Abstract
Most tumours contain a heterogeneous population of cancer cells, which harbour a range of genetic mutations and have probably undergone deregulated differentiation programmes that allow them to adapt to tumour microenvironments. Another explanation for tumour heterogeneity might be that the cells within a tumour are derived from tumour-initiating cells through diverse differentiation programmes. Tumour-initiating cells are thought to constitute one or more distinct subpopulations within a tumour and to drive tumour initiation, development and metastasis, as well as to be responsible for their recurrence after therapy. Recent studies have raised crucial questions about the nature, frequency and importance of melanoma-initiating cells. Here, we discuss our current understanding of melanoma-initiating cells and outline several approaches that the scientific community might consider to resolve the controversies surrounding these cells.
Keywords: melanoma, stem cell, tumour-initiating cell, cancer stem cell
Glossary
Glossary.
ABCB5 ATP-binding cassette subfamily B member 5
BRAF V-raf murine sarcoma viral oncogene homologue B1
CD cluster of differentiation
c-kit cellular homologue of the feline sarcoma viral oncogene v-kit
c-Met mesenchymal–epithelial transition factor
ENL eleven nineteen leukaemia
Eμ-MYC transgenic mice in which the immunoglobulin heavy chain enhancer (Eμ) drives the expression of c-myc in cells of the B lineage
GNAQ guanine nucleotide binding protein (G protein), q polypeptide
GRM1 glutamate receptor, metabotropic 1
HGF/SF hepatocyte growth factor/scatter factor
HRAS human homologue of the Harvey rat sarcoma RAS viral oncogene homologue
HSC haematopoietic stem cell
IL-2Rγ interleukin-2/common receptor gamma chain
INK4 inhibitors of CDK4
MEK mitogen-activated protein kinase kinase
MLL-AF9 a fusion protein that results from the translocation t(9;11) (p22;q23) observed in acute myelogenous leukaemia; AF9 shares 56% homology with ENL
MLL-Enl a fusion protein that results from the translocation t(11;19), observed in recurrent mixed lymphocyte leukaemias; the fusion protein involves the mammalian homologue of Drosophila trithorax (MLL) and the transcription factor ENL
mTORC1 mammalian target of rapamycin complex 1
NOD non-obese diabetic
NRAS neuroblastoma homologue of the RAS viral oncogene
Prom1 prominin 1
PTEN phosphatase and tensin homologue
SCID severe combined immunodeficiency
Wnt Wingless and Int
Introduction
Here, we review recent developments in the field of melanoma-initiating cells (MICs). Our aim is not to review the entire MIC or melanoma stem-cell literature, as both have recently been extensively reviewed (La Porta, 2007; Schatton & Frank, 2008; Shackleton et al, 2009). Instead, our aim is to highlight the questions that have arisen from those studies, to stimulate the discussion of ways in which to resolve the existing controversies.
This article focuses on MICs, rather than on melanoma stem cells. It is not yet clear whether melanoma follows a cancer stem-cell model; several key questions remain to be answered in this respect. Do melanomas contain a subpopulation of cells with the propensity to differentiate into different cell lineages? Do any of those lineages show infinite proliferation capacities? Are melanoma tumours organized hierarchically into epigenetically distinct subpopulations that allow tumorigenic cells to be distinguished from non-tumorigenic cells? For the purposes of this analysis, we define MICs as a distinct, well-characterized subpopulation of melanoma cells that reproducibly retain specific properties when re-purified and can reconstitute a tumour from a single cell in an animal model. MICs might not have the propensity to differentiate into different cell lineages, which would distinguish them from stem cells. Recent studies raise serious questions about the nature of these cells (Sidebar A), prompting us to analyse them in detail and to propose how they might be answered. Once they are addressed, we will be in a better position to ask a bigger question: Do genuine melanoma stem cells exist and can some MICs be considered melanoma stem cells?
Sidebar A | In need of answers.
What proportion of cells in a melanoma tumour—when removed from the host and dispersed into single cells—can regenerate the tumour when serially transplanted into an animal model?
What are the characteristics of the cells that constitute this fraction?
Which genes are uniquely expressed or modified in this population—for example, epigenetically or by post-translational modifications of the protein product—to confer specific activation/inactivation patterns?
Do MICs express surface markers that would allow their identification within the tumour mass?
Does a genetic model support the changes seen in MICs?
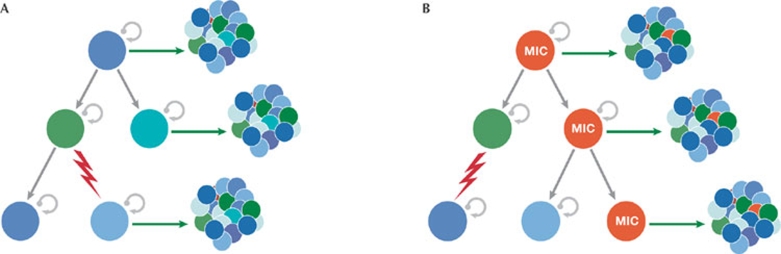
According to most of the published literature, MICs are a distinct subset of the total number of tumour cells that can maintain tumour growth indefinitely (Fig 1). However, although some studies have shown the existence of MICs, as detailed below, the identification of genes that are necessary for MIC generation is lacking and the proportion of a melanoma mass that is made up of MICs is unclear. Published reports are markedly discordant on this issue, with some reporting the frequency of MICs to be as low as 0.01% and others as high as 25%. In addition, it is equally important to define markers that are characteristic of MICs with a high propensity for infinite proliferation and tumour formation.
Figure 1.
Models of cancer growth. (A) The stochastic model predicts that all cancer cells (coloured circles) derived from a heterogeneous human tumour population have the capacity to proliferate, regenerate and maintain tumour growth in vivo. (B) The cancer stem-cell model suggests that only a subpopulation of cells within the tumour, the tumour-initiating cells—MICs (red circles) in the case of melanoma—has the capacity to regenerate and maintain a tumour in vivo (green arrows). The bulk of the heterogeneous tumour cell population does not share these properties and lacks the ability to be tumorigenic. The red zig-zag arrow indicates the genetic and/or epigenetic changes that drive the oncogenic programme. Circular arrows represent the proliferative cells. MIC, melanoma-initiating cell.
Notably, if a subset of melanoma tumour cells is endowed with the capacity to regenerate tumours, it is crucially important that they be characterized, as these cells would constitute targets for new therapies. Accordingly, the molecular characterization of MICs offers great promise for understanding the fundamentals of tumour development, progression and drug resistance. The characterization of markers that distinguish MICs from the rest of the melanoma mass and of genes that allow MIC self-renewal will undoubtedly lead to new diagnostics and treatments. Indeed, the major obstacle that we face today in analysing MICs is a lack of reliable biomarkers to identify them in the melanoma tumour mass and of assays to reproducibly measure the tumorigenic potential of MICs.
A brief history of MICs
Tumour-initiating cells were first reported for acute myeloid leukaemia (Bonnet & Dick, 1997; Kleinsmith & Pierce, 1964). Since then, they have been reported to promote solid tumour development, including brain (Singh et al, 2004), colon (O'Brien et al, 2007) and breast (Al-Hajj et al, 2003) and, most recently, the onset of melanoma. Highly aggressive melanoma cells have molecular signatures that are reminiscent of those of pluripotent stem cells (Bittner et al, 2000; Hendrix et al, 2007). Furthermore, stem-cell- and progenitor-cell-associated proteins have been reported to be expressed in melanoma cells, including testicle cancer antigens (Simpson et al, 2005), bone morphogenetic proteins (Rothhammer et al, 2007), Notch receptors (Balint et al, 2005), Wnt proteins (Weeraratna et al, 2002), and the ABCB5, CD133, CD166, CD34, nestin, and c-kit stem-cell antigens (Fang et al, 2005; Frank et al, 2005; Hendrix et al, 2003; Klein et al, 2007; van Kempen et al, 2000). In vitro studies and xenograft assays have shown the ability of melanoma cell populations that express these markers in various combinations to grow in three-dimensional cultures and in immunocompromised mice, respectively. However, in all cases, the limited purity of these cultures has prevented the isolation of a MIC population, which is required for rigorous molecular analysis. The field is in great need of genetically based evidence to identify the genes that have important roles in the generation of MICs. Some studies report the isolation of melanoma-initiating cells to 90% purity; however, one cannot obviate the contribution of the remaining 10% of cells—which could be as many as 100,000—to the establishment of tumours after injection into these mouse models.
The transplantation of primary human tumour cells into immunocompromised mice that lack lymphoid cells allows the analysis of tumour growth in vivo. This approach has been used to characterize leukaemia stem cells (Krivtsov et al, 2006; Williams et al, 2007) and to analyse the number of cells that are necessary to form a tumour; a small subset of cells—normally between 0.0001%–0.1% of the tumour mass—is required to generate a new tumour in most immunodeficient mice. Similar frequencies have been reported for melanoma by Schatton et al (2008), who identified MICs based on the expression of ABCB5—a member of the ABC cassette family of transporter proteins. By serially transplanting human melanoma cancer cells into NOD/SCID immunodeficient mice, this group reported that—within 8 weeks of transplantation—only 1 in 1 million (0.000001%) human melanoma cells showed tumour-initiating properties. The authors concluded that human cancer-initiating cells that are able to produce tumours in immunodeficient mice represent a biologically distinct population with stem-cell-like properties and that are characterized by the expression of the ABCB5 antigen (Schatton et al, 2008). Schatton and colleagues also pointed out that the expression of ABCB5 was responsible for the relative chemoresistance of melanoma-initiating cells, a finding that is potentially important for the treatment of melanoma. However, in a more recent study, the Morrison group found that by altering the transplant assay conditions, namely by injecting cells in the presence of matrigel into a more severely immunocompromised strain of mice—NOD/SCID IL-2Rγ−/− mice, which lack lymphoid and natural killer cells—the number of cells needed to form a tumour was markedly lower. Their findings suggest that MICs could be as frequent as 1 in 4 cells, as opposed to the 1 in 837,000 found in NOD/SCID mice (Quintana et al, 2008). The authors also reported that the relative number of cells that are able to form tumours changes with the length of time that a mouse is monitored; if a low number of cells are injected tumours can be seen, although after longer periods of time. Furthermore, when Quintana and colleagues used NOD/SCID IL-2Rγ−/− mice to assess the role of markers that had been reported previously to be exclusive of MICs in tumour formation, they did not observe a substantial difference in tumorigenic potential between marker-positive and marker-negative cells. Indeed, they were unable to identify any markers that were characteristic of a subpopulation of melanoma cells with unique melanoma-initiating ability.
The Quintana study raises several questions. Why is there such a marked difference in the incidence of MICs compared with previous melanoma studies? What are the markers that distinguish MICs? Which of the above studies is more representative of human melanomas? Many of these questions can be addressed by considering the nature of the mouse model used in the different studies. It seems that the more immnuocompromised the model, the higher the frequency of MICs and the less likely that MICs are characterized by the expression of a specific marker. In a sense, a highly immunocompromised mouse could be compared to an in vivo colony forming assay; in both cases, there are few limitations on the growth potential of the cells and, thus, a larger number of cells will be able to proliferate. Accordingly, in neither case do cells require mechanisms that allow them to evade a functional immune system. Some members of the stem-cell and melanoma communities would probably debate this conclusion; however, it raises a fundamental question: Can MICs be analysed properly in immunocompromised mice and are these mice the best model to advance the development of new therapies against human melanoma? Although additional studies are required, we suggest that such systems might not be adequate to thoroughly understand MICs or melanoma biology. The inherent heterogeneity of melanomas and the low incidence of MICs reported in some studies, in addition to the reported set of variable MIC cell-surface markers, suggest that the MIC compartment probably consists of various subpopulations of cells that have different properties.
Before offering possible solutions, one needs to understand clearly the technical obstacles that the community has faced—and will continue to face—in studying human tumours in mouse models. Immunocompromised mouse strains have been commonly used to study human tumours and, more recently, MICs by xenotransplantation. The idea is that the abrogation of the endogenous murine adaptive immune response would provide an environment conducive to the engraftment of tumour-initiating cells. The studies discussed above clearly illustrate that the lack of immune system probably provides an environment that might not be of physiological relevance. However, addressing this serious problem is technically challenging. The use of syngeneic mouse models is one option that will need to be considered to circumvent this crucial obstacle.
Syngeneic mouse melanoma models
The syngeneic mouse models of cancer bypass the concern of immune rejection by the resident adaptive immune system of a mouse in the context of human tumour cells. The studies of haematopoietic malignancies using syngeneic mouse models have put forward a wide range of tumour-initiating cell—often referred to as cancer stem cells—frequencies. For example, some leukaemia and lymphoma models—such as Eμ-MYC and MLL-Enl—suggest a frequency of 1 in 1 × 106 cells or higher (Kelly et al, 2007; Kennedy et al, 2007), whereas the expression of a relevant oncogene can increase this frequency to up to 1 in 4 cells, as shown for the MLL-AF9 model. In this latter model, the leukaemia stem cells arise from the myeloid lineage and have acquired mutations that drive an aberrant self-renewal programme and other biological features of haematopoietic stem cells (Somervaille & Cleary, 2006). Therefore, one could expect to identify higher frequencies of stem cells if they acquire initial modifications that allow them to drive their transforming programme. This issue has been addressed extensively in recent reviews that reassess the concept of cancer stem cells (Adams & Strasser, 2008; Williams & Sherr, 2008). By analogy, one might argue that the high incidence of MICs observed in recent xenotransplantation studies could suggest that they had already acquired modifications that conferred on them an aberrant self-renewal programme. Having said this, we expect that melanomas will show a similar variation in the frequency for tumour-initiating cells as leukaemias. The establishment of appropriate animal systems to study this question will be crucial in accurately determining the abundance and, more importantly, the characteristics of MICs.
Animal models of melanoma for syngeneic transfer studies are beginning to emerge from recent developments in genetically modified mice (Table 1). In two recent examples, mice were engineered to have genetic profiles that were homologous to genetic changes found in human melanomas. Dhomen et al (2009) showed that approximately 70% of mice carrying a V600E mutation in BRAF developed melanomas that reproduced the histological and molecular features of human melanomas. Similarly, Dankort et al (2009) showed that, after the induction of BRAF(V600E) expression, mice developed benign melanocytic hyperplasias that did not progress to melanoma in 15–20 months. By contrast, the expression of this mutant BRAF in combination with the silencing of the PTEN tumour suppressor, elicited the development of a metastatic melanoma with 100% penetrance, which, after a short latency period, metastasized to the lymph nodes and lungs. In this model, melanoma development was prevented by inhibitors of mTORC1 (rapamycin) or MEK1/2 (PD325901) but was re-established after the cessation of drug administration, suggesting the existence of long-lived melanoma-initiating cells (Dankort et al, 2009).
Table 1.
Available mouse models of melanoma
| Mouse model | Genetic modification | Incidence of melanoma | Metastasis | Reference |
|---|---|---|---|---|
| BRAFV600E/CreERT2 | BRAF(V600E) mutation | 60% | None | Dhomen et al, 2009 |
| Tyr::CreER; BRAFCA; Ptenlox/lox | Normal BRAF expression before Cre-mediated recombination, after which BRAF(V600E) is expressed at physiological levels | 100% | Lymph nodes, lung | Dankort et al, 2009 |
| Tyr::NRASQ61K INK4a−/− | Mutation of NRAS (NRASQ61K) and deletion of p16INK4a | 90% at 6 months | Lymph nodes, lung, liver | Ackermann et al, 2005 |
| INK4a−/− Arf −/−:Tyr-HRAS (G12V) | Activated human HRAS gene (G12V) that is expressed in melanocytes, and deletion of p16INK4a and p19ARF | 60% at 6 months | None | Chin et al, 1997 |
| Tg(Grm1)EPv (E) | Transgenic line with GRM1 expression driven by the dopachrome tautomerase promoter | 100% | Limited | Pollock et al, 2003 |
| Tg(HGF/SF) | Transgenic mice expressing HGF/SF in the skin | 50% at 12 months | Limited | Noonan et al, 2001 |
BRAF, V-raf murine sarcoma viral oncogene homologue B1; GRM1, glutamate receptor, metabotropic 1; HGF/SF, hepatocyte growth factor/scatter factor; HRAS, human homologue of the Harvey rat sarcoma v-Ras viral oncogene homologue; INK4, inhibitors of CDK4; NRAS, neuroblastoma homologue of the v-Ras viral oncogene.
Other models that are available for such studies include the NRAS Q61K/INK4a−/− (Ackermann et al, 2005) and HRAS (G12V)/INK4a−/− (Chin et al, 1997) mouse strains. Although both models develop spontaneous melanomas, only the NRASQ61K was reported to result in tumour metastasis (Ackermann et al, 2005). Another mouse melanoma model involves the overexpression of the HGF/SF gene—the ligand of the receptor tyrosine kinase c-Met—that, in combination with treating newborn mice with ultraviolet light, results in the development of melanomas (Noonan et al, 2001). An additional model was engineered by Chen and colleagues, who generated mice that developed multiple melanomas owing to an insertion in the third intron of grm1, which encoded the metabotropic glutamate receptor 1 (Pollock et al, 2003). Recent studies have revealed that the GRM1 pathway is activated in uveal melanomas and blue nevi by somatic mutations in the Gnaq gene (Van Raamsdonk et al, 2009), emphasizing that the Grm1 model recapitulates a subset of human melanomas.
Among the syngeneic mouse melanoma models are also the B16 and 1735p models, which were developed in the 1970s (Fidler, 1978; Fidler et al, 1981; Kripke, 1979; Talmadge et al, 1982). Although they are well characterized, a general concern when using them and their related cell systems is the extensive set of modifications that the cells suffered in the course of many passages in cell culture and in mice that they were subject to when establishing the model.
One would predict that these models would allow the isolation and characterization of putative initiating cell populations, which could be assessed further within the same genetic background with a fully competent immune system. However, it should be noted that although these models can provide an important source of tumour-initiating cells, they might not fully represent the timely occurrence of genetic changes in human melanomas. For example, although BRAF mutations have been reported to occur in normal nevi, the inactivation of PTEN is probably a later event. Nevertheless, these models offer an unprecedented source of cells that can be isolated, manipulated in culture and placed back into immunocompetent mice, thereby allowing the study of specific gene mutations that are thought to have important roles in MIC biology. In addition, these systems allow the assessment of drugs that would selectively affect the MICs and not the bulk of melanoma cells. The different genetic changes in each of these mouse melanoma models offer an opportunity to identify and characterize the common, as well as unique, features of MICs. Such knowledge would further advance our ability to identify and characterize their human counterparts.
Humanized mouse models
One of the main advantages of the use of humanized mouse models of cancer is the origin of the tumours that are studied. Humanized mice allow the assessment of the growth potential of human melanoma cells. Hence, unlike with syngeneic mouse models, there is no uncertainty with respect to the significance of the tumour in question for the human disease. However, it must be noted that several tumour-intrinsic issues—such as the tumour stage, the amount of genetic damage, the aggressiveness in vivo, the site of presentation and isolation (if one is studying a primary or a recurrent/relapsed tumour), and inter-patient variation—could also affect the MIC frequency that is detected.
Two additional and crucial advantages of humanized models of cancer relate to the presence of a human haematopoietic system. Human myeloid cells and inflammatory cytokines will support tumour development in a biologically appropriate manner (Melinkova & Bar-Eli, 2009). Human cytokines can function with murine cytokine receptors, whereas murine cytokines do not normally trigger signals in human cytokine receptors. Therefore, the introduction of human tumour cells into immunodeficient mice could lead to an anomalous cytokine response and perhaps select for specific requirements for tumour engraftment that need not be physiologically relevant. Indeed, the requirement to introduce melanoma cells in matrigel aggregates, as described recently (Quintana et al 2008), is suggestive of such deficiencies in the recipient mice that were used. In addition, some important immunological functions are completely missing in such animals. The presence of human haematopoietic components will allow a proper immunoselection of the MICs and derived tumour cells, such that the frequency of MICs obtained from these studies might be different from what is estimated using human tumours in mice and more in line with what is discussed in the context of syngeneic systems. The presence of human lymphoid cells could also significantly decrease the possibility of xenorejection by endogenous haematopoietic mouse components.
The ability to generate mice that harbour a human haematopoietic system and a human tumour will provide a more physiologically relevant—albeit restricted—system in which to define and characterize the biology of MICs. The generation of such a system would entail the establishment of a human haematopoietic system within an immunocompromised animal before the transplantation of human tumour cells obtained from the same patient. Importantly, the human immune system should be tolerant of the human cancer cells to avoid an allogeneic reaction and rejection of the tumour graft. Humanized chimaeric mice—which are generated by intra-hepatic transplantation of human fetal liver-derived HSCs or cord-blood-derived HSCs (Vormoor et al, 1994)—have been developed and could be used to address this issue. At least 1 × 106 cells are required for engraftment, and reconstitution is still a rare event (Berges et al, 2006). Another caveat is that the primary HSCs need to be implanted fresh, without previous testing, and therefore the results from one cohort of mice are restricted to that experiment, as no additional HSCs can be obtained from that source (Dick et al, 1997). In addition, the resultant human lymphoid development yields human T and B cells that remain in the thymus or bone marrow and in the spleen (Hogan et al, 1997), complicating the use of such mice to study the response of the human haematopoietic compartment to various challenges, including tumour development. Another point of concern is that the chances of obtaining tumour cells and HSCs from the same patient under the current protocols are slim, such that an allogeneically mismatched situation is the best that this technology can achieve so far. Although the introduction of allogeneically mismatched tumours and HSCs into a mouse is likely to be problematic and skew the results, this approach might have some advantages over using mice devoid of lymphoid cells and natural killer cells. Nonetheless, this is not the ideal situation, and technological advances are being developed that could help to overcome some of these obstacles and lead to the development of human ‘xenochimaeric' mice that have a haematopoietic system and tumour derived from the same patient. One such approach involves the generation of conditionally immortalized HSC cell lines with the aim of developing cell lines that can reconstitute mice and be preserved in laboratories. These can then be used to generate many cohorts of mice over time and allow the comparison of data obtained from different experiments, as the mice would have the same immune system. Furthermore, the ability to develop such HSC cell lines from melanoma patients will provide an improved system for the development of syngeneic haematopoietic-derived stromal elements in the tumour and could ultimately provide a more accurate view of MIC frequencies.
Additional approaches for studying MICs
A complementary approach for studying MICs is the transduction of melanocytes with specific genes. By implementing a forward genetic approach to identify melanocytes that acquire stem-cell properties, including those associated with tumour-initiating cells such as transformation, one might be able to identify the genes involved in these processes. In such a screen, melanocytes would be subject to genetic manipulations such as the transduction of tagged cDNA libraries. Those cells that passed the selection process would then be characterized and individual genes assessed for their relative contribution to the acquisition of stem-cell properties. It should be noted that such an approach relies on the premise that MICs emerge from melanocytes, a point that has yet to be proven. It is also possible that MICs could arise from other cellular lineages, including neuroectodermal stem cells, melanoblasts, less mature melanocyte progenitors, non-melanocytic cells or from cell fusion. Such possibilities need to be considered equally and could be addressed, in part, when we acquire better knowledge of the properties of MICs based on their characterization from human tumours.
Another possibility would be the isolation of melanoma stem cells from melanoma tumours or cell lines. Until now, the approaches to isolate potential MICs have relied on the fractionation of tumour cells to obtain rare cell populations that have tumour-initiating cell properties, as determined using three-dimensional cultures or mouse transplantation models. The identification of additional factors that are characteristic of MICs such as cell surface markers or factors that confer selective growth properties, would allow the better development of rigorous procedures to isolate and study MICs. The identification of such factors would also allow their manipulation during different phases of melanocyte differentiation and transformation. Ultimately—and ideally—one would be able to re-engineer MICs by introducing or activating specific genes that confer an oncogenic phenotype in normal melanocytes. A genuine tumorigenic factor would be amenable to genetic manipulation in a mouse model, allowing an assessment of whether it is required for melanoma development. An excellent example of the use of genetic approaches to confirm the requirement of single genes in colorectal tumour cell stemness has recently been published (Botchkina et al, 2009).
It is also important to recognize that attempts to analyse MICs in cell culture could be impeded by a lack of proper physiological conditions. For example, recent studies of glioma stem cells have revealed the importance of hypoxia and hypoxia-induced factors—including HIF2α—in glioma stem-cell development (Li et al, 2009). The role that tumour stromal cells have in MIC development deserves equal consideration. In this regard, keratinocytes and fibroblasts have been proposed to have a role in the maintenance of MICs (Nishimura et al, 1999), providing another example of additional supporting conditions that are necessary for the propagation and development of these cultures in vitro. Therefore, the use of physiologically relevant oxygen conditions and cellular contexts in the culture conditions should increase our ability to isolate and characterize MICs.
Another aspect of MIC physiology that could perhaps be approached in vitro is the characterization of MIC cell surface markers. Several cell surface markers have been reported to be expressed on cultured MICs in variable levels and combinations, suggesting that MICs are a heterogeneous population of cells. However, as stated above, a clear requirement for specific genes in MIC function must be shown through genetic approaches. Such analyses can be complex, as illustrated by the study of CD133 expression—which is reportedly also a MIC marker—in colon cancer. The properties of intestinal tumour-initiating cells have been suggested to evolve as a tumour develops (Zhu et al, 2009). In that study, all neoplastic cells were shown to arise from CD133+ cells—also known as Prom1+ cells—in a mouse model that has an inducible Cre-nLacZ gene in the Prom1 locus, although only 7% of LacZ+ tumour cells retained CD133 expression (Zhu et al, 2009). If this model applies to MICs, it would suggest that dynamic changes occur in the relative expression of these markers, and raises the possibility that isolating CD133+ cells from tumours might not accurately identify the original tumour-initiating cell population. A second level of complication is that some markers—such as CD133—could be expressed on many cell types and not restricted to tumour-initiating cells. A recent study addressed this question by using a NOD/SCID immunocompromised reporter mouse in which the expression of LacZ was driven by the endogenous CD133 promoter (Shmelkov et al, 2008). CD133 expression was shown not to be restricted to stem cells and the tumour burden not to depend on cells expressing CD133. Thus, careful monitoring of markers, possibly of more than one marker at time, might be necessary.
Summary
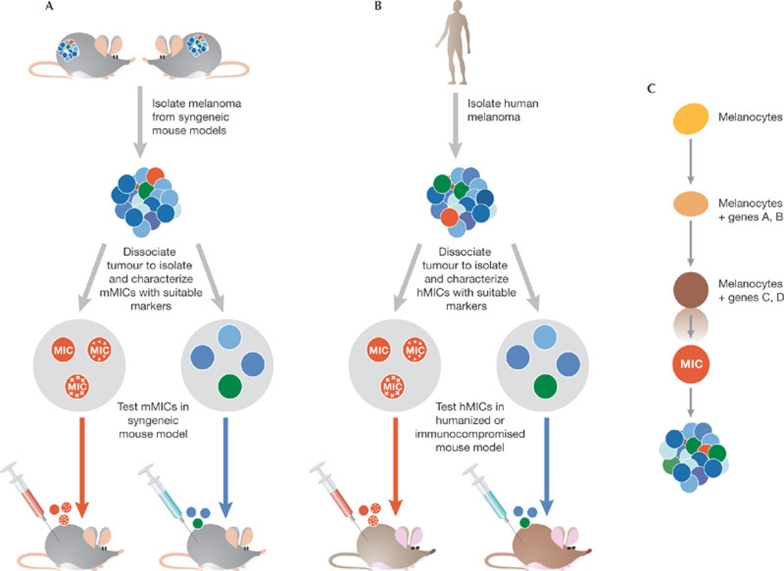
So far, our knowledge of tumour-initiating cells is derived from seminal studies of normal haematopoiesis, which have led to the identification of murine and human HSCs. Those studies provided the blueprint for a hierarchical organization of tumour-initiating cells in the heterogeneous population found in many neoplasms. Nonetheless, we do not have rigorous evidence to suggest that MICs behave in a similar manner to the tumour-initiating cells that have been identified in haematopietic malignancies, nor can we suggest that melanoma stem cells exist. One of the initial aims should be to attain the ability to isolate a homogeneous population of tumour-initiating cells based on defined surface-molecule expression patterns, which are crucial to advancing the studies of MICs and will allow us to address the possibility that some MICs might, in fact, be melanoma stem cells. In addition, the ability of this population of tumour-initiating cells to recapitulate a heterogeneous tumour on transplantation into naive animals is essential to show multipotency and the presence of a true hierarchical relationship between MICs and other cells in the tumour mass. An outline of the main options available as we move forward to achieve this goal is provided in Fig 2.
Figure 2.
Available models to study melanoma-initiating cells. (A) Murine tumour models: their use would entail the isolation and dissociation of melanomas and the identification of markers to isolate MICs from the other populations. Once the mouse MICs have been isolated, they would be subject to characterization followed by injection of a limited number of cells back into the syngeneic mouse strain to monitor tumorigenesis and metastasis. (B) Human tumour models: their use would entail the isolation of melanomas from melanoma patients, dissociation of the tumour and isolation of MICs based on defined markers. Isolated MICs will be characterized in culture and by the transplantation of a limited amount of the purified cell population into either a humanized mouse—with a matched HLA haplotype—or immunocompromised mouse, to monitor tumorigenesis and metastasis. (C) Forward genetic approach to identify crucial genes that drive the conversion of melanocytes into MICs. The transduction of genes into melanocytes would allow selection of the populations that acquire properties associated with tumour-initiating cells, including their transformation in culture, which should be subsequently confirmed in mouse models. HLA, human leukocyte antigen; hMIC, human MIC; MIC, melanoma-initiating cell; mMIC, mouse MIC.
Here, we urge a reassessment of the mouse models that should be considered appropriate for studying MICs. We emphasize the need for markers that define specific subpopulations for further characterization supported by genetic studies. We ask whether monitoring melanoma cell proliferation in mice lacking an immune system is the best way to identify and characterize the subpopulations of melanoma cells in which gene expression is altered owing to epigenetic changes. We also note that some recent studies could be interpreted as evidence against the existence of a MIC population, owing to the ability of almost every tumour cell to drive tumour growth and the lack of markers that distinguish MICs. Unless such markers are identified and their role in the development of melanoma demonstrated, these model systems are of limited value in determining whether MICs and melanoma stem cells exist.
MICs are likely to show heterogeneity similar to that of normal HSCs. Some MICs might be able to proliferate and reconstitute heterogeneous tumours on serial transplantation, whereas other MICs might have a more limited potential in a serial passage setting. The possible similarities with HSCs will only be fully evident after the development of reliable readout assays for tumour repopulation. Such in vivo assays will require defined and widely agreed on endpoints to define the tumour-forming capacity or self-renewal of a potential cell population. The establishment of these systems could also require the advent of improved mouse models to study human cancer, as well as the generation of a new set of antibodies that can detect antigens found on the surface of MICs.
In summary, we propose that MIC analysis be standardized once specific markers that allow the characterization of a defined MIC subpopulation are developed. The comparison of mouse melanoma markers with those expressed in human tumours will provide the ultimate proof of the validity and utility of mouse markers to identify human MICs. Only then will we be in a strong position to identify drugs that selectively target the more active population of human melanoma cells and, hence, improve therapeutic outcomes.
Yosef Refaeli
Anindita Bhoumik
Dennis R. Roop
Ze'ev A. Ronai
Acknowledgments
We thank R. Oshima, M. McMahon, G. Merlino and D. Fisher for critical reading of the manuscript. Support by National Cancer Institute grants CA099961 (to Z.R.), CA051995 (to Z.R.), CA117802 (to Y.R.), CA52607 (to D.R.R.) and CA105491 (to D.R.R.) is gratefully acknowledged. Y.R. is supported by a Translational Research Award from the Leukemia and Lymphoma Society.
References
- Ackermann J, Frutschi M, Kaloulis K, McKee T, Trumpp A, Beermann F (2005) Metastasizing melanoma formation caused by expression of activated N-RasQ61K on an INK4a-deficient background. Cancer Res 65: 4005–4011 [DOI] [PubMed] [Google Scholar]
- Adams JM, Strasser A (2008) Is tumor growth sustained by rare cancer stem cells or dominant clones? Cancer Res 68: 4018–4021 [DOI] [PubMed] [Google Scholar]
- Al-Hajj M, Wicha MS, Benito-Hernandez A, Morrison SJ, Clarke MF (2003) Prospective identification of tumorigenic breast cancer cells. Proc Natl Acad Sci USA 100: 3983–3988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balint K, Xiao M, Pinnix CC, Soma A, Veres I, Juhasz I, Brown EJ, Capobianco AJ, Herlyn M, Liu ZJ (2005) Activation of Notch1 signaling is required for beta-catenin-mediated human primary melanoma progression. J Clin Invest 115: 3166–3176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berges BK, Wheat WH, Palmer BE, Connick E, Akkina R (2006) HIV-1 infection and CD4 T cell depletion in the humanized Rag2−/−gamma c−/− (RAG-hu) mouse model. Retrovirology 3: 76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bittner M et al. (2000) Molecular classification of cutaneous malignant melanoma by gene expression profiling. Nature 406: 536–540 [DOI] [PubMed] [Google Scholar]
- Bonnet D, Dick JE (1997) Human acute myeloid leukemia is organized as a hierarchy that originates from a primitive hematopoietic cell. Nat Med 3: 730–737 [DOI] [PubMed] [Google Scholar]
- Botchkina IL, Rowehl RA, Rivadeneira DE, Karpeh MS Jr, Crawford H, Dufour A, Ju J, Wang Y, Leyfman Y, Botchkina GI (2009) Phenotypic subpopulations of metastatic colon cancer stem cells: genomic analysis. Cancer Genomics Proteomics 6: 19–29 [PubMed] [Google Scholar]
- Chin L, Pomerantz J, Polsky D, Jacobson M, Cohen C, Cordon-Cardo C, Horner JW II, DePinho RA (1997) Cooperative effects of INK4a and ras in melanoma susceptibility in vivo. Genes Dev 11: 2822–2834 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dankort D, Curley DP, Cartlidge RA, Nelson B, Karnezis AN, Damsky WE Jr, You MJ, DePinho RA, McMahon M, Bosenberg M (2009) Braf(V600E) cooperates with Pten loss to induce metastatic melanoma. Nat Genet 41: 544–552 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhomen N, Reis-Filho JS, da Rocha Dias S, Hayward R, Savage K, Delmas V, Larue L, Pritchard C, Marais R (2009) Oncogenic Braf induces melanocyte senescence and melanoma in mice. Cancer Cell 15: 294–303 [DOI] [PubMed] [Google Scholar]
- Dick JE, Bhatia M, Gan O, Kapp U, Wang JC (1997) Assay of human stem cells by repopulation of NOD/SCID mice. Stem Cells 15 (Suppl 1): 199–203; discussion 204–207 [DOI] [PubMed] [Google Scholar]
- Fang D, Nguyen TK, Leishear K, Finko R, Kulp AN, Hotz S, Van Belle PA, Xu X, Elder DE, Herlyn M (2005) A tumorigenic subpopulation with stem cell properties in melanomas. Cancer Res 65: 9328–9337 [DOI] [PubMed] [Google Scholar]
- Fidler IJ (1978) Tumor heterogeneity and the biology of cancer invasion and metastasis. Cancer Res 38: 2651–2660 [PubMed] [Google Scholar]
- Fidler IJ, Gruys E, Cifone MA, Barnes Z, Bucana C (1981) Demonstration of multiple phenotypic diversity in a murine melanoma of recent origin. J Natl Cancer Inst 67: 947–956 [PubMed] [Google Scholar]
- Frank NY, Margaryan A, Huang Y, Schatton T, Waaga-Gasser AM, Gasser M, Sayegh MH, Sadee W, Frank MH (2005) ABCB5-mediated doxorubicin transport and chemoresistance in human malignant melanoma. Cancer Res 65: 4320–4333 [DOI] [PubMed] [Google Scholar]
- Hendrix MJ, Seftor EA, Hess AR, Seftor RE (2003) Vasculogenic mimicry and tumour-cell plasticity: lessons from melanoma. Nat Rev Cancer 3: 411–421 [DOI] [PubMed] [Google Scholar]
- Hendrix MJ, Seftor EA, Seftor RE, Kasemeier-Kulesa J, Kulesa PM, Postovit LM (2007) Reprogramming metastatic tumour cells with embryonic microenvironments. Nat Rev Cancer 7: 246–255 [DOI] [PubMed] [Google Scholar]
- Hogan CJ, Shpall EJ, McNiece I, Keller G (1997) Multilineage engraftment in NOD/LtSz-scid/scid mice from mobilized human CD34+ peripheral blood progenitor cells. Biol Blood Marrow Transplant 3: 236–246 [PubMed] [Google Scholar]
- Kelly PN, Dakic A, Adams JM, Nutt SL, Strasser A (2007) Tumor growth need not be driven by rare cancer stem cells. Science 317: 337 [DOI] [PubMed] [Google Scholar]
- Kennedy JA, Barabe F, Poeppl AG, Wang JC, Dick JE (2007) Comment on “Tumor growth need not be driven by rare cancer stem cells”. Science 318: 1722; author reply 1722 [DOI] [PubMed] [Google Scholar]
- Klein WM, Wu BP, Zhao S, Wu H, Klein-Szanto AJ, Tahan SR (2007) Increased expression of stem cell markers in malignant melanoma. Mod Pathol 20: 102–107 [DOI] [PubMed] [Google Scholar]
- Kleinsmith LJ, Pierce GB Jr (1964) Multipotentiality of single embryonal carcinoma cells. Cancer Res 24: 1544–1551 [PubMed] [Google Scholar]
- Kripke ML (1979) Speculations on the role of ultraviolet radiation in the development of malignant melanoma. J Natl Cancer Inst 63: 541–548 [DOI] [PubMed] [Google Scholar]
- Krivtsov AV et al. (2006) Transformation from committed progenitor to leukaemia stem cell initiated by MLL-AF9. Nature 442: 818–822 [DOI] [PubMed] [Google Scholar]
- La Porta CA (2007) Drug resistance in melanoma: new perspectives. Curr Med Chem 14: 387–391 [DOI] [PubMed] [Google Scholar]
- Li Z et al (2009) Hypoxia-inducible factors regulate tumorigenic capacity of glioma stem cells. Cancer Cell 15: 501–513 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melnikova V, Bar-Eli M (2009) Inflammation and melanoma metastasis. Pigment Cell Melanoma Res 22: 257–267 [DOI] [PubMed] [Google Scholar]
- Nishimura EK, Yoshida H, Kunisada T, Miyachi Y, Nishikawa S-I (1999)
- Microenvironmentally accordant expression of cadherins associated with melanocyte migration and differentiation. Pigment Cell Res 7 (Suppl): 62 [Google Scholar]
- Noonan FP, Recio JA, Takayama H, Duray P, Anver MR, Rush WL, De Fabo EC, Merlino G (2001) Neonatal sunburn and melanoma in mice. Nature 413: 271–272 [DOI] [PubMed] [Google Scholar]
- O'Brien CA, Pollett A, Gallinger S, Dick JE (2007) A human colon cancer cell capable of initiating tumour growth in immunodeficient mice. Nature 445: 106–110 [DOI] [PubMed] [Google Scholar]
- Pollock PM et al. (2003) Melanoma mouse model implicates metabotropic glutamate signaling in melanocytic neoplasia. Nat Genet 34: 108–112 [DOI] [PubMed] [Google Scholar]
- Quintana E, Shackleton M, Sabel MS, Fullen DR, Johnson TM, Morrison SJ (2008) Efficient tumour formation by single human melanoma cells. Nature 456: 593–598 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothhammer T, Bataille F, Spruss T, Eissner G, Bosserhoff AK (2007) Functional implication of BMP4 expression on angiogenesis in malignant melanoma. Oncogene 26: 4158–4170 [DOI] [PubMed] [Google Scholar]
- Schatton T, Frank MH (2008) Cancer stem cells and human malignant melanoma. Pigment Cell Melanoma Res 21: 39–55 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schatton T et al. (2008) Identification of cells initiating human melanomas. Nature 451: 345–349 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shackleton MJ, Quintana E, Fullen DR, Sabel MS, Johnson TM (2009) Melanoma: do we need a hatchet or a scalpel? Arch Dermatol 145: 307–308 [DOI] [PubMed] [Google Scholar]
- Shmelkov SV et al. (2008) CD133 expression is not restricted to stem cells, and both CD133+ and CD133– metastatic colon cancer cells initiate tumors. J Clin Invest 118: 2111–2120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simpson AJ, Caballero OL, Jungbluth A, Chen YT, Old LJ (2005) Cancer/testis antigens, gametogenesis and cancer. Nat Rev Cancer 5: 615–625 [DOI] [PubMed] [Google Scholar]
- Singh SK, Hawkins C, Clarke ID, Squire JA, Bayani J, Hide T, Henkelman RM, Cusimano MD, Dirks PB (2004) Identification of human brain tumour initiating cells. Nature 432: 396–401 [DOI] [PubMed] [Google Scholar]
- Somervaille TC, Cleary ML (2006) Identification and characterization of leukemia stem cells in murine MLL-AF9 acute myeloid leukemia. Cancer Cell 10: 257–268 [DOI] [PubMed] [Google Scholar]
- Talmadge JE, Wolman SR, Fidler IJ (1982) Evidence for the clonal origin of spontaneous metastases. Science 217: 361–363 [DOI] [PubMed] [Google Scholar]
- van Kempen LC, van den Oord JJ, van Muijen GN, Weidle UH, Bloemers HP, Swart GW (2000) Activated leukocyte cell adhesion molecule/CD166, a marker of tumor progression in primary malignant melanoma of the skin. Am J Pathol 156: 769–774 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Raamsdonk CD, Bezrookove V, Green G, Bauer J, Gaugler L, O'Brien JM, Simpson EM, Barsh GS, Bastian BC (2009) Frequent somatic mutations of GNAQ in uveal melanoma and blue naevi. Nature 457: 599–602 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vormoor J, Lapidot T, Pflumio F, Risdon G, Patterson B, Broxmeyer HE, Dick JE (1994) SCID mice as an in vivo model of human cord blood hematopoiesis. Blood Cells 20: 316–320; discussion 320–322 [PubMed] [Google Scholar]
- Weeraratna AT, Jiang Y, Hostetter G, Rosenblatt K, Duray P, Bittner M, Trent JM (2002) Wnt5a signaling directly affects cell motility and invasion of metastatic melanoma. Cancer Cell 1: 279–288 [DOI] [PubMed] [Google Scholar]
- Williams RT, Sherr CJ (2008) The INK4-ARF (CDKN2A/B) locus in hematopoiesis and BCR-ABL-induced leukemias. Cold Spring Harb Symp Quant Biol 73: 461–467 [DOI] [PubMed] [Google Scholar]
- Williams RT, den Besten W, Sherr CJ (2007) Cytokine-dependent imatinib resistance in mouse BCR-ABL+, Arf-null lymphoblastic leukemia. Genes Dev 21: 2283–2287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu L, Gibson P, Currle DS, Tong Y, Richardson RJ, Bayazitov IT, Poppleton H, Zakharenko S, Ellison DW, Gilbertson RJ (2009) Prominin 1 marks intestinal stem cells that are susceptible to neoplastic transformation. Nature 457: 603–607 [DOI] [PMC free article] [PubMed] [Google Scholar]