctions among Drosophila embryonic myoblast fusion mutants are readily apparent, following examination using electron microscopy, which has identified fusion-specific cellular structures and has allowed categorization of the fusion process into discrete steps (Doberstein et al, 1997). Thus, we chose to pursue electron-microscopy level analysis of SCAR complex and D-WIP/Wsp mutants to assess the consequences of separate and simultaneous genetic disruption of Arp2/3 NPF activity.
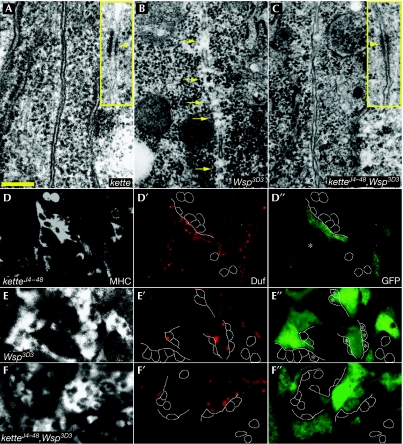
We used embryos homozygous for the amorphic ketteJ4–48 allele (Hummel et al, 2000; Schroter et al, 2004) to represent strong SCAR-complex loss-of-function conditions, and Wsp3D3 homozygotes to represent disruption of Wsp function. Wsp3D3 encodes an inactive Wsp gene product that also interferes with the activity of maternally contributed Wsp (Schafer et al, 2007; see supplementary information online for further phenotypic analysis). The ketteJ4–48 embryos (Fig 3A) show all of the established cytological features of fusing myoblasts (Doberstein et al, 1997), including the electron-dense plaques that appear in the aligned membranes of attached cells just before the opening of the fusion pores (Fig 3A, inset). Importantly, however, in none of approximately 100 attached myoblast pairs examined by electron microscopy were any discontinuities, indicative of fusion-pore formation, observed in the paired myoblast membranes of kette mutants. These observations imply, therefore, that SCAR-complex stimulation of Arp2/3-based actin polymerization is required at a relatively advanced stage of the fusion process, possibly as an accessory to the establishment of fusion pores.
Figure 3.
SCAR and Wsp pathway mutants arrest at distinct stages of the myoblast fusion process. (A–C) Electron microscopy micrographs of attached pairs of myoblasts. (A) Closely aligned and fully intact membranes in a ketteJ4–48-mutant embryo. The inset shows an electron-dense plaque (arrowhead), commonly observed in the apposed myoblast membranes in these embryos. (B) Multiple gaps (arrows) form in the apposed myoblast membranes of a Wsp3D3-mutant embryo. (C) Attached myoblast membranes remain intact in a ketteJ4–48,Wsp3D3 double-mutant embryo. Fusion-related structures, including electron-dense plaques (inset), are present, as in the kette mutant alone. (D–F) Monitoring cytoplasmic transfer in SCAR and Wsp pathway mutants after expression of GFP in myotubes through rp298/Duf–GAL4∷UAS–GFP (Menon & Chia, 2001). The mutants shown include ketteJ4–48 (D–D″), Wsp3D3 (E–E″) and ketteJ4–48,Wsp3D3 double mutant (F–F″). All genotypes were stained with anti-MHC (grey), to determine myoblast cell outlines, anti-Duf (red), to mark sites of arrested fusion, and anti-GFP (green). Asterisks (E″) mark GFP-positive FCMs in Wsp3D3. Scale bar, 200 nm (A). FCM, fusion-competent myoblast; GFP, green fluorescent protein; MHC, myosin heavy chain; SCAR, suppressor of cyclic AMP receptor; UAS, upstream activation sequence; Wsp, Wiskott–Aldrich syndrome protein.
A clearly distinct picture is revealed following the examination of the arrested myoblast fusion in Wsp3D3 homozygous embryos (Fig 3B). Roughly half of all of the attached myoblast pairs in these embryos (n∼100) show multiple, small (70–100 nm) breaches distributed along the length of apposed myoblast membranes. This is fully consistent with our previous report on embryos lacking either Wsp or D-WIP function (Massarwa et al, 2007), and implies a requirement for the Wsp-associated molecular machinery during the expansion of nascent fusion pores.
These phenotypic differences therefore allow for the assessment of an epistatic relationship between the SCAR and Wsp systems. To this end, we examined homozygous ketteJ4–48,Wsp3D3 double-mutant embryos (Schafer et al, 2007) in which both NPF systems are compromised simultaneously. We found that the ketteJ4–48,Wsp3D3 double-mutant phenotype is identical to the mutant phenotype of ketteJ4–48 alone: fusion-related structures are present, but none of the apposed membranes (in ∼50 myoblast pairs examined) show any sign of fusion-pore formation (Fig 3C). The kette phenotype is therefore epistatic to Wsp, supporting a step-wise model in which the SCAR-complex activity precedes the requirement for D-WIP/Wsp.
We have previously shown that the presence of partial membrane openings between attached myoblasts, such as in D-WIP-mutant embryos, can be verified by monitoring the transfer of cytoplasmic material from one cell to the other (Massarwa et al, 2007). We have now used this assay to complement the electron microscopy observations on kette- and Wsp-mutant embryos. Although no indication of green fluorescent protein (GFP) transfer from myotubes to myoblasts can be detected in ketteJ4–48 mutants (Fig 3D–D″), at least half of the attached myoblasts in Wsp3D3 homozygotes clearly contain GFP (Fig 3E–E″), which is consistent with the, achievement of cytoplasmic continuity. Markedly, and in full correspondence with the electron microscopy observations, we failed to observe any instance of GFP transfer in homozygous ketteJ4–48,Wsp3D3 double-mutant embryos (Fig 4F–F″). This independent demonstration of genetic epistasis emphasizes the sequential requirement for the two Arp2/3 NPF systems during embryonic myoblast fusion.
Figure 4.
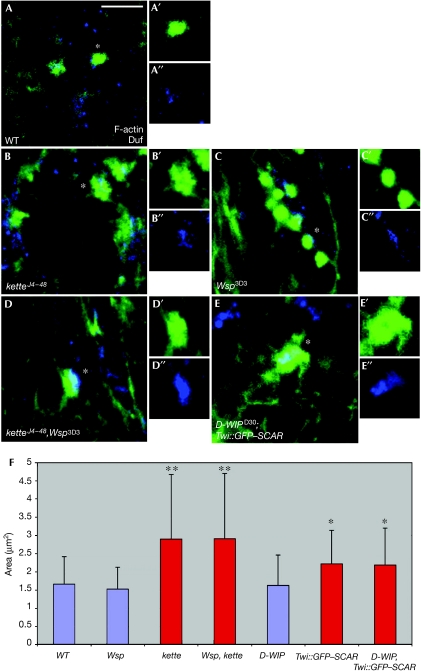
F-actin foci form and persist despite disruption of both the SCAR and Wsp NPF pathways. (A–E) Phalloidin-staining structures (green) in stage-14 wild-type (A), ketteJ4–48 (B), Wsp3D3 (C), ketteJ4–48,Wsp3D3 (D) and D-WIPD30; twist-GAL4∷GFP–SCAR (E) embryos. All embryos were also stained with anti-Duf (blue) to visualize myotube surfaces and fusion sites, and anti-MHC to visualize myoblasts (data not shown). For each genotype, isolated phalloidin and anti-Duf stainings of single representative F-actin foci (asterisks) are also shown. Note enlarged and irregular morphology of foci in kette and double-mutant embryos, as opposed to the round shape of wild-type and Wsp foci. A′,B′,C′, Phalloidin; A′′,B′′,C′′, Duf. Scale bar, 10 μm. (F) Bar diagram showing quantification of F-actin foci diameters. Asterisks indicate mutant genotypes in which the difference from wild type was statistically significant. Blue and red colouring are used to designate round versus spread-out irregular focus morphologies, respectively. GFP, green fluorescent protein; MHC, myosin heavy chain; NFP, nucleation-promoting factor; SCAR, suppressor of cyclic AMP receptor; Twi, twist; Wsp, Wiskott–Aldrich syndrome protein; WT, wild type.
We wish to emphasize the significance of these observations, in light of the recent controversy surrounding the phenotype of embryos defective in D-WIP function (Menon & Chia, 2007). Our data continue to show the formation of small membrane pores, and a corresponding transfer of cytoplasmic GFP between attached myoblasts, specifically in D-WIP- and Wsp-mutant embryos, but not in any other mutant background. Furthermore, we now show the suppression of both the perforated membrane phenotype and of GFP transfer in ketteJ4–48,Wsp3D3 double mutants. We believe that these observations provide significant support for our assertion that when D-WIP/Wsp function is compromised, fusion is arrested only after cytoplasmic continuity between fusing myoblasts has been achieved.
Actin foci at fusion sites are not formed by SCAR and Wsp
Several recent studies have documented conspicuous F-actin concentrations, commonly referred to as ‘actin foci', at the interface of fusing myoblasts in Drosophila embryos (Kesper et al, 2007; Kim et al, 2007; Richardson et al, 2007). The temporal profile of appearance and dissolution of the actin foci in wild-type embryos closely parallels the dynamics of myoblast fusion (Richardson et al, 2007), suggesting that these structures have an important function in the process. The substantial functional requirements for components of the Arp2/3 machinery in embryonic myoblast fusion naturally raise the possibility that construction of the fusion-associated foci depends on Arp2/3-based actin polymerization.
We examined this issue by using single- and double-mutant allelic combinations of zygotic loss-of-function conditions for both NPF systems (Fig 4). Consistent with published reports (Richardson et al, 2007), we found that disruption of SCAR-complex function results in the formation of enlarged, irregularly shaped foci at sites of myotube–myoblast attachment. Homozygous ketteJ4–48-mutant embryos show foci, the area of which is nearly twice the size of wild-type foci (2.89±1.77 versus 1.65±0.76 μm2, P=5.66E−08, n=67). Enlarged foci (2.21±0.92 μm2, P=0.007, n=162) are also characteristic of embryos expressing the dominant-negative GFP–SCAR construct in muscle tissue. We chose to disrupt SCAR function in this manner, as it generates stronger fusion phenotypes than those obtained with zygotic SCAR alleles (supplementary Fig 1 online). To assess the requirement for Wsp activity, we examined actin-focus morphology in Wsp3D3 homozygotes and in embryos homozygous for D-WIPD30 (Massarwa et al, 2007), a complete deletion of the D-WIP locus. We found that actin foci form under both these circumstances, but do not grow to larger dimensions than those present in wild-type embryos (1.52±0.6 and 1.62±0.83 μm2, respectively; also see, Richardson et al, 2008).
Next we sought to determine whether actin foci form after simultaneous disruption of both NPF systems. To this end, we examined both ketteJ4–48,Wsp3D3 and D-WIPD30; twist-GAL4∷UAS–GFP–SCAR double-mutant embryos. In both cases, we observed enlarged actin foci (2.9±1.79 μm2, P=3.72E−07 and 2.18±1.01 μm2, P=0.015, respectively), resembling those present in the corresponding single mutants of SCAR-complex elements. The molecular basis for the construction of fusion-associated actin foci therefore remains unknown. Furthermore, the analysis of single- and double-mutant combinations is consistent with the epistatic relationship between the NPF systems derived from the electron microscopy and GFP transfer studies, supporting our assertion that the SCAR and D-WIP/Wsp systems act separately and sequentially during myoblast fusion.
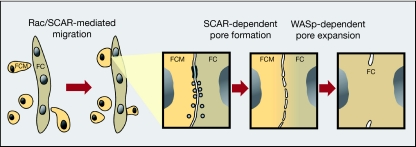
In this study, we have assessed and compared directly the contributions of the two primary Arp2/3 NPFs—SCAR/WAVE and WASp, to the process of myoblast fusion during Drosophila embryogenesis. Our analysis leads us to draw the following conclusions. (i) The SCAR complex is utilized twice during embryonic myogenesis. This NPF system initially promotes the migration of myoblasts towards growing myotubes in a Rac-dependent manner, which is consistent with the widespread involvement of SCAR/WAVE elements in regulating morphological transitions and associated changes in migratory behaviour (Croft & Olson, 2008). A second utilization of SCAR-complex activity occurs subsequently to myoblast–myotube attachment, during the fusion process itself (also see, Schroter et al, 2004; Richardson et al, 2007). (ii) The Wsp NPF system is assigned a single, independent and relatively late role, following fusion-pore formation. As initially suggested by our previous study (Massarwa et al, 2007), we propose that forces generated through Wsp-based actin polymerization are used to mediate fusion-pore expansion. (iii) The formation of fusion-associated F-actin foci does not depend on SCAR and Wsp activity, implying that a nucleation system distinct from Arp2/3 operates in this context.
A model summarizing our interpretation of the myogenic functions performed by the two Arp2/3 NPFs is shown in Fig 5. The model predicts a stepwise progression, in which the two systems have independent and sequential roles, within a single physiological setting. A central feature of this model, which we believe to be significant for the in vivo roles ascribed to the Arp2/3 system, is that NPFs that use a common molecular mechanism to target the same actin-polymerization machinery, and do so in close spatial and temporal proximity, can still mediate distinct cellular functions. Our model should be viewed in the broader context of studies carried out in other systems, addressing coordinated activity of Arp2/3 NPFs (Weaver et al, 2002; Sun et al, 2006). Deciphering the activation mechanisms that allow SCAR/WAVE and WASp to carry out distinct roles in muscle fusion through the common Arp2/3 actin nucleator is a future challenge.
Figure 5.
Model of the functional contributions of the SCAR and WASp pathways to myoblast fusion in Drosophila embryos. A SCAR-complex-dependent morphological transition underlies the acquisition of migratory behaviour by myoblasts, which are now able to attach to founder cells and/or myotubes. Upon the initiation of fusion, the SCAR complex is again used to promote the formation of pores in the apposed cell membranes. Once initial pores are formed, Wsp, recruited by D-WIP, mediates pore expansion, presumably through forces derived from Arp2/3-based actin polymerization. Note, an alternative model for the contribution of the two pathways has been recently proposed (Berger et al, 2008; see supplementary information online for further discussion). D-WIP, D-WASp-interacting protein; FC, founder cell; FCM, fusion-competent myoblast; SCAR, suppressor of cyclic AMP receptor; WASp, Wiskott–Aldrich syndrome protein.
Methods
Drosophila genetics. See specific references and Flybase (http://flybase.bio.indiana.edu/) for details regarding all genetic loci and mutant alleles described throughout this study.
Embryo processing, immunohistochemistry and microscopy. Embryos were processed for viewing by fluorescent and electron microscopy as described previously (Massarwa et al, 2007). See supplementary information online for further technical details and a list of antibodies used. Fluorescent images were collected on a Zeiss (Jena, Germany) LSM510 confocal system. The actin-foci measurements were performed using the Overlay function of the Zeiss LSM software (also see Richardson et al, 2007).
Supplementary information is available at EMBO reports online (http://www.emboreports.org).
Supplementary Material
Supplementary Information
Acknowledgments
We are grateful to M. Baylies, P. Fisher, M. Frasch, C. Klämbt, D. Menon, S. Önel, R. Renkawitz-Pohl, M. Ruiz-Gomez, T. Volk and the Bloomington Stock Center for stocks and reagents. We thank M. Haskel for contributing to single-copy abundant mRNA-complex phenotypic analysis, O. Bachar for drawing the model in Fig 5, and all the members of our lab for discussion and support. This study was financially supported by grants from the M.L. Ralph Designated Funds and the Israel Science Foundation to B-Z.S. and E.S. B-Z.S is an incumbent of the Hilda and Cecil Lewis chair in Molecular Genetics.
Footnotes
The authors declare that they have no conflict of interest.
References
- Ben-Yaacov S, Le Borgne R, Abramson I, Schweisguth F, Schejter ED (2001) Wasp, the Drosophila Wiskott–Aldrich syndrome gene homologue, is required for cell fate decisions mediated by Notch signaling. J Cell Biol 152: 1–13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berger S, Schafer G, Kesper DA, Holz A, Eriksson T, Palmer RH, Beck L, Klambt C, Renkawitz-Pohl R, Onel SF (2008) WASP and SCAR have distinct roles in activating the Arp2/3 complex during myoblast fusion. J Cell Sci 121: 1303–1313 [DOI] [PubMed] [Google Scholar]
- Bompard G, Caron E (2004) Regulation of WASP/WAVE proteins: making a long story short. J Cell Biol 166: 957–962 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Croft DR, Olson MF (2008) Regulating the conversion between rounded and elongated modes of cancer cell movement. Cancer Cell 14: 349–351 [DOI] [PubMed] [Google Scholar]
- Doberstein SK, Fetter RD, Mehta AY, Goodman CS (1997) Genetic analysis of myoblast fusion: blown fuse is required for progression beyond the prefusion complex. J Cell Biol 136: 1249–1261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goley ED, Welch MD (2006) The ARP2/3 complex: an actin nucleator comes of age. Nat Rev Mol Cell Biol 7: 713–726 [DOI] [PubMed] [Google Scholar]
- Hakeda-Suzuki S, Ng J, Tzu J, Dietzl G, Sun Y, Harms M, Nardine T, Luo L, Dickson BJ (2002) Rac function and regulation during Drosophila development. Nature 416: 438–442 [DOI] [PubMed] [Google Scholar]
- Hummel T, Leifker K, Klambt C (2000) The Drosophila HEM-2/NAP1 homolog KETTE controls axonal pathfinding and cytoskeletal organization. Genes Dev 14: 863–873 [PMC free article] [PubMed] [Google Scholar]
- Kaksonen M, Toret CP, Drubin DG (2006) Harnessing actin dynamics for clathrin-mediated endocytosis. Nat Rev Mol Cell Biol 7: 404–414 [DOI] [PubMed] [Google Scholar]
- Kesper DA, Stute C, Buttgereit D, Kreiskother N, Vishnu S, Fischbach KF, Renkawitz-Pohl R (2007) Myoblast fusion in Drosophila melanogaster is mediated through a fusion-restricted myogenic-adhesive structure (FuRMAS). Dev Dyn 236: 404–415 [DOI] [PubMed] [Google Scholar]
- Kim S, Shilagardi K, Zhang S, Hong SN, Sens KL, Bo J, Gonzalez GA, Chen EH (2007) A critical function for the actin cytoskeleton in targeted exocytosis of prefusion vesicles during myoblast fusion. Dev Cell 12: 571–586 [DOI] [PubMed] [Google Scholar]
- Massarwa R, Carmon S, Shilo BZ, Schejter ED (2007) WIP/WASp-based actin-polymerization machinery is essential for myoblast fusion in Drosophila. Dev Cell 12: 557–569 [DOI] [PubMed] [Google Scholar]
- Menon SD, Chia W (2001) Drosophila rolling pebbles: a multidomain protein required for myoblast fusion that recruits D-Titin in response to the myoblast attractant Dumbfounded. Dev Cell 1: 691–703 [DOI] [PubMed] [Google Scholar]
- Menon SD, Chia W (2007) Actin on multiple fronts to generate a muscle fiber. Dev Cell 12: 479–481 [DOI] [PubMed] [Google Scholar]
- Nose A, Isshiki T, Takeichi M (1998) Regional specification of muscle progenitors in Drosophila: the role of the msh homeobox gene. Development 125: 215–223 [DOI] [PubMed] [Google Scholar]
- Richardson BE, Beckett K, Nowak SJ, Baylies MK (2007) SCAR/WAVE and Arp2/3 are crucial for cytoskeletal remodelling at the site of myoblast fusion. Development 134: 4357–4367 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardson BE, Nowak SJ, Baylies MK (2008) Myoblast fusion in fly and vertebrates: new genes, new processes and new perspectives. Traffic 9: 1050–1059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schafer G, Weber S, Holz A, Bogdan S, Schumacher S, Muller A, Renkawitz-Pohl R, Onel SF (2007) The Wiskott–Aldrich syndrome protein (WASP) is essential for myoblast fusion in Drosophila. Dev Biol 304: 664–674 [DOI] [PubMed] [Google Scholar]
- Schroter RH, Lier S, Holz A, Bogdan S, Klambt C, Beck L, Renkawitz-Pohl R (2004) kette and blown fuse interact genetically during the second fusion step of myogenesis in Drosophila. Development 131: 4501–4509 [DOI] [PubMed] [Google Scholar]
- Sun Y, Martin AC, Drubin DG (2006) Endocytic internalization in budding yeast requires coordinated actin nucleation and myosin motor activity. Dev Cell 11: 33–46 [DOI] [PubMed] [Google Scholar]
- Takenawa T, Suetsugu S (2007) The WASP-WAVE protein network: connecting the membrane to the cytoskeleton. Nat Rev Mol Cell Biol 8: 37–48 [DOI] [PubMed] [Google Scholar]
- Weaver AM, Heuser JE, Karginov AV, Lee WL, Parsons JT, Cooper JA (2002) Interaction of cortactin and N-WASp with Arp2/3 complex. Curr Biol 12: 1270–1278 [DOI] [PubMed] [Google Scholar]
- Zallen JA, Cohen Y, Hudson AM, Cooley L, Wieschaus E, Schejter ED (2002) SCAR is a primary regulator of Arp2/3-dependent morphological events in Drosophila. J Cell Biol 156: 689–701 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Information