Abstract
Background
Hepatic glucose uptake is enhanced by portal delivery of glucose which creates a negative arterio-portal substrate gradient. Hepatic amino acid (AA) utilization may be regulated by the same phenomenon, but this has not been proven.
Objective
We aimed to assess hepatic AA balance and protein synthesis with or without a negative arterio-portal AA gradient.
Design
Somatostatin was infused IV, and insulin and glucagon were replaced intraportally at 4- and 3-fold basal rates, respectively, in 3 groups (n=9 each) of conscious dogs with catheters for hepatic balance measurement. Arterial glucose concentrations were clamped at 9 mM. An AA mixture was infused IV to maintain basal concentrations (EuAA), intraportally to mimic the post-meal AA increase (PoAA), or IV (PeAA) to match the hepatic AA load in PoAA. Protein synthesis was assessed with a primed, continuous [14C]leucine infusion.
Results
Net hepatic glucose uptake in PoAA was ≤50% of that in EuAA and PeAA (P<0.05). The hepatic intracellular leucine concentration was 2- to 2.5-fold greater in PoAA and PeAA than EuAA (P<0.05); net hepatic leucine uptake and 14C leucine utilization were ≈2-fold greater (P<0.05) and albumin synthesis was 30% greater (P<0.05) in PoAA than EuAA and PeAA, Phosphorylation of ribosomal protein S6 (downstream of the mammalian target of Rapamycin complex 1 [mTORC1]) was significantly increased in PoAA, but not PeAA, vs EuAA.
Conclusions
Portal, but not peripheral, AA delivery significantly enhanced hepatic protein synthesis under conditions where AA, glucose, insulin and glucagon did not differ at the liver, an effect apparently mediated by mTORC1 signalling.
Keywords: liver, protein synthesis, portal vein, amino acids
INTRODUCTION
Protein synthesis in the liver and intestines is considerably higher per gram tissue than in most other tissues, accounting for 25% of whole-body protein synthesis (1). Forty percent of the protein synthesized in the liver is secreted, with albumin alone accounting for almost 40% of secreted proteins. Following a mixed meal, the liver is exposed to high levels of amino acids (AAs) and insulin. In adult humans, increasing amino acid (AA) levels by intravenous infusion results in a stimulation of protein synthesis across the splanchnic bed (2).
Splanchnic metabolism includes both gut and hepatic metabolism, for which regulatory pathways could be different. Studies conducted in mature animals with intravenous infusion of AAs have shown that AAs and insulin have no stimulatory effect on liver protein synthesis (3–5). Conversely, in the neonate, hepatic protein synthesis can be stimulated by the rise in AAs (6–7). Davis et al. (7) have observed a developmental decline in AA-induced stimulation of hepatic protein synthesis in the growing animal. Similarly, aging is associated with a decline in the anabolic response of skeletal muscle to food intake and AAs (8–10). From these studies, one might infer that there is a decreased sensitivity of the liver to AAs during aging. However, this inference is inconsistent with findings in adult human volunteers, in whom dietary AAs have been shown to increase hepatic secretory protein synthesis (11–14).
One difference between the animal and human studies cited above is the route of administration of AAs: peripherally in most of the animal studies versus orally in most of the human investigations. We hypothesized that, in the mature organism, the route of delivery of AAs is critical in the stimulation of hepatic protein synthesis. In support of this hypothesis, human studies have shown that albumin synthesis is stimulated with oral meal feeding whereas it is not responsive to intravenous nutrients (4, 12). Furthermore, we have shown that the net hepatic uptake of glutamine and the net fractional extractions of glutamine and serine are significantly increased during the portal vein administration of AAs compared to a peripheral infusion matching hepatic AA loads (15). From the latter study, it is also tempting to hypothesize that the portal delivery of the AAs directed the AAs to hepatic protein synthesis. We therefore designed the present study to compare, using the adult conscious dog, the rate of hepatic protein synthesis observed during portal or peripheral delivery of an AA mixture that reproduces the normal elevation of AAs seen in the portal vein in the post-prandial state. Because protein metabolism is sensitive to AA availability, the concentration and the infusion rate of the AA mixtures were adjusted to maintain the same hepatic AA load between groups under pancreatic clamp conditions. The synthesis rate of resident or exported proteins was then assessed, as was the intracellular signalling pathway leading to protein synthesis.
MATERIALS AND METHODS
Animals and Surgical Procedures
Experiments were performed on twenty seven 42-h fasted conscious adult male mongrel dogs (20–25 kg) that had been fed once daily a standard meat and kibble diet (31% protein, 52% carbohydrate, 11% fat, and 6% fiber based on dry weight, Kal Kan; Vernon, CA; and Purina Lab Canine Diet no. 5006, Purina Mills; St. Louis, MO). The dogs were housed in a facility that met American Association for the Accreditation of Laboratory Animal Care guidelines, and protocols were approved by the Vanderbilt University Medical Center Animal Care Committee. At least 16 days before experimentation, a laparotomy was performed with animals under general anesthesia. Silastic catheters (Dow Corning; Midland, MI) for blood sampling were placed into the portal vein, a hepatic vein, and a femoral artery, and infusion catheters were inserted into a jejunal vein and a splenic vein as previously described (16). Ultrasonic flow probes (Transonic Systems; Ithaca, NY) were placed around the portal vein and hepatic artery. On the day of the experiment, the catheters were exteriorized under local anesthesia, and intravenous access was established in three peripheral veins. Dogs were used for an experiment only if they met established criteria for good health (17).
Experimental design
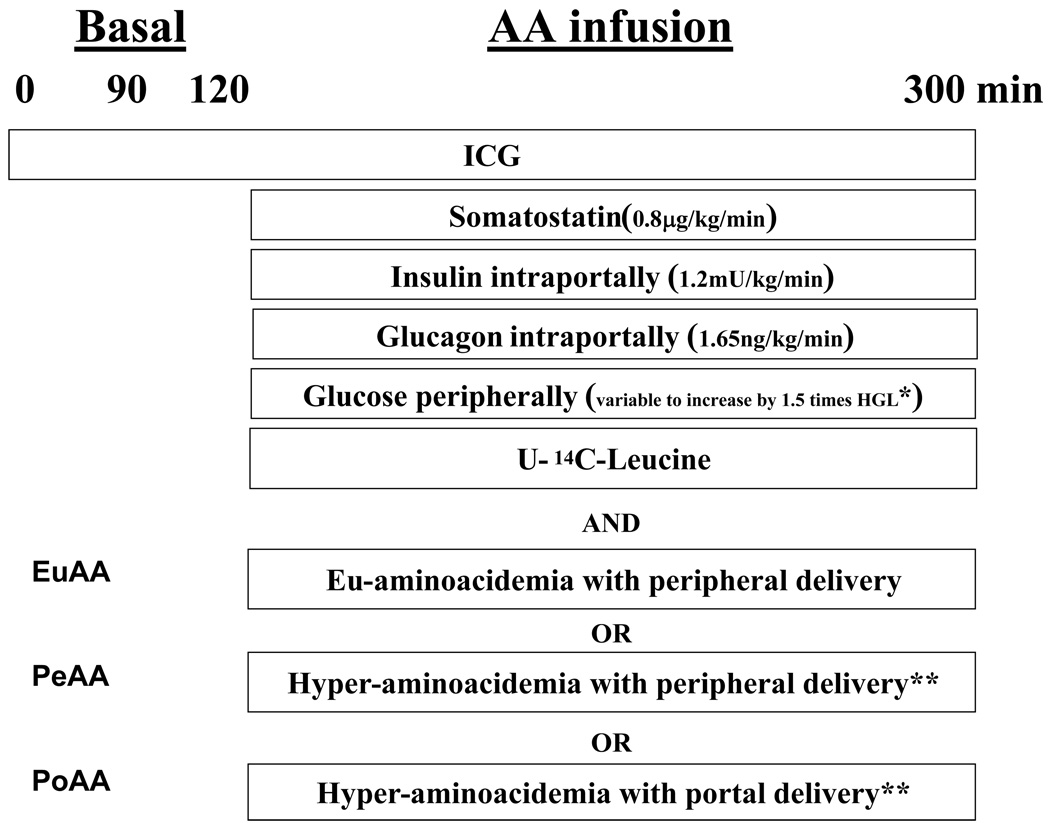
In each of the three groups (n = 9 dogs each), the protocol consisted of an equilibration period (0–90 min) and a basal period (90–120 min), followed by an experimental period (120–300 min) (Figure. 1). At time (t) = 0 min, a continuous infusion of indocyanine green dye (0.08 mg/min, Sigma Chemical; St. Louis, MO) was started. At t = 120 min, the timer was stopped briefly. A peripheral venous infusion of somatostatin (0.8 µg·kg−1·min−1) was begun to inhibit endogenous pancreatic hormone secretion. Two minutes later, an intraportal insulin infusion (1.2 mU·kg−1·min−1; to produce 3- to 4-fold basal circulating concentrations) was initiated. Two minutes after the insulin infusion started, an intraportal glucagon infusion (1.65 ng·kg−1·min−1; to produce 3-fold basal concentrations) was started. Two minutes later, dextrose and AA infusions were initiated, and the timer was restarted. Over many years, we have demonstrated that this staggered approach to initiating infusions is effective in controlling insulin and glucagon concentrations. A dextrose solution was infused peripherally at variable rates starting at t = 120 min to clamp the arterial plasma glucose level at 9.0 mM. The infusion rate of glucose was adjusted in response to the plasma glucose concentration, which was measured every 5 min. The euaminoacidemia group (EuAA) received a mixture of AAs peripherally to maintain the plasma AAs at their post-absorptive levels since insulin is known to decrease plasma AA levels; the portal group (PoAA) received AAs intraportally to mimic a meal, and the peripheral group (PeAA) received AAs peripherally. Because protein metabolism is sensitive to the AA availability, the concentration of the AA mixtures were adjusted to maintain hepatic AA load equivalence for each amino acid in the PoAA and PeAA groups. AA concentrations of the infusates (Table 1) were determined in pilot experiments, and the mixture was infused at the rate of 0.034 ml kg−1·min−1. Protein synthesis was assessed in all groups by a primed (7.6 µCi/kg), continuous infusion (0.76 µCi/kg/min) of [U-14C]leucine (Moravek Biomedicals, Brea, CA) beginning at 120 min. Blood was collected on EDTA. At the end of the 300 min experimental period, animals were sacrificed and samples of each liver lobe were rapidly freeze clamped and stored at −80°C.
Figure 1.
Experimental design. * HGL= Hepatic glucose load; ** same amino acid hepatic load.
Table 1.
Concentration of the aminoacid mixture infused in the three experimental groups
| Amino acid infusate concentration µM |
|||
|---|---|---|---|
| EuAA | PeAA | PoAA | |
| Arginine | 32.1 | 62.0 | 40.2 |
| Histidine | 6.7 | 11.6 | 11.4 |
| Isoleucine | 21.7 | 74.6 | 32.8 |
| Leucine | 45.7 | 137.2 | 70.1 |
| Lysine | 54.7 | 102.6 | 64.3 |
| Methionine | 11.6 | 31.5 | 14.4 |
| Threonine | 35.9 | 84.0 | 60.8 |
| Tryptophane | 8.2 | 11.8 | 13.7 |
| Valine | 27.3 | 102.5 | 56.0 |
| Phenylalanine | 9.7 | 36.3 | 21.8 |
| Alanine | 35.9 | 107.8 | 88.0 |
| Asparagine | 15.5 | 30.6 | 16.7 |
| Aspartate | 2.4 | 8.0 | 3.3 |
| Glutamate | 16.3 | 54.4 | 21.8 |
| Glutamine | 153.3 | 178.0 | 150.6 |
| Glycine | 51.4 | 45.3 | 31.0 |
| Serine | 26.1 | 66.6 | 39.8 |
| Tyrosine | 13.2 | 17.7 | 11.9 |
| Cysteine | 6.6 | 17.1 | 21.5 |
| Proline | 27.8 | 93.8 | 52.1 |
Analytical procedures
Plasma glucose, insulin and glucagon levels were measured as previously described (18). Plasma amino acids were analyzed by reverse-phase HPLC of their phenyl isothiocyanate derivatives (PicoTag, Waters, Woburn, MA). Free and bound leucine specific radioactivities were determined as described by Donahue et al (19). Plasma albumin was isolated by ethanol extraction from trichloroacetic acid precipitated plasma proteins as described previously (4).
Methods of calculation
Hepatic load (µmol kg−1·min−1)
The load of a substrate or hormone reaching the liver (HL) was calculated as
where Fa and Fp are the arterial and portal flows (ml/kg/min) and Ca and Cp are the concentrations of the variable in question in arterial and portal blood or plasma, as appropriate.
Estimated hepatic plasma sinusoidal hormone concentrations were calculated as HL/total hepatic plasma flow.
Net Hepatic Substrate Balance (nmol kg−1·min−1)
Whole blood glucose concentrations and plasma AA concentrations were used, along with blood or plasma flow, as appropriate, to assess net hepatic substrate balance (NHSB). NHSB was determined as
where Fh represents blood or plasma flow in the hepatic vein (the sum of Fa and Fp) and Ch represents the substrate concentrations in the hepatic vein. A positive value represents net production and a negative value represents net uptake of the substrate by the liver.
Hepatic leucine uptake (nmol kg−1·min−1)
Calculations were based on the differences between the specific radioactivity (Sa) of [14C] leucine entering and exiting the liver (Sain and Sah) and the tracee concentration across the liver (Cin and Ch).
where Saart, Saport are the hepatic artery and portal vein leucine specific activities and Fart and Fport are the hepatic and portal vein blood flows, respectively.
where Cart and Cport are the hepatic artery and portal vein tracee concentrations.
where Sa liver is the liver intracellular specific radioactivity of the tracer.
Protein synthesis rate
Protein synthesis was calculated using the continuous infusion of [14C]leucine as a tracer. Briefly, a primed continuous infusion of [14C]leucine was used to rapidly reach a [14C]leucine Sa plateau in the plasma. A single sample of the product (liver proteins or albumin) was then used and not the incremental increase in specific activity of protein-bound leucine across multiple time points. Liver protein and albumin synthesis rates (Ks in % per day) were calculated using the following equation:
where Sab, Satissue are the specific radioactivities at time t of the protein-bound leucine and the specific radioactivity of the leucine precursor pool between time 0 and time t. The Sa of the tissue fluid was used as the Sa of the precursor pool since Ahlman et al (5) showed it to be a reliable surrogate measure of leucyl-tRNA specific radioactivity (the real precursor pool for protein synthesis) in a swine model. Protein synthesis was measured in the seven lobes of the liver since it appeared from our preliminary studies that slight differences in the rate of protein synthesis might occur among lobes. Because labelled albumin appears in the blood stream with a lag time of 20 minutes after the beginning of the tracer infusion (secretion time determined in a pilot experiment), the calculation of albumin synthesis was identical to that of protein synthesis except that it was calculated with a t = t-20, 20 representing the average excretion time in minutes of newly synthesized plasma proteins by the liver.
Measurement of ribosomal protein S6, ribosomal protein S6 kinase 1 (S6K1), eukaryotic initiation factor (eIF)4E binding protein 1 (4E-BP1), and eIF4G Phosphorylation
Phosphorylation of ribosomal protein (rp) S6 on Ser235/236 and Ser240/244 and eIF4G phosphorylation on Ser1108 was measured by Western blot analysis as described previously (20) using anti- phospho-rpS6(Ser235/236) and anti-phospho-rpS6(Ser240/244) antibodies or anti-phospho-eIF4G(Ser1108) antibodies, respectively (Cell Signalling Technology, Beverly, MA). Phosphorylation of S6K1 and 4E-BP1 was measured as changes in migration during gel electrophoresis as described previously (20). Briefly, frozen liver samples were homogenized in 7 volumes of buffer consisting of 20 mM HEPES (pH 7.4), 100 mM KCl, 0.2 mM EDTA, 2 mM EGTA, 50 mM NaF, 50 mM β-glycerophosphate, 0.1 mM phenylmethylsulfonyl fluoride, 1 mM benzamidine, and 0.5 mM sodium vanadate using a Polytron homogenizer. The homogenate was centrifuged at 1,000 × g for 3 min and the supernatant was subjected to Western blot analysis using antibodies that recognize the protein only when it is phosphorylated on specific amino acid residues (rpS6 and eIF4G) or antibodies that recognize the protein regardless of phosphorylation state.
Statistical Analysis
Data are expressed as means ± SE and analyzed by XLStat (Addinsoft NY, USA, version 7.5.2). For net hepatic glucose balance, albumin synthesis, blood flows and sinusoidal hormone concentrations, the statistical evaluation of the data was performed by a 2-way repeated-measures analysis of variance to test the group and time effects and time × group interaction. When significant, the Bonferroni test was employed for post hoc analysis. Differences were considered significant when P < 0.05.
For protein metabolism (hepatic leucine balance, hepatic leucine utilization and hepatic protein synthesis rates), statistical evaluation of the data was performed by one-way ANOVA to analyse the effect of the route of delivery of AAs. Within the same animal of each group, values were calculated as the mean of individual values recorded between 210 and 300 min. When a significant overall effect was detected, differences among individual means were assessed with the Bonferroni test to determine significant differences. Differences were considered significant when P<0.05.
RESULTS
Hormone concentrations, blood flows and glucose balance data
Arterial blood flows were not different between groups but increased slightly during the experimental period. Portal blood flows were also not different between groups but significantly decreased during the experimental period (Table 2). As a result, total hepatic blood flow changed minimally (<10%) in all groups (not shown). Hepatic sinusoidal insulin concentrations increased by a physiological amount during the experimental period (150–300 min) (Table 2), with no significant differences among the groups. Hepatic sinusoidal glucagon concentrations increased ≈3-fold during the experimental period (150–300 min) compared to the basal period (90–120 min) and were similar in all the groups (Table 2).
Table 2.
Hepatic plasma flows, hepatic sinusoidal insulin and glucagon concentrations
| Basal | Amino acid infusion | |||||||
|---|---|---|---|---|---|---|---|---|
| Time (min) | 90 | 120 | 150 | 210 | 240 | 270 | 300 | Variance analysis |
| Arterial flows (ml/kg/min) | ||||||||
| EuAA | 3.1±0.2 | 3.0±0.2 | 4.0±0.3 | 4.3±0.4 | 4.1±0.4 | 4.5±0.4 | 4.5±0.4 | Group effect = NS |
| PeAA | 3.1±0.2 | 3.1±0.2 | 3.9±0.3 | 4.2±0.2 | 3.8±0.3 | 4.1±0.2 | 4.3±0.3 | Time effect P<0.001 |
| PoAA | 3.0±0.3 | 3.2±0.3 | 3.7±0.5 | 4.5±0.4 | 4.1±0.4 | 4.0±0.4 | 4.1±0.3 | Time×Group =NS |
| Portal flows (ml/kg/min) | ||||||||
| EuAA | 14.6±1.0 | 14.9±1.0 | 13.0±0.7 | 12.5±0.6 | 12.6±0.8 | 13.1±1.0 | 13.1±0.9 | Group effect = NS |
| PeAA | 13.6±0.8 | 14.0±0.9 | 12.4±0.9 | 12.4±0.8 | 12.6±0.8 | 12.9±0.8 | 12.9±0.9 | Time effect P<0.006 |
| PoAA | 13.7±0.6 | 14.2±0.6 | 12.7±0.8 | 12.4±0.7 | 12.7±0.6 | 12.8±0.6 | 12.9±0.7 | Time×Group =NS |
| Hepatic sinusoidal insulin (mU/ml) | ||||||||
| EuAA | 17.1±1.6 | 15.4±2.4 | 75.8±9.6 | 74.7±5.7 | 60.1±8.8 | 63.9±9.2 | 74.9±10.7 | Group effect = NS |
| PeAA | 16.1±2.1 | 20.0±5.3 | 63.5±4.8 | 72.1±11.2 | 70.5±5.7 | 73.4±4.2 | 82.7±4.4 | Time effect P<0.0001 |
| PoAA | 16.8±3.1 | 20.7±3.8 | 88.2±18.4 | 74±8.1 | 69.8±7.3 | 83.7±6.3 | 78.2±9.2 | Time×Group =NS |
| Hepatic sinusoidal glucagon (pg/ml) | ||||||||
| EuAA | 53.2± 4.7 | 49.6± 9.0 | 144.6±16.0 | 138.7±16.5 | 134.0±11.3 | 142.7±13.7 | 124.0±16.2 | Group effect = NS |
| PeAA | 42.8±6.9 | 41.6±6.2 | 110.3±16.5 | 112.2±5.3 | 113.0±5.4 | 103.1±4.3 | 107.7±7.7 | Time effect P<0.001 |
| PoAA | 30.7±4.2 | 32.4±3.8 | 122.3±15.7 | 90.6±7.8 | 96.5±10.5 | 98.2±7.8 | 106.6±13.6 | Time×Group =NS |
Data are expressed as means ± SE (n=9 in each group. The statistical evaluation of the data was performed by a 2-way repeated-measures analysis of variance to test the group and time effects and time × group interaction. When significant, the Bonferonni test was employed for post hoc analysis. Differences were considered significant when P < 0.05. NS= non significant
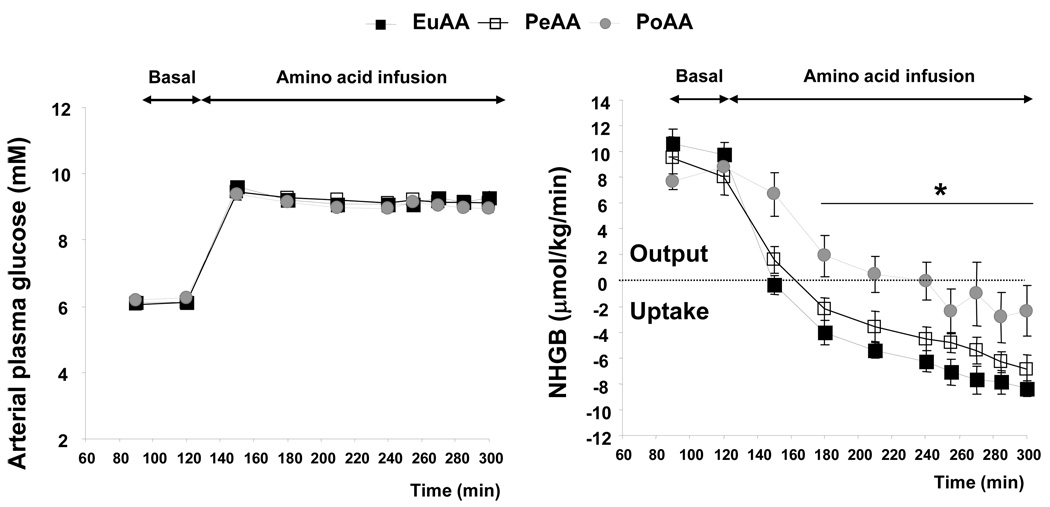
The arterial plasma glucose concentration during the basal period and during the AA infusion period did not differ among groups (Figure. 2). The hepatic glucose load increased significantly during glucose infusion and reached 178±10, 175±6 and 172±6 µmol/kg/min in EuAA, PeAA and PoAA, respectively. In the basal period, the animals of the 3 groups exhibited similar net hepatic glucose output at a rate of 10.2±0.8, 8.8±1.4 and 8.2±0.5 µmol/kg/min in EuAA, PeAA and PoAA, respectively (Figure 2). During the AA infusion period, they shifted to net hepatic glucose uptake (NHGU). With a mean rate of −3.6±1.5 µmol/kg/min (between 210–300 min), NHGU in the PoAA group was significantly lower when compared to the EuAA (−7.7±0.7 µmol/kg/min) and PeAA (−5.7±0.9 µmol/kg/min) groups (Figure 2).
Figure 2.
Portal amino acid infusion reduced net hepatic glucose uptake (NHGU). Amino acids were infused as described in Figure 1 and Table 1. Arterial, portal and hepatic blood samples were drawn at time indicated in the figure. Glucose concentration was determined and net hepatic glucose balance (NHGB) was calculated as described in “Materials and Methods”. Results represent the mean ± SE of 9 animals for each group. The statistical evaluation of the data was performed by a 2-way repeated-measures analysis of variance to test the group and time effects and time × group interaction. When significant, the Bonferroni test was employed for post hoc analysis. Differences were considered significant when P < 0.05.
Time effect P<0.0001, Group effect P<0.0001 and Time × Group interaction P<0.011.* PoAA significantly different from both EuAA and PeAA. PeAA not significantly different from EuAA
Amino acid concentrations and arterio-portal venous gradient
Hepatic AA loads were similar in the 3 groups (except for arginine) during the basal period (Table 3). Hepatic AA loads in the EuAA group were maintained at the basal values during the experimental period for each amino acid. Hepatic amino acid loads increased in the PeAA and PoAA groups when compared to EuAA (except for Gly, Ala and Cys) but were similar in the PeAA and PoAA groups (except for Trp) (Table 3). During the AA infusion, the total hepatic load of AAs (sum of all AA loads) increased significantly but similarly in PeAA and PoAA compared to EuAA (82.3± 4.3 and 82.5±7.7 compared to 52.6±3.4 µmol/kg/min, respectively). In order to maintain the matching of hepatic AA loads in PeAA and PoAA, the portal infusion of AAs resulted in arterial AA concentrations lower than with the ones generated with the peripheral AA infusion used in the PeAA group (except for Trp and Asp) (Table 3). The PeAA and PoAA groups differed by the generation of a negative arterio-portal venous gradient (A-P gradient) of AAs (i.e., more negative when AAs were infused intraportally; Table 3). The A-P gradient in the PeAA group for most of the AAs was no different from that observed in the post-absorptive state (i.e., the EuAA group).
Table 3.
Arterial and portal amino acid concentrations, hepatic aminoacid load and amino acid arterio-portal gradient in the three experimental groups EuAA, PeAA and PoAA
| Arterial concentration µM |
Portal concentration µM |
Hepatic load (µmol/kg/min) |
ArterioPortal gradient µM |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| AA infusion | AA infusion | Basal | AA infusion | AA infusion | |||||||
| Mean | SE | Mean | SE | Mean | SE | Mean | SE | Mean | SE | ||
| ALA | EuAA | 459.2 | 42.0 a | 542.8 | 44.7 a | 7.0 | 0.8 a | 9.1 | 1.0 a | −83.7 | 8.2 a |
| PeAA | 539.6 | 57.3 b | 601.9 | 95.5 b | 6.0 | 0.6 a | 9.6 | 1.0 a | −62.3 | 13.8 a | |
| PoAA | 470.2 | 40.4 a | 777.4 | 101.6 c | 7.8 | 1.0 a | 11.0 | 1.1 a | −307.2 | 19.4 b | |
| ARG | EuAA | 157.8 | 13.5 ab | 146.8 | 13.6 a | 2.4 | 0.2 a | 2.6 | 0.3 a | 10.9 | 3.0 a |
| PeAA | 183.7 | 19.0 a | 169.6 | 26.9 a | 1.5 | 0.2 b | 2.8 | 0.3 a | 14.1 | 5.0 a | |
| PoAA | 121.9 | 8.1 b | 233.7 | 35.4 b | 1.8 | 0.1 ab | 2.9 | 0.3 a | −111.9 | 17.5 b | |
| ASN | EuAA | 78.2 | 6.5 a | 70.1 | 6.7 a | 1.3 | 0.1 a | 1.3 | 0.2 a | 8.1 | 3.4 a |
| PeAA | 107.7 | 10.2 b | 101.8 | 15.3 b | 1.2 | 0.1 a | 1.7 | 0.2 b | 5.9 | 2.4 a | |
| PoAA | 60.4 | 4.7 a | 128.3 | 18.7 b | 1.3 | 0.1 a | 1.7 | 0.2 b | −67.9 | 7.7 b | |
| ASP | EuAA | 7.3 | 1.8 a | 8.7 | 1.9 a | 0.2 | 0.0 a | 0.1 | 0.0 a | −1.3 | 0.8 a |
| PeAA | 17.7 | 2.3 b | 17.9 | 2.3 b | 0.2 | 0.0 a | 0.3 | 0.0 b | −0.2 | 1.2 a | |
| PoAA | 12.2 | 1.5 ab | 21.2 | 2.9 b | 0.2 | 0.0 a | 0.3 | 0.0 b | −9.0 | 1.6 b | |
| CYS | EuAA | 36.6 | 4.1 a | 37.2 | 3.2 a | 0.5 | 0.0 a | 0.6 | 0.1 a | −0.7 | 1.7 a |
| PeAA | 55.7 | 6.2 b | 53.1 | 7.7 b | 0.6 | 0.1 a | 0.9 | 0.1 a | 2.5 | 1.6 a | |
| PoAA | 24.1 | 5.1 a | 54.3 | 9.4 b | 0.4 | 0.1 a | 0.7 | 0.1 a | −30.2 | 5.7 b | |
| GLN | EuAA | 754.1 | 32.1 a | 648.3 | 28.7 a | 13.4 | 1.2 a | 12.7 | 1.0 a | 105.7 | 8.3 a |
| PeAA | 1005.6 | 67.6 b | 934.4 | 111.6 b | 15.8 | 1.3 a | 16.4 | 0.6 ab | 62.4 | 19.3 a | |
| PoAA | 781.3 | 25.5 a | 989.7 | 125.2 b | 17.5 | 1.7 a | 19.1 | 2.2 b | −208.4 | 32.6 b | |
| GLU | EuAA | 74.7 | 4.2 a | 80.8 | 4.5 a | 0.7 | 0.1 a | 0.9 | 0.1 a | −6.0 | 1.6 a |
| PeAA | 131.4 | 13.8 b | 133.4 | 16.4 b | 0.7 | 0.1 a | 1.4 | 0.2 b | −2.1 | 3.5 a | |
| PoAA | 79.4 | 5.8 a | 132.5 | 17.5 b | 0.8 | 0.1 a | 1.5 | 0.1 b | −53.1 | 8.6 b | |
| GLY | EuAA | 319.3 | 34.9 a | 266.6 | 32.0 a | 4.6 | 0.4 a | 4.7 | 0.5 a | 52.7 | 13.8 a |
| PeAA | 313.6 | 21.6 a | 301.5 | 41.5 b | 4.1 | 0.4 a | 5.0 | 0.5 a | 12.2 | 11.4 b | |
| PoAA | 206.2 | 16.8 b | 317.4 | 47.2 b | 4.6 | 0.4 a | 4.6 | 0.5 a | −111.2 | 22.3 c | |
| HIS | EuAA | 57.3 | 3.2 a | 56.2 | 3.4 a | 1.0 | 0.1 a | 1.0 | 0.1 a | 1.1 | 1.6 a |
| PeAA | 87.9 | 13.2 b | 86.2 | 11.0 b | 1.0 | 0.1 a | 1.4 | 0.2 b | 1.7 | 1.8 a | |
| PoAA | 59.4 | 6.1 a | 87.8 | 12.9 b | 0.9 | 0.1 a | 1.3 | 0.1 b | −28.4 | 4.1 b | |
| ISO | EuAA | 63.3 | 6.6 a | 56.1 | 5.7 a | 1.1 | 0.1 a | 1.0 | 0.1 a | 7.2 | 2.4 a |
| PeAA | 169.5 | 24.2 b | 152.8 | 28.1 b | 1.3 | 0.2 a | 2.6 | 0.4 b | 16.7 | 5.5 b | |
| PoAA | 89.4 | 6.3 a | 177.1 | 24.2 b | 1.3 | 0.1 a | 2.3 | 0.2 b | −87.8 | 8.0 c | |
| LEU | EuAA | 232.6 | 14.7 a | 219.0 | 16.3 a | 3.4 | 0.3 a | 3.8 | 0.3 a | 13.5 | 6.7 a |
| PeAA | 412.3 | 45.8 b | 392.1 | 65.2 b | 3.0 | 0.3 a | 6.5 | 0.8 b | 20.2 | 8.0 a | |
| PoAA | 211.5 | 19.1 a | 382.2 | 55.8 b | 2.7 | 0.3 a | 5.2 | 0.6 b | −170.6 | 17.1 b | |
| LYS | EuAA | 214.2 | 14.7 a | 199.5 | 20.0 a | 2.7 | 0.3 a | 2.4 | 0.3 a | 9.8 | 4.9 a |
| PeAA | 364.9 | 26.4 b | 361.5 | 46.0 b | 2.8 | 0.2 a | 5.2 | 0.3 b | 3.0 | 11.3 a | |
| PoAA | 231.0 | 19.2 a | 390.6 | 50.3 b | 2.6 | 0.6 a | 5.4 | 0.5 b | −159.6 | 20.6 b | |
| MET | EuAA | 55.6 | 2.9 a | 50.5 | 2.8 a | 0.8 | 0.1 a | 0.9 | 0.1 a | 5.1 | 2.0 a |
| PeAA | 99.9 | 14.5 b | 93.9 | 18.0 b | 0.9 | 0.1 a | 1.5 | 0.2 b | 5.9 | 3.2 a | |
| PoAA | 44.4 | 4.5 a | 87.8 | 13.2 b | 0.9 | 0.1 a | 1.5 | 0.2 b | −43.5 | 4.7 b | |
| PHE | EuAA | 50.0 | 2.8 a | 45.1 | 2.7 a | 0.9 | 0.1 a | 0.8 | 0.1 a | 4.9 | 2.1 a |
| PeAA | 109.4 | 6.9 b | 107.5 | 14.6 b | 0.9 | 0.1 a | 1.8 | 0.1 b | 1.9 | 2.4 a | |
| PoAA | 49.2 | 5.3 a | 112.6 | 15.8 b | 0.9 | 0.1 a | 1.5 | 0.2 b | −63.3 | 7.5 b | |
| PRO | EuAA | 120.5 | 9.0 a | 126.6 | 7.8 a | 2.0 | 0.3 a | 2.2 | 0.4 a | −3.1 | 2.6 a |
| PeAA | 357.3 | 32.6 b | 362.5 | 57.6 b | 2.5 | 0.2 a | 5.9 | 0.6 b | −5.2 | 8.9 a | |
| PoAA | 183.0 | 12.0 c | 350.5 | 49.6 b | 2.8 | 0.3 a | 4.7 | 0.5 b | −167.5 | 15.8 b | |
| SER | EuAA | 126.5 | 7.2 a | 113.8 | 6.1 a | 2.0 | 0.2 a | 2.0 | 0.2 a | 12.7 | 2.8 a |
| PeAA | 233.8 | 33.7 b | 210.6 | 36.0 b | 2.0 | 0.3 a | 3.6 | 0.5 b | 23.2 | 5.2 b | |
| PoAA | 114.2 | 7.4 a | 258.1 | 39.0 b | 2.1 | 0.2 a | 3.3 | 0.3 b | −143.9 | 17.9 c | |
| THR | EuAA | 199.0 | 13.7 a | 233.3 | 15.6 a | 3.9 | 0.4 a | 3.9 | 0.4 a | −20.1 | 4.4 a |
| PeAA | 346.3 | 47.0 b | 355.8 | 57.2 b | 2.9 | 0.3 a | 5.9 | 0.9 b | −9.5 | 9.0 b | |
| PoAA | 258.2 | 22.3 a | 380.8 | 50.1 b | 3.6 | 0.6 a | 5.4 | 0.7 b | −122.6 | 15.4 c | |
| TRP | EuAA | 51.5 | 5.8 a | 46.2 | 5.0 a | 0.6 | 0.1 a | 0.5 | 0.1 a | 3.2 | 2.0 a |
| PeAA | 120.0 | 17.1 b | 118.2 | 20.0 b | 1.0 | 0.1 a | 2.0 | 0.3 b | 1.8 | 2.3 a | |
| PoAA | 146.5 | 14.7 b | 185.3 | 30.3 c | 1.2 | 0.1 a | 2.7 | 0.3 c | −38.8 | 8.5 b | |
| TYR | EuAA | 53.8 | 4.6 a | 48.7 | 4.4 a | 0.9 | 0.1 a | 0.9 | 0.1 ab | 5.1 | 2.7 a |
| PeAA | 42.1 | 4.6 a | 35.3 | 4.9 a | 0.7 | 0.1 a | 0.6 | 0.1 a | 6.8 | 1.1 a | |
| PoAA | 30.8 | 2.9 b | 66.9 | 10.0 b | 0.8 | 0.1 a | 0.9 | 0.1 b | −36.0 | 4.0 b | |
| VAL | EuAA | 217.0 | 10.8 a | 202.5 | 9.2 a | 4.1 | 0.2 a | 3.6 | 0.3 a | 14.5 | 4.0 a |
| PeAA | 465.9 | 24.2 b | 442.8 | 54.7 b | 3.7 | 0.3 a | 7.3 | 0.3 b | 23.0 | 6.9 a | |
| PoAA | 309.4 | 23.1 c | 462.4 | 65.2 b | 3.5 | 0.4 a | 6.6 | 0.6 b | −153.0 | 14.7 b | |
Data are expressed as means ± SE (n=9) in each group. The basal data are the mean of the values at 90 and 120 min. The AA infusion data are the mean of the determinations between 150 and 300 min. The statistical evaluation of the data was performed by a one way measures analysis of variance. Bonferroni adjusted data are shown. Differences were considered significant when P < 0.05. Values with different letters are significantly different within the same column, P <0.05.
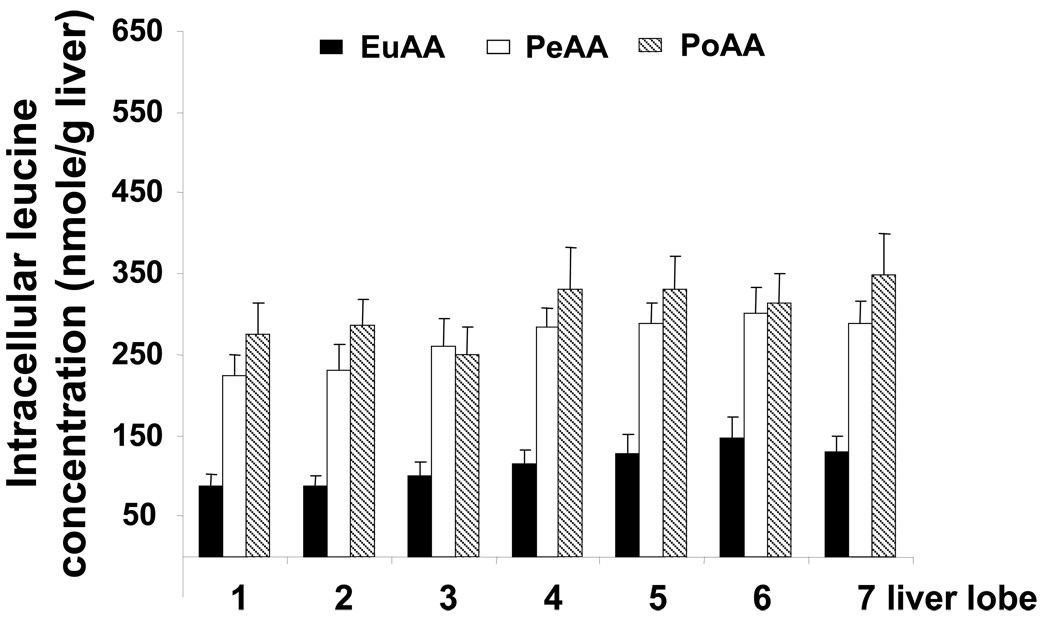
In the liver, the intracellular leucine concentration increased significantly in the PeAA and PoAA groups compared to EuAA (Figure 3). This increase ranged from 2 to 2.5 times depending of the liver lobe considered, but the same intracellular leucine concentration was observed whatever the route of delivery of the AAs (PeAA or PoAA) (Figure 3).
Figure 3.
Intracellular leucine concentration in the liver lobes of the dogs within the three experimental groups. At the end of the infusion period, liver samples were quickly removed and frozen in liquid nitrogen and stored at −80°C until analyzed. Liver samples were prepared as described in “Materials and Methods” and leucine was determined as described in Donahue et al. (19). The results represent the mean ± SE of 9 animals for each group. The statistical evaluation of the data was performed by a 2-way repeated-measures analysis of variance to test the group and lobe effects and lobe × group interaction. When significant, the Bonferroni test was employed for post hoc analysis. Differences were considered significant when P < 0.05.
Group effect P<0.0001, Lobe effect P<0.036 and no significant Lobe × Group interaction. EuAA significantly different from PoAA and PeAA.; PeAA not significantly different from PoAA.
Hepatic leucine balance and hepatic protein synthesis
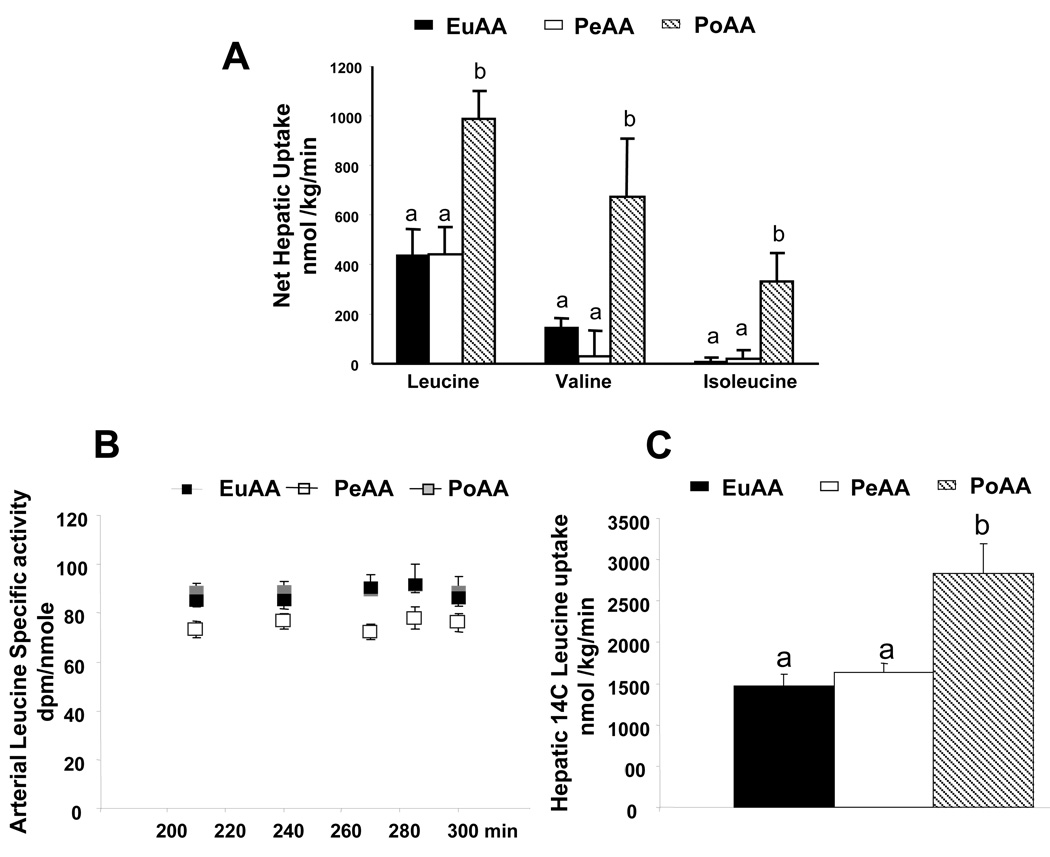
During the experimental period, no significant differences in the net hepatic leucine, valine or isoleucine balances was seen with the peripheral AA infusion. By contrast, portal AA infusion to create the same hepatic leucine, valine and isoleucine loads increased net hepatic leucine, valine and isoleucine uptake in comparison with PeAA (Figure 4A). Similar results were observed with other AAs including tryptophan, lysine, glutamine, glutamate and tyrosine (data not shown).
Figure 4.
Arterial specific radioactivity of 14C leucine, net hepatic branched chain amino acid balance and net 14C leucine utilization. Amino acids were infused as described in the legend to Figure 1 and Table 1. Arterial, portal and hepatic blood samples were drawn at the time indicated in the figure. Leucine and leucine SA were determined as described in “Materials and Methods”. The results represent the mean ± SE of 9 animals for each group. The statistical evaluation of the data was performed by a one-way measures analysis of variance to test the group effect. The Bonferroni test was employed for post hoc analysis. Values with different letters are significantly different, P <0.05.
Arterial plasma leucine specific radioactivity was constant and similar among the 3 groups throughout the experimental period (210 to 300 min) and this confirms that a steady state of leucine kinetics was achieved in all groups (Figure 4B). Leucine specific radioactivities were similar in portal plasma, 74.4± 3.9; 71.6± 4.2 and 71.3± 4.3 dpm/nmol in EuAA, PeAA and PoAA, respectively (mean values between 210–300min). Hepatic tracer balance showed similar results to the net hepatic leucine balance. Only the portal infusion of AA was associated with increased leucine uptake into the liver compared to EuAA (Figure 4C).
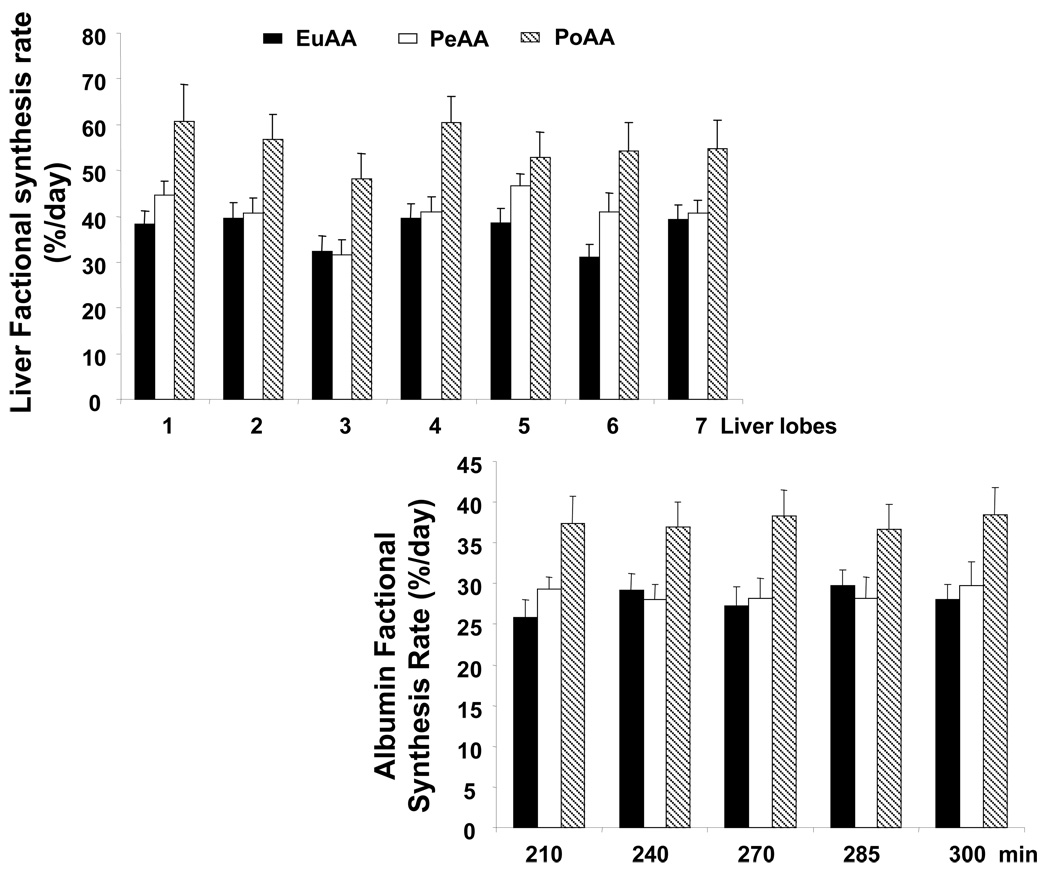
Liver protein synthesis was measured in the 7 lobes at 300 min (i.e., after 180 min of AA infusion). Hepatic leucine specific radioactivities were 29.4±1.9, 41.6±2.8 and 36.8±3.9 dpm/nmol in EuAA, PeAA and PoAA, respectively. Peripheral delivery of AAs did not increase hepatic protein synthesis significantly, whereas portal delivery stimulated the fractional synthesis in all the liver lobes (Figure 5). The stimulatory effect of portal delivery of AAs was also found on exported hepatic proteins. Indeed, albumin synthesis was significantly increased by 30% whereas peripheral AA infusion had no significant effect compared to EuAA (Figure 5).
Figure 5.
Hepatic and albumin fractional protein synthesis rates. Amino acids were infused as described in the legend to Figure 1 and Table 1. Arterial, portal and hepatic blood samples were drawn at times indicated in the figure. At the end of the infusion period, liver samples were quickly removed and frozen in liquid nitrogen and stored at −80°C until analyzed. Liver samples were prepared as described in “Materials and Methods” and leucine and leucine SA were determined as described in Donahue et al. (19). Liver protein synthesis was analyzed on the seven hepatic lobes in each dog. Albumin synthesis was determined kinetically from 210 to 300 min. The results represent the mean ± SE of 9 animals for each group.
For liver synthesis, the statistical evaluation of the data was performed by a 2-way measures analysis of variance to test the group and lobe effects and lobe × group interaction. When significant, the Bonferroni test was employed for post hoc analysis. Differences were considered significant when P < 0.05. Group effect P<0.0001; Lobe effect P<0.025 and no significant interaction interaction. PoAA significantly different from EuAA and PeAA. PeAA not significantly different to EuAA
For albumin synthesis, the statistical evaluation of the data was performed by a 2-way repeated-measures analysis of variance to test the group and time effects and time × group interaction. When significant, the Bonferroni test was employed for post hoc analysis. Differences were considered significant when P < 0.05. No significant time effect; Group effect P<0.0001 and no significant interaction. PoAA significantly different from EuAA and PeAA. PeAA not significantly different from EuAA
Signalling pathways
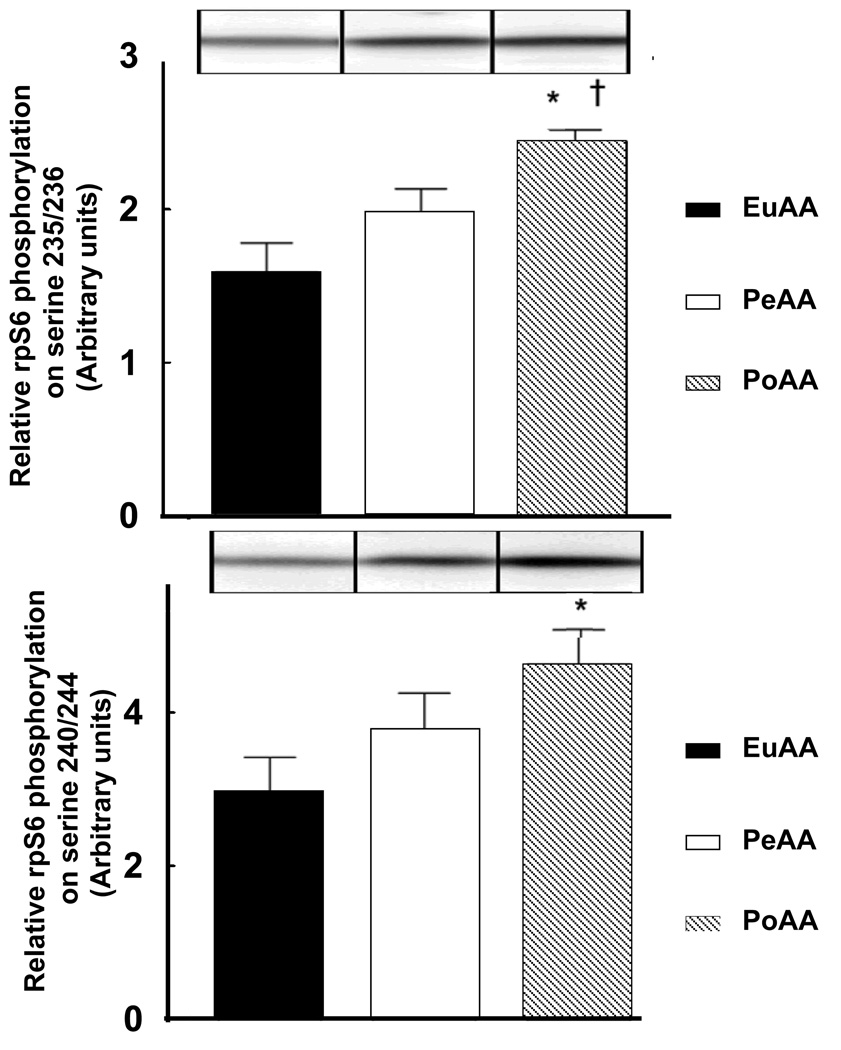
Because of its central role in mediating increases in protein synthesis, mTORC1 signaling was assessed in the livers of each group. As an index of alterations in mTORC1 signalling, the phosphorylation status of the downstream target, rpS6, on Ser235/236 and Ser240/244 was examined by Western blot analysis. This target was chosen because it was expected to be less subject to transient changes over such a long experimental protocol. As shown in Figure 6, AA infused peripherally had no significant effect on phosphorylation of rpS6 on residues Ser240/244, whereas there was a trend (P=0.07) for increased rpS6 phosphorylation on residues Ser235/236. In contrast, AA infused portally led to a significant increase in phosphorylation of rpS6 at both Ser235/236 and Ser240/244 compared to EuAA values. In addition, portal AA delivery produced a significant (P<0.05) increase in phosphorylation on Ser235/236 and tended (P=0.1) to increase phosphorylation on Ser240/244 compared to peripheral delivery. Activation of mTORC1 was also monitored by assessment of the phosphorylation status of S6K1 as well two other proximal targets of the kinase, i.e. 4E-BP1 and eIF4G. Although all three mTORC1 substrates showed the same pattern of increased phosphorylation as rpS6 in response to portal versus peripheral delivery of AA, the magnitude of the change at the 180 min time point was less than that of rpS6 and did not reach statistical significance. For example, phosphorylation of 4E-BP1, expressed as a percentage of the protein present in the hyperphosphorylated γ-form relative to the total protein, was 59.5±0.8, 61.37±0.7, and 84.2 ±3.8 for control, AA infused peripherally, and AA infused portally. Likewise, phosphorylation of eIF4G on SAer1108, expressed in arbitrary units, was 0.27±0.03, 0.36±0.04, and 0.42±0.1 for control, AA infused peripherally, and AA infused portally, respectively.
Figure 6.
Amino acids infused portally, but not peripherally, activate mTORC1 signaling as indicated by increased phosphorylation of rpS6. Amino acids were infused as described in Figure 1 and Table 1. At the end of the infusion period, liver samples were quickly removed and frozen in liquid nitrogen and stored at −80°C until analyzed. Phosphorylation of rpS6 on (A) Ser235/236 and (B) Ser240/244 was measured by Western blot analysis as described under “Materials and Methods”. In addition, samples were analyzed for eEF2 content to demonstrate equal loading of protein onto the gel (see insert to panel A). eEF2 was used as a loading control because its expression did not change under the experimental conditions used in this study. The results represent the mean ± SE of 9 animals for each group. Representative Western blots are shown in the insets to each panel. The statistical evaluation of the data was performed by a one-way measures analysis of variance to test the group effect. The Bonferroni test was employed for post hoc analysis. * P<0.002 vs. EuAA; † P<0.05 vs. peripheral amino acid condition.
DISCUSSION
Peripheral delivery of AAs to produce plasma concentrations seen postprandially did not stimulate hepatic protein synthesis significantly compared with euaminoacidemia, but synthesis of resident liver protein and albumin increased significantly (30%) with portal AA delivery. The stimulation of hepatic protein synthesis with portal delivery of AAs could not be attributed to differences in the hepatic AA load or in insulin, glucagon or glucose concentrations. Thus the route of delivery of AAs is critical for the stimulation of liver protein synthesis in the adult dog.
In support of our observation, human studies have shown that albumin synthesis is stimulated with oral meal feeding, whereas it is not responsive to intravenous nutrients (4, 12). Moreover, 20 to 96% of enterally administered AA are utilized by the splanchnic bed, showing the crucial role of the splanchnic tissues in the distribution and availability of dietary AAs to peripheral tissues (21–24). Interestingly, recent studies conducted in the pig show that the dietary requirement for AAs (lysine, methionine, leucine, valine, isoleucine, and threonine) is lower with total parenteral nutrition than with enteral nutrition (21, 25–28), consistent with an increased utilization of the dietary AAs by the gut and liver. Complementary results have shown that dietary AAs are the preferential source of substrate for hepatic protein synthesis (29–31). In the post-prandial state, the AA concentrations are higher in portal vein than in hepatic artery blood (32). However, a difference in AA availability could not explain our results since the hepatic loads of AAs were carefully matched. It is possible that the portal delivery of AA initiates a signal to the liver that stimulates hepatic AA utilization, i.e., protein synthesis. Several studies conducted in our laboratory suggest the existence of an AA-initiated portal signal.
Previously we carried out a series of studies to examine the role of AAs and their route of delivery (peripheral vein vs portal vein infusions) on NHGU in the conscious dog. With a fixed hormonal milieu (4× basal insulin; 1× basal glucagon) and a portal glucose infusion, portal gluconeogenic AA infusion reduced NHGU and net hepatic glycogen synthesis by 50% and 30%, respectively (33). This decrease could have originated from the hepatic AA load itself (i.e, competition between substrates for hepatic uptake) or it could have been specific to the intra-portal route of AA delivery. To answer this question (34), we studied a group of dogs under identical conditions to our previous studies except that the gluconeogenic AAs were delivered peripherally at a rate that would maintain the hepatic AA load equivalent to that existing in the previous studies. Peripheral delivery of AA did not reduce NHGU initiated by portal glucose delivery. This observation is thus consistent with the hypothesis that intra-portal delivery of AAs generates a signal that competes with or modulates the signal that enhances NHGU during portal glucose delivery. In the present experiments, similar conclusions can be drawn, as the portal delivery of all AAs also decreased NHGU when compared to the peripheral AA infusion. In the study of Moore et al. (33), with the peripheral AA infusion, the net hepatic uptake of glucose and AAs (in carbon equivalents) together equalled the net hepatic glycogen deposition and lactate release. With the portal AA infusion, only 70% of their uptake could be accounted for glycogen synthesis and lactate release. Our present study strongly suggests that at least a portion of the remaining 30% of carbon extracted by the liver was then redirected to protein synthesis. Taken together, our data indicate that a portal signal may activate liver protein synthesis. The portal concentrations of almost all of the AAs were statistically indistinguishable in PoAA and PeAA, suggesting that the negative A-P gradient of AAs initiates the signal.
Whether portal AAs modulate the activity of the autonomic nervous system to increase hepatic protein synthesis is unknown. However, the involvement of neuronal input in controlling liver glucose metabolism has already been shown (see 35 for a review)), and Niijima et al. (36–37) have described hepato-portal sensors for 15 different AAs serving as stimulators or inhibitors of the vagal afferent discharge rate. Moreover, Watanabe et al. (38) postulated that plasma protein synthesis is enhanced by vagal-nerve stimulation.
The present study shows that portal, but not peripheral, delivery of AAs leads to a significant increase in phosphorylation of rpS6 on Ser235/236 and Ser240/244. Both sets of sites are phosphorylated by the rpS6 kinase, S6K1 (39). Because mTOR phosphorylates S6K1 (40), an event that is critical for maximal activation of the kinase, this finding suggests that portal AA delivery increases mTORC1 signalling in liver to a greater extent than peripheral delivery. Phosphorylation of S6K1 and 4E-BP1 was maximally increased 60 min after re-feeding fasted rats, and returned toward control values within 180 min (41). In contrast, phosphorylation of rpS6 was maintained through the 180 min time point. Therefore, because the liver samples in the current study were collected following 180 min of amino acid infusion, rpS6 phosphorylation should be a better indicator of mTORC1 activation. Previous studies by our laboratory and others have provided evidence in support of the concept that albumin mRNA is translationally regulated in response to the availability of nutrients. Yap et al. (42) showed that, following a 24 h fast in rats, albumin mRNA shifts out of polysomes and accumulates in 30–50S ribonucleoprotein complexes. Moreover, within 1 h of feeding a complete meal, the albumin mRNA redistributes back into polysomes. Studies in perfused rat liver (43) suggested that the translation of albumin mRNA is repressed by deficiency of essential AAs. Kuwahata et al (44), using acute liver injury in rats as an experimental model, showed that polysome-associated albumin mRNA increases in proportion to the content of branched-chain AAs during total parenteral nutrition. Finally, a cDNA microarray analysis of polysome-associated mRNAs over a time course following meal feeding to fasted rats demonstrated translational control of albumin mRNA (43). Taken together, these data indicate that the mTORC1 signaling pathway is intimately involved in regulating albumin mRNA translation, which helps explain the increase in albumin production observed in PoAA. Leucine modulates the activity of the signalling pathway leading to the initiation of protein translation (45–47). However, intracellular leucine concentrations were identical in the PoAA and PeAA livers, suggesting that the stimulation of the mTOR pathways in the liver is also under the control of signal inputs that are different from the leucine pathways and initiated specifically by the portal AA infusion. In addition to actions on the mTOR signalling pathway, portal vs peripheral infusion of AAs might impact upon liver protein synthesis via other mechanisms, such as the metabolic sensors AMP-activated protein kinase or general control nonderepressible 2 kinase (GCN2) (48).
Although mTORC1 is a central mediator of the increase in protein synthesis engendered by increased provision of amino acids, other signalling pathways may also be involved in the response. For example, GCN2 is activated in response to deprivation of essential amino acids (49). Upon activation, GCN2 phosphorylates the α-subunit of eIF2 leading to inhibition of the first step in translation initiation. Thus, eIF2α phosphorylation is increased in livers from rats fed a diet lacking either Trp or Leu compared to animals fed a complete meal or a diet lacking the nonessential amino acid Gly (50). However, in the present study there was no difference in eIF2α phosphorylation in livers from dogs infused with amino acids peripherally compared to portally (0.82±0.04 vs 0.79±0.08 arbitrary units, respectively). The basis for the lack of change in eIF2α phosphorylation in the present study is unknown, but is likely related to amino acids being provided in a balanced manner (i.e. one or more essential amino acids were not limiting). In this regard, in previous studies (e.g. 51), no change in eIF2α phosphorylation was observed in liver in response to an overnight fast or after refeeding overnight fasted animals.
In conclusion, portal delivery of AAs stimulated hepatic synthesis of resident and exported proteins whereas peripheral delivery of AAs did not, despite similar AA concentrations within the liver. Our results suggest that a portal signal was generated with the portal infusion of AAs which was necessary to fully stimulate hepatic AA utilization and hepatic protein synthesis. Thus, enteral nutrition may be preferred over parenteral in stimulating or maintaining liver protein synthesis in humans. In contrast, an enhanced splanchnic extraction of AAs may be deleterious in sustaining AA provision to peripheral tissues such as skeletal muscle. This has been suspected, for example, to be responsible for the defect in the post-prandial stimulation of muscle protein synthesis during aging. When muscle protein synthesis is of key importance, our findings suggest that a peripheral infusion of AAs may be advantageous over a portal AA infusion to spare AAs.
AKNOWLEDGEMENTS
These studies were supported by NIH Grant RO1 DK 43706 (ADC), the Vanderbilt Diabetes Research and Training Center Grant SP-60-AM20593 and NIH grant DK-13499 (LSJ).
The authors’ responsibilities were as follows—D. Dardevet: planned and implemented the study, analyzed statistics, interpreted data, and wrote the manuscript; S. Kimball: sample analysis and critical revision of the manuscript; L. Jefferson: sample analysis and critical revision of the manuscript; A. Cherrington: critical revision of the manuscript; D. Rémond: analyzed statistics and critical revision of the manuscript; C. DiCostanzo: sample analysis; M. Moore: study design and critical revision of the manuscript.
Footnotes
None of the authors had a conflict of interest.
REFERENCES
- 1.Barle H, Nyberg B, Essén P, et al. The synthesis rates of total liver protein and plasma albumin determined simultaneously in vivo in humans. Hepatology. 1997;25:154–158. doi: 10.1002/hep.510250128. [DOI] [PubMed] [Google Scholar]
- 2.Nygren J, Nair KS. Differential regulation of protein dynamics in splanchnic and skeletal muscle beds by insulin and amino acids in healthy human subjects. Diabetes. 2003;52:1377–1385. doi: 10.2337/diabetes.52.6.1377. [DOI] [PubMed] [Google Scholar]
- 3.Mosoni L, Patureau Mirand P, Houlier ML, Arnal M. Age-related changes in protein synthesis measured in vivo in rat liver and gastrocnemius muscle. Mech Ageing Dev. 1993;68:209–220. doi: 10.1016/0047-6374(93)90152-h. [DOI] [PubMed] [Google Scholar]
- 4.Ballmer PE, McNurlan MA, Essen P, Anderson SE, Garlick PJ. Albumin synthesis rates measured with [(2)H(5)ring]phenylalanine are not responsive to short-term intravenous nutrients in healthy humans. J Nutr. 1995;125:512–519. doi: 10.1093/jn/125.3.512. [DOI] [PubMed] [Google Scholar]
- 5.Ahlman B, Charlton M, Fu AZ, Berg C, OBrien P, Nair KS. Insulin's effect on synthesis rates of liver proteins - A swine model comparing various precursors of protein synthesis. Diabetes. 2001;50:947–954. doi: 10.2337/diabetes.50.5.947. [DOI] [PubMed] [Google Scholar]
- 6.Davis TA, Fiorotto ML, Beckett PR, et al. Differential effects of insulin on peripheral and visceral tissue protein synthesis in neonatal pigs. Am J Physiol - Endocrinol Metab. 2001;280:E770–E779. doi: 10.1152/ajpendo.2001.280.5.E770. [DOI] [PubMed] [Google Scholar]
- 7.Davis TA, Fiorotto ML, Burrin DG, et al. Stimulation of protein synthesis by both insulin and amino acids is unique to skeletal muscle in neonatal pigs. Am J Physiol - Endocrinol Metab. 2002;282:E880–E890. doi: 10.1152/ajpendo.00517.2001. [DOI] [PubMed] [Google Scholar]
- 8.Mosoni L, Valluy MC, Serrurier B, et al. Altered response of protein synthesis to nutritional state and endurance training in old rats. Am J Physiol. 1995;268:E328–E335. doi: 10.1152/ajpendo.1995.268.2.E328. [DOI] [PubMed] [Google Scholar]
- 9.Dardevet D, Sornet C, Balage M, Grizard J. Stimulation of in vitro rat muscle protein synthesis by leucine decreases with age. J Nutr. 2000;130:2630–2635. doi: 10.1093/jn/130.11.2630. [DOI] [PubMed] [Google Scholar]
- 10.Rieu I, Balage M, Sornet C, et al. Leucine supplementation improves muscle protein synthesis in elderly men independently of hyperaminoacidaemia. J Physiol. 2006;575:305–315. doi: 10.1113/jphysiol.2006.110742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.De Feo P, Horber FF, Haymond MW. Meal stimulation of albumin synthesis - A significant contributor to whole body protein synthesis in humans. Am J Physiol. 1992;263:E794–E799. doi: 10.1152/ajpendo.1992.263.4.E794. [DOI] [PubMed] [Google Scholar]
- 12.Hunter KA, Ballmer PE, Anderson SE, Broom J, Garlick PJ, McNurlan MA. Acute stimulation of albumin synthesis rate with oral meal feeding in healthy subjects measured with [ringH-2(5)]phenylalanine. Clin Sci. 1995;88:235–242. doi: 10.1042/cs0880235. [DOI] [PubMed] [Google Scholar]
- 13.Volpi E, Lucidi P, Cruciani G, et al. Contribution of amino acids and insulin to protein anabolism during meal absorption. Diabetes. 1996;45:1245–1252. doi: 10.2337/diab.45.9.1245. [DOI] [PubMed] [Google Scholar]
- 14.Cayol M, Boirie Y, Rambourdin F, et al. Influence of protein intake on whole body and splanchnic leucine kinetics in humans. Am J Physiol. 1997;272:E584–E591. doi: 10.1152/ajpendo.1997.272.4.E584. [DOI] [PubMed] [Google Scholar]
- 15.Moore MC, Hsieh PS, Flakoll PJ, Neal DW, Cherrington AD. Net hepatic gluconeogenic amino acid uptake in response to peripheral versus portal amino acid infusion in conscious dogs. J Nutr. 1999b;129:2218–2224. doi: 10.1093/jn/129.12.2218. [DOI] [PubMed] [Google Scholar]
- 16.Dardevet D, Moore MC, Neal D, Dicostanzo CA, Snead W, Cherrington AD. Insulin-independent effects of GLP-1 on canine liver glucose metabolism: duration of infusion and involvement of hepatoportal region. Am J Physiol - Endocrinol Metab. 2004;287:E75–E81. doi: 10.1152/ajpendo.00035.2004. [DOI] [PubMed] [Google Scholar]
- 17.Myers SR, McGuinness OP, Neal DW, Cherrington AD. Intraportal glucose delivery alters the relationship between net hepatic glucose uptake and the insulin concentration. J Clin Invest. 1991;87:930–939. doi: 10.1172/JCI115100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pagliassotti MJ, Holste LC, Moore MC, Neal DW, Cherrington AD. Comparison of the time courses of insulin and the portal signal on hepatic glucose and glycogen metabolism in the conscious dog. J Clin Invest. 1996;97:81–91. doi: 10.1172/JCI118410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Donahue EP, Brown LL, Flakoll PJ, Abumrad NN. Rapid measurement of leucine-specific activity in biological fluids by ion-exchange chromatography and post-column ninhydrin detection. Journal of Chromatography - Biomedical Applications. 1991;571:29–36. doi: 10.1016/0378-4347(91)80431-b. [DOI] [PubMed] [Google Scholar]
- 20.Williamson DL, Bolster DR, Kimball SR, Jefferson LS. Time course changes in signaling pathways and protein synthesis in C2C12 myotubes following AMPK activation by AICAR. Am J Physiol - Endocrinol Metab. 2006;291:E80–E89. doi: 10.1152/ajpendo.00566.2005. [DOI] [PubMed] [Google Scholar]
- 21.Burrin DG, Davis TA. Proteins and amino acids in enteral nutrition. Curr Opin Clin Nutr Metab Care. 2004;7:79–87. doi: 10.1097/00075197-200401000-00013. [DOI] [PubMed] [Google Scholar]
- 22.Ferrannini E, DeFronzo RA, Gusberg R, et al. Splanchnic amino acid and glucose metabolism during amino acid infusion in dogs. Diabetes. 1988;37:237–245. doi: 10.2337/diab.37.2.237. [DOI] [PubMed] [Google Scholar]
- 23.Biolo G, Tessari P, Inchiostro S, et al. Leucine and phenylalanine kinetics during mixed meal ingestion - A multiple tracer approach. Am J Physiol. 1992;262:E455–E463. doi: 10.1152/ajpendo.1992.262.4.E455. [DOI] [PubMed] [Google Scholar]
- 24.Matthews DE, Marano MA, Campbell RG. Splanchnic bed utilization of leucine and phenylalanine in humans. Am J Physiol. 1993;264:E109–E118. doi: 10.1152/ajpendo.1993.264.1.E109. [DOI] [PubMed] [Google Scholar]
- 25.Bertolo RFP, Chen CZL, Law G, Pencharz PB, Ball RO. Threonine requirement of neonatal piglets receiving total parenteral nutrition is considerably lower than that of piglets receiving an identical diet intragastrically. J Nutr. 1998;128:1752–1759. doi: 10.1093/jn/128.10.1752. [DOI] [PubMed] [Google Scholar]
- 26.House JD, Pencharz PB, Ball RO. Lysine requirement of neonatal piglets receiving total parenteral nutrition as determined by oxidation of the indicator amino acid L-[1-C-14]phenylalanine. Am J Clin Nutr. 1998;67:67–73. doi: 10.1093/ajcn/67.1.67. [DOI] [PubMed] [Google Scholar]
- 27.Elango R, Pencharz PB, Ball RO. The branched-chain amino acid requirement of parenterally fed neonatal piglets is less than the enteral requirement. J Nutr. 2002;132:3123–3129. doi: 10.1093/jn/131.10.3123. [DOI] [PubMed] [Google Scholar]
- 28.Shoveller AK, Brunton JA, Pencharz PB, Bal RO. The methionine requirement is lower in neonatal piglets fed parenterally than in those fed enterally. J Nutr. 2003;133:1390–1397. doi: 10.1093/jn/133.5.1390. [DOI] [PubMed] [Google Scholar]
- 29.Berthold HK, Jahoor F, Klein PD, Reeds PJ. Estimates of the effect of feeding on whole-body protein degradation in women vary with the amino acid used as tracer. J Nutr. 1995;125:2516–2527. doi: 10.1093/jn/125.10.2516. [DOI] [PubMed] [Google Scholar]
- 30.Cayol M, Boirie Y, Prugnaud J, Gachon P, Beaufrère B, Obled C. Precursor pool for hepatic protein synthesis in humans : effects of tracer route infusion and dietary proteins. Am J Physiol. 1996;270:E980–E987. doi: 10.1152/ajpendo.1996.270.6.E980. [DOI] [PubMed] [Google Scholar]
- 31.Stoll B, Burrin DG, Henry J, Yu H, Jahoor F, Reeds PJ. Dietary amino acids are the preferential source of hepatic protein synthesis in piglets. J Nutr. 1998;128:1517–1524. doi: 10.1093/jn/128.9.1517. [DOI] [PubMed] [Google Scholar]
- 32.Moore MC, Pagliassotti MJ, Swift LL, et al. Disposition of a mixed meal by the conscious dog. Am J Physiol. 1994;266:E666–E675. doi: 10.1152/ajpendo.1994.266.4.E666. [DOI] [PubMed] [Google Scholar]
- 33.Moore MC, Flakoll PJ, Hsieh PS, et al. Hepatic glucose disposition during concomitant portal glucose and amino acid infusions in the dog. Am J Physiol - Endocrinol Metab. 1998;274:E893–E902. doi: 10.1152/ajpendo.1998.274.5.E893. [DOI] [PubMed] [Google Scholar]
- 34.Moore MC, Hsieh PS, Flakoll PJ, Neal DW, Cherrington AD. Differential effect of amino acid infusion route on net hepatic glucose uptake in the dog. Am J Physiol. 1999a;276:E295–E302. doi: 10.1152/ajpendo.1999.276.2.E295. [DOI] [PubMed] [Google Scholar]
- 35.Dardevet D, Moore MC, Remond D, Everett-Grueter CA, Cherrington AD. Regulation of hepatic metabolism by enteral delivery of nutrients. Nutr Res Rev. 2006;19:161–173. doi: 10.1017/S0954422407315175. [DOI] [PubMed] [Google Scholar]
- 36.Niijima A, Meguid MM. Parenteral nutrients in rat suppresses hepatic vagal afferent signals from portal vein to hypothalamus. Surgery. 1994;116:294–301. [PubMed] [Google Scholar]
- 37.Niijima A, Meguid MM. An electrophysiological study on amino acid sensors in the hepatoportal system in the rat. Obes Res. 1995;3:741S–745S. doi: 10.1002/j.1550-8528.1995.tb00494.x. [DOI] [PubMed] [Google Scholar]
- 38.Watanabe Y, Takahashi A, Shimazu T. Neural control of biosynthesis and secretion of serum transferrin in perfused rat liver. Biochem J. 1990;267:545–548. doi: 10.1042/bj2670545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pende M, Um SH, Mieulet V, et al. S6K1(−/−)/S6K2(−/−) mice exhibit perinatal lethality and rapamycin-sensitive 5'-terminal oligopyrimidine mRNA translation and reveal a mitogen-activated protein kinase-dependent S6 kinase pathway. Mol Cell Biol. 2004;24:3112–3124. doi: 10.1128/MCB.24.8.3112-3124.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Richardson CJ, Schalm SS, Blenis J. PI3-kinase and TOR: PIKTORing cell growth. Semin Cell Dev Biol. 2004;15:147–159. doi: 10.1016/j.semcdb.2003.12.023. [DOI] [PubMed] [Google Scholar]
- 41.Reiter AK, Crozier SJ, Kimball SR, Jefferson LS. Meal feeding alters translational control of gene expression in rat liver. J Nutr. 2005;135:367–375. doi: 10.1093/jn/135.3.367. [DOI] [PubMed] [Google Scholar]
- 42.Yap SH, Strair RK, Shafritz DA. Effect of a short term fast on the distribution of cytoplasmic albumin messenger ribonucleic acid in rat liver. Evidence for formation of free albumin messenger ribonucleoprotein particles. J Biol Chem. 1978;253:4944–4950. [PubMed] [Google Scholar]
- 43.Flaim KE, Liao WS, Peavy DE, Taylor JM, Jefferson LS. The role of amino acids in the regulation of protein synthesis in perfused rat liver II Effects of amino acid deficiency on peptide chain initiation, polysomal aggregation, and distribution of albumin mRNA. J Biol Chem. 1982;257:2939–2946. [PubMed] [Google Scholar]
- 44.Kuwahata M, Oka T, Asagi K, Kohri H, Kato A, Natori Y. Effect of branched-chain amino acids on albumin gene expression in the liver of galactosamine-treated rats. J Nutr Biochem. 1998;9:209–214. [Google Scholar]
- 45.Anthony JC, Anthony TG, Kimball SR, Vary TC, Jefferson LS. Orally administered leucine stimulates protein synthesis in skeletal muscle of postabsorptive rats in association with increased elF4F formation. J Nutr. 2000a;130:139–145. doi: 10.1093/jn/130.2.139. [DOI] [PubMed] [Google Scholar]
- 46.Anthony JC, Yoshizawa F, Anthony TG, Vary TC, Jefferson LS, Kimball SR. Leucine stimulates translation initiation in skeletal muscle of postabsorptive rats via a rapamycin-sensitive pathway. J Nutr. 2000b;130:2413–2419. doi: 10.1093/jn/130.10.2413. [DOI] [PubMed] [Google Scholar]
- 47.Greiwe JS, Kwon G, McDaniel ML, Semenkovich CF. Leucine and insulin activate p70 S6 kinase through different pathways in human skeletal muscle. Am J Physiol - Endocrinol Metab. 2001;281:E466–E471. doi: 10.1152/ajpendo.2001.281.3.E466. [DOI] [PubMed] [Google Scholar]
- 48.Kimball SR. Regulation of translation initiation by amino acids in eukaryotic cells. Prog Mol Subcell Biol. 2001;26:155–184. doi: 10.1007/978-3-642-56688-2_6. [DOI] [PubMed] [Google Scholar]
- 49.Sood R, Porter AC, Olsen DA, Cavener DR, Wek RC. A mammalian homologue of GCN2 protein kinase important for translational control by phosphorylation of eukaryotic initiation factor-2alpha. Genetics. 2000;154:787–801. doi: 10.1093/genetics/154.2.787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Anthony TG, Reiter AK, Anthony JC, Kimball SR, Jefferson LS. Deficiency of dietary EAA preferentially inhibits mRNA translation of ribosomal proteins in liver of meal-fed rats. Am J Physiol. 2001;281:E430–E439. doi: 10.1152/ajpendo.2001.281.3.E430. [DOI] [PubMed] [Google Scholar]
- 51.Yoshizawa F, Kimball SR, Jefferson LS. Modulation of translation initiation in rat skeletal muscle and liver in response to food intake. Biochem Biophys Res Commun. 1997;240:825–831. doi: 10.1006/bbrc.1997.7652. [DOI] [PubMed] [Google Scholar]