Abstract
The phosphoserine/threonine binding protein 14-3-3 stimulates the catalytic activity of protein kinase C-ɛ (PKCɛ) by engaging two tandem phosphoserine-containing motifs located between the PKCɛ regulatory and catalytic domains (V3 region). Interaction between 14-3-3 and this region of PKCɛ is essential for the completion of cytokinesis. Here, we report the crystal structure of 14-3-3ζ bound to a synthetic diphosphorylated PKCɛ V3 region revealing how a consensus 14-3-3 site and a divergent 14-3-3 site cooperate to bind to 14-3-3 and so activate PKCɛ. Thermodynamic data show a markedly enhanced binding affinity for two-site phosphopeptides over single-site 14-3-3 binding motifs and identifies Ser 368 as a gatekeeper phosphorylation site in this physiologically relevant 14-3-3 ligand. This dual-site intra-chain recognition has implications for other 14-3-3 targets, which seem to have only a single 14-3-3 motif, as other lower affinity and cryptic 14-3-3 gatekeeper sites might exist.
Keywords: 14-3-3, isothermal titration calorimetry, PKCɛ, crystal structure
Introduction
The 14-3-3 family are phosphoserine/threonine binding modules that recognize proteins containing either a mode 1 motif (RSXpSXP, in which pS indicates phosphorylated serine) or mode 2 motif (RXF/YXpSXP; Muslin et al, 1996; Yaffe et al, 1997). 14-3-3 proteins can recognize non-phosphorylated targets and phosphoserine/threonine-containing sequences distinct from the canonical mode 1 and 2 motifs, generating a large and diverse array of potential substrates (Waterman et al, 1998; Fuglsang et al, 1999; Henriksson et al, 2002). 14-3-3 proteins are typically homodimeric and adopt a horseshoe-shaped structure (Liu et al, 1995; Xiao et al, 1995). The highly basic phosphoserine-binding pockets from each protomer face each other within the 14-3-3 dimer, allowing interactions from dimeric single-site partners as well as potentially two-site monomeric partners (Yaffe et al, 1997). Structural studies so far have focused on symmetric 14-3-3 complexes with either single-site peptides or dimeric protein partners (Yaffe et al, 1997; Petosa et al, 1998; Obsil et al, 2001; Ottmann et al, 2007).
The 14-3-3 isoforms show distinct regulatory functions towards various target proteins (reviewed by Bridges & Moorhead, 2005). Although several known 14-3-3 binding partners seem to contain a single binding motif, many have more than one confirmed 14-3-3 binding sites, including the serine kinase c-Raf, FOXO4, CDC25 and protein kinase C-ɛ (PKCɛ; Tzivion et al, 1998; Zeng et al, 1998; Obsil et al, 2003; Saurin et al, 2008). The presence of several 14-3-3 binding motifs within a single polypeptide raises the possibility of an intra-chain dual 14-3-3 binding site that engages both phosphoserine-binding clefts within a 14-3-3 dimer. Indeed, chemically linked 14-3-3 binding motifs have been shown to display positive cooperativity and enhanced binding compared with individual mono-phosphorylated motifs (Yaffe et al, 1997). For two-site dual 14-3-3 ligands, phosphorylation of one of the binding sites has been proposed to act as a ‘gatekeeper' insofar as it is required for 14-3-3 binding but is not sufficient for full biological activity by itself (Yaffe, 2002). Despite considerable biological evidence suggesting that 14-3-3 might commonly use tandem intra-chain sites on binding partners (Obsil et al, 2003; Saurin et al, 2008), so far there has been no crystallographic or biophysical description of a biologically relevant tandem intra-chain 14-3-3 site.
We recently described the functional importance of a multi-phosphorylated PKCɛ V3 region that binds to 14-3-3 (Saurin et al, 2008). The actions of p38 and glycogen synthase kinase-3β (GSK3β) produce a mode 1 motif at residues 343–348 (RSKpSAP) of PKCɛ and autophosphorylation produces a divergent mode 2 motif at residues 364–370 (RKALpSFD) lacking proline at +2 and an aromatic residue at the −2 position (Durgan et al, 2008; Saurin et al, 2008; Fig 1A). The 14-3-3 interaction mediated by these motifs is crucial for the exit from cytokinesis, as shown by PKCɛ mutants S346A/S368A or R343A, which prevent the ability of PKCɛ to recover cytokinesis defects after PKCɛ knockout or knockdown (Saurin et al, 2008). Here, we report a structural and thermodynamic characterization of the PKCɛ V3 region, a physiological 14-3-3 ligand that contains tandem intra-chain 14-3-3 binding sites with a ‘gatekeeper' phosphorylation site as first postulated by Yaffe (2002).
Figure 1.
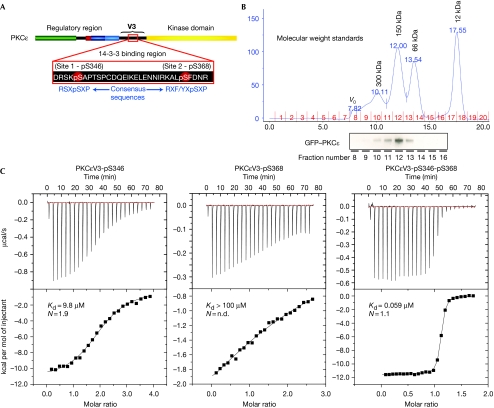
Stoichiometry and thermodynamics of the 14-3-3–PKCɛ interaction. (A) Schematic showing PKCɛ primary structure, 14-3-3 phosphoserine-binding motifs and idealized 14-3-3 consensus sequences underneath. (B) The 14-3-3ζ–GFP-PKCɛ complex migrates by size exclusion chromatography with an apparent molecular weight that is consistent with a stoichiometry of one GFP–PKCɛ monomer (110 kDa) to one 14-3-3ζ dimer (58 kDa). (C) Isothermal titration calorimetry experiments showing binding curves for PKCɛV3 monophosphopeptides and diphosphopeptide. Raw data are shown in the top panels with fitted curves below. GFP, green fluorescent protein, PKCɛ, protein kinase C-ɛ.
Results And Discussion
14-3-3ζ–PKCɛ complex stoichiometry
To confirm the composition of a complex containing a full-length PKCɛ bound to 14-3-3ζ, we assembled green fluorescent protein (GFP)-tagged PKCɛ with 14-3-3ζ according to published protocols (Saurin et al, 2008). The complex was then fractionated by using size exclusion chromatography and probed with a PKCɛ antibody, showing a molecular weight of 150 kDa consistent with a 14-3-3ζ dimer bound to one molecule of PKCɛ (Fig 1B), which is in agreement with published data for a 14-3-3β–PKCɛ complex (Saurin et al, 2008).
We then investigated the stoichiometry of a smaller analogous complex between 14-3-3ζ and a biotinylated PKCɛ V3 region diphosphopeptide (PKCɛV3-b-pS346-pS368) spanning residues 342–372. By using an avidin displacement assay, we found that this complex showed a stoichiometry of 0.96 (±0.15) peptide molecules per 14-3-3 dimer (see Methods). Furthermore, it was possible to disrupt the association of PKCɛ and 14-3-3 in cell extracts by adding the PKCɛV3-b-pS346-pS368 peptide (supplementary Fig S1 online). Thus, the diphosphorylated PKCɛ V3 peptide retains the essential features of the intact 14-3-3–PKCɛ complex.
Tandem phosphoserine sites enhance 14-3-3 binding
To probe the thermodynamics of the 14-3-3–PKCɛ interaction, we synthesized PKCɛ-V3 region peptides spanning residues 342–372 and characterized them using isothermal titration calorimetry (ITC). The peptides corresponded to monophosphorylated (PKCɛV3-pS346 and PKCɛV3-pS368), diphosphorylated (PKCɛV3-pS346-pS368) or unphosphorylated (PKCɛV3-n) species. The ITC results are summarized in Table 1. The monophosphorylated PKCɛV3-pS346 (defined hereafter as site 1) showed a modest affinity (dissociation constant Kd=9.8 μM), consistent with it conforming to an optimal mode 1 14-3-3 binding motif. By contrast, binding of the PKCɛV3-pS368 peptide (defined hereafter as site 2) was too weak to accurately determine the dissociation constant. The unphosphorylated peptide PKCɛV3-n showed no detectable binding. Combining site 1 and site 2 within a diphosphopeptide markedly enhanced its affinity for 14-3-3 (Kd=59 nM), resulting in a 166-fold increase in affinity over site 1 alone (Fig 1C). This shows that Ser 368 of site 2 shows characteristics of a gatekeeper phosphorylation site, being necessary for high-affinity binding but with almost no intrinsic affinity for 14-3-3 by itself. The apparent stoichiometry from our ITC experiments indicates that a 14-3-3 dimer interacts with two molecules of PKCɛV3-pS346 site-1 phosphopeptide and one molecule of PKCɛV3-pS346-pS368 diphosphopeptide (Table 1). The bidentate interaction is likely to be the only stable conformation in vivo, as mutation of either binding site renders the 14-3-3–PKCɛ interaction undetectable (Saurin et al, 2008).
Table 1.
PKCɛ V3 peptide binding constants for 14-3-3ζ
| Peptide | Kd* (μM) | N† |
|---|---|---|
| PKCɛV3-n | ND | – |
| PKCɛV3-pS346 (site 1) | 9.8±0.44 | 1.9±0.0083 |
| PKCɛV3-pS368 (site 2) | >100 | – |
| PKCɛV3-pS346-pS368 | 0.059±0.0030 | 1.1±0.0011 |
| PKCɛV3-pS346-pS350-pS368 | 2.0±0.20 | 1.5±0.013 |
| PKCɛV3-site2-site2 | 0.75±0.051 | 1.3±0.0042 |
| PKCɛV3-site1-site1‡ | 0.00058±0.00043 | 0.69±0.066 |
| 0.0072±0.0045 | 0.49±0.062 | |
| PKCɛV3-G6 | 6.9±0.53 | 2.0±0.021 |
| PKCɛV3-G10 | 0.12±0.0090 | 0.88±0.0023 |
| PKCɛV3-G14 | 0.13±0.012 | 0.93±0.0029 |
| ND, not detectable; PKCɛ, protein kinase C-ɛ. | ||
| *KD=1/KA. | ||
| †N=stoichiometry of binding (peptide to 14-3-3 dimer). | ||
| ‡Both KD1 and KD2 estimates possible, see supplementary information online. | ||
| Each experiment was carried out for a minimum of three times on independent samples. | ||
To create a GSK3β recognition motif (SXXXpS; Fiol et al, 1990) and hence trigger phosphorylation at Ser 346, a priming phosphorylation is required at Ser 350 (Saurin et al, 2008). The peptide PKCɛV3-pS346-pS350-pS368 was therefore synthesized to mimic the triphosphorylated V3 region of PKCɛ. PKCɛV3-pS346-pS350-pS368 showed a Kd of 2.0 μM for 14-3-3 (supplementary Fig S2A online), a significantly higher affinity than PKCɛV3-pS346 site 1 but still 34-fold weaker than that of the diphosphopeptide (Table 1). The weaker affinity indicates that this phosphorylation compromises the high-affinity tandem interaction with 14-3-3. Consequently, selective dephosphorylation of Ser 350 might be important for 14-3-3 activation of PKCɛ, although the phosphorylation state of Ser 350 during cytokinesis is uncharacterized.
To determine whether two weak tandem sites are sufficient to produce an enhanced 14-3-3 binding interaction, we carried out similar ITC experiments on a diphosphopeptide with the site-2 sequence RKALpSFD at both ends of the peptide (PKCɛV3-site2-site2). This peptide had a Kd of 750 nM and is a significant improvement in affinity over the virtually undetectable binding of a single pS368 site 2. Furthermore, it is almost tenfold weaker than for the PKCɛV3-pS346-pS368 diphosphopeptide. By contrast, ITC binding curves obtained from a peptide with two optimal 14-3-3 binding sites (with sequence RSKpSAP, designated PKCɛV3-site1-site1) showed approximately 100-fold higher affinity than PKCɛV3-pS346-pS368 binding. This high affinity interaction was strong enough to observe two separate binding events (Kd1=0.58 nM and Kd2=7.2 nM; supplementary Fig S2B,C). These experiments lead us to conclude that the affinity of the PKCɛV3 region has been selected to allow regulated 14-3-3 binding through gatekeeper phosphorylation, rather than to maximize 14-3-3 affinity.
Structure of a 14-3-3–diphosphopeptide complex
To investigate this interaction further we then crystallized and determined the structure of the 14-3-3–PKCɛV3-pS346-pS368 complex at a resolution of 2.2 Å (see supplementary Table S1 online for details). Crystals contained two 14-3-3 dimers within the asymmetric unit, each of which bound a phosphopeptide ligand. As one 14-3-3 dimer showed clearer electron density for the bound diphosphopeptide, we used this complex for the structural description below. Fortuitously, we did not observe an admixture of the diphosphopeptide bound in both possible orientations to a 14-3-3 dimer.
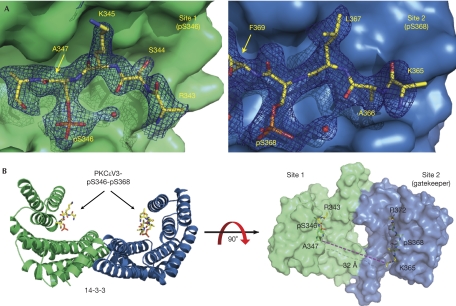
The refined model includes mainchain atoms for PKCɛV3 residues 343–347 (site 1) and 365–372 (site 2), including both pS346 and pS368. Side-chain density for residues Lys 345 and Leu 367 assisted with the assignment of pS346 and pS368 to each 14-3-3 protomer (Fig 2A). The presence of an ordered site 2, despite its almost undetectable affinity for 14-3-3 by itself, indicates that it is highly likely to be connected to site 1 through the same diphosphopeptide, which is consistent with our thermodynamic data and the apparent stoichiometry of the complex. However, the intervening linker sequence (residues 348–364) is not ordered, leaving a gap of 32 Å from the end of site 1 to the start of site 2 (Fig 2B). A tight turn is likely to connect site 1 and site 2, as the PKCɛV3 site-1 sequence has a consensus proline (Pro 348) at the +2 position, previously shown to kink the path of the peptide mainchain by 180° (Rittinger et al, 1999).
Figure 2.
Structure of the 14-3-3–PKCɛV3 complex. (A) The electron density (2mFo-DFc contoured at σ=1.2) for the 14-3-3–PKCɛV3-pS346-pS368 diphosphopeptide complex is shown. (B) Surface representation of the 14-3-3 dimer (protomers are shown in blue and green, respectively) with the PKCɛV3-pS346-pS368 peptide shown as sticks in yellow, and the location and distance spanned by the missing connecting linker indicated. The figure was prepared using PyMOL (Delano, 2002). PKCɛ, protein kinase C-ɛ.
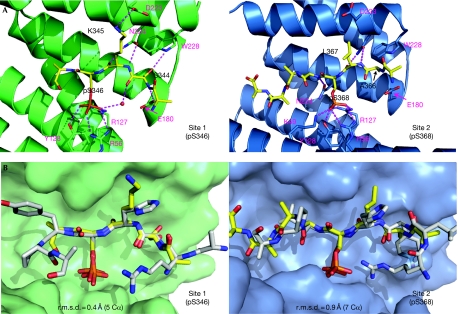
The r.m.s. difference for site 1 compared with a mode 1 structure (0.4 Å over 5 Cα for Protein Data Bank (PDB) code 1QJB) is lower than that of site 2 compared with a mode 2 structure (0.9 Å over 7 Cα for PDB code 1QJA), reflecting the greater similarity of site 1 to a consensus 14-3-3 sequence (Fig 3B). The 14-3-3ζ dimer is similar to previously reported 14-3-3 structures (supplementary Fig S3 online).
Figure 3.
Close-up of the molecular contacts made by each phosphoserine binding site and structural comparisons. (A) Hydrogen bonds are shown for site 1 (left panel) and site 2 (right panel) with selected interaction residues labelled in pink (14-3-3) and black (PKCɛ). (B) Comparison of the PKCɛV3 site 1 with a mode 1 14-3-3 peptide (left panel, grey, PDB code 1QJB) and site 2 with a mode 2 peptide (right panel, grey, PDB code 1QJA). PKCɛV3-pS346-pS368 is shown in yellow, 14-3-3 chain A is green and chain B is blue. Residue numbers are labelled as in panel (A). The figure was prepared using PyMOL (Delano, 2002). PDB, protein data bank; PKCɛ, protein kinase C-ɛ.
The 14-3-3 side chains coordinating each phosphoserine moiety include R5614-3-3, R12714-3-3 and Y12814-3-3 as previously reported for other phosphoserine peptides (Fig 3A). The side chain of K4914-3-3 also coordinates pS368 in site 2 but is poorly ordered beyond the Cγ atom for site 1 and was omitted from the final model. Side chains of N22414-3-3 and N17314-3-3 form a hydrogen bond with mainchain atoms from the −1 and +1 residues relative to each phosphoserine, which is also consistent with previous 14-3-3 structures (Yaffe et al, 1997).
The greater affinity of 14-3-3 for a PKCɛV3-pS346 site-1 peptide over a PKCɛV3-pS368 site-2 peptide can be rationalized by the greater number of hydrogen bonds formed by interaction with the site-1 peptide (Fig 3A). These include hydrogen bonds from the E18014-3-3 side chain to S344PKCɛV3 mainchain nitrogen and the S344PKCɛV3 side chain to Nɛ of W22814-3-3. The latter interaction is not possible in site 2 as the serine is replaced by alanine (Ala 367). Furthermore, K345PKCɛV3 from site 1 forms a salt bridge with D22314-3-3. For the site 2-bound protomer, the D22314-3-3 side chain is torsioned away from the binding pocket, presumably because the equivalent site 2 residue to Lys 345 is Leu 367 (Fig 3A). Neither the R343PKCɛV3 nor the R364PKCɛV3 side chain is included in the final model, similar to several other reported peptide-bound 14-3-3 structures, however, a role in phosphoserine coordination is likely (Yaffe et al, 1997; Petosa et al, 1998).
The weaker affinity of the triphosphorylated PKCɛV3-pS346-pS350-pS368 peptide for 14-3-3 might be explained by the predicted tight turn induced by P348PKCɛV3, placing pS350PKCɛV3 in close proximity to the pS346PKCɛV3 binding pocket and thereby potentially perturbing pS346PKCɛV3 coordination by 14-3-3.
A shortened linker abolishes high-affinity binding
To determine how linker length influences 14-3-3–PKCɛ binding, residues 350–363 of PKCɛV3-pS346-pS368 were replaced with 6, 10 and 14 glycine residues, (peptides PKCɛV3-pS346-G6-pS368, PKCɛV3-pS346-G10-pS368 and PKCɛV3-pS346-G14-pS368). This was predicted to give maximal linker lengths of 22.8, 38 and 58.2 Å, respectively (calculated from a model peptide in an extended conformation). ITC binding curves showed that the G14 and G10 linked peptides retained high-affinity binding for 14-3-3 (Kd=120 and 130 nM, respectively), consistent with linker lengths greater than 32 Å allowing two-site binding (Fig 2B). By contrast, the G6 linked peptide binding affinity (Kd=6.9 μM) was comparable with that measured for the PKCɛV3-pS346 monophosphorylated peptide (Table 1; supplementary Fig S4 online). We also observed a concomitant increase in stoichiometry from one to two molecules of the G6 linker peptide bound per 14-3-3 dimer. These data indicate that a minimal linker sequence of approximately ten residues is required between the two half sites to generate a tandem intramolecular 14-3-3 site. The PKCɛ linker is somewhat longer and has a predicted short helix (Ile 356 to Leu 367) and several acidic residues that might influence linker conformation after PKCɛ phosphorylation.
Wider implications for tandem 14-3-3 binding sites
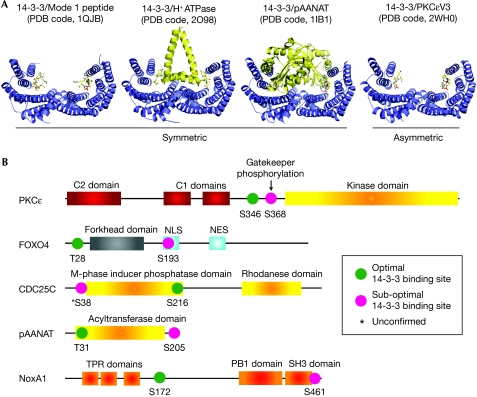
Previous studies by Yaffe et al (1997) chemically linked two identical 14-3-3 sites from c-Raf to mimic a tandem intra-chain 14-3-3 site. This synthetic substrate showed a 30-fold higher affinity for 14-3-3 over a single 14-3-3 site. This led to the ‘gatekeeper' phosphorylation hypothesis for biologically relevant tandem-site 14-3-3 ligands (Yaffe, 2002). Our data provide compelling evidence for this postulate by showing that a divergent 14-3-3 site is required for high-affinity binding, but has a barely detectable interaction on its own. Our asymmetrically bound 14-3-3 ligand structure reveals the conformation of such a gatekeeper site in contrast to previous symmetric 14-3-3–peptide structures (Fig 4A). We also show that the affinity of the tandem 14-3-3 site can be attenuated by a third phosphorylation, allowing further regulatory inputs to potentially alter complex formation and PKCɛ activation. PKCɛ thus provides a good example of how divergence from a consensus 14-3-3 motif within a gatekeeper site opens up the target to a wider range of modulators, either serine/threonine kinases or phosphatases, that control 14-3-3 binding. This is in marked contrast to the more limited array of regulators that can act on a strict consensus 14-3-3 site.
Figure 4.
Distinct binding modes for 14-3-3 partner proteins and selected examples of proteins with known two-site intra-chain 14-3-3 binding sites. (A) Structures of inter-chain (symmetric) complexes with a 14-3-3 dimer (blue ribbon) compared with the intra-chain (asymmetric) structure described for the PKCɛV3 ligand. (B) The domain structure and location of consensus and divergent 14-3-3 sites within selected intra-chain two-site 14-3-3 partners. Optimal motifs are defined as those containing at least three out of four consensus mode 1 or mode 2 residues (Peng et al, 1997; Obsil et al, 2003; Ganguly et al, 2005; Kim et al, 2007; Saurin et al, 2008). PDB, protein data bank; PKCɛ, protein kinase C-ɛ.
The implications of two-site intra-chain 14-3-3 binding extend beyond PKCɛ given that many confirmed 14-3-3 targets contain multiple 14-3-3 binding motifs, some of which conform to a mode 1/mode 2 consensus but many of which do not (Fig 4B). A similar affinity enhancement might be anticipated in these examples, in which a weaker divergent 14-3-3 site is present. For multi-site 14-3-3 ligands such as c-Raf the situation is likely to be more complicated. For example, c-Raf contains two optimal 14-3-3 binding sites and more than 10 sequences, which could form imperfect 14-3-3 motifs. Although c-Raf is able to bind to dimerization-deficient 14-3-3 mutants, its activation is impaired (Tzivion et al, 1998), suggesting that tandem binding indeed has a crucial role in regulating the interaction. Importantly, many of the known 14-3-3 targets with a single reported 14-3-3 interaction motif possibly contain as yet unidentified motifs crucial to their association with 14-3-3. Our data, therefore, suggest a significant role for two-site intra-chain binding across a spectrum of 14-3-3 interaction partners, and highlight the importance of identifying cryptic motifs and assessing their roles in a physiological setting.
Methods
Protein purification. BL21(DE3) Escherichia coli cells expressing hexa-histidine-tagged 14-3-3ζ (His-14-3-3) were induced at OD600 0.8 with 0.4 mM isopropyl-β-D-1-thiogalactopyranoside for 5 h at 37°C. Cells were lysed in buffer A (20 mM HEPES (pH 7.4), 100 mM NaCl, 1 mM tris (2-carboxyethyl) phosphine) with complete EDTA-free protease inhibitors (Roche Diagnostics, West Sussex, UK) by sonication. The supernatant was applied to a Ni2+-loaded HiTrap chelating column (GE Healthcare, Buckinghamshire, UK). His-14-3-3 was eluted with buffer A with 200 mM imidazole (pH 7.4). Gel filtration was carried out on a HiLoad 26/60 Superdex 200 column (GE Healthcare) in buffer A.
Stoichiometry measurements. Human embryonic kidney-293 cells producing tetracycline-induced GFP–PKCɛ were treated with 400 nM phorbol-12-myristate-13-acetate and 100 nM calyculin A phosphatase inhibitor for 20 min. Cell lysate was added to Ni–nitrilotriacetic acid beads pre-bound to 14-3-3ζ. The 14-3-3ζ–PKCɛ complex was eluted with imidazole and separated on a Superdex 200 10/30 GL column (GE Healthcare) in 50 mM Tris (pH 7.5), 150 mM NaCl, 0.5% TritonX-100 and 10 mM NaF. Molecular-weight standards were used as references. Eluted 14-3-3ζ–PKCɛ complex was detected using Western blotting with a PKCɛ antibody (Santa Cruz, Santa Cruz, CA, USA).
Peptide stoichiometry was determined using the EZ Biotin Quantitation Kit (Pierce Chemicals, IL, USA). A fivefold excess of biotinylated PKCɛV3-b-pS346-pS368 peptide was incubated with 14-3-3ζ followed by gel filtration in PBS buffer (5.3 mM Na2HPO4, 1.8 mM KH2PO4, 140 mM NaCl, 3.5 mM KCl, (pH 7.2)). The complex was added to 4′-hydroxyazobenzene-2-carboxylic acid–avidin solution in PBS. The absorbance was measured at 500 nm and the biotin/protein ratio calculated according to the manufacturer's instructions.
ITC. His-14-3-3 and HPLC-purified synthetic peptides were dialysed overnight in PBS+1 mM tris (2-carboxyethyl) phosphine. Peptides were injected in 1.4 μl increments into 200 μl 14-3-3ζ using concentrations ranging from 100 to 1,400 μM injectant and from 10 to 100 μM in the cell. All measurements were conducted a minimum of three times on independent samples in an iTC200 (Microcal, Milton Keynes, UK).
Crystallization. The 14-3-3ζ–PKCɛV3-pS346-pS368 complex was crystallized by vapour diffusion. A total of 1 μl of the 14-3-3ζ–PKCɛV3-pS346-pS368 complex, at a concentration of 42 mg/ml, was added to 1 μl 22% (w/v) polyethylene glycol 3350 (PEG3350), 100 mM calcium acetate, 50 mM NaF and incubated at 20°C for 4 weeks. The crystals were crushed and seeded in sitting drops with 1 μl of protein complex at 32 mg/ml and 1 μl 18% (w/v) PEG3350, 50 mM calcium acetate, 50 mM NaF and incubated at 20°C for 2 weeks to give diffraction quality crystals. The crystals were soaked step-wise in a final solution of 10% (v/v) glycerol, 20% (w/v) PEG3350, 50 mM calcium acetate and 50 mM NaF before cryo-cooling in liquid nitrogen.
X-ray data collection and structural refinement. The native data set for 14-3-3ζ–PKCɛV3-pS346-pS368 was collected to an effective resolution of 2.25 Å. The data were processed and scaled using XDS (Kabsch, 1988, 1993). Phaser (McCoy et al, 2007) was used for molecular replacement. Pseudo-merohedral twinning was detected using phenix.xtriage and least squares twin refinement was carried out using phenix.refine (Adams et al, 2002), with manual rebuilding carried out using Coot (Emsley & Cowtan, 2004). The coordinates have been deposited in the PDB with accession number 2WH0.
Supplementary information is available at EMBO reports online (http://www.emboreports.org).
Supplementary Material
supplementary information S1–S4
Acknowledgments
Thanks to the Cancer Research-UK Peptide Synthesis Laboratory, to J. Endicott and J. Welburn for advice, and A. Aitken for the 14-3-3 cDNA. We also thank S. Mouilleron, J. Murray-Rust and E. Lorentzen for providing X-ray crystallography expertize, and R. George for help with the ITC data. We acknowledge support from EU FP6 #LSHB-CT-2004-503467.
Footnotes
The authors declare that they have no conflict of interest.
References
- Adams PD, Grosse-Kunstleve RW, Hung LW, Ioerger TR, McCoy AJ, Moriarty NW, Read RJ, Sacchettini JC, Sauter NK, Terwilliger TC (2002) PHENIX: building new software for automated crystallographic structure determination. Acta Crystallogr D Biol Crystallogr 58: 1948–1954 [DOI] [PubMed] [Google Scholar]
- Bridges D, Moorhead GB (2005) 14-3-3 proteins: a number of functions for a numbered protein. Sci STKE 2005: re10. [DOI] [PubMed] [Google Scholar]
- Delano WL (2002) The PyMOL Molecular Graphics System. Palo Alto, CA USA: Delano Scientific. http://www.pymol.org [Google Scholar]
- Durgan J, Cameron AJ, Saurin AT, Hanrahan S, Totty N, Messing RO, Parker PJ (2008) The identification and characterization of novel PKCepsilon phosphorylation sites provide evidence for functional cross-talk within the PKC superfamily. Biochem J 411: 319–331 [DOI] [PubMed] [Google Scholar]
- Emsley P, Cowtan K (2004) Coot: model-building tools for molecular graphics. Acta Crystallogr D Biol Crystallogr 60: 2126–2132 [DOI] [PubMed] [Google Scholar]
- Fiol CJ, Wang A, Roeske RW, Roach PJ (1990) Ordered multisite protein phosphorylation. Analysis of glycogen synthase kinase 3 action using model peptide substrates. J Biol Chem 265: 6061–6065 [PubMed] [Google Scholar]
- Fuglsang AT, Visconti S, Drumm K, Jahn T, Stensballe A, Mattei B, Jensen ON, Aducci P, Palmgren MG (1999) Binding of 14-3-3 protein to the plasma membrane H(+)-ATPase AHA2 involves the three C-terminal residues Tyr(946)-Thr-Val and requires phosphorylation of Thr(947). J Biol Chem 274: 36774–36780 [DOI] [PubMed] [Google Scholar]
- Ganguly S, Weller JL, Ho A, Chemineau P, Malpaux B, Klein DC (2005) Melatonin synthesis: 14-3-3-dependent activation and inhibition of arylalkylamine N-acetyltransferase mediated by phosphoserine-205. Proc Natl Acad Sci USA 102: 1222–1227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henriksson ML, Francis MS, Peden A, Aili M, Stefansson K, Palmer R, Aitken A, Hallberg B (2002) A nonphosphorylated 14-3-3 binding motif on exoenzyme S that is functional in vivo. Eur J Biochem 269: 4921–4929 [DOI] [PubMed] [Google Scholar]
- Kabsch W (1988) Evaluation of single-crystal X-ray diffraction data from a position-sensitive detector. J Appl Cryst 21: 916–924 [Google Scholar]
- Kabsch W (1993) Automatic processing of rotation diffraction data from crystals of initially unknown symmetry and cell constants. J Appl Cryst 26: 795–800 [Google Scholar]
- Kim JS, Diebold BA, Babior BM, Knaus UG, Bokoch GM (2007) Regulation of Nox1 activity via protein kinase A-mediated phosphorylation of NoxA1 and 14-3-3 binding. J Biol Chem 282: 34787–34800 [DOI] [PubMed] [Google Scholar]
- Liu D, Bienkowska J, Petosa C, Collier RJ, Fu H, Liddington R (1995) Crystal structure of the zeta isoform of the 14-3-3 protein. Nature 376: 191–194 [DOI] [PubMed] [Google Scholar]
- McCoy AJ, Grosse-Kunstleve RW, Adams PD, Winn MD, Storoni MC, Read RJ (2007) Phaser crystallographic software. J Appl Cryst 40: 658–674 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muslin AJ, Tanner JW, Allen PM, Shaw AS (1996) Interaction of 14-3-3 with signaling proteins is mediated by the recognition of phosphoserine. Cell 84: 889–897 [DOI] [PubMed] [Google Scholar]
- Obsil T, Ghirlando R, Klein DC, Ganguly S, Dyda F (2001) Crystal structure of the 14-3-3zeta:serotonin N-acetyltransferase complex. A role for scaffolding in enzyme regulation. Cell 105: 257–267 [DOI] [PubMed] [Google Scholar]
- Obsil T, Ghirlando R, Anderson DE, Hickman AB, Dyda F (2003) Two 14-3-3 binding motifs are required for stable association of forkhead transcription factor FOXO4 with 14-3-3 proteins and inhibition of DNA binding. Biochemistry 42: 15264–15272 [DOI] [PubMed] [Google Scholar]
- Ottmann C et al. (2007) Structure of a 14-3-3 coordinated hexamer of the plant plasma membrane H+-ATPase by combining X-ray crystallography and electron cryomicroscopy. Mol Cell 25: 427–440 [DOI] [PubMed] [Google Scholar]
- Peng CY, Graves PR, Thoma RS, Wu Z, Shaw AS, Piwnica-Worms H (1997) Mitotic and G2 checkpoint control: regulation of 14-3-3 protein binding by phosphorylation of Cdc25C on serine-216. Science 277: 1501–1505 [DOI] [PubMed] [Google Scholar]
- Petosa C, Masters SC, Bankston LA, Pohl J, Wang B, Fu H, Liddington RC (1998) 14-3-3zeta binds a phosphorylated Raf peptide and an unphosphorylated peptide via its conserved amphipathic groove. J Biol Chem 273: 16305–16310 [DOI] [PubMed] [Google Scholar]
- Rittinger K, Budman J, Xu J, Volinia S, Cantley LC, Smerdon SJ, Gamblin SJ, Yaffe MB (1999) Structural analysis of 14-3-3 phosphopeptide complexes identifies a dual role for the nuclear export signal of 14-3-3 in ligand binding. Mol Cell 4: 153–166 [DOI] [PubMed] [Google Scholar]
- Saurin AT, Durgan J, Cameron AJ, Faisal A, Marber MS, Parker PJ (2008) The regulated assembly of a PKCepsilon complex controls the completion of cytokinesis. Nat Cell Biol 10: 891–901 [DOI] [PubMed] [Google Scholar]
- Tzivion G, Luo Z, Avruch J (1998) A dimeric 14-3-3 protein is an essential co-factor for Raf kinase activity. Nature 394: 88–92 [DOI] [PubMed] [Google Scholar]
- Waterman MJ, Stavridi ES, Waterman JL, Halazonetis TD (1998) ATM-dependent activation of p53 involves dephosphorylation and association with 14-3-3 proteins. Nat Genet 19: 175–178 [DOI] [PubMed] [Google Scholar]
- Xiao B, Smerdon SJ, Jones DH, Dodson GG, Soneji Y, Aitken A, Gamblin SJ (1995) Structure of a 14-3-3 protein and implications for coordination of multiple signalling pathways. Nature 376: 188–191 [DOI] [PubMed] [Google Scholar]
- Yaffe MB (2002) How do 14-3-3 proteins work?—Gatekeeper phosphorylation and the molecular anvil hypothesis. FEBS Lett 513: 53–57 [DOI] [PubMed] [Google Scholar]
- Yaffe MB, Rittinger K, Volinia S, Caron PR, Aitken A, Leffers H, Gamblin SJ, Smerdon SJ, Cantley LC (1997) The structural basis for 14-3-3:phosphopeptide binding specificity. Cell 91: 961–971 [DOI] [PubMed] [Google Scholar]
- Zeng Y, Forbes KC, Wu Z, Moreno S, Piwnica-Worms H, Enoch T (1998) Replication checkpoint requires phosphorylation of the phosphatase Cdc25 by Cds1 or Chk1. Nature 395: 507–510 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
supplementary information S1–S4