Abstract
Context
As we identify genes involved in psychiatric disorders, the next step will be to study how the risk associated with susceptibility genes manifests across development and in conjunction with the environment. We describe analyses aimed at characterizing the pathway of risk associated with GABRA2, a gene previously associated with adult alcohol dependence, in a community sample of children followed longitudinally from childhood through young adulthood.
Objective
To test for an association between GABRA2 and trajectories of externalizing behavior from adolescence to young adulthood and for moderation of genetic effects by parental monitoring.
Design
Data were analyzed from the Child Development Project, with yearly assessments conducted since that time. A saliva sample was collected for DNA at the 2006 follow-up, with a 93% response rate in the target sample. Growth mixture modeling was conducted using Mplus to identify trajectories of externalizing behavior and to test for effects of GABRA2 sequence variants and parental monitoring.
Setting
Nashville and Knoxville, Tennessee, and Bloomington, Indiana.
Participants
A community-based sample of families enrolled at 3 sites as children entered kindergarten in 1987 and 1988. Analyses for the white subset of the sample (n=378) are reported here.
Main Outcome Measures
Parental monitoring measured at 11 years of age; Child Behavior Checklist youth reports of externalizing behavior at ages 12, 14, 15, 16, 17, 19, 20, 21, and 22 years.
Results
Two classes of externalizing behavior emerged: a stable high externalizing class and a moderate decreasing externalizing behavior class. The GABRA2 gene was associated with class membership, with subjects who showed persistent elevated trajectories of externalizing behavior more likely to carry the genotype previously associated with increased risk of adult alcohol dependence. A significant interaction with parental monitoring emerged; the association of GABRA2 with externalizing trajectories diminished with high levels of parental monitoring.
Conclusions
These analyses underscore the importance of studying genetic effects across development and of identifying environmental factors that moderate risk.
Considerable advances in genetics at the level of understanding the structure of the human genome and associated diversity as well as in genotyping and analysis methods, have ushered in an era where identifying genes underlying complex disorders is no longer a distant possibility.1 The number of articles published regarding such associations is rapidly escalating, and associations are replicating across independent samples. The growth of genomewide association studies and large consortia of researchers who are pooling data to create more powerful gene-finding samples only promises to continue this trend.2,3
The necessity for very large sample sizes to identify genes of small effect, such as those believed to be involved in the predisposition to most psychiatric conditions, has necessarily limited the amount of phenotypic information collected in most large gene-finding projects. Historically, most samples collected for gene identification were cross-sectional in nature and focused on affected individuals and their family members and/or control subjects. Although this is a justifiable strategy for identifying initial associations with disease outcome, psychiatric disorders often represent the eventual end point of a trajectory of risk-related behavior that begins much earlier in development. Accordingly, identifying genes involved in psychiatric outcomes opens a door of opportunity for many interesting and important research questions to be addressed about the risk associated with that gene.4 What is the spectrum of behavioral phenotypes associated with the gene? How does that genetic risk manifest across developmental stages? Are there important environmental factors that moderate the risk associated with the gene? These are only a few of the many questions that we must now address about risk-related processes and underlying mechanisms by which genetic susceptibility translates into eventual association with psychiatric disease.
Addressing these questions will necessitate different study designs than those traditionally used for gene identification. Prospective studies will be necessary to understand how risk unfolds across developmental stages. Longitudinal designs will allow us to go beyond snapshots of gene-behavior association and to study genetic influences on trajectories of risk. It will also be important to go beyond clinical samples to study how genetic variants affect risk in population-based samples. This will be critical in understanding how we might eventually use this information to inform prevention and early intervention programs. Finally, it will be essential to integrate genetic findings with the considerable literature on environmental influences on psychiatric outcome. Although family, twin, and adoption studies have convincingly demonstrated that most psychiatric disorders show a significant degree of genetic influence,5 forming a compelling rationale for gene identification efforts, they have also been pivotal in demonstrating that environmental influences play a considerable role.6 For many common psychiatric conditions including substance dependence, major depression, and anxiety disorders, heritability rarely exceeds 50%,7 underscoring the importance of parallel research endeavors aimed at identifying the critical relevant environments. More recently, studies have focused on understanding how genetic influences and environmental factors interact.8-12
Here we present analyses aimed at delineating the pathways of risk associated with GABRA2 OMIM 137140. This gene was originally associated with adult alcohol dependence in the Collaborative Study on the Genetics of Alcoholism (COGA) project.13 The association with adult alcohol dependence has been replicated in several independent samples.14-17 Subsequent analyses of GABRA2 in the COGA sample also yielded evidence of association with other forms of drug dependence,18,19 antisocial personality disorder,20 and childhood conduct disorder,19 leading to the hypothesis that GABRA2 may be involved in the predisposition to alcohol dependence through general externalizing pathways.21 This is further supported by the fact that there is evidence of association between GABRA2 and an electrophysiological endophenotype in the COGA sample.13
Parallel to the advances in identifying specific genes involved in alcohol dependence, research efforts have identified a number of environmental factors that influence alcohol use.22-25 One environmental factor shown to be particularly important in adolescent substance use is parental monitoring, with a substantial body of literature consistently demonstrating that more parental monitoring is associated with reduced risk of smoking, alcohol use, and other deviant and risky behaviors among adolescents such as delinquency and aggression.26-28 Importantly, parental monitoring has been shown to moderate the importance of genetic effects on substance use across adolescence.29,30 In a population-based sample of twins aged 14 and 17 years, as parental monitoring increased, genetic effects on substance use significantly decreased.30 Although this study of gene-environment interaction tested only for changes in heritability as a function of the environment, it suggests that parental monitoring is an important candidate environment to test for moderating effects associated with specific identified susceptibility genes.
Herein we describe analyses of the GABRA2 gene in a representative community-based sample of children followed up from kindergarten to 22 years of age, with comprehensive developmental assessments, including environmental information. We used growth mixture modeling (GMM) to identify discrete patterns of externalizing behavior within the Child Development Project (CDP) sample, and then we tested whether variation in the probability of trajectory class membership could be explained by GABRA2 genotypes and/or whether this association was moderated by parental monitoring.
Methods
Sample
Participants in the CDP were originally recruited from 3 cities (Nashville and Knoxville, Tennessee, and Bloomington, Indiana) during kindergarten preregistration in 1987 and 1988. Within each site, about 6 schools that served families from a range of socioeconomic status groups were selected to participate, explicitly including schools in economically at-risk neighborhoods. Most of the sample was enrolled by randomly approaching parents at preregistration and inviting them to participate in a longitudinal study of child development, with-approximately 75% agreement. Because a small percentage of children in the targeted schools do not preregister (15%), a similar proportion of the CDP sample was recruited on the first day of school or through a letter or telephone call to maintain representativeness of the school population. This procedure produced a participant sample that validly represented the broader population demographically and behaviorally, as determined by teacher and sociometric ratings of the entire population at those sites. The original CDP sample consisted of 585 children (52% male; 81% European American, 17% African American, and 2% belonging to other ethnic groups). More than 20% of participants were born into single-parent families, and more than half lived with single or divorced parents before adulthood. Data collection began the summer before the participants entered kindergarten (at about 5 years of age) and follow-ups have been conducted annually and remain ongoing. Through newsletters, birthday cards, handwritten thank-you notes, postmaster notification of changed addresses, tracking through named relatives and friends, and Web-based searches, we have maintained high rates of participation over time. Ninety percent (n=526) of the original 585 participants took part in at least 1 assessment in early adulthood (ages, 19-23 years). The 10% that attrited had been slightly higher in kindergarten teacher–reported externalizing behavior and lower in socioeconomic status than the retained sample, but the groups did not differ in other measures, including ethnicity. We have routinely tested for site and cohort (1987 vs 1988) main effects and interactions, but none of these tests yielded significant site or cohort effects.
The DNA was collected from CDP participants from February 2006 to July 2007. For many participants, DNA collection took place at an annual follow-up visit. Following completion of phenotypic assessments, participants were invited to provide a DNA sample. However for some participants, especially for the considerable number who lived away from the main research offices, DNA was collected in the context of a special visit. The DNA was collected via saliva sample using Oragene collection kits under the supervision of a specially trained interviewer. Participants received an additional $20 for participating in the DNA collection portion of the project and provided separate consent for the genotyping component of the project. Saliva samples were subsequently labeled anonymously and mailed to Washington University in St Louis, Missouri, where DNA extraction and genotyping occurred. The DNA samples were obtained from 452 individuals, representing 93% of the target sample of regular CDP participants. The institutional review boards at all sites approved the study.
Phenotypes
Externalizing Behavior
The CDP collected data on externalizing behavior using Achenbach's Youth/Young Adult Self-Reports at ages 12, 14, 15, 16, 17, 19, 20, 21, and 22 years.31,32 This widely used assessment battery33 consists of 113 items in the Youth version and 123 items in the Young Adult version, for which the participant indicates whether the behavior is not true, somewhat or sometimes true, or very or often true (scored 0, 1, and 2, respectively). The Externalizing Scale consists of 30 items in the Youth version and 28 items in the Young Adult version comprising both delinquency (eg, “I don't feel guilty after doing something I shouldn't”; “I cut classes or skip school”) and aggression measures (eg, “I am mean to others”; “I get in many fights”). These measures have been shown to have excellent psychometric properties including high test-retest reliability, content validity, criterion-related validity, and construct validity.34,35 Values of α in the present study ranged from .84 to .88.
Parental Monitoring
Monitoring items were included in the mother interview conducted in the summer following fifth grade when the participants were aged 11 years. We use monitoring as reported at this age because the reports preceded the assessments of externalizing behavior analyzed here and we were interested in how monitoring may interact with genetic susceptibilities to predict subsequent trajectories of externalizing. Nine items, each rated on a 5-point scale, were combined to create a composite scale.36 These items asked the mother to report how often (1) she thinks her child goes to places he or she is asked not to go to; (2) a parent or other adult is present when the child is at a friend's house; (3) she would know if the child played with children who get in trouble; (4) the child calls, leaves a note, or communicates with her if he or she is going out when at home without an adult; (5) she talks with her child about what he or she does with friends when away from home; and if she knows (6) where the child is, (7) who he or she is with, and (8) when he or she will return when not at home; and (9) whether she knows the first and last names of the friends the child is with. The internal consistency for these 9 items was α=.73, and the average interitem correlation was r=0.40. In no case did the removal of an item result in an overall increase in α of more than .01. Although we refer to this measure as parental monitoring, it likely reflects both parental efforts (and skills) at obtaining information and the child's willingness to inform the parent.37,38
Genotyping
Genotyping was conducted with a modified single-nucleotide extension reaction, with allele detection by mass spectrometry (Sequenom MassArray system; Sequenom, San Diego, California). Polymerase chain reaction and extension primers (available on request) were designed using MassArray Assay Design Version 3.1.2.5 (Sequenom). All studies of GABRA2 and alcohol dependence, along with data from The International HapMap Project, have identified 2 linkage disequilibrium blocks in GABRA2.39 Moreover, all significant associations with alcohol dependence have been described with single-nucleotide polymorphisms (SNPs) in the larger haplotype block that extends downstream from intron 3. In the CDP sample, we genotyped 10 SNPs in GABRA2, selected based on evidence of association with alcohol dependence in the COGA sample. All SNPs genotyped in the CDP were located in the previously associated haplotype block. The overall genotyping success rate was 98.4%. A total of 24 biological and 12 technical replicates were genotyped and produced a concordance rate of 100%. All 10 SNPs were successfully genotyped for 96% of the samples. Because allele frequencies and linkage disequilibrium structures often differ across populations, we limited all analyses and data in this study to the subsample of white individuals (n=378; 190 men, 188 women). Haploview40 was used to estimate linkage disequilibrium across the genotyped SNPs. As in previously genotyped samples, linkage disequilibrium was very high, with r2 ranging from 0.81 to 1.00 (mean r2=0.91).41 This indicates that the SNPs are highly correlated and do not represent independent tests of association. Table 1 displays descriptive information on each of these SNPs, including chromosomal position estimates from the SNP database (dbSNP) and minor allele frequencies. All SNPs were in Hardy-Weinberg equilibrium.
Table 1. Markers Genotyped in GABRA2.
| Markera | Positionb | Alleles, Minor/Majorc | MAFd |
|---|---|---|---|
| rs497068 | 45945434 | C/T | 0.1423 |
| rs548583 | 45958101 | T/C | 0.1419 |
| rs279871 | 46000490 | G/A | 0.1430 |
| rs279858 | 46009350 | G/A | 0.1429 |
| rs279845 | 46024480 | A/T | 0.1453 |
| rs1440130 | 46028010 | C/T | 0.1451 |
| rs279826 | 46028966 | G/A | 0.1454 |
| rs279827 | 46029459 | G/A | 0.1453 |
| rs279828 | 46029567 | C/A | 0.1453 |
| rs279836 | 46033827 | A/T | 0.1441 |
Abbreviations: MAF, minor allele frequency; SNP single-nucleotide polymorphism.
Markers are shown as rs numbers from the SNP database (dbSNP).
Position is in nucleotides from chromosome 4pter, as shown in dbSNP (build 129) or by blasting against the National Center for Biotechnology Information Human Genome assembly (build 36.3).
Markers rs497068, rs548583, rs279871, and rs279858 were genotyped on the minus strand of chromosome 4; all other SNPs were typed on the plus strand.
Minor allele frequency in the present sample.
Statistical Analysis
Growth mixture modeling42,43 was used to identify homogeneous subgroups of individuals manifesting distinct patterns of change in their externalizing behavior from early adolescence through young adulthood (ie, from 12 to 22 years of age). Conventional growth curve modeling assumes a mean pattern of change in behavior within the population, with individual differences expressed in terms of normal variability around specified growth parameters (ie, intercept and slope coefficients that define the level and shape of the change44). Growth mixture modeling is a widely used extension of this procedure that allows for the possibility of 2 or more discrete subgroups of individuals within a population, each having unique mean trajectories.45 Individuals are classified into groups by probability of class membership conditioned on their response pattern across the 9 repeated measurements of self-reported externalizing behavior. To determine the influence of genotype on trajectory class membership within the resulting GMM, probability of class membership was regressed on genotype. For these analyses, each of the SNPs were coded 0, 1, or 2, reflecting an additive genotypic model. This coding is in reference to the number of copies of the minor allele for all SNPs. These analyses were subsequently extended to the multivariate framework to test for moderation of genotypic effects by parental monitoring. All models in this study were fitted in Mplus version 5.0,46 allowing for single-stage modeling of classification into trajectories and testing of genetic/interaction effects. This method provides the advantage of using the probability of class membership as a continuous outcome variable rather than first classifying individuals into classes and using the resultant classes as discrete outcome variables in subsequent analyses, which results in a loss of information (and statistical power) that can systematically distort the characteristics of the latent classes.47 Odds ratios reflect pairwise comparisons of classification status (ie, the trajectories into which individuals are grouped on the basis of their highest probability of membership), but probability of class membership is the dependent variable modeled in analyses. Missing data are accommodated in Mplus via robust maximum likelihood estimation, which takes advantage of all available data rather than deleting cases with partially missing data in a listwise manner. A set of standard indices was used to assess relative model fit, quality of classification, and the following direct comparisons between models: Bayesian information criterion,48,49 Akaike information criterion,50 entropy coefficients,51 and the Lo-Mendel-Rubin likelihood ratio test.52
Results
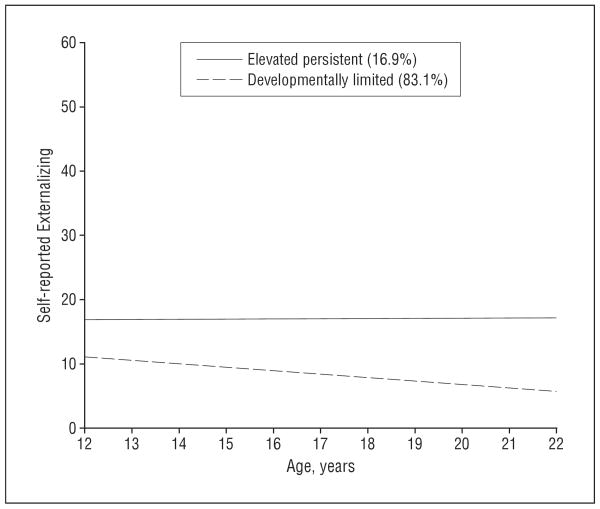
Consideration of several selection criteria suggested that a 2-class solution fit the externalizing data best. As shown in Table 2, there was a significant decrease in both Bayesian information criterion (20 203.81 to 20 154.60) and Akaike information criterion (20 146.50 to 20 085.01) between a single class and the 2-class solution. Likewise, the likelihood ratio test comparing the fit of 1 vs 2 trajectories favored the 2-class solution (P<.001). In relation to the addition of a third distinct pattern of externalizing behavior, the Bayesian information criterion failed to decrease (20 159.52), the overall quality of classification was reduced (entropy went from 0.83 to 0.80), and though Akaike information criterion did decrease, the magnitude of the change between coefficients for the 2- and 3-class solutions was much smaller than corresponding to the previous increment. In addition, the Lo-Mendel-Rubin likelihood ratio test indicated that the addition of a third class would not result in significant improvement in model fit (P=.52). The Figure depicts these 2 GMM-estimated externalizing trajectories. Most of the sample displayed a developmentally limited pattern of externalizing behavior (83.1%), peaking at or before the initial wave of assessment (at 12 years of age), with a steady linear decline thereafter (βintercept=11.1, P<.001; βslope=−0.54, P<.001). A smaller proportion of the sample (16.9%) had higher initial levels of externalizing behavior that persisted across the period from early adolescence into young adulthood (βintercept=16.8, P<.001; βslope=0.04; P=.79 [not significant]). Table 3 displays results of a series of 2-class GMMs wherein trajectory class membership was regressed on genotype. Findings demonstrate that adolescents' odds of membership in the elevated persistent externalizing trajectory increased with each additional copy of the minor allele. For example, for rs497068, the first SNP listed in the table, 9.3% of individuals carrying no copies of the minor allele displayed elevated persistent antisocial behavior, 15.2% of individuals carrying 1 copy of the minor allele displayed elevated persistent externalizing behavior, and 21.1% of individuals carrying 2 copies of the minor allele displayed elevated persistent externalizing behavior. Corresponding odds ratios ranged from 2.1 to 2.7 (all P≤.001). A parallel analysis with trajectory class membership regressed on parental monitoring yielded no evidence of a main effect of parental monitoring (P=.44).
Table 2. Fit Indices for Linear Growth Mixture Modeling Solutions.
| Latent Trajectory Classes, No. | BICa | AICa | Entropyb | LMR-LRTc |
|---|---|---|---|---|
| 1 | 20203.81 | 20146.50 | ||
| 2 | 20154.60 | 20085.01 | 0.83 | <.001 |
| 3 | 20159.52 | 20077.64 | 0.80 | .52 |
Abbreviations: AIC, Akaike information criteria; BIC, Bayesian information criteria; LMR-LRT, Lo-Mendel-Rubin likelihood ratio test.
These indices balance model complexity and goodness of fit to the sample data, with smaller values denoting better fit.
An indicator of how well a model predicts profile membership, with values closer to 1 denoting greater precision.
A direct test comparing models with k and k–1 classes, wherein P≤.05 denotes significant improvement in fit, indicating that the model with k–1 classes should be rejected in favor of the model with k classes.
Figure.
Two-class linear solution for growth mixture model of self-reported externalizing behavior from 12 to 22 years of age. Percentages in key indicate the percentage of the sample that was classified in that trajectory.
Table 3. Percentage of Individuals Belonging to Each Trajectory Class by Genotype and Associated P Values for Main Effects of the GABRA2 SNPs on Trajectory Class Membership.
| Individuals by Genotype, %b | ||||||||
|---|---|---|---|---|---|---|---|---|
| 0 | 1 | 2 | Main Effects | |||||
| SNPa | DL | EP | DL | EP | DL | EP | P Value | OR (95% CI) |
| rs497068 | 90.7 | 9.3 | 84.8 | 15.2 | 78.9 | 21.1 | <.001 | 2.1 (1.3-3.2) |
| rs548583 | 90.7 | 9.3 | 84.7 | 15.3 | 78.7 | 21.3 | .001 | 2.2 (1.4-3.4) |
| rs279871 | 90.7 | 9.3 | 84.9 | 15.1 | 79.1 | 20.9 | .001 | 2.2 (1.4-3.6) |
| rs279858 | 92.8 | 7.2 | 85.2 | 14.8 | 78.4 | 21.6 | <.001 | 2.1 (1.4-3.3) |
| rs279845 | 90.5 | 9.5 | 84.9 | 15.1 | 78.7 | 21.3 | .001 | 2.5 (1.4-4.3) |
| rs1440130 | 93.8 | 6.2 | 83.4 | 16.6 | 80.3 | 19.7 | <.001 | 2.5 (1.4-4.3) |
| rs279826 | 93.8 | 6.2 | 83.8 | 16.2 | 80.0 | 20.0 | <.001 | 2.5 (1.4-4.3) |
| rs279827 | 93.8 | 6.2 | 83.2 | 16.8 | 80.8 | 19.2 | .001 | 2.5 (1.4-4.3) |
| rs279828 | 93.5 | 6.5 | 84.0 | 16.0 | 80.0 | 20.0 | <.001 | 2.5 (1.4-4.3) |
| rs279836 | 90.5 | 9.5 | 84.8 | 15.2 | 78.9 | 21.1 | .001 | 2.7 (1.5-4.7) |
Abbreviations: CI, confidence interval; DL, developmentally limited; EP, elevated, persistent; OR, odds ratio; SNP, single-nucleotide polymorphism.
SNPs are in strong linkage disequilibrium and do not represent independent tests.
Genotype designation (0, 1, 2) reflects the number of copies of the minor allele.
In a subsequent set of 2-class GMMs, trajectory class membership was regressed on (1) genotype at each of the 10 SNPs, (2) parental monitoring, and (3) the interaction between (the product of) the specified SNP and parental monitoring. Results of these multivariate analyses are depicted in Table 4. Parental monitoring significantly moderated the influence of genotype on trajectory class membership (P= .007-.03). Table 4 shows class membership by genotype split by parental monitoring. Parental monitoring is divided into high and low groups based on a median split in Table 4 for illustrative purposes, although analyses were conducted using the full quasi-continuous monitoring variable, as described in the methods. The influence of GABRA2 on externalizing trajectory was considerably pronounced under conditions of lower parental monitoring and was diminished under conditions of higher parental monitoring. For example, for SNP rs497068 (again selected for illustration based on being the first SNP in the Table), the percentage of individuals in the elevated persistent externalizing class as a function of number of copies of the minor allele was 10.3%, 12.0%, 16.3%, for individuals with 0, 1, or 2 copies, respectively, under conditions of high parental monitoring. However, under conditions of low parental monitoring, the corresponding figures were 6.9%, 18.5%, and 27.7%, illustrating a more dramatic increase in elevated persistent externalizing behavior as a function of GABRA2 genotype.
Table 4. Percentage of Individuals Belonging to Each Trajectory Class as a Function of Genotype and Parental Monitoring and Associated P Values for Tests of Interaction.
| Individuals by Genotype, %b | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| High Parental Monitoringc | Low Parental Monitoringc | ||||||||||||
| 0 | 1 | 2 | 0 | 1 | 2 | Interaction Effects | |||||||
| SNPa | DL | EP | DL | EP | DL | EP | DL | EP | DL | EP | DL | EP | P Value |
| rs497068 | 89.7 | 10.3 | 88.0 | 12.0 | 83.7 | 16.3 | 93.1 | 6.9 | 81.5 | 18.5 | 72.3 | 27.7 | .01 |
| rs548583 | 89.7 | 10.3 | 87.8 | 12.2 | 84.0 | 16.0 | 93.1 | 6.9 | 81.5 | 18.5 | 71.1 | 28.9 | .007 |
| rs279871 | 89.7 | 10.3 | 88.0 | 12.0 | 84.3 | 15.7 | 93.1 | 6.9 | 81.7 | 18.3 | 72.3 | 27.7 | .02 |
| rs279858 | 94.4 | 5.6 | 87.8 | 12.2 | 82.7 | 17.3 | 92.3 | 7.7 | 82.5 | 17.5 | 72.3 | 27.7 | .02 |
| rs279845 | 89.7 | 10.3 | 88.0 | 12.0 | 84.0 | 16.0 | 92.3 | 7.7 | 81.5 | 18.5 | 71.7 | 28.3 | .03 |
| rs1440130 | 97.0 | 3.0 | 85.7 | 14.3 | 83.3 | 16.7 | 92.0 | 8.0 | 80.2 | 19.8 | 76.0 | 24.0 | .02 |
| rs279826 | 97.0 | 3.0 | 85.7 | 14.3 | 83.0 | 17.0 | 92.0 | 8.0 | 81.3 | 18.7 | 75.5 | 24.5 | .03 |
| rs279827 | 97.1 | 2.9 | 84.7 | 15.3 | 83.9 | 16.1 | 91.3 | 8.7 | 81.3 | 18.7 | 76.0 | 24.0 | .03 |
| rs279828 | 96.9 | 3.1 | 87.0 | 13.0 | 82.1 | 17.9 | 91.3 | 8.7 | 80.2 | 19.8 | 76.5 | 23.5 | .03 |
| rs279836 | 89.7 | 10.3 | 88.0 | 12.0 | 83.7 | 16.3 | 92.9 | 7.1 | 81.5 | 18.5 | 72.3 | 27.7 | .02 |
Abbreviations: DL, developmentally limited; EP, elevated, persistent; SNP, single-nucleotide polymorphism.
SNPs are in strong linkage disequilibrium and do not represent independent tests.
Genotype designation (0, 1, 2) reflects the number of copies of the minor allele.
For illustrative purposes, the high vs low parental monitoring designation was derived via median split.
Comment
As we successfully identify genes involved in psychiatric disorders, the next important step will be to characterize the pathways of risk associated with identified genes. This must involve studying how these genes affect risk across development and how the risk associated with susceptibility genes may change as a function of the environment. Here we describe analyses from one such effort. An association between GABRA2 and increased risk for adult alcohol dependence had been established across multiple studies. We extended these findings by genotyping GABRA2 in an independent community sample of children, followed longitudinally from childhood through young adulthood. Using data on externalizing behavior as reported at 9 time points between ages 12 and 22 years, we used person-oriented latent class analysis to identify 2 classes of trajectories of externalizing behavior; most of the sample (83%) showed a decrease in externalizing behavior from early adolescence to adulthood, while 17% of the sample showed consistent elevated levels of externalizing behavior that persisted into adulthood. The individuals showing this pattern of persistently high externalizing behavior were significantly more likely to carry the variant of GABRA2 that was originally associated with increased risk for adult alcohol dependence in the COGA sample13 (though we note that there is inconsistency as to the risk allele across studies).39 Our findings extend the association with GABRA2 to a nonselected community-based sample, confirm broad-based involvement in general externalizing behavior, and demonstrate that this gene is associated not only cross-sectionally with behavioral outcome, but with different trajectories of behavior extending from adolescence to young adulthood.
The trajectories of externalizing behavior identified in our sample have interesting connections with the broader literature on antisocial behavior across development. Considerable literature exists on the differentiation between adolescent-limited and life-course–persistent antisocial behavior, a seminal developmental taxonomy proposed by Moffitt.53 This taxonomy differentiates individuals showing patterns of delinquency and more severe antisocial behavior, so we do not presuppose to make direct comparisons with the trajectories identified in our community-based sample. However, we note that there are interesting similarities that reflect aspects of Moffitt's observations on antisocial behavior. Moffitt suggested that some degree of externalizing behavior in adolescence is normative and hypothesized that this reflects a reaction to the maturity gap that exists as a result of the fact that puberty and biological maturation precede the granting of adult privileges and responsibilities.53 The majority class identified in our sample shows a pattern in which externalizing behavior is highest early in adolescence (though with mean levels still in the normal range), and there is a gradual decline in externalizing behavior as individuals age toward young adulthood. In addition, parallel to Moffitt's theory, we find a group of individuals who persist in their externalizing behavior across time. Though we do not suggest that all of these individuals are necessarily “life-course persistent” antisocial individuals, it is interesting nonetheless that in a community sample of general externalizing behavior, as assessed using a measure that includes both potentially clinically symptomatic conduct problems (eg, getting into fights, running away from home, stealing) as well as less severe behaviors such as arguing, being loud, and bragging, we observe patterns of both persistence and normative patterns of both persistence in externalizing behavior and normative declines in externalizing behavior as the individual matures.
What might be the mechanism by which GABRA2 affects risk for externalizing behavior? All of the outcomes that have been associated with GABRA2 (adult alcohol dependence, drug dependence, adult antisocial behavior, childhood conduct problems, adolescent externalizing behavior) are characterized by aspects of impulsivity. Accordingly, it seems plausible that genetic variants in GABRA2 may be involved in a general predisposition toward behavioral disinhibition. The association of GABRA2 with an electrophysiological endophenotype13 may further support this hypothesis, as it has been suggested that the electrophysiological abnormalities evident in individuals at risk for various forms of externalizing psychopathology represent a deficit of central nervous system inhibition and/or an excess of central nervous system excitation.54 This central nervous system hyperexcitability reflects a disequilibrium in the homeostatic mechanisms responsible for maintaining a balance between excitation and inhibition. Variations in GABRA2 may be involved in creating this homeostatic imbalance, which may in turn increase risk for externalizing behavior.
Importantly, we find evidence that the association between GABRA2 and trajectories of externalizing behavior is moderated by parental monitoring; the effect of the genotype on externalizing behavior is stronger under conditions of lower parental monitoring and weaker under conditions of higher parental monitoring. This finding is consistent with the evidence of moderation from twin studies in which heritable influences were found to be higher under conditions of lower parental monitoring and lower under conditions of higher parental monitoring.30 However, that demonstration of moderation was only at the level of latent genetic influences—that is, the heritability of the trait was shown to differ, as inferred by comparing monozygotic and dizygotic twin correlations; no specific genes were measured. Herein, we find that the effect of a specific measured gene varies as a function of parental monitoring in the predicted way. These findings underscore the utility of using results from twin studies about how genetic influences act (as inferred by monozygotic/dizygotic twin correlation comparisons) to develop testable hypotheses in relation to the effect associated with specific measured genes.
How does one interpret the interaction observed between GABRA2 and parental monitoring? Several different models have been proposed for how social context may alter genetic expression; for example, the environment may trigger, enhance, or compensate for a genetic predisposition.55 We hypothesize that the effect of parental monitoring likely operates through another proposed mechanism by controlling the expression of a genetic predisposition by changing the opportunity to engage in behavior to which an individual may be predisposed. Conditions of high parental monitoring may constrain the behavior of youth, precluding adolescents with high-risk GABRA2 variants from having the opportunity to engage in antisocial behaviors. Conversely, conditions of low parental monitoring may provide greater opportunity for engagement in antisocial acts among those predisposed to such behavior. Many of the gene-environment interactions that have been described in substance use and antisocial behavior in the twin literature appear to act through social control mechanisms. For example, the heritability of alcohol use and externalizing behavior in adolescents is also lower in rural environments compared with urban settings, presumably due to enhanced community monitoring and more restricted access to alcohol, leading to reduced opportunity to express genetic predispositions.8,56,57
Although the GABRA2–parental monitoring interaction described here is consistent with the mechanisms suggested by the moderation of latent genetic influences from the twin literature, it is notable that we conceptualize the relationship between this particular gene and environment differently from that reported for other specific gene-environment interactions in the literature. For example, with respect to the interaction between monoamine oxidase A and physical maltreatment in the development of antisocial behavior, the original report stated, “MAOA [monoamine oxidase A] was found to moderate the effect of maltreatment.”12(p851) Similarly, the original description of interaction between life stress and the serotonin transporter (5-HTT) gene on depression was titled, “Influence of Life Stress on Depression: Moderation by a Polymorphism in the 5-HTT Gene.”11 Thus, in both of these cases, the genotype was characterized as the moderator of an association between an environmental risk factor and behavioral outcome. In principle, gene-environment interaction can be conceptualized either as genetic moderation of environmental effects or environmental moderation of genetic effects.58 In the case of the interaction between GABRA2, parental monitoring, and externalizing behavior reported here, we conceptualize parental monitoring as a context that moderates the likelihood that individuals carrying the high-risk genotype at GABRA2 will display externalizing psychopathology. Thus, we theorize that parental monitoring acts as the moderator rather than the genotype acting as the moderator. We believe that in the case of GABRA2, parental monitoring, and the development of externalizing behavior, this represents the more plausible pathway of risk. In addition, we add a cautionary note that although we refer to the interaction as a gene-environment interaction, we cannot rule out the possibility that it represents an epistatic association (ie, gene-gene interaction). There was no association between GABRA2 and the measure of parental monitoring analyzed in our sample; however, it is possible that other genetic variants influence adolescents' behavior in a way that elicits differential parental monitoring, in which case the interaction could represent epistasis. However, even if this was the case, we still believe that mediation of the effect through the construct of parental monitoring (though it may be genetically influenced59,60) would be of importance.
Our study has several limitations. Owing to the modest sample size, we did not have sufficient power to test for sex differences. Some studies have suggested that girls are more susceptible to environmental influences during adolescence,61,62 and potential sex differences in the effect reported here should be explored in larger samples. Additionally, we used maternal reports of parental monitoring in this study. Previous studies have found that differences in how monitoring is measured (eg, parent vs child report, observation) can lead to different conclusions, including in genetic studies.63 Accordingly, the robustness of this finding across different measures of monitoring should be explored.
In conclusion, we find evidence that GABRA2 is associated with different developmental patterns of externalizing behavior from adolescence to young adulthood. Importantly, we also find evidence that the effect of this gene on externalizing behavior can vary as a function of parental monitoring. Identifying how specific environmental variables moderate risk associated with identified genes will be necessary to advance our ability to design more effective prevention and intervention programs for individuals at risk. These analyses underscore the importance of studying genetic effects across development and of identifying environmental factors that moderate risk.
Acknowledgments
Funding/Support: The Child Development Project has been funded by grants MH42498, MH56961, MH57024, MH057024-07S1 (supplemental funds to collect DNA), and MH57095 from the National Institute of Mental Health; HD30572 from the Eunice Kennedy Shriver National Institute of Child Health and Human Development; and DA016903 from the National Institute on Drug Abuse. Drs Latendresse and Dick are also supported by AA-15416 from the National Institute of Alcohol Abuse and Alcoholism to Dr Dick. Dr Dodge acknowledges the support of Senior Scientist Award DA015226 and Center grant DA017589 from the National Institute on Drug Abuse 5R01DA16903.
Footnotes
Financial Disclosure: Dr Goate reports being listed as an inventor on patent US 20070258898, held by Perlegen Sciences, Inc, covering the use of certain single-nucleotide polymorphisms in diagnosing, prognosing, and treating addiction. No other authors report any financial disclosures.
Previous Presentations: These data were presented at the Association for Psychological Science meeting May 2008; Chicago, Illinois; and the Behavior Genetics Association Meeting; June 2008; Louisville, Kentucky.
References
- 1.Manolio TA, Brooks LD, Collins FSA. HapMap harvest of insights into the genetics of common disease. J Clin Invest. 2008;118(5):1590–1605. doi: 10.1172/JCI34772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wellcome Trust Case Control Consortium. Genome-wide association study of 14,000 cases of seven common diseases and 3,000 shared controls. Nature. 2007;447(7145):661–678. doi: 10.1038/nature05911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Manolio TA, Rodriguez LL, Brooks L, Abecasis G, GAIN Collaborative Research Group. Ballinger D, Daly M, Donnelly P, Faraone SV, Collaborative Association Study of Psoriasis. Frazer K, Gabriel S, Gejman P, International Multi-Center ADHD Genetics Project. Guttmacher A, Harris EL, Insel T, Kelsoe JR, Molecular Genetics of Schizophrenia Collaboration. Lander E, McCowin N, Mailman MD, Nabel E, Ostell J, Pugh E, Sherry S, Sullivan PF, Bipolar Genome Study. Thompson JF, Warram J, Major Depression Stage 1 Genomewide Association in Population-Based Samples Study. Wholley D, Milos PM, Collins FS, Genetics of Kidneys in Diabetes (GoKinD) Study New models of collaboration in genome-wide association studies: the Genetic Association Information Network. Nat Genet. 2007;39(9):1045–1051. doi: 10.1038/ng2127. [DOI] [PubMed] [Google Scholar]
- 4.Dick DM, Rose RJ, Kaprio J. The next challenge for psychiatric genetics: characterizing the risk associated with identified genes. Ann Clin Psychiatry. 2005;18(4):223–231. doi: 10.1080/10401230600948407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Plomin R, DeFries JC, McClearn GE, McGuffin P. Behavioral Genetics. London, England: Worth; 2001. [Google Scholar]
- 6.Reiss D, Plomin R, Hetherington EM. Genetics and psychiatry: an unheralded window on the environment. Am J Psychiatry. 1991;148(3):283–291. doi: 10.1176/ajp.148.3.283. [DOI] [PubMed] [Google Scholar]
- 7.Kendler KS, Prescott C, Myers J, Neale MC. The structure of genetic and environmental risk factors for common psychiatric and substance use disorders in men and women. Arch Gen Psychiatry. 2003;60(9):929–937. doi: 10.1001/archpsyc.60.9.929. [DOI] [PubMed] [Google Scholar]
- 8.Dick DM, Rose RJ, Viken RJ, Kaprio J, Koskenvuo M. Exploring gene-environment interactions: socioregional moderation of alcohol use. J Abnorm Psychol. 2001;110(4):625–632. doi: 10.1037//0021-843x.110.4.625. [DOI] [PubMed] [Google Scholar]
- 9.Button TM, Scourfield J, Martin N, Purcell S, McGuffin P. Family dysfunction interacts with genes in the causation of antisocial symptoms. Behav Genet. 2005;35(2):115–120. doi: 10.1007/s10519-004-0826-y. [DOI] [PubMed] [Google Scholar]
- 10.Heath AC, Eaves LJ, Martin NG. Interaction of marital status and genetic risk for symptoms of depression. Twin Res. 1998;1(3):119–122. doi: 10.1375/136905298320566249. [DOI] [PubMed] [Google Scholar]
- 11.Caspi A, Sugden K, Moffitt TE, Taylor A, Craig IW, Harrington H, McClay J, Mill J, Martin J, Braithwaite A, Poulton R. Influence of life stress on depression: moderation by a polymorphism in the 5-HTT gene. Science. 2003;301(5631):386–389. doi: 10.1126/science.1083968. [DOI] [PubMed] [Google Scholar]
- 12.Caspi A, McClay J, Moffitt TE, Mill J, Martin J, Craig IW, Taylor A, Poulton R. Role of genotype in the cycle of violence in maltreated children. Science. 2002;297(5582):851–854. doi: 10.1126/science.1072290. [DOI] [PubMed] [Google Scholar]
- 13.Edenberg HJ, Dick DM, Xuei X, Tian H, Almasy L, Bauer LO, Crowe RR, Goate A, Hesselbrock V, Jones K, Kwon J, Li TK, Nurnberger JI, Jr, O'Connor SJ, Reich T, Rice J, Schuckit MA, Porjesz B, Foroud T, Begleiter H. Variations in GABRA2, encoding the alpha 2 subunit of the GABA(A) receptor, are associated with alcohol dependence and with brain oscillations. Am J Hum Genet. 2004;74(4):705–714. doi: 10.1086/383283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Covault J, Gelernter J, Hesselbrock V, Nellissery M, Kranzler HR. Allelic and haplotypic association of GABRA2 with alcohol dependence. Am J Med Genet B Neuropsychiatr Genet. 2004;129B(1):104–109. doi: 10.1002/ajmg.b.30091. [DOI] [PubMed] [Google Scholar]
- 15.Fehr C, Sander T, Tadic A, Lenzen KP, Anghelescu I, Klawe C, Dahmen N, Schmidt LG, Szegedi A. Confirmation of association of the GABRA2 gene with alcohol dependence by subtype-specific analysis. Psychiatr Genet. 2006;16(1):9–17. doi: 10.1097/01.ypg.0000185027.89816.d9. [DOI] [PubMed] [Google Scholar]
- 16.Soyka M, Preuss UW, Hesselbrock V, Zill P, Koller G, Bondy B. GABA-A2 receptor subunit gene (GABRA2) polymorphisms and risk for alcohol dependence. J Psychiatr Res. 2008;42(3):184–191. doi: 10.1016/j.jpsychires.2006.11.006. [DOI] [PubMed] [Google Scholar]
- 17.Xu K, Westly E, Taubman J, Astor W, Lipsky RH, Goldman D. Linkage disequilibrium relationships among GABRA cluster genes located on chromosome 4 with alcohol dependence in two populations. Alcohol Clin Exp Res. 2004;28:48A. [Google Scholar]
- 18.Agrawal A, Edenberg HJ, Foroud T, Bierut LJ, Dunne G, Hinrichs AL, Nurnberger JI, Crowe R, Kuperman S, Schuckit MA, Begleiter H, Porjesz B, Dick DM. Association of GABRA2 with drug dependence in the Collaborative Study of the Genetics of Alcoholism sample. Behav Genet. 2006;36(5):640–650. doi: 10.1007/s10519-006-9069-4. [DOI] [PubMed] [Google Scholar]
- 19.Dick DM, Bierut L, Hinrichs AL, Fox L, Bucholz KK, Kramer J, Kuperman S, Hesselbrock V, Schuckit M, Almasy L, Tischfield J, Porjesz B, Begleiter H, Nurnberger J, Jr, Xuei X, Edenberg HJ, Foroud T. The role of GABRA2 in risk for conduct disorder and alcohol and drug dependence across developmental stages. Behav Genet. 2006;36(4):577–590. doi: 10.1007/s10519-005-9041-8. [DOI] [PubMed] [Google Scholar]
- 20.Dick DM, Agrawal A, Schuckit M, Bierut L, Hinrichs A, Fox L, Mullaney J, Cloninger CR, Hesselbrock V, Nurnberger JI, Jr, Almasy L, Foroud T, Porjesz B, Edenberg H, Begleiter H. Marital status, alcohol dependence, and GABRA2: evidence for gene-environment correlation and interaction. J Stud Alcohol. 2006;67(2):185–194. doi: 10.15288/jsa.2006.67.185. [DOI] [PubMed] [Google Scholar]
- 21.Dick DM. Identification of genes influencing a spectrum of externalizing psychopathology. Curr Dir Psychol Sci. 2007;16(6):331–335. [Google Scholar]
- 22.Marshal MP, Chassin L. Peer influence on adolescent alcohol use: the moderating role of parental support and discipline. Appl Dev Sci. 2000;4(2):80–88. [Google Scholar]
- 23.Johnstone BM. Sociodemographic, environmental, and cultural influences on adolescent drinking behavior. In: Zucker R, Boyd G, Howard J, editors. The Development of Alcohol Problems: Exploring the Biopsychosocial Matrix of Risk. Rockville, MD: NIH Publication; 1994. pp. 169–203. [Google Scholar]
- 24.Windle M. Parental, sibling, and peer influences on adolescent substance use and alcohol problems. Appl Dev Sci. 2000;4(2):98–110. [Google Scholar]
- 25.Leventhal T, Brooks-Gunn J. The neighborhoods they live in: the effects of neighborhood residence on child and adolescent outcomes. Psychol Bull. 2000;126(2):309–337. doi: 10.1037/0033-2909.126.2.309. [DOI] [PubMed] [Google Scholar]
- 26.Barnes GM, Farrell MP. Parental support and control as predictors of adolescent drinking, delinquency, and related problem behaviors. J Marriage Fam. 1992;54:763–776. [Google Scholar]
- 27.Steinberg L, Fletcher A, Darling N. Parental monitoring and peer influences on adolescent substance use. Pediatrics. 1994;93(6 pt 2):1060–1064. [PubMed] [Google Scholar]
- 28.Chilcoat HD, Anthony JC. Impact of parental monitoring on initiation of drug use through late childhood. J Am Acad Child Adolesc Psychiatry. 1996;35(1):91–100. doi: 10.1097/00004583-199601000-00017. [DOI] [PubMed] [Google Scholar]
- 29.Dick DM, Pagan JL, Viken R, Purcell S, Kaprio J, Pulkkinen L, Rose RJ. Changing environmental influences on substance use across development. Twin Res Hum Genet. 2007;10(2):315–326. doi: 10.1375/twin.10.2.315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dick DM, Viken R, Purcell S, Kaprio J, Pulkkinen L, Rose RJ. Parental monitoring moderates the importance of genetic and environmental influences on adolescent smoking. J Abnorm Psychol. 2007;116(1):213–218. doi: 10.1037/0021-843X.116.1.213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Achenbach TM. Manual for the Youth Self-Report and 1991 Profile. Burlington: Dept of Psychiatry, University of Vermont; 1991. [Google Scholar]
- 32.Achenbach TM. Manual for the Young Adult Self-report and Young Adult Behavior Checklist. Burlington: Dept of Psychiatry, University of Vermont; 1997. [Google Scholar]
- 33.Ivanova MY, Achenbach TM, Rescorla LA, Dumenci L, Almqvist F, Bilenberg N, Bird H, Broberg AG, Dobrean A, Döpfner M, Erol N, Forns M, Hannesdottir H, Kanbayashi Y, Lambert MC, Leung P, Minaei A, Mulatu MS, Novik T, Oh KJ, Roussos A, Sawyer M, Simsek Z, Steinhausen HC, Weintraub S, Winkler Metzke C, Wolanczyk T, Zilber N, Zukauskiene R, Verhulst FC. The generalizability of the Youth Self-Report syndrome structure in 23 societies. J Consult Clin Psychol. 2007;75(5):729–738. doi: 10.1037/0022-006X.75.5.729. [DOI] [PubMed] [Google Scholar]
- 34.Achenbach TM, Rescorla LA. Manual for ASEBA Adult Forms & Profiles. Burlington: University of Vermont, Research Center for Children, Youth, & Families; 2003. [Google Scholar]
- 35.Achenbach TM, Rescorla LA. Manual for ASEBA School-Age Forms & Profiles. Burlington: University of Vermont, Research Center for Children, Youth, & Families; 2001. [Google Scholar]
- 36.Pettitt GS, Bates JE, Dodge KA, Meece DW. The impact of after-school peer contact on early adolescent externalizing problems is moderated by parental monitoring, perceived neighborhood safety, and prior adjustment. Child Dev. 1999;70(3):768–778. doi: 10.1111/1467-8624.00055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Stattin H, Kerr M. Parental monitoring: a reinterpretation. Child Dev. 2000;71(4):1072–1085. doi: 10.1111/1467-8624.00210. [DOI] [PubMed] [Google Scholar]
- 38.Kerr M, Stattin H. What parents know, how they know it, and several forms of adolescent adjustment: further support for a reinterpretation of monitoring. Dev Psychol. 2000;36(3):366–380. [PubMed] [Google Scholar]
- 39.Enoch MA. The role of GABA(A) receptors in the development of alcoholism. Pharmacol Biochem Behav. 2008;90(1):95–104. doi: 10.1016/j.pbb.2008.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Barrett JC, Fry B, Maller J, Daly MJ. Haploview: analysis and visualization of LD and haplotype maps. Bioinformatics. 2005;21(2):263–265. doi: 10.1093/bioinformatics/bth457. [DOI] [PubMed] [Google Scholar]
- 41.Hedrick P, Kumar S. Mutation and linkage disequilibrium in human mtDNA. Eur J Hum Genet. 2001;9(12):969–972. doi: 10.1038/sj.ejhg.5200735. [DOI] [PubMed] [Google Scholar]
- 42.Muthén B, Shedden K. Finite mixture modeling with mixture outcomes using the EM algorithm. Biometrics. 1999;55(2):463–469. doi: 10.1111/j.0006-341x.1999.00463.x. [DOI] [PubMed] [Google Scholar]
- 43.Jung T, Wickrama KAS. An introduction to latent class growth analysis and growth mixture modeling. Soc Pers Psychol Compass. 2007;1:1–9. [Google Scholar]
- 44.McArdle JJ, Epstein D. Latent growth curves within developmental structural equation models. Child Dev. 1987;58(1):110–133. [PubMed] [Google Scholar]
- 45.Muthén BO, Muthén LK. Integrating person-centered and variable centered analyses: growth mixture modeling with latent trajectory classes. Alcohol Clin Exp Res. 2000;24(6):882–891. [PubMed] [Google Scholar]
- 46.Mplus Users Guide [computer program] Los Angeles, CA: Muthén LK and Muthén BO; 2006. [Google Scholar]
- 47.Yamaguchi K. Multinomial logit latent-class regression models: an analysis of the predictors of gender-role attitudes among Japanese women. Am J Sociol. 2000;105(6):1702–1740. [Google Scholar]
- 48.Kass RE, Raftery AE. Bayes factors. J Am Stat Assoc. 1995;90:773–795. [Google Scholar]
- 49.Keribin C. Consistent estimation of the order of mixture models. Indian J Stat. 1997;62:49–66. [Google Scholar]
- 50.Akaike H. A new look at the statistical model identification. IEEE Trans Automatic Control. 1974;19(6):716–723. [Google Scholar]
- 51.Muthén BO. Latent variable analysis: growth mixture modeling and related techniques for longitudinal data. In: Kaplan D, editor. Handbook of Quantitative Methodology for the Social Sciences. Thousand Oaks, CA: Sage Publications; 2004. pp. 345–368. [Google Scholar]
- 52.Lo Y, Mendell N, Rubin D. Testing the number of components in a normal mixture. Biometrika. 2001;88(3):767–778. [Google Scholar]
- 53.Moffitt TE. Adolescence-limited and life-course-persistent antisocial behavior: a developmental taxonomy. Psychol Rev. 1993;100(4):674–701. [PubMed] [Google Scholar]
- 54.Begleiter H, Porjesz B. What is inherited in the predisposition toward alcoholism? a proposed model. Alcohol Clin Exp Res. 1999;23(7):1125–1135. doi: 10.1111/j.1530-0277.1999.tb04269.x. [DOI] [PubMed] [Google Scholar]
- 55.Shanahan MJ, Hofer SM. Social context in gene-environment interactions: retrospect and prospect. J Gerontol B Psychol Sci Soc Sci. 2005;60(1):65–76. doi: 10.1093/geronb/60.special_issue_1.65. [DOI] [PubMed] [Google Scholar]
- 56.Rose RJ, Dick DM, Viken And RJ, Kaprio J. Gene-environment interaction in patterns of adolescent drinking: regional residency moderates longitudinal influences on alcohol use. Alcohol Clin Exp Res. 2001;25(5):637–643. [PubMed] [Google Scholar]
- 57.Legrand LN, Keyes M, McGue M, Iacono WG, Krueger RF. Rural environments reduce the genetic influence on adolescent substance use and rule-breaking behavior [published online ahead of print October 1, 2007] Psychol Med. 2008;38(9):1341–1350. doi: 10.1017/S0033291707001596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kendler KS, Eaves LJ. Models for the joint effect of genotype and environment on liability to psychiatric illness. Am J Psychiatry. 1986;143(3):279–289. doi: 10.1176/ajp.143.3.279. [DOI] [PubMed] [Google Scholar]
- 59.Plomin R, Bergeman CS. The nature of nurture: genetic influence on “environmental” measures. Behav Brain Sci. 1991;14:373–427. [Google Scholar]
- 60.Reiss D. Genetic influence on family systems: implications for development. J Marriage Fam. 1995;57:543–560. [Google Scholar]
- 61.Rose RJ, Dick DM, Viken RJ, Pulkkinen L, Kaprio J. Drinking or abstaining at age 14: a genetic epidemiological study. Alcohol Clin Exp Res. 2001;25(11):1594–1604. [PubMed] [Google Scholar]
- 62.Dick DM, Pagan JL, Holliday C, Viken R, Pulkkinen L, Kaprio J, Rose RJ. Gender differences in friends' influences on adolescent drinking: a genetic epidemiological study. Alcohol Clin Exp Res. 2007;31(12):2012–2019. doi: 10.1111/j.1530-0277.2007.00523.x. [DOI] [PubMed] [Google Scholar]
- 63.Neiderhiser JM, Reiss D, Pedersen N, Lichtenstein P, Spotts EL, Hansson K, Cederblad M, Ellhammer O. Genetic and environmental influences on mothering of adolescents: a comparison of two samples. Dev Psychol. 2004;40(3):335–351. doi: 10.1037/0012-1649.40.3.335. [DOI] [PMC free article] [PubMed] [Google Scholar]