Abstract
Centromeric constitutive heterochromatin is marked by DNA methylation and dimethylated histone H3 Lys 9 (H3K9me2) in Arabidopsis. RNA-directed DNA methylation (RdDM) is a process that uses 24-nucleotide (nt) small interfering RNAs (siRNAs) to induce de novo methylation to its homologous DNA sequences. Despite the presence of centromeric 24-nt siRNAs, mutations in genes required for RdDM do not appreciably influence the methylation of centromeric repeats. The mechanism by which constitutive heterochromatin is protected from RdDM remains puzzling. Here, we report that the vegetative cell nuclei (VN) of the male gametophyte (pollen) invariably undergo extensive decondensation of centromeric heterochromatin and lose centromere identity. VN show greatly reduced H3K9me2, phenocopying nuclei carrying a mutation in the chromatin remodeller DECREASE IN DNA METHYLATION 1 (DDM1). However, unlike the situation in ddm1 nuclei, the decondensed heterochromatin retains dense CG methylation and transcriptional silencing, and, unexpectedly, is subjected to RdDM-dependent hypermethylation in non-CG contexts. These findings reveal two assembly orders of silent heterochromatin and implicate the condensed form in blocking the RdDM machinery.
Keywords: RdDM, constitutive heterochromatin, CenH3, H3 Lys 9 methylation, pollen
Introduction
Constitutively heterochromatic centromeric regions in plants and animals are rich in highly repeated tandem arrays of satellite DNA and moderately repeated sequences, such as transposons, and show a low level of transcription. Constitutive heterochromatin remains condensed after mitosis, contains special forms of histones and is associated with repressive chromatin marks, including DNA cytosine methylation (Richards & Elgin, 2002) and histone H3 Lys 9 methylation (Jenuwein & Allis, 2001). Centromeric heterochromatin is required for establishing centromeres and for correct chromosome segregation in fission yeast (Folco et al, 2008).
In Arabidopsis, 5-methylcytosine is detected in the sequence contexts CG, CHG and CHH (in which H is A, T or C). METHYLTRANSFERASE 1 (MET1), a homologue of the mammalian DNMT1, is responsible for the maintenance of CG methylation (Kankel et al, 2003). Most of the CHG and a small fraction of CHH methylation are achieved by CHROMOMETHYLASE 3 (CMT3; Bartee et al, 2001). DOMAINS REARRANGED METHYLTRANSFERASE 2 (DRM2) encodes a protein homologous to the mammalian de novo DNMT3 and is required for RNA-directed DNA methylation (RdDM) in all sequence contexts (Cao & Jacobsen, 2002). Two functionally diversified plant-specific RNA polymerases, Pol IV and V, have specific functions in RdDM (Herr et al, 2005; Onodera et al, 2005; Kanno et al, 2005b). Pol IV is involved in producing or amplifying small interfering RNAs (siRNAs), whereas Pol V, together with DRM2, functions downstream of siRNA production (Matzke & Birchler, 2005). The RdDM machinery uses 24-nt siRNAs to guide methylation of homologous DNA sequences (Xie et al, 2004).
Unlike the situation in animals, in which gametes represent the direct products of meiosis, higher plants form multicellular haploid male and female gametophytes by additional post-meiotic mitotic divisions. The male gametophyte (pollen) has two sperm cells and one vegetative cell. The first male post-meiotic division is asymmetric, and leads to the formation of the generative (germ) and the terminally differentiated vegetative (somatic) cell lines. The two daughter cells have two distinct fates: the generative cell undergoes mitosis once again, giving rise to two sperm cells, whereas the vegetative cell supports pollen-tube elongation for delivering the sperm cells to the female gametophyte and degenerates before fertilization (McCormick, 2004). Previous studies have revealed unique epigenetic processes in the female gametophyte, but the features of the epigenome in pollen are relatively poorly understood. Here, we aimed to investigate the epigenetic states of centromeric heterochromatin in sperm cell nuclei (SN) and vegetative cell nuclei (VN) of Arabidopsis pollen.
Results
Decondensation of 180CEN repeats in VN
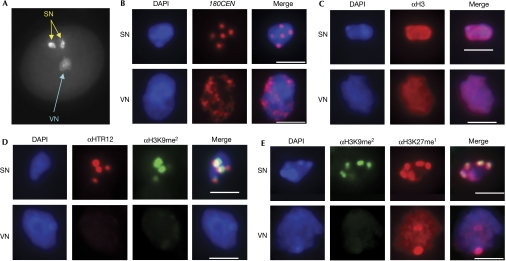
The first indication of differential chromatin states in the two types of nucleus is that VN show more diffuse and fainter staining with 4',6-diamidino-2-phenylindole than SN (Fig 1A). To address whether invisible chromocentres in VN reflect the dispersal of centromeric heterochromatin, we analysed 180-bp centromeric (180CEN) repeats in pollen nuclei by using fluorescence in situ hybridization (FISH; Fig 1B). The 180CEN repeat probe decorated condensed centromeric foci in SN, whereas dispersed FISH signals were detected in VN, indicating that centromeric heterochromatin is decondensed in VN.
Figure 1.
Cytological characterization of centromeric heterochromatin in sperm and vegetative nuclei of wild-type (Col-0) mature pollen. (A) A DAPI-stained mature pollen grain. (B) FISH image of SN and VN of pollen with a probe for 180CEN repeats. Approximately 1,000 vegetative nuclei were examined and all of them showed extremely dispersed FISH signals. Immunostaining of (C) bulk histone H3; (D) CenH3 (HTR12) and H3K9me2; and (E) H3K9me2 and H3K27me1 in SN and VN. Immunostaining patterns similar to those shown were observed in all approximately 100 nuclei examined. (B–E) Nuclei were counterstained with DAPI (blue). Scale bars, 3 μm. DAPI, 4',6-diamidino-2-phenylindole; FISH, fluorescence in situ hybridization; SN, sperm nuclei; VN, vegetative nuclei.
Similar large-scale heterochromatin decondensation occurs during dedifferentiation of leaf mesophyll cells into protoplasts or floral transition (Tessadori et al, 2007a, 2007b). These findings indicate that constitutive heterochromatin undergoes decondensation in other cell types in Arabidopsis.
Disruption of CenH3 chromatin in VN
Next, we characterized HTR12 (Talbert et al, 2002), the centromere-specific histone H3 variant (CenH3), by immunolocalization using the antibody against HTR12, together with the antibody against canonical histone H3 that we used as a positive control. Both SN and VN produced robust signals with the antibody against bulk H3 (Fig 1C). By contrast, faint scattered CenH3 signals were barely detectable in VN under conditions that produced strong signals of centromeric foci in SN (Fig 1D). Given that CenH3 is a conserved epigenetic mark for centromeres and is required for mitotic progression, the loss of CenH3 indicates that centromeres are disrupted in VN. These findings are in contrast to those for normal interphase nuclei, in which chromosomes maintain centromeres as detected by condensed foci containing 180CEN repeats and CenH3.
Loss of H3K9me2 in VN
Notably, DECREASE IN DNA METHYLATION 1 (DDM1) is the only gene identified so far in which mutations cause marked decondensation of 180CEN repeats in a sub-population of nuclei from sporophytic tissues (Probst et al, 2003), similar to that observed in the wild-type VN. The fact that ddm1 causes a decrease in heterochromatic H3K9me2 (Soppe et al, 2002), together with the recent finding of the absence of detectable DDM1 in VN (Slotkin et al, 2009), motivated us to investigate whether the decondensed heterochromatin in VN also coincides with the loss of H3K9me2 using immunostaining with the antibody against H3K9me2. We found that the H3K9me2 signals are greatly reduced or are undetectable in VN under conditions that gave intense signals in SN, which overlap substantially with CenH3 foci (Fig 1D). Next, we analysed H3K27me1, which is also enriched at heterochromatic chromocentres but also labels euchromatin to a lesser extent. It is of note that unlike H3K9me2, ddm1 does not reduce H3K27me1 in sporophytic nuclei (Mathieu et al, 2005). The antibody against H3K27me1 detected robust, dispersed signals and a prominent focal signal, which presumably represents nucleolus organizer regions (N. Chumak & H.T., unpublished data), in VN as intensively as in SN, in which the H3K27me1 signals co-localize with the H3K9me2 signals (Fig 1E). These results suggest that H3K9me2, but not H3K27me1, is lost in VN.
Non-CG hypermethylation at centromeric DNA in VN
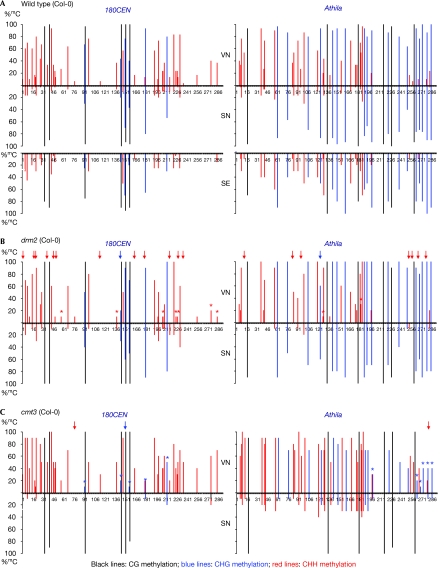
Our finding of the loss of H3K9me2 in VN, which phenocopies ddm1, led us to investigate DNA methylation, which is also decreased in ddm1 nuclei. To analyse DNA sequences from SN and VN separately, we developed a method for fractionating each type of nucleus using fluorescence-activated cell sorting based on differences in their DNA content and granularity (supplementary Fig S1 online). We used bisulphite sequencing to analyse cytosine methylation in the sorted wild-type SN and VN at a single unique allele of the 180CEN repeat (Saze et al, 2003) and of the Athila transposon (Lindroth et al, 2001), which are located close to the centromeres of chromosomes I and IV, respectively (Fig 2A). Contrary to the expectation from the absence of DDM1 in VN, nearly complete levels (90–100% 5-methylcytosine (mC)) of CG methylation and intermediate levels (20–100% mC) of CHG methylation were detected in the 180CEN and Athila sequences from both SN and VN. More surprisingly, compared with the relatively low levels (0–30% mC) of CHH methylation in SN, we found a marked increase in CHH methylation, and a noticeable increase in CHG methylation in VN. Specifically, all CHH sites in both the 180CEN (39 sites) and Athila (30 sites) sequences from VN showed a markedly higher percentage of mC than those from SN. In additon, all six CHG sites in the 180CEN, and 14 out of 18 CHG sites in the Athila sequences from VN showed a higher percentage of mC than those from SN. Parallel bisulphite-sequencing analysis with wild-type seedlings also showed a relatively lower percentage of mC at most of the CHH sites, compared with that in VN, suggesting that the infrequent CHH methylation at the 180CEN and Athila regions is a default state in Arabidopsis. A similar decondensation of 180CEN repeats and non-CG hypermethylation were observed in VN of another wild-type Arabidopsis thaliana ecotype, Wassilevskija (supplementary Fig S2A,B online). This shows that the phenomena are not limited to the Col-0 background.
Figure 2.
Bisulphite sequencing analysis of cytosine methylation in wild-type (Col-0), drm2 and cmt3 lines. The graphs show the percentage of methylation (%mC) at individual cytosines in cloned 180CEN (left) and Athila (right) sequences from (A) sorted wild-type vegetative (top) and sperm (middle) nuclei, and seedings (bottom); (B) sorted drm2; and (C) cmt3 vegetative (top) and sperm (bottom) nuclei. Black lines, CG methylation; blue lines, CHG methylation; and red lines, CHH methylation. We define ‘hypermethylated non-CG sites in VN in pollen' as the CHH or CHG sites that are methylated in more cloned DNA sequences from VN than from SN. Note that all CHH sites in both the 180CEN (39 sites) and Athila (30 sites) sequences are hypermethylated in the wild-type VN. Red and blue arrows above the methylation lines indicate the CHH and CHG sites, respectively, at which hypermethylation is lost in mutant VN compared with wild-type VN. Red and blue asterisks mark the CHH and CHG sites, respectively, showing more than a 50% reduction in %mC in mutant VN compared with wild-type VN. Cytosine positions are indicated by numbers. The results are from 30 and 20 cloned sequences for wild-type pollen nuclei and seedlings, respectively, and 10 cloned sequences for drm2 and cmt3 pollen nuclei. Original data are shown in supplementary Fig S4 online. Cmt3, chromomethylase 3; drm2, domains rearranged methyltransferase 2; SE, seedlings; SN, sperm nuclei; VN, vegetative nuclei.
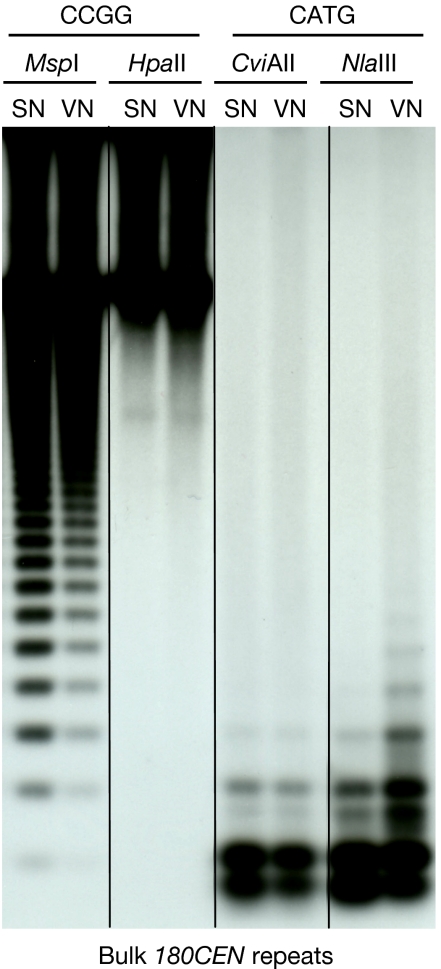
Next we analysed cytosine methylation at bulk 180CEN repeats from sorted SN and VN by Southern hybridization probed with 180CEN DNA (Fig 3). Two pairs of isoschizomers, HpaII/MspI and CviAII/NlaIII, were used to analyse CG/CHG and CHH methylation, respectively. DNA from both SN and VN showed evidence of heavy CG and CHG methylation at HpaII/MspI sites. A shift of the hybridization signals to bands of higher molecular weight in the MspI-digested VN DNA, compared with SN DNA, indicates CHG hypermethylation in VN. These findings show that heterochromatin can undergo decondensation (Fig 1B) with a concomitant loss of H3K9me2 (Fig 1D, E), leaving dense CG and CHG methylation. By contrast, digestions using CviAII and NlaIII showed negligible methylation in SN and noticeably enhanced methylation in VN, suggesting that a subset of 180CEN repeats is subjected to CHH hypermethylation in VN.
Figure 3.
Southern hybridization analysis of cytosine methylation at bulk 180CEN repeats in sperm and vegetative nuclei of wild-type (Col-0) mature pollen. A subclass of 180CEN repeats contains one MspI/HpaII (CCGG) site and two CviAII/NlaIII (CATG) sites. Genomic DNA (30 ng) extracted from sorted SN or VN was digested using MspI, HpaII, CviAII or NlaIII, separated by gel electrophoresis and probed with 180CEN DNA. MspI and HpaII both cut CCGG but differ in that MspI is inhibited by methylation of outer C, whereas HpaII is inhibited by methylation of inner C. CviAII and NlaIII both cut CATG, but differ in that only NlaIII is inhibited by methylation. SN, sperm nuclei; VN, vegetative nuclei.
Non-CG hypermethylation at centromeric DNA by DRM2
A characteristic of RdDM is CHH methylation, which is catalysed by DRM2. Therefore, we examined whether DRM2 is involved in the non-CG hypermethylation in VN by using an equivalent bisulphite sequencing analysis with the sorted drm2 pollen nuclei. To evaluate the effect of mutation, we compared the number of hypermethylated non-CG sites and percentage of mC between the wild-type and mutant VN. We defined ‘hypermethylated non-CG sites in VN in pollen' as the CHH or CHG sites that are methylated in more cloned DNA sequences from VN than from SN. We found a marked decrease in the number of hypermethylated non-CG sites in both the 180CEN and Athila sequences analysed from drm2 VN (Fig 2B). Specifically, 12 CHH sites and 1 CHG site in the 180CEN sequences, and 7 CHH sites and 1 CHG site in the Athila sequences showed loss of hypermethylation in drm2 in comparison with wild-type VN. In addition, many of the CHH sites that remained methylated in drm2 VN showed a lower percentage of mC than in wild-type VN. A similar methylation defect was detected in drm1 drm2 VN in the Wassilevskija background (supplementary Fig S2C online). These findings suggest that DRM2 is involved in de novo non-CG hypermethylation in the VN in pollen.
It is noteworthy that there were still hypermethylated non-CG sites in both the sequences analysed from the VN of the drm2 and drm1 drm2 mutants. DRM2 and CMT3 have a redundant function in accomplishing non-CG methylation (Chan et al, 2006). To test whether CMT3 is also involved in the de novo methylation process, we analysed the DNA sequences from the sorted cmt3 SN and VN in the same manner (Fig 2C). As expected from a defect of cmt3 in maintaining CHG methylation, a significant decrease in the percentage of mC was detected at all CHG sites in both the cmt3 SN and VN compared with the wild type in the 180CEN and Athila sequences. Nevertheless, all but one CHG sites at both regions in cmt3 VN showed a higher percentage of mC than that in cmt3 SN. This shows that cmt3 VN do not lose CHG hypermethylation. Similarly, CHH hypermethylation remained unaffected in cmt3 VN and, remarkably, many CHH sites showed an even higher percentage of mC than in wild-type VN. These findings suggest that CMT3 is not required for de novo non-CG methylation in wild-type VN. The lack of one DNA methyltransferase activity can activate alternative methylation pathways, resulting in aberrant de novo methylation (Mathieu et al, 2007). Thus, the partial loss of non-CG hypermethylation in the drm2 mutants and the enhanced CHH hypermethylation in the cmt3 mutant could be explained by drm2 promoting CMT3 and, conversely, cmt3 promoting DRM2, for the de novo methylation of centromeric repeats.
Induction of RdDM on centromeric DNA in VN
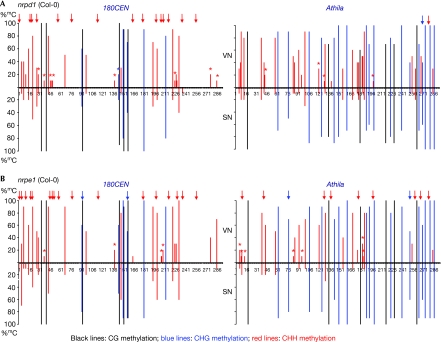
NUCLEAR RNA POLYMERASE 4 SUBUNIT 1 (NRPD1) and NUCLEAR RNA POLYMERASE 5 SUBUNIT 1 (NRPE1) encode the largest subunits of Pol IV and Pol V, respectively (Onodera et al, 2005). As a second test for the involvement of RdDM in non-CG hypermethylation in the VN, we analysed DNA from the SN and VN of nrpd1 and nrpe1 pollen (Fig 4). Similarly to the drm2 mutants, we found a substantial decrease in the number of hypermethylated non-CG sites and the percentage of mC in the 180CEN sequences from both the nrpd1 and nrpe1 VN. Interestingly, only nrpe1 VN showed a similar obvious methylation defect in the Athila region. This more subtle or non-existent effect of nrpd1 on the Athila region compared with the 180CEN sequences raises the possibility that Pol IV is essential for transcribing the 180CEN, but not the Athila region to produce siRNAs that trigger RdDM.
Figure 4.
Bisulphite sequencing analysis of cytosine methylation in nrpd1 and nrpe1 lines. The graphs show the percentage of methylation (%mC) at individual cytosines in cloned 180CEN (left) and Athila (right) sequences from sorted vegetative (top) and sperm (bottom) nuclei of (A) nrpd1 and (B) nrpe1 pollen. Black lines, CG methylation; blue lines, CHG methylation; and red lines, CHH methylation. Red and blue arrows above the methylation lines indicate the CHH and CHG sites, respectively, at which hypermethylation is lost in mutant VN compared with wild-type VN. Red and blue asterisks mark the CHH and CHG sites, respectively, showing more than a 50% reduction in %mC in mutant VN compared with wild-type VN. The results are from 10 cloned sequences for both mutants. Original data are shown in supplementary Fig S4 online. nrpd1, nuclear rna polymerase 4 subunit 1; nrpe1, nuclear rna polymerase 5 subunit 1; SN, sperm nuclei; VN, vegetative nuclei.
Therefore, we conclude that RdDM is responsible for the induction of de novo methylation at centromeric DNA.
Discussion
The SUCROSE NONFERMENTABLE 2 (SNF2)-like chromatin-remodelling factor, DDM1, is the main regulator of constitutive heterochromatin and transposable elements (TEs). Centromeric repeats and most TEs lose DNA methylation, repressive histone modifications and 24-nt TE siRNAs in ddm1 mutants (Lippman et al, 2004). Interestingly, Slotkin et al (2009) have reported recently that DDM1 is lost in VN in wild-type pollen, coincident with transcriptional reactivation, transposition and DNA hypomethylation of TEs. However, unlike in ddm1 mutants, bulk 180CEN repeats remain heavily methylated (Fig 3) and transcriptionally silent in VN (supplementary Fig S3 online; Slotkin et al, 2009).
An intriguing question is how downregulation of DDM1 could result in differential methylation effects on centromeric repeats and non-centromeric TEs in VN. The most likely timing of DDM1 shutdown takes place after the first pollen mitotic division has given rise to the generative and vegetative cells. At this stage, CG methylation is propagated by the ‘maintenance methylase' activity of MET1, which depends on DDM1. The ddm1-induced DNA hypomethylation of centromeric repeats probably results from passive demethylation, which could account for why CG methylation remains in VN, as they do not divide any more in pollen. Conversely, an active demethylation process seems to be involved in the loss of DNA methylation in TE sequences in the VN (Slotkin et al, 2009). It remains unclear how a DNA demethylase recognizes its target TEs and why centromeric repeats are not susceptible to demethylation. It should be noted that the N-terminal amino acids of CenH3 are different from those of canonical H3 in animals and plants. Thus, it is conceivable that the presence or absence of specific marks on CenH3 chromatin at centromeric repeats inhibits the demethylation machinery.
In contrast to the dispersal of chromocentres containing pericentromeric 5S ribosomal DNA clusters in Pol IV mutants, which correlates with the loss of cytosine methylation in all sequence contexts, chromocentres containing 180CEN repeats remain condensed and the methylation of 180CEN repeats is not appreciably affected in the mutants (Onodera et al, 2005). Our finding of infrequent CHH methylation, the characteristic of RdDM, at 180CEN repeats in seedlings and SN of pollen reinforces the idea that RdDM does not work efficiently for constitutive heterochromatin. Nevertheless, abundant 24-nt siRNAs corresponding to 180CEN repeats are produced in Arabidopsis and the RdDM components RDR2 and DCL3 contribute to the siRNA production (May et al, 2005). Why the 180CEN siRNAs do not efficiently trigger RdDM of 180CEN repeats remains unknown.
Speculation
Facultative heterochromatin undergoes transition from a silent to an active state and vice versa, whereas constitutive heterochromatin is continuously silent. Hence, it makes sense that de novo DNA methylation and demethylation control the reversible switch of gene expression in facultative heterochromatin, and that the self-enforcing mechanisms of DNA and H3K9 methylation act on constitutive heterochromatin. Given the recent hypothesis regarding the interaction of the RdDM machinery with DNA glycosylases (Kanno et al, 2005a), one could imagine that the RdDM of constitutive heterochromatin might disturb the stable propagation of DNA methylation and the silent state. Thus, it is reasonable to speculate that constitutive heterochromatin uses various mechanisms for blocking RdDM. We propose that a specific chromatin mark—or marks—dictated by DDM1 (for example, H3K9me2) prevents access of the downstream RdDM complex containing DRM2 and Pol V. Downregulation of DDM1 in VN in pollen leads to the demethylation of heterochromatic H3K9me2, which then causes disruption of centromeric heterochromatin and CenH3 chromatin at centromeres, allowing the RdDM of centromeric repeats.
Methods
Plant material and growth. We carried out all analyses using wild-type Arabidopsis thaliana ecotypes Columbia (Col-0) and Wassilevskija (WS). All mutants used are in the Col-0 background, with the exception of drm1 drm2, which is in the WS background. The cmt3 mutant was originally in the WS background and was out-crossed six times to the Col-0 ecotype. We used the following alleles: ddm1-10 (SALK_000590), drm1-1 drm2-1 (Cao & Jacobsen, 2002), drm2-2 (SALK_150863), nrpd1-4 (formerly nrpd1a-4; SALK_083051), nrpe1/drd3-1 (formerly nrpd1b; Kanno et al, 2005b) and cmt3-i11 (Bartee et al, 2001). Seedlings were cultivated under sterile conditions on solid ½ MS medium in a 22°C chamber under a 16/8 h light/dark photoperiod for 20 days.
Immunostaining and FISH. Immunostaining was carried out according to Soppe et al (2002) with modifications, and FISH was carried out according to Pedrosa et al (2002), as described in the supplementary information online.
Sorting the sperm and vegetative nuclei of pollen. Pollen nuclei were prepared from mature pollen grains, stained with SYBR Green I (Roche Diagnostics GmbH, Mannheim, Germany) or propidium iodide, and SN and VN were sorted as described in the supplementary information online.
Bisulphite sequencing analysis of cytosine methylation. Genomic DNA extracted from the sorted pollen nuclei or seedlings was treated with bisulphite using the EpiTect bisulfite kit (Qiagen, Hilden, Germany). A single unique allele of the 180CEN repeat and of the Athila region were amplified by PCR of the bisulphite-treated DNA, cloned and sequenced as described in the supplementary information online. Complete bisulphite reactions were confirmed by sequencing a region of an unmethylated endogenous DDM1 gene or a cloned exogenous 180CEN repeat mixed with genomic DNA.
Southern hybridization analysis of cytosine methylation. Southern hybridizations with a probe for bulk 180CEN repeats were carried out as described in the supplementary information online. To verify that digestions were complete, digested DNA was subjected to PCR to amplify a region of an unmethylated DDM1 gene, containing CCGG and CATG sites. Hybridization signals were visualized using the Amersham Gene Images AlkPhos Direct labelling and detection system (GE Healthcare, Little Chalfont, Buckinghamshire, UK).
Supplementary information is available at EMBO reports online (http://www.emboreports.org).
Supplementary Material
Supplementary Information
Acknowledgments
We thank G. Stengl for fluorescence-activated cell sorting, I. Schubert for anti-CenH3, T. Jenuwein for providing anti-H3K27me1, R. Tsien for pmRFP1, O. Mittelsten Scheid for drm1 drm2, M. Siomos for reviewing the paper, and M. Matzke for ddm1, drm2, nrpd1, nrpe1 and discussion. H.T. thanks O. Esina for inspiration. This work was supported by a grant from the UK Biotechnology and Biological Sciences Research Council to D.T.
Footnotes
The authors declare that they have no conflict of interest.
References
- Bartee L, Malagnac F, Bender J (2001) Arabidopsis cmt3 chromomethylase mutations block non-CG methylation and silencing of an endogenous gene. Genes Dev 15: 1753–1758 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao X, Jacobsen SE (2002) Role of the Arabidopsis DRM methyltransferases in de novo DNA methylation and gene silencing. Curr Biol 12: 1138–1144 [DOI] [PubMed] [Google Scholar]
- Chan SW, Henderson IR, Zhang X, Shah G, Chien JS, Jacobsen SE (2006) RNAi, DRD1, and histone methylation actively target developmentally important non-CG DNA methylation in Arabidopsis. PLoS Genet 2: e83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Folco HD, Pidoux AL, Urano T, Allshire RC (2008) Heterochromatin and RNAi are required to establish CENP-A chromatin at centromeres. Science 319: 94–97 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herr AJ, Jensen MB, Dalmay T, Baulcombe DC (2005) RNA polymerase IV directs silencing of endogenous DNA. Science 308: 118–120 [DOI] [PubMed] [Google Scholar]
- Jenuwein T, Allis CD (2001) Translating the histone code. Science 293: 1074–1080 [DOI] [PubMed] [Google Scholar]
- Kankel MW, Ramsey DE, Stokes TL, Flowers SK, Haag JR, Jeddeloh JA, Riddle NC, Verbsky ML, Richards EJ (2003) Arabidopsis MET1 cytosine methyltransferase mutants. Genetics 163: 1109–1122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanno T, Aufsatz W, Jaligot E, Mette MF, Matzke M, Matzke AJ (2005a) A SNF2-like protein facilitates dynamic control of DNA methylation. EMBO Rep 6: 649–655 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanno T, Huettel B, Mette MF, Aufsatz W, Jaligot E, Daxinger L, Kreil DP, Matzke M, Matzke AJ (2005b) Atypical RNA polymerase subunits required for RNA-directed DNA methylation. Nat Genet 37: 761–765 [DOI] [PubMed] [Google Scholar]
- Lindroth AM, Cao X, Jackson JP, Zilberman D, McCallum CM, Henikoff S, Jacobsen SE (2001) Requirement of CHROMOMETHYLASE3 for maintenance of CpXpG methylation. Science 292: 2077–2080 [DOI] [PubMed] [Google Scholar]
- Lippman Z et al. (2004) Role of transposable elements in heterochromatin and epigenetic control. Nature 430: 471–476 [DOI] [PubMed] [Google Scholar]
- Mathieu O, Probst AV, Paszkowski J (2005) Distinct regulation of histone H3 methylation at lysines 27 and 9 by CpG methylation in Arabidopsis. EMBO J 24: 2783–2791 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathieu O, Reinders J, Caikovski M, Smathajitt C, Paszkowski J (2007) Transgenerational stability of the Arabidopsis epigenome is coordinated by CG methylation. Cell 130: 851–862 [DOI] [PubMed] [Google Scholar]
- Matzke MA, Birchler JA (2005) RNAi-mediated pathways in the nucleus. Nat Rev Genet 6: 24–35 [DOI] [PubMed] [Google Scholar]
- May BP, Lippman ZB, Fang Y, Spector DL, Martienssen RA (2005) Differential regulation of strand-specific transcripts from Arabidopsis centromeric satellite repeats. PLoS Genet 6: 705–713 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCormick S (2004) Control of male gametophyte development. Plant Cell 16: 142–153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Onodera Y, Haag JR, Ream T, Nunes PC, Pontes O, Pikaard CS (2005) Plant nuclear RNA polymerase IV mediates siRNA and DNA methylation-dependent heterochromatin formation. Cell 120: 613–622 [DOI] [PubMed] [Google Scholar]
- Pedrosa A, Sandal N, Stougaard J, Schweizer D, Bachmair A (2002) Chromosomal map of the model legume Lotus japonicus. Genetics 161: 1661–1672 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Probst AV, Fransz PF, Paszkowski J, Mittelsten Scheid O (2003) Two means of transcriptional reactivation within heterochromatin. Plant J 33: 743–749 [DOI] [PubMed] [Google Scholar]
- Richards EJ, Elgin SC (2002) Epigenetic codes for heterochromatin formation and silencing: rounding up the usual suspects. Cell 108: 489–500 [DOI] [PubMed] [Google Scholar]
- Saze H, Mittelsten Scheid O, Paszkowski J (2003) Maintenance of CpG methylation is essential for epigenetic inheritance during plant gametogenesis. Nat Genet 34: 65–69 [DOI] [PubMed] [Google Scholar]
- Slotkin RK, Vaughn M, Borges F, Tanurdzić M, Becker JD, Feijó JA, Martienssen RA (2009) Epigenetic reprogramming and small RNA silencing of transposable elements in pollen. Cell 136: 461–472 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soppe WJ, Jasencakova Z, Houben A, Kakutani T, Meister A, Huang MS, Jacobsen SE, Schubert I, Fransz PF (2002) DNA methylation controls histone H3 lysine 9 methylation and heterochromatin assembly in Arabidopsis. EMBO J 21: 6549–6559 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talbert PB, Masuelli R, Tyagi AP, Comai L, Henikoff S (2002) Centromeric localization and adaptive evolution of an Arabidopsis histone H3 variant. Plant Cell 14: 1053–1066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tessadori F, Chupeau MC, Chupeau Y, Knip M, Germann S, van Driel R, Fransz P, Gaudin V (2007a) Large-scale dissociation and sequential reassembly of pericentric heterochromatin in dedifferentiated Arabidopsis cells. J Cell Sci 120: 1200–1208 [DOI] [PubMed] [Google Scholar]
- Tessadori F, Schulkes RK, van Driel R, Fransz P (2007b) Light-regulated large-scale reorganization of chromatin during the floral transition in Arabidopsis. Plant J 50: 848–857 [DOI] [PubMed] [Google Scholar]
- Xie Z, Johansen LK, Gustafson AM, Kasschau KD, Lellis AD, Zilberman D, Jacobsen SE, Carrington JC (2004) Genetic and functional diversification of small RNA pathways in plants. PLoS Biol 2: e104. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Information