Abstract
Cell division cycle 5-like protein (Cdc5L) is a core component of the putative E3 ubiquitin ligase complex containing Prp19/Pso4, Plrg1 and Spf27. This complex has been shown to have a role in pre-messenger RNA splicing from yeast to humans; however, more recent studies have described a function for this complex in the cellular response to DNA damage. Here, we show that Cdc5L interacts physically with the cell-cycle checkpoint kinase ataxia-telangiectasia and Rad3-related (ATR). Depletion of Cdc5L by RNA-mediated interference methods results in a defective S-phase cell-cycle checkpoint and cellular sensitivity in response to replication-fork blocking agents. Furthermore, we show that Cdc5L is required for the activation of downstream effectors or mediators of ATR checkpoint function such as checkpoint kinase 1 (Chk1), cell cycle checkpoint protein Rad 17 (Rad17) and Fanconi anaemia complementation group D2 protein (FancD2). In addition, we have mapped the ATR-binding region in Cdc5L and show that a deletion mutant that is unable to interact with ATR is defective in the rescue of the checkpoint deficiency in Cdc5L-depleted cells. These findings show a new function for Cdc5L in the regulation of the ATR-mediated cell-cycle checkpoint in response to genotoxic agents.
Keywords: ATR, Cdc5L, checkpoint, DNA damage
Introduction
Precursor messenger RNA-processing factor 19 (Prp19)/Pso4 and cell division cycle 5-like protein (Cdc5L) are highly conserved eukaryotic proteins that have a defined role in precursor messenger RNA (pre-mRNA) splicing from yeast to humans (Cheng et al, 1993; Tarn et al, 1993; Grey et al, 1996; McDonald et al, 1999; Ajuh et al, 2000; Ohi & Gould, 2002). However, recent findings indicate that these proteins also have a distinct and direct role in the cellular response to DNA damage. By using biochemical approaches, we isolated a four-protein complex composed of Prp19/Pso4, Cdc5L, pleiotropic regulator 1 (Plrg1) and spliceosome-associated protein 27 (SPF27; Pso4 complex), which is required for the processing of DNA interstrand cross-links (ICLs) in vitro (Zhang et al, 2005a). We have also shown that Prp19/Pso4 is modified by ubiquitination in response to DNA damage and that this modification reduces its affinity for other members of the Pso4 complex (Lu & Legerski, 2007). Others have shown that Prp19/Pso4 interacts with the terminal deoxynucleotidyl transferase, and that depletion of Prp19/Pso4 results in the accumulation of double-strand breaks, apoptosis and decreased survival on exposure of cells to ionizing radiation (Mahajan & Mitchell, 2003). Prp19/Pso4 has also been shown to be a DNA-binding protein and is required for the recruitment of the DNA-repair protein Metnase to sites of double-strand breaks (Mahajan & Mitchell, 2003; Beck et al, 2008). Prp19/Pso4 is highly conserved in eukaryotes, and in budding yeast pso4 mutants show broad hypersensitivity to DNA damage-inducing agents, but are particularly sensitive to psoralen and other ICL-inducing drugs (Henriques et al, 1989; Grey et al, 1996). Taken together, these findings clearly indicate that the Pso4 complex has a role in the DNA-damage response (DDR), although the nature of its function in these pathways remains to be determined.
The only identified catalytic centre in any of the four Pso4 complex members is a U-box domain located in the amino terminus of Prp19. U-box domains have been shown to contain E3 ubiquitin ligase activity (Hatakeyama et al, 2001; Zhang et al, 2005a); such activity has also been shown for Prp19 in vitro (Ohi et al, 2003; Loscher et al, 2005; Vander Kooi et al, 2006) and this function is required for pre-mRNA splicing in vivo (Ohi et al, 2003; Loscher et al, 2005). Two members of the Pso4 complex—Prp19 and Plrg1—contain WD-40 domains, which have been shown to represent scaffolds for protein–protein interactions (Gettemans et al, 2003), and often function as substrate adaptors and as components of E3 ubiquitin ligases.
Ataxia-telangiectasia and Rad3-related protein (ATR) is a crucial signalling kinase that mediates pleiotropic responses to DNA damage, including activation of cell-cycle checkpoints, DNA repair pathways, transcription and apoptosis (reviewed in Zhou & Elledge, 2000; Abraham, 2001; Durocher & Jackson, 2001; Shiloh, 2003). ATR is a member of a family of large phosphatidylinositol-3-OH kinase-like kinases that also include the DNA-dependent protein kinase catalytic subunit (DNA-PKcs) and ataxia-telangiectasia mutated protein (ATM). ATR is activated particularly by agents that induce replication-fork stalling such as ultraviolet irradiation, hydroxyurea and interstrand cross-linking drugs. In response to stalled replication forks encountered during S phase of the cell cycle, ATR is recruited to damaged sites by its cofactor, ATR-interacting protein (ATRIP), which binds to replication protein A (RPA)-coated single-stranded DNA (Cortez et al, 2001; Zou & Elledge, 2003). Once recruited to the replication fork, it acts in combination with other effectors and mediators such as the 9–1–1 complex, claspin and TopBP1, to activate downstream branched S-phase checkpoint pathways involving Chk1–Cdc25A/C and Nbs1–Smc1 (Abraham, 2001; Bartek et al, 2004; Lambert & Carr, 2005; Harper & Elledge, 2007).
Results
Cdc5L and ATR interact
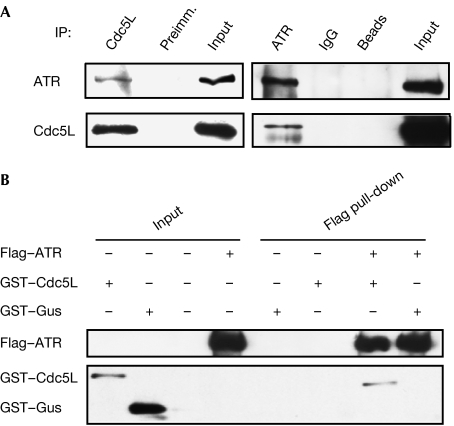
In our previous study on the involvement of the Pso4 complex in ICL repair, we showed that Cdc5L interacted with the WRN (for Werner syndrome) protein (Zhang et al, 2005a). This finding prompted us to examine whether other DNA repair or cell-cycle checkpoint factors interact with Cdc5L. As shown in Fig 1A, reciprocal co-immunoprecipitations of Cdc5L and ATR showed that these two proteins associate physically in HeLa cell extracts. Pre-treatment of cells with ultraviolet irradiation did not affect the interaction (data not shown). Furthermore, pull-down assays performed with purified recombinant proteins confirmed this interaction and indicated that it is probably direct (Fig 1B; supplementary Fig S1 online).
Figure 1.
Cdc5L interacts with ATR. (A) Immunoblot showing that Cdc5L and ATR co-immunoprecipitate from HeLa nuclear extracts. (B) Cdc5L and ATR interact as determined in a pull-down assay with purified recombinant proteins. ATR, ataxia-telangiectasia and Rad3-related protein; Cdc5L, cell division cycle 5-like protein; GST, glutathione-S-transferase; Gus, β-glucuronidase; IP, immunoprecipitation; Preimm., pre-immune serum.
Cdc5L is required for the S-phase cell-cycle checkpoint
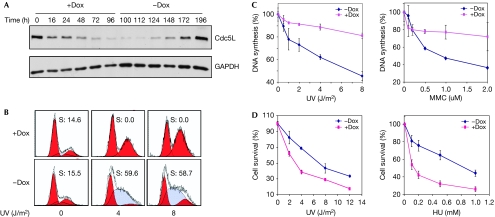
To study the effects of Cdc5L on cell-cycle regulation, we prepared a clone of HCT-116 cells that contained an inducible transgene expressing a short hairpin RNA (shRNA) targeted to Cdc5L. Induction of the transgene by doxycycline resulted in strong depletion of Cdc5L between 48 and 72 h after addition of the drug (Fig 2A). To determine whether Cdc5L has a role in the S-phase checkpoint mediated by ATR, we depleted Cdc5L by induction of the shRNA transgene and exposed the cells to specific doses of ultraviolet irradiation (Fig 2B). This experiment showed that the increase in S-phase cells typical of an ultraviolet radiation-induced checkpoint was greatly reduced in cells depleted of Cdc5L. Depletion of Cdc5L did not affect the cell-cycle distribution of untreated cells (supplementary Fig S2 online), indicating that the differential effects on the cell cycle were in response to ultraviolet irradiation. To verify the role of Cdc5L in the S-phase checkpoint, we monitored DNA synthesis after exposure to DNA damage (Painter & Young, 1980) and found that the depleted cells were severely deficient in the normal reduction observed in DNA synthesis in response to ultraviolet radiation or mitomycin C (MMC; Fig 2C). In addition, the Cdc5L-depleted cells were hypersensitive to both ultraviolet radiation and hydroxyurea (Fig 2D). Together, these results show that Cdc5L is required for the S-phase checkpoint in response to replication-fork blocking lesions, which is consistent with the physical interaction with ATR described earlier.
Figure 2.
Depletion of Cdc5L results in a defective S-phase checkpoint and hypersensitivity in response to DNA damage. (A) Dox was added to HCT-116 cells expressing the Cdc5L shRNA for the indicated time periods. At the 100-h time point Dox was removed and cells were kept in fresh medium for the indicated time periods. (B) Cell-cycle profiles of HCT-116 cells with or without the depletion of Cdc5L. Cells were incubated for 48 h with or without Dox, treated with the indicated dose of ultraviolet (UV) radiation and, 16 h later, analysed by FACS for cell-cycle distribution. Numbers inside the panels indicate the percentage of S-phase cells as determined by the ModFit modelling programme. (C) Quantification of DNA-synthesis assays of HCT-116 cells with or without the depletion of Cdc5L and after being exposed to the indicated genotoxic agents. (D) HCT-116 cells were treated with Dox for 48 h and then exposed to the indicated genotoxic agent; cell survival was determined by a clonogenic assay. Cdc5L, cell division cycle 5-like protein; Dox, doxycycline; FACS, fluorescence-activated cell sorting; GAPDH, glyceraldehyde 3-phosphate dehydrogenase; HU, hydroxyurea; MMC, mitomycin C; shRNA, short hairpin RNA.
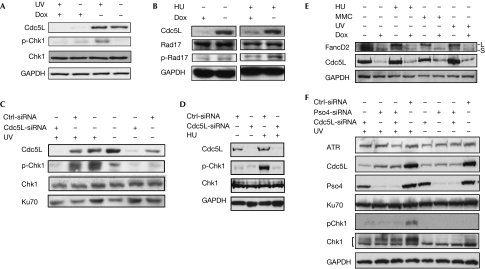
The ATR-dependent checkpoint pathway is mediated by several proteins that act downstream of this kinase (Bartek et al, 2004; Lambert & Carr, 2005; Harper & Elledge, 2007). We therefore examined several of these effector proteins to determine whether Cdc5L has a role in their activation, as suggested by the cell-cycle checkpoint studies described earlier. As shown in Fig 3A,B, depletion of Cdc5L resulted in decreased phosphorylation of checkpoint kinase 1 (Chk1) and cell cycle checkpoint protein Rad 17 (Rad17) in response to replication-fork blocking agents. As a control, and for verification of these results, we used transient knockdown of Cdc5L by small interfering RNA (siRNA) targeted to a sequence distinct from the inducible shRNA used in the previous experiments. Compared with a nonspecific control siRNA, the phosphorylation of Chk1 was strongly depressed by transfection of the Cdc5L siRNA (Fig 3C,D). ATR has also been shown to be required for the monoubiquitination of Fanconi anaemia complementation group D2 protein (FancD2) in response to replication-fork blocking agents (Andreassen et al, 2004). Interestingly, depletion of Cdc5L caused a marked decrease in FancD2 expression levels (Fig 3E). Nevertheless, it can also be noted that the ratio of monoubiquitinated FancD2 to the unmodified protein is also reduced by Cdc5L depletion. Together, these results indicate that Cdc5L is required for the DNA damage-induced modification of effector proteins downstream of ATR, and further support a role for Cdc5L in ATR-mediated checkpoint activation.
Figure 3.
Cdc5L is required for the activation of ATR-mediated checkpoint pathways. (A,B) Immunoblots showing that Cdc5L is required for the phosphorylation of Chk1 and Rad17 in HCT-116 cells. The ultraviolet (UV) radiation dose and HU concentration were 20 J/m2 and 2 μM, respectively. Cells were fixed for analysis 1 h after ultraviolet radiation treatment and 3 h after the introduction of HU. (C–E) Knockdown of Cdc5L by siRNA in HeLa cells results in defective phosphorylation of Chk1, and monoubiquitylation of FancD2. In (E) ‘L' and ‘S' refer to the ubiquitinated and unmodified forms of FancD2, respectively. (F) Depletion of Prp19/Pso4 destabilizes Cdc5L and causes impairment of ATR activation. Experiments were carried out as described in (C); Ku70 is shown as a loading control. ATR, ataxia-telangiectasia and Rad3-related protein; Cdc5L, cell division cycle 5-like protein; Chk1, checkpoint kinase 1; Ctrl, control; Dox, doxycycline; FancD2, Fanconi anaemia complementation group D2 protein; GAPDH, glyceraldehyde 3-phosphate dehydrogenase; HU, hydroxyurea; MMC, mitomycin C; Prp19, precursor messenger RNA-processing factor 19; Rad17, cell cycle checkpoint protein Rad 17; siRNA, small interfering RNA.
Finally, as described in the Introduction, Cdc5L interacts with the ubiquitin E3 ligase Prp19/Pso4. Therefore, we examined whether Prp19/Pso4 was also required for the activation of ATR as indicated by Chk1 phosphorylation (Fig 3F). Depletion of Prp19/Pso4 did impair the activation of ATR; however, depletion of Prp19/Pso4 also resulted in the destabilization of Cdc5L. Interestingly, depletion of Cdc5L did not seem to affect the stabilization of Prp19/Pso4. Thus, it is unclear from these experiments whether Prp19/Pso4 is required directly for ATR activation or whether the observed result is due to the destabilization of Cdc5L.
Cdc5L and ATR interact
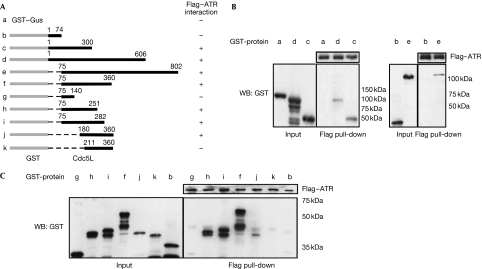
As described earlier, Cdc5L and ATR interact physically. To map the site of this interaction, we prepared various truncation constructs of Cdc5L and examined them for interaction with purified Flag–ATR in pull-down assays. As shown in Fig 4, this deletion analysis indicated that a strong binding site for ATR is present between residues 75–251 (construct h) of human CDC5L. A weaker interaction was observed with construct j, suggesting that residues 180–251 contain a minimal binding site for ATR. The additional removal of residues 180–210 (construct k) abolished the interaction, indicating that this region is important for ATR binding. Interestingly, this region of Cdc5L is completely conserved between the Xenopus, chicken, mouse and human homologues, and is also highly conserved with the budding yeast homologue Cef1 (supplementary Fig S3 online).
Figure 4.
Mapping of the ATR-binding site in Cdc5L. (A) Schematic showing results of ATR binding to various deletion constructs of Cdc5L. (B,C) Western blots (WB) showing pull-down assays between purified Flag–ATR and deletion constructs of GST–Cdc5L. The uppermost band in each lane represents the full-length recombinant protein, whereas the lower bands represent degradation products. ATR, ataxia-telangiectasia and Rad3-related protein; Cdc5L, cell division cycle 5-like protein; GST, glutathione-S-transferase; Gus, β-glucuronidase.
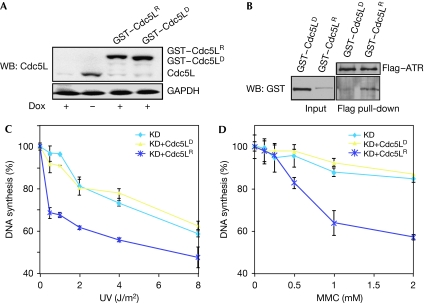
To confirm that this region mediated an interaction with ATR, we prepared a construct of Cdc5L that was lacking residues 180–210 (GST–Cdc5LD), and expressed this construct and a wild-type control (GST–Cdc5LR) in the HCT-116 cell clone expressing the inducible shRNA (Fig 5A). Both of these constructs were modified to render them refractory to the shRNA, as shown by their expression in the presence of doxycycline. Pull-down assays showed that the GST–Cdc5LD mutant did not interact with ATR (Fig 5B), thus confirming that residues 180–210 are required for ATR binding. Next, we examined DNA synthesis after DNA damage in the HCT-116 clones stably expressing these constructs. As shown in Fig 5C,D, the Cdc5LR construct, but not the Cdc5LD construct, was able to rescue the defect in the S-phase checkpoint after exposure to either ultraviolet radiation or MMC. Finally, we also tested whether this ATR-interacting region affected the interaction between Cdc5L and Prp19/Pso4 and found that it did not (supplementary Fig S4 online), thus indicating that the impaired ability of the Cdc5LD mutant to activate ATR was not due to disruption of the association between these proteins. Together, these results indicate that interaction between Cdc5L and ATR is required for activation of the S-phase checkpoint in response to replication-fork blocking lesions.
Figure 5.
Deletion of the ATR-interacting region in Cdc5L results in a failure to rescue the S-phase checkpoint. (A) Expression of Cdc5L alleles in HCT-116 cells containing an inducible Cdc5L shRNA. GST–Cdc5LR indicates an allele of Cdc5L that is refractory to the shRNA. GST–Cdc5LD contains a deletion of the ATR-interacting domain located in amino-acid residues 181–210; this allele is also refractory to the shRNA. (B) GST–Cdc5LD does not interact with ATR as indicated in the pull-down assay. (C,D) The GST–Cdc5LD allele is unable to rescue the S-phase checkpoint defect in HCT-116 cells expressing the Cdc5L shRNA, as indicated by quantification of DNA synthesis. ‘KD' indicates knockdown of endogenous Cdc5L by an inducible shRNA. ATR, ataxia-telangiectasia and Rad3-related protein; Cdc5L, cell division cycle 5-like protein; Dox, doxycycline; GAPDH, glyceraldehyde 3-phosphate dehydrogenase; GST, glutathione-S-transferase; KD, knockdown; MMC, mitomycin C; shRNA, short hairpin RNA; UV, ultraviolet radiation; WB, western blot.
Discussion
As indicated in the Introduction, a growing amount of evidence suggests that Cdc5L and Prp19/Pso4 are involved in DDR in mammalian cells. The results presented here extend these findings and show that Cdc5L interacts with ATR, and that this interaction is required for activation of this kinase with respect to downstream mediators and for implementation of the S-phase cell-cycle checkpoint. These findings are, to our knowledge, the first to suggest that Cdc5L is involved in regulation of the cell cycle in response to genotoxic stress.
The exact biochemical functions of Cdc5L and Prp19/Pso4, and by implication the Pso4 complex, in the DDR remain unclear. One possibility is that as Prp19/Pso4 is a DNA-binding protein, it might have a role in recruiting other repair and/or checkpoint proteins to sites of DNA damage, as shown by its interaction with the repair protein Metnase (Beck et al, 2008). Furthermore, Cdc5L contains two Myb-like domains at its N terminus and might therefore also have DNA-binding activity (Neubauer et al, 1998; Ajuh et al, 2000). Thus, Cdc5L could have a role in recruiting proteins such as ATR and WRN to sites of DNA damage. However, a more probable, but not mutually exclusive, hypothesis is that the Pso4 complex functions as an E3 ubiquitin ligase in the DDR. The role of ubiquitination in the DDR has been shown by the recent demonstration of requirements for the E3 ligases Cul4, ring-finger protein 8 and CHIP (for carboxyl terminus of Hsc70-interacting protein) in both DNA repair and checkpoint pathways (Groisman et al, 2003; Sugasawa et al, 2005; Huen et al, 2007; Kolas et al, 2007; Mailand et al, 2007; Wang & Elledge, 2007; Parsons et al, 2008). Both CHIP and Prp19 contain U-box domains that are required for the transfer of ubiquitin from an E2 ligase to the substrate (Hatakeyama et al, 2001; Zhang et al, 2005b). The U-box domain is a modified RING motif that lacks the full complement of zinc-ion-binding ligands (Aravind & Koonin, 2000) and has clearly been shown to function as an E3 ligase. Although numerous substrates have been identified for CHIP (Boudrez et al, 2000; Dickey et al, 2007; Xia et al, 2007; Parsons et al, 2008), few, if any, substrates have been identified for Prp19/Pso4, although it has been shown that this protein can undergo autoubiquitination in vitro (Ohi et al, 2005). Furthermore, we have shown that this modification occurs in vivo in response to DNA damage (Lu & Legerski, 2007). The role of Cdc5L in the Pso4 complex is not known, but an obvious possibility is that it might act as an adaptor protein to recruit substrates to the E3 ligase complex. The concept that the Pso4 complex acts as an E3 ubiquitin ligase can readily explain its pleitropic roles in both pre-mRNA splicing and the DDR.
Methods
Pull-down assay. For pull-down assays Flag-tagged ATR protein expressed in human embryonic kidney-293 cells was bound on Flag-beads overnight at 4°C. The bound Flag–ATR was washed with TBS (Tris buffer saline) buffer and incubated with TBS with 1 M NaCl on ice for 2 min to remove non-specifically bound proteins (see supplementary Fig S1 online). The salt was removed by washing with TBS three times. Otherwise, the assay was carried out as described previously (Zhang et al, 2005a).
Clonogenic assay. Cdc5L was depleted in HCT-116 cells by adding doxycycline to a final concentration of 2 μg/ml. At 48 h after induction of the shRNA, cells were treated with ultraviolet radiation and hydroxyurea at various doses. After treatment, the cells were trypsinized and plated with fresh media without antibiotics. After 12–15 days, colonies were fixed and stained with crystal violet (0.25% crystal violet, 0.2% paraformaldehyde, 72% methanol and 18% water) and counted.
Evaluation of DNA synthesis. HCT-116 cells were incubated with [14C]thymidine for 24 h. After replacing the medium, cells were exposed to various doses of ultraviolet radiation. The cells were returned to the incubator for 60 min and pulse-labelled in medium containing [3H]thymidine for an additional 120 min (Painter & Young, 1980). The samples were collected and counted by scintillation. The ratio of incorporated 3H to 14C was used for quantification to standardize the variation in DNA recovery. For MMC treatment, cells were incubated with the drug for 3 h and pulse-labeled for further 3 h.
Cdc5L depletion by RNA-mediated interference. Double-stranded DNA with the sequence 5′-GGAAGAGAGGAGTTGATTATATTCAAGAGATATAATCAACTCCTCTCTTCC-3′ with corresponding sticky ends was cloned into pEntry-H1-TO (Invitrogen, Carlsbad, CA, USA) and transfected into HCT-116 cells that stably express the tetracycline repressor protein. 48 h after transfection, zeocin was added to the medium, and separated colonies were picked and amplified. Cdc5L knockdown in HeLa cells was achieved by using the siRNA sequence 5′-GGAAGAGAGGAGUUGAUUA-3′ (Dharmacon, Lafayette, CO, USA). Further methods are detailed in the supplementary information online.
Supplementary information is available at EMBO reports online (http://www.emboreports.org).
Supplementary Material
Supplementary Information
Acknowledgments
This work was supported by National Cancer Institute grant CA097175. DNA sequencing resources were supported by the Cancer Center Support grant CA16672. We thank F. Culajay and J. Liu for providing technical support.
Footnotes
The authors declare that they have no conflict of interest.
References
- Abraham RT (2001) Cell cycle checkpoint signaling through the ATM and ATR kinases. Genes Dev 15: 2177–2196 [DOI] [PubMed] [Google Scholar]
- Ajuh P, Kuster B, Panov K, Zomerdijk JC, Mann M, Lamond AI (2000) Functional analysis of the human CDC5L complex and identification of its components by mass spectrometry. EMBO J 19: 6569–6581 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andreassen PR, D'Andrea AD, Taniguchi T (2004) ATR couples FANCD2 monoubiquitination to the DNA-damage response. Genes Dev 18: 1958–1963 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aravind L, Koonin EV (2000) The U box is a modified RING finger—a common domain in ubiquitination. Curr Biol 10: R132–R134 [DOI] [PubMed] [Google Scholar]
- Bartek J, Lukas C, Lukas J (2004) Checking on DNA damage in S phase. Nat Rev Mol Cell Biol 5: 792–804 [DOI] [PubMed] [Google Scholar]
- Beck BD, Park SJ, Lee YJ, Roman Y, Hromas RA, Lee SH (2008) Human Pso4 is a metnase (SETMAR)-binding partner that regulates metnase function in DNA repair. J Biol Chem 283: 9023–9030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boudrez A, Beullens M, Groenen P, Van Eynde A, Vulsteke V, Jagiello I, Murray M, Krainer AR, Stalmans W, Bollen M (2000) NIPP1-mediated interaction of protein phosphatase-1 with CDC5L, a regulator of pre-mRNA splicing and mitotic entry. J Biol Chem 275: 25411–25417 [DOI] [PubMed] [Google Scholar]
- Cheng SC, Tarn WY, Tsao TY, Abelson J (1993) PRP19: a novel spliceosomal component. Mol Cell Biol 13: 1876–1882 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cortez D, Guntuku S, Qin J, Elledge SJ (2001) ATR and ATRIP: partners in checkpoint signaling. Science 294: 1713–1716 [DOI] [PubMed] [Google Scholar]
- Dickey CA et al. (2007) The high-affinity HSP90–CHIP complex recognizes and selectively degrades phosphorylated tau client proteins. J Clin Invest 117: 648–658 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durocher D, Jackson SP (2001) DNA-PK, ATM and ATR as sensors of DNA damage: variations on a theme? Curr Opin Cell Biol 13: 225–231 [DOI] [PubMed] [Google Scholar]
- Gettemans J, Meerschaert K, Vandekerckhove J, De Corte V (2003) A kelch beta propeller featuring as a G beta structural mimic: reinventing the wheel? Sci STKE 2003: PE27. [DOI] [PubMed] [Google Scholar]
- Grey M, Dusterhoft A, Henriques JA, Brendel M (1996) Allelism of PSO4 and PRP19 links pre-mRNA processing with recombination and error-prone DNA repair in Saccharomyces cerevisiae. Nucleic Acids Res 24: 4009–4014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groisman R, Polanowska J, Kuraoka I, Sawada J, Saijo M, Drapkin R, Kisselev AF, Tanaka K, Nakatani Y (2003) The ubiquitin ligase activity in the DDB2 and CSA complexes is differentially regulated by the COP9 signalosome in response to DNA damage. Cell 113: 357–367 [DOI] [PubMed] [Google Scholar]
- Harper JW, Elledge SJ (2007) The DNA damage response: ten years after. Mol Cell 28: 739–745 [DOI] [PubMed] [Google Scholar]
- Hatakeyama S, Yada M, Matsumoto M, Ishida N, Nakayama KI (2001) U box proteins as a new family of ubiquitin-protein ligases. J Biol Chem 276: 33111–33120 [DOI] [PubMed] [Google Scholar]
- Henriques JA, Vicente EJ, Leandro da Silva KV, Schenberg AC (1989) PSO4: a novel gene involved in error-prone repair in Saccharomyces cerevisiae. Mutat Res 218: 111–124 [DOI] [PubMed] [Google Scholar]
- Huen MS, Grant R, Manke I, Minn K, Yu X, Yaffe MB, Chen J (2007) RNF8 transduces the DNA-damage signal via histone ubiquitylation and checkpoint protein assembly. Cell 131: 901–914 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolas NK et al. (2007) Orchestration of the DNA-damage response by the RNF8 ubiquitin ligase. Science 318: 1637–1640 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambert S, Carr AM (2005) Checkpoint responses to replication fork barriers. Biochimie 87: 591–602 [DOI] [PubMed] [Google Scholar]
- Loscher M et al. (2005) The U-box E3 ligase SNEV interacts with PSMB4, the beta7 subunit of the 20S proteasome. Biochem J 388: 593–603 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu X, Legerski RJ (2007) The Prp19/Pso4 core complex undergoes ubiquitylation and structural alterations in response to DNA damage. Biochem Biophys Res Commun 354: 968–974 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahajan KN, Mitchell BS (2003) Role of human Pso4 in mammalian DNA repair and association with terminal deoxynucleotidyl transferase. Proc Natl Acad Sci USA 100: 10746–10751 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mailand N, Bekker-Jensen S, Faustrup H, Melander F, Bartek J, Lukas C, Lukas J (2007) RNF8 ubiquitylates histones at DNA double-strand breaks and promotes assembly of repair proteins. Cell 131: 887–900 [DOI] [PubMed] [Google Scholar]
- McDonald WH, Ohi R, Smelkova N, Frendewey D, Gould KL (1999) Myb-related fission yeast cdc5p is a component of a 40S snRNP-containing complex and is essential for pre-mRNA splicing. Mol Cell Biol 19: 5352–5362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neubauer G, King A, Rappsilber J, Calvio C, Watson M, Ajuh P, Sleeman J, Lamond A, Mann M (1998) Mass spectrometry and EST-database searching allows characterization of the multi-protein spliceosome complex. Nat Genet 20: 46–50 [DOI] [PubMed] [Google Scholar]
- Ohi MD, Gould KL (2002) Characterization of interactions among the Cef1p–Prp19p-associated splicing complex. RNA 8: 798–815 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohi MD, Vander Kooi CW, Rosenberg JA, Chazin WJ, Gould KL (2003) Structural insights into the U-box, a domain associated with multi-ubiquitination. Nat Struct Biol 10: 250–255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohi MD, Vander Kooi CW, Rosenberg JA, Ren L, Hirsch JP, Chazin WJ, Walz T, Gould KL (2005) Structural and functional analysis of essential pre-mRNA splicing factor Prp19p. Mol Cell Biol 25: 451–460 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Painter RB, Young BR (1980) Radiosensitivity in ataxia-telangiectasia: a new explanation. Proc Natl Acad Sci USA 77: 7315–7317 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parsons JL, Tait PS, Finch D, Dianova II, Allinson SL, Dianov GL (2008) CHIP-mediated degradation and DNA damage-dependent stabilization regulate base excision repair proteins. Mol Cell 29: 477–487 [DOI] [PubMed] [Google Scholar]
- Shiloh Y (2003) ATM and related protein kinases: safeguarding genome integrity. Nat Rev Cancer 3: 155–168 [DOI] [PubMed] [Google Scholar]
- Sugasawa K et al. (2005) UV-induced ubiquitylation of XPC protein mediated by UV-DDB-ubiquitin ligase complex. Cell 121: 387–400 [DOI] [PubMed] [Google Scholar]
- Tarn WY, Lee KR, Cheng SC (1993) The yeast PRP19 protein is not tightly associated with small nuclear RNAs, but appears to associate with the spliceosome after binding of U2 to the pre-mRNA and prior to formation of the functional spliceosome. Mol Cell Biol 13: 1883–1891 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vander Kooi CW, Ohi MD, Rosenberg JA, Oldham ML, Newcomer ME, Gould KL, Chazin WJ (2006) The Prp19 U-box crystal structure suggests a common dimeric architecture for a class of oligomeric E3 ubiquitin ligases. Biochemistry 45: 121–130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang B, Elledge SJ (2007) Ubc13/Rnf8 ubiquitin ligases control foci formation of the Rap80/Abraxas/Brca1/Brcc36 complex in response to DNA damage. Proc Natl Acad Sci USA 104: 20759–20763 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia T et al. (2007) Chaperone-dependent E3 ligase CHIP ubiquitinates and mediates proteasomal degradation of soluble guanylyl cyclase. Am J Physiol Heart Circ Physiol 293: H3080–H3087 [DOI] [PubMed] [Google Scholar]
- Zhang N, Kaur R, Lu X, Shen X, Li L, Legerski RJ (2005a) The Pso4 mRNA splicing and DNA repair complex interacts with WRN for processing of DNA interstrand cross-links. J Biol Chem 280: 40559–40567 [DOI] [PubMed] [Google Scholar]
- Zhang M, Windheim M, Roe SM, Peggie M, Cohen P, Prodromou C, Pearl LH (2005b) Chaperoned ubiquitylation—crystal structures of the CHIP U box E3 ubiquitin ligase and a CHIP–Ubc13–Uev1a complex. Mol Cell 20: 525–538 [DOI] [PubMed] [Google Scholar]
- Zhou BB, Elledge SJ (2000) The DNA damage response: putting checkpoints in perspective. Nature 408: 433–439 [DOI] [PubMed] [Google Scholar]
- Zou L, Elledge SJ (2003) Sensing DNA damage through ATRIP recognition of RPA-ssDNA complexes. Science 300: 1542–1548 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Information