Abstract
Methods of crossmatch testing prior to kidney transplantation are not standardized and there are limited large-scale data on the use and outcomes implications of crossmatch modality. Data describing the most sensitive crossmatch modality for crossmatch-negative kidney transplants were drawn from the Organ Procurement and Transplant Network Registry. Within the cohort transplanted in 1999−2005, we identified patient and transplant characteristics predictive of each testing modality by multivariate logistic regression. We assessed associations of crossmatch modality with rejection risk by logistic regression and with graft survival by Cox's hazards analysis. Among 230,995 transplants, use of flow cytometry with T-and B-lymphocytes (T&B FC) increased progressively in 1987−2005. Among the recent transplants performed in 1999−2005 (n=64,320), negative T&B FC crossmatch was associated with 15% lower relative risk of first-year acute rejection (adjusted HR 0.85, 95% CI 0.80−0.89) compared to negative T-antihuman-globulin and B-National Institutes of Health/Wash (T AHG &B) crossmatch. Five-year graft survival after transplant with negative T&B FC (82.6%) was modestly better than after negative T AHG &B (81.4%, P= 0.008) or T AHG crossmatch (81.1%, P< 0.0001), but on adjusted analysis was significantly different only among recipients from deceased donors and patients aged > 60 years. Many subgroups for whom negative T&B FC crossmatch predicted lower rejection risk (Caucasians, deceased donor recipients, re-transplants) were not more likely to be crossmatched by this method. We conclude that current practice patterns have not aligned utilization of T&B FC crossmatch with associated benefits. Prospective evaluation of the relationship of crossmatch modality with outcomes is warranted.
Introduction
Since the mid 1960s scientists have known that the presence of antibodies in kidney transplant recipients specific for donor human leukocyte antigens (HLA) is associated with an increased frequency of rejection and graft loss.11,17,21,25 In order to detect such antibodies and avoid the adverse results, serologists modified the complement-dependent microcytotoxicity assay that was used for HLA typing into a donor-recipient crossmatch.24 Recognizing that early rejection episodes still occur, even with a negative cross-match, the basic complement-dependent micro-cytotoxicity technique was modified by adding a wash step to increase specificity and an anti-human globulin (AHG) step to increase sensitivity.1,4 Sensitivity was further increased through the use of flow cytometry (FC) which can detect the presence of recipient antibodies on the surface of donor lymphocytes independent of complement binding.6,22 The most common targets used with all these techniques have been T and B lymphocytes.
Through the years, immunogeneticists have favored one crossmatch technique and target cell combination over others based on their own data, experience and personal biases. In this era of evidence-based medicine, it is important to compare results of kidney transplantation with the utilization of different crossmatch techniques. While the highest level of clinical evidence derives from randomized clinical trials, such studies are expensive to conduct on a large-scale and have not been performed to assess crossmatch-related outcomes to date.
To advance the understanding of practice patterns in crossmatch use and associated graft outcomes, we retrospectively studied a large sample of kidney transplants in the United States recorded in the Organ Procurement and Transplantation Network (OPTN) registry. The purposes of this study are to examine (1) the time-related trends in the utilization of these techniques/target cells, (2) the correlation between crossmatch modality utilization and recipient/transplant characteristics in recent nation-nal practice, and (3) the associations between crossmatch modality use and transplant outcomes.
Methods
Data source, inclusion criteria, definitions and outcomes
Data were drawn from the OPTN Standard Transplant Analysis and Research Files. At the time of transplantation, information is transmitted from the transplant center to the OPTN on the crossmatch techniques, targets used, and the types of antibodies detected (IgG only, IgM only, both or undefined). Reports are submitted at six months, and annually thereafter to the OPTN concerning the condition of the transplant recipients, including acute rejection episodes and graft failure. Patients with reported non-negative crossmatch results (positive, weak, not done, or indeterminate) and those receiving extrarenal transplants were excluded from the study. We restricted the analysis to crossmatches performed for the detection of IgG antibodies. In those cases where more than one technique/cell type combination was used per individual, we considered the most sensitive crossmatch modality according to the ranking: anti-IgG T&B FC > T AHG plus B-cell crossmatch by any technique other than FC (henceforth called T AHG &B) >T AHG without B-cell crossmatch (henceforth called T AHG). This hierarchy was established based on American Society for Histocompatibility and Immunogenetics/College of American Pathologists survey data.20,26
To describe usage patterns over time, we examined the most sensitive negative crossmatch modality performed for all transplants in 1987−2005. Since 1987, both immunosuppressive therapy and the quality of crossmatch techniques have improved. For these reasons we limited the study of clinical correlates of crossmatch modality and associations with graft outcomes to transplants performed in 1999−2005.
We considered acute rejection and graft failure as post-transplant outcomes of interest. Acute rejection is clinically defined by the reporting transplant program and may include biopsy-confirmed episodes as well as clinical diagnoses treated without biopsy. Graft loss is defined as a permanent return of the transplant patient to dialysis, re-listing for transplant or re-transplantation according to OPTN reports, censored for patient death. We also considered the timing of graft failure, distinguishing early events within the first year and events between three and five years after transplant.
Crossmatch Techniques
All crossmatch techniques and target sources have been described in the literature.20,26 Nonetheless, some aspects of these tests are highlighted here:
The complement-dependent microcytotoxi-city assay ends with a complement incubation followed by the addition of a supravital dye.
In the wash technique, following the recipient serum-donor cell incubation, a wash step is added to remove nonspecifically bound antibodies and increase specificity.
In the AHG technique, following the wash step, the cells are incubated with AHG. T-cells do not have significant immunoglobulin on their membranes; therefore, T-cells that have not bound recipient antibodies will not bind AHG but T-cells that have bound recipient antibodies will bind AHG. The bound AHG is more effective at binding complement than the bound recipient antibodies, thus increasing the sensitivity of the assay. Because of the presence of immunoglobulins on the surface of B-cells, this technique is infrequently used in the B-cell crossmatch in kidney transplantation.
In the FC crossmatch, following the recipient serum-donor cell incubation, cells are incubated with an antihuman IgG or IgM fluorescein conjugated antibodies to identify cells that have bound antibodies.
In this study the designation FC crossmatch refers only to tests performed with IgG.
Statistical Analysis
Statistical analysis was performed using SAS software (version 9.1, SAS Institute Inc., Cary, NC). Chi-square test was used to conduct bivariate comparisons of the distribution of patient and transplant factors according to the most sensitive negative crossmatch type done before transplantation. Missing data were identified as distinct variable categories. We used logistic regression to construct multivariate models in which associations of patient/transplant factors with crossmatch technique are adjusted for other observed factors (adjusted odds ratio, aOR). An association with an odds ratio < 1.0 indicates characteristics associated with reduced likelihood of testing by the modeled crossmatch modality compared to the indicated reference group, and an odds ratio > 1.0 indicates patients’ characteristics associated with increased likelihood of use of the crossmatch test of interest. We modeled associations (aOR) of crossmatch modality with risk of acute rejection within the first year after transplantation using multivariable logistic regression within the full sample and subgroups stratified by clinical characteristics.
The relationship between crossmatch modality and death-censored graft loss was examined by the Kaplan-Meier method (Log-Rank test). We employed multivariable Cox's regression to estimate adjusted associations (adjusted hazards ratio, aHR) of crossmatch modality with the risk of death-censored graft failure within the full sample and clinical subgroups. Both the Cox proportional hazards and logistic regression models were adjusted for donor/recipient demographics, panel reactive antibodies, retransplantation, duration of dialysis, HLA mismatching, duration of cold ischemia, donor quality and year of transplant. Standard criteria donor, expanded criteria donors and donation after cardiac death have been previously defined.3,19
To further minimize the risks of potential bias in the relationship of crossmatch modality selection with graft outcomes, we performed propensity adjustments. Predicted probabilities of testing by T&B FC crossmatch and T AHG crossmatch based on observed covariates were computed with logistic regression. The resulting propensity scores were then entered as adjustment covariates in the final Cox regret-ssions. Statistical significance was set at P ≤ 0.05.
Results
Time-related utilization
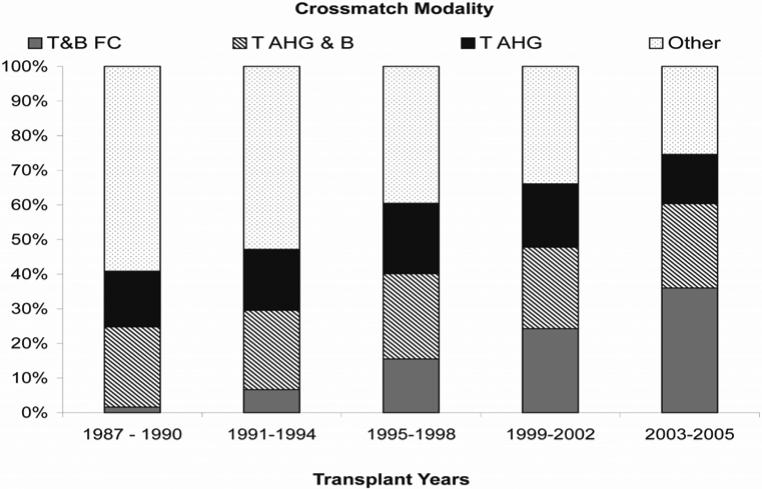
Among 597,930 crossmatch tests performed for detection of IgG antibody in 1987−2005, 1031 (0.2%) had missing results, 867 (0.1%) were indeterminate, 17,240 (2.9%) were positive and 578,792 (96.8%) were negative. Individual tests were considered in terms of combination modalities, as defined above. Time-related trends in the most sensitive crossmatch modality performed for crossmatch-negative transplants in 1987−2005 are shown in Figure 1. T&B FC utilization increased from 2% of these transplants in 1987−1990 to 36% in 2003−2005, while T AHG & B crossmatch utilization remained constant at approximately 25% during these same time period. T AHG crossmatch use also remained constant at approximately 15%. It should be noted that in 2003−2005, approximately 25% of these crossmatches still employed other modalities.
Figure 1.
Trends in the crossmatch utilization according to the most sensitive modality performed among crossmatch-negative kidney transplants in 1987−2005.
Computations are based on the most sensitive crossmatch modality performed for detection of IgG antibodies among all crossmatch-negative, kidney-only transplants in 1987−2005.
AHG: Anti-human globulin, FC: Flow Cytometry, T&B: T and B lymphocyte target cells
In 1999−2005 there were 92,023 kidney transplants performed with negative crossmatches for detection of IgG antibodies. Table 1 displays the utilization frequencies of the most sensitive negative crossmatch techniques/target cell type among these transplants. In subsequent analyses we considered the subset of these crossmatch modalities that were performed in > 10% of transplants, as per the distribution in Table 1 – specifically: T&B FC (N=27,129, 29.5%), T AHG & B (N=22,052, 24.0%) and T AHG (N=15,138, 16.5%).
Table 1.
Distribution of the most sensitive crossmatch modalities performed among crossmatch negative kidney transplants in 1999−205 (N=92,023)
| B-Cell Crossmatch Technique | |||||
|---|---|---|---|---|---|
| Flow | AHG | NIH/Wash | No B-Cell Crossmatch | ||
| T-Cell Crossmatch Technique | Flow | 27,129A (29.48%) | 94 (0.10%) | 3,011 (3.27%) | 3,189 (3.47%) |
| AHG | 76 (0.08%) | 4,602 (5.00%) | 22,052B (23.96%) | 15,139C (16.45%) | |
| NIH/Wash | 39 (0.04%) | 68 (0.07%) | 7,176 (7.80%) | 2,966 (3.22%) | |
| No T-Cell Crossmatch | 43 (0.05%) | 59 (0.06%) | 715 (0.78%) | 5,665 (6.16%) | |
AHG: Anti-human globulin, FC: Flow Cytometry, NIH: National Institutes of Health Lymphocytotoxicity Crossmatch Assay, T&B: T and B lymphocyte target cells, Wash: Lymphocytotoxicity Crossmatch assay
T&B FC Group
T AHG &B Group
T AHG Group
Clinical correlates of crossmatch modality use
In this section we focused on the 64,320 transplants performed after T&B FC, T AHG & B or T AHG as the most sensitive negative crossmatch modality. The distributions of T&B FC, T AHG & B, and T AHG crossmatches used for transplants within clinical subgroups are shown in Table 2. Adjusted OR for associations between recipient/transplant clinical characteristics and utilization of T&B FC, T AHG & B or T AHG crossmatches are shown in Table 3. African American recipients and recipients of living donor kidney transplants showed increased utilization of T&B FC and T AHG & B crossmatches. Recipients with panel reactive antibodies > 10% and recipients receiving kidneys with cold ischemia time > 12 hours also showed an increased utilization of T&B FC crossmatch. Recipients younger than 18 years and recipients of kidneys from expanded criteria donors showed increased utilization of T AHG &B crossmatch. Recipients older than 60 years and recipients receiving kidneys donated after cardiac death showed an increased utilization of T AHG crossmatch.
Table 2.
Distributions of T&B FC, T AHG & B, and T AHG techniques as the most sensitive crossmatch modalities within clinical subgroups, 1999−2005
| Total N=64,319 | T&B FC | T AHG &B | T AHG | P-value2 | |
|---|---|---|---|---|---|
| N=27,129 (%)1 | N=22,052 (%)1 | N=15,139 (%)1 | |||
| Recipient Race | |||||
| Caucasian | 37,403 | 38.9 | 35.3 | 25.8 | <.0001 |
| African-American | 14,225 | 43.0 | 36.5 | 20.5 | |
| Hispanic or other | 12,691 | 50.9 | 28.7 | 20.4 | |
| Recipient Age (yrs) | |||||
| 0−18 | 3,730 | 42.9 | 37.0 | 20.1 | <.0001 |
| 19−30 | 6,902 | 44.3 | 36.2 | 19.5 | |
| 31−45 | 17,598 | 42.8 | 34.9 | 22.3 | |
| 46−60 | 23,979 | 41.9 | 34.1 | 24.0 | |
| Over 60 | 12,110 | 40.4 | 31.8 | 27.8 | |
| Transplant Number | |||||
| First transplant | 57,506 | 41.9 | 34.3 | 23.8 | <.0001 |
| Re-transplant | 6,813 | 44.7 | 34.0 | 21.3 | |
| Peak PRA (%) | |||||
| 0−10 | 47,511 | 39.7 | 35.2 | 25.1 | <.0001 |
| 11−50 | 7,304 | 46.0 | 32.0 | 22.0 | |
| Over 50 | 6,301 | 51.8 | 32.8 | 15.4 | |
| Missing | 3,203 | 51.5 | 28.3 | 20.2 | |
| HLA Matching | |||||
| 0 ABDR MM | 6,781 | 43.4 | 34.3 | 22.3 | <.0001 |
| 0 DR MM | 12,912 | 42.8 | 34.7 | 22.5 | |
| DR MM | 44,354 | 41.7 | 34.2 | 24.1 | |
| Missing | 272 | 54.4 | 31.6 | 14.0 | |
| Donor Type and Quality | |||||
| Living Donor | 26,054 | 44.6 | 37.1 | 18.3 | <.0001 |
| SCD | 30,515 | 41.2 | 32.5 | 26.3 | |
| ECD | 6,187 | 37.5 | 33.4 | 29.1 | |
| DCD | 1563 | 39.0 | 25.5 | 35.4 | |
| Cold Ischemia Time (hours) | |||||
| 0−12 | 23,483 | 40.9 | 40.1 | 19.0 | <.0001 |
| 13−24 | 18,068 | 41.5 | 33.2 | 25.3 | |
| Over 24 | 7,206 | 45.8 | 28.4 | 25.9 | |
| Missing - Living | 10,631 | 46.3 | 29.7 | 24.0 | |
| Missing - Deceased | 4,931 | 36.8 | 29.1 | 34.2 | |
| Transplant Year | |||||
| 1999−2000 | 14,215 | 34.7 | 36.8 | 28.5 | <.0001 |
| 2001−2002 | 18,834 | 38.2 | 34.7 | 27.1 | |
| 2003−2005 | 31,270 | 48.0 | 32.9 | 19.2 |
Indicates percentage of specified patient group crossmatched by indicated modalities (“row percentage”). The study sample was limited to transplants with negative crossmatch results.
P-values were derived from Chi-square test comparing distributions of characteristics across crossmatch technique.
DCD: donation after cardiac death, ECD: expanded criteria donor, MM: mismatch, SCD: standard criteria donor
Table 3.
Associations of recipient, donor and transplant characteristics with the most sensitive crossmatch technique used prior to transplant, 1999−2005
| T&B FC | T AHG &B | T AHG | ||||
|---|---|---|---|---|---|---|
| aOR (95% CI) | P-value | aOR (95% CI) | P-value | aOR (95% CI) | P-value | |
| Recipient Race | ||||||
| Caucasian | Reference | Reference | Reference | |||
| African-American | 1.17 (1.12 − 1.23) | <.0001 | 1.13 (1.08 − 1.18) | <.0001 | 0.69 (0.66 − 0.73) | <.0001 |
| Hispanic or other | 1.49 (1.42 − 1.56) | <.0001 | 0.76 (0.73 − 0.80) | <.0001 | 0.79 (0.75 − 0.84) | <.0001 |
| Recipient Age (yrs) | ||||||
| 0−18 | 0.94 (0.88 − 1.01) | 0.101 | 1.10 (1.02 − 1.18) | 0.013 | 0.96 (0.88 − 1.05) | 0.4095 |
| 19−45 | Reference | Reference | Reference | |||
| 46−60 | 1.00 (0.96 − 1.03) | 0.796 | 0.97 (0.93 − 1.01) | 0.0958 | 1.06 (1.01 − 1.10) | 0.0188 |
| >60 | 0.97 (0.92 − 1.02) | 0.1963 | 0.89 (0.85 − 0.93) | <.0001 | 1.20 (1.14 − 1.27) | <.0001 |
| Transplant Number | ||||||
| First transplant | Reference | Reference | Reference | |||
| Re-transplant | 0.98 (0.93 − 1.04) | 0.477 | 1.00 (0.94 − 1.06) | 0.9908 | 1.03 (0.97 − 1.10) | 0.3432 |
| Peak PRA | ||||||
| 0−10% | Reference | Reference | Reference | |||
| 11−50% | 1.33 (1.26 − 1.40) | <.0001 | 0.89 (0.84 − 0.94) | <.0001 | 0.79 (0.74 − 0.84) | <.0001 |
| >50% | 1.68 (1.59 − 1.78) | <.0001 | 0.94 (0.88 − 1.00) | 0.037 | 0.49 (0.46 − 0.53) | <.0001 |
| Missing | 1.21 (1.12 − 1.31) | <.0001 | 0.68 (0.62 − 0.73) | <.0001 | 1.28 (1.16 − 1.40) | <.0001 |
| HLA Matching | ||||||
| 0 ABDR MM | 1.05 (0.99 − 1.10) | 0.1057 | 1.06 (1.00 − 1.12) | 0.0549 | 0.87 (0.82 − 0.93) | <.0001 |
| 0 DR MM | 1.08 (1.03 − 1.12) | 0.0005 | 1.03 (0.99 − 1.07) | 0.1791 | 0.86 (0.82 − 0.91) | <.0001 |
| DR MM | Reference | Reference | Reference | |||
| Missing | 1.53 (1.20 − 1.95) | 0.0007 | 0.88 (0.68 − 1.14) | 0.3196 | 0.59 (0.41 − 0.84) | 0.003 |
| Transplant Year | ||||||
| 1999−2000 | 0.57 (0.54 − 0.59) | <.0001 | 1.15 (1.10 − 1.20) | <.0001 | 1.80 (1.71 − 1.89) | <.0001 |
| 2001−2002 | 0.68 (0.65 − 0.71) | <.0001 | 1.05 (1.01 − 1.09) | 0.0267 | 1.63 (1.56 − 1.71) | <.0001 |
| 2003−2005 | Reference | Reference | Reference | |||
| Cold Ischemia Time | ||||||
| 0−12 Hours | Reference | Reference | Reference | |||
| 12−24 Hours | 1.30 (1.23 − 1.37) | <.0001 | 0.88 (0.83 − 0.93) | <.0001 | 0.85 (0.80 − 0.90) | <.0001 |
| >24 Hours | 1.54 (1.44 − 1.64) | <.0001 | 0.70 (0.65 − 0.75) | <.0001 | 0.88 (0.82 − 0.95) | 0.0006 |
| Missing - LD | 0.94 (0.86 − 1.03) | 0.1885 | 1.24 (1.13 − 1.36) | <.0001 | 0.71 (0.64 − 0.79) | <.0001 |
| Missing - DD | 1.13 (1.08 − 1.19) | <.0001 | 0.58 (0.55 − 0.61) | <.0001 | 1.86 (1.75 − 1.98) | <.0001 |
| Donor Type & Quality | ||||||
| Living Donor | 1.50 (1.41 − 1.59) | <.0001 | 1.33 (1.25 − 1.41) | <.0001 | 0.37 (0.34 − 0.40) | <.0001 |
| SCD | Reference | Reference | Reference | |||
| ECD | 0.94 (0.86 − 1.03) | 0.1821 | 1.13 (1.03 − 1.24) | 0.0068 | 0.93 (0.85 − 1.03) | 0.1512 |
| DCD | 0.87 (0.79 − 0.97) | 0.0127 | 0.73 (0.65 − 0.82) | <.0001 | 1.61 (1.44 − 1.80) | <.0001 |
The study sample was limited to transplants with negative crossmatch results. Subgroups were defined according to levels of clinical factors. For example, African American and Hispanic/Other are racial groups compared to Caucasian race as a reference group. An aOR <1.0 indicates that the indicated crossmatch test (T&B FC, T AHG &B, or T AHG) was less likely to be used in the indicated group compared to the reference group. An aOR >1.0 indicates that the test was more likely to be used in the indicated group compared to the reference group. P-values reflect the significance of difference in utilization of a crossmatch test in the indicated group compared to the reference group.
aOR: adjusted Odds Ratio, DD: deceased donor, LD: living donor
Associations of graft outcomes with crossmatch modality and recipient/transplant characteristics
Acute rejection risk
Acute rejection within the first year after transplantation occurred among 14.9% of the full sample transplanted in 1999−2005. Unadjusted rejection rates according to crossmatch modality were 13.3%, 16.1% and 16.1%, respectively, among patients crossmatched by T&B FC, T AHG & B, and T AHG methods. After adjustment for other factors, there was an approximate 15% reduction in the adjusted relative risk of acute rejection (aOR 0.85, 95% CI 0.80−0.89) within the full sample when transplants were performed after negative T&B FC crossmatch compared to after negative T AHG &B crossmatch (Table 4). Within subgroups defined by clinical recipient and transplant characteristics, the adjusted risk of rejection after negative T&B FC compared to T AHG &B crossmatch was not significantly different among African Americans, recipients aged 0−18 years and recipients of kidneys from living donors. Risk of rejection was not significantly different after negative T AHG compared to T AHG & B crossmatch within the full sample, but results within subgroups were variable – specifically, omission of B-cell cross-match was associated with increased risk of acute rejection compared to T AHG & B in patients with panel reactive antibodies > 10%, but was associated with lower rejection risk among Hispanic recipients and transplants with 0 ABDR mismatches, from deceased donors or from donors after cardiac death.
Table 4.
Associations of crossmatch modality with first-year acute rejection risk1 within the full sample of transplants performed in 1999−2005 and within clinical subgroups
| T&B FC2 | T AHG2 | |||
|---|---|---|---|---|
| Sample | aOR (95% CI) | P-Value | aOR (95% CI) | P-Value |
| Full sample | 0.85 (0.80 − 0.89) | <.0001 | 0.96 (0.91 − 1.02) | 0.2239 |
| Subgroup | ||||
| Recipient Race | ||||
| Caucasian | 0.81 (0.75 − 0.87) | <.0001 | 1.01 (0.94 − 1.09) | 0.7873 |
| African-American | 1.00 (0.90 − 1.11) | 0.9753 | 0.95 (0.84 − 1.07) | 0.4047 |
| Hispanic or other | 0.73 (0.64 − 0.83) | <.0001 | 0.77 (0.66 − 0.90) | 0.0013 |
| Recipient Age | ||||
| 0−18 | 1.12 (0.91 − 1.39) | 0.2837 | 1.21 (0.95 − 1.56) | 0.1274 |
| 19−45 | 0.88 (0.81 − 0.95) | 0.0019 | 0.97 (0.88 − 1.06) | 0.4637 |
| 46−60 | 0.79 (0.72 − 0.86) | <.0001 | 0.94 (0.85 − 1.04) | 0.2192 |
| >60 | 0.80 (0.70 − 0.92) | 0.002 | 0.90 (0.78 − 1.05) | 0.1824 |
| Peak PRA (%) | ||||
| 0−10 | 0.84 (0.79 − 0.90) | <.0001 | 0.90 (0.84 − 0.97) | 0.0038 |
| 11−50 | 0.96 (0.82 − 1.12) | 0.5582 | 1.31 (1.10 − 1.55) | 0.0023 |
| >50 | 0.80 (0.68 − 0.93) | 0.0043 | 1.23 (1.02 − 1.50) | 0.0341 |
| Transplant Number | ||||
| First transplant | 0.87 (0.82 − 0.92) | <.0001 | 0.96 (0.90 − 1.02) | 0.1759 |
| Re-transplant | 0.73 (0.62 − 0.86) | 0.0001 | 1.06 (0.89 − 1.27) | 0.5125 |
| HLA Matching | ||||
| 0 ABDR MM | 0.79 (0.65 − 0.96) | 0.0172 | 0.73 (0.58 − 0.92) | 0.0082 |
| 0 DR MM | 0.83 (0.73 − 0.94) | 0.0037 | 0.90 (0.78 − 1.04) | 0.1689 |
| DR MM | 0.86 (0.81 − 0.91) | <.0001 | 1.01 (0.94 − 1.08) | 0.8871 |
| Donor Type | ||||
| Living | 0.93 (0.85 − 1.01) | 0.0748 | 1.05 (0.95 − 1.16) | 0.3783 |
| Deceased3 | 0.80 (0.74 − 0.85) | <.0001 | 0.92 (0.85 − 0.99) | 0.0301 |
| Donor Donor Quality | ||||
| SCD | 0.81 (0.75 − 0.88) | <.0001 | 0.98 (0.90 − 1.06) | 0.549 |
| ECD | 0.76 (0.65 − 0.90) | 0.0011 | 0.79 (0.67 − 0.94) | 0.0075 |
| DCD | 0.75 (0.49 − 1.13) | 0.1673 | 0.56 (0.36 − 0.87) | 0.0089 |
Rejection within the first year after transplant was defined by individual transplant programs, as reported to the OPTN
The study sample was limited to transplants with negative crossmatch results. T & B FC and T AHG-only crossmatch were compared to the reference group, T AHG &B crossmatch. An aOR <1.0 indicates that risk of rejection in association with the modeled crossmatch test was lower compared to T AHG &B in a particular sample. An aOR >1.0 indicates that risk of rejection in association with the modeled crossmatch test was higher compared to T AHG &B in a particular sample. P-values reflect the significance of difference in rejection risk associated with the indicated crossmatch test compared to T AHG &B within a specified sample.
Deceased donor is a composite of SCD, ECD and DCD
aOR: adjusted odds ratio
Risk of graft failure
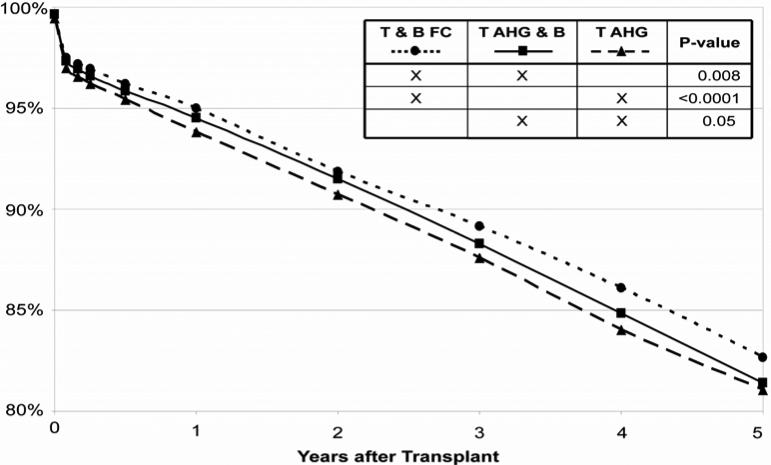
The five-year unadjusted graft survival rates for 64,320 transplants carried out between 1999 and 2005, stratified for T&B FC, T AHG & B and T AHG crossmatch are shown in Figure 2. Five-year, cumulative graft survival after transplant with negative T&B FC crossmatch as the most sensitive technique (82.6%) was modestly better than after negative T AHG & B crossmatch (81.4%; P= 0.008) or T AHG crossmatch (81.1%; P< 0.0001). Five-year cumulative graft survival was not appreciably different after negative T AHG &B compared to T AHG crossmatch (81.4% versus 81.1%, P= 0.05).
Figure 2.
Kidney graft survival according to the most sensitive modality performed among crossmatch-negative kidney transplants, 1999−2005.
P-value by the Log-Rank test. “X” symbols in the legend indicate the tests being compared.
Five-year, cumulative graft survival after transplant with negative T&B FC crossmatch as the most sensitive technique (82.6%) was modestly better than after negative T AHG &B crossmatch (81.4%; P=0.008) or T AHG crossmatch (81.1%; P<0.0001). Five-year cumulative graft survival was not appreciably different after negative T AHG &B compared to T AHG crossmatch (81.4% versus 81.1%, P=0.05).
Adjusted associations of crossmatch modality with death-censored graft loss up to five years after transplant are presented in Table 5. Use of T&B FC crossmatch was associated with a non-significant trend towards modestly improved adjusted graft survival in the full sample (aHR 0.95, 95% CI 0.89−1.01, P=0.07). Analysis of early (one year) and later (three to five years) graft survival in recipients transplanted after negative T&B FC compared to T AHG & B crossmatch also showed no significant differences (data not shown).
Table 5.
Associations of crossmatch modality with risk of death-censored graft failure1 within the full sample of transplants performed in 1999−2005 and within clinical subgroups
| T&B FC2 | T AHG2 | |||
|---|---|---|---|---|
| Sample | aHR (95% CI) | P-Value | aHR (95% CI) | P-Value |
| Full sample | 0.95 (0.89 − 1.01) | 0.0702 | 1.03 (0.97 − 1.10) | 0.3156 |
| Subgroup | ||||
| Recipient Race | ||||
| Caucasian | 0.94 (0.86 − 1.02) | 0.1421 | 1.12 (1.03 − 1.23) | 0.0106 |
| African-American | 0.92 (0.83 − 1.02) | 0.0968 | 0.92 (0.82 − 1.03) | 0.1598 |
| Hispanic or other | 1.03 (0.89 − 1.21) | 0.674 | 0.97 (0.80 − 1.16) | 0.7049 |
| Recipient Age | ||||
| 0−18 | 1.12 (0.89 − 1.40) | 0.3335 | 0.99 (0.77 − 1.28) | 0.9301 |
| 19−45 | 0.96 (0.88 − 1.05) | 0.3387 | 1.01 (0.91 − 1.11) | 0.8844 |
| 46−60 | 0.94 (0.85 − 1.05) | 0.2822 | 1.05 (0.94 − 1.18) | 0.3957 |
| > 60 | 0.85 (0.73 − 0.99) | 0.0361 | 1.03 (0.88 − 1.20) | 0.6995 |
| Peak PRA (%) | ||||
| 0−10 | 0.95 (0.89 − 1.03) | 0.2071 | 0.99 (0.92 − 1.07) | 0.8528 |
| 11−50 | 0.97 (0.82 − 1.15) | 0.6882 | 1.22 (1.02 − 1.46) | 0.033 |
| > 50 | 0.88 (0.75 − 1.02) | 0.0956 | 1.14 (0.95 − 1.36) | 0.1707 |
| Transplant Number | ||||
| First transplant | 0.95 (0.89 − 1.02) | 0.131 | 1.02 (0.95 − 1.10) | 0.5042 |
| Re-transplant | 0.91 (0.77 − 1.07) | 0.2461 | 1.11 (0.93 − 1.32) | 0.2639 |
| HLA Matching | ||||
| 0 ABDR MM | 1.12 (0.90 − 1.41) | 0.3083 | 1.13 (0.88 − 1.46) | 0.3384 |
| 0 DR MM | 0.92 (0.80 − 1.06) | 0.2515 | 1.13 (0.97 − 1.30) | 0.1187 |
| DR MM | 0.94 (0.87 − 1.01) | 0.0736 | 1.01 (0.93 − 1.09) | 0.8269 |
| Donor Type | ||||
| Living | 0.99 (0.89 − 1.11) | 0.9141 | 1.12 (0.98 − 1.28) | 0.0874 |
| Deceased2 | 0.92 (0.86 − 0.99) | 0.0193 | 1.00 (0.93 − 1.08) | 0.9808 |
| Donor Quality | ||||
| SCD | 0.92 (0.85 − 1.00) | 0.0514 | 1.00 (0.92 − 1.09) | 0.9887 |
| ECD | 0.97 (0.83 − 1.13) | 0.6754 | 1.03 (0.88 − 1.21) | 0.7108 |
| DCD | 0.94 (0.61 − 1.47) | 0.7976 | 0.82 (0.53 − 1.26) | 0.3575 |
Graft failure out to five years post-transplant was defined by OPTN records as permanent return to dialysis, relisting for retransplant, or retransplant
The study sample was limited to transplants with negative crossmatch results. T & B FC and T AHG-only crossmatches were each compared to the reference group, T AHG &B crossmatch. An aHR <1.0 indicates that risk of graft failure in association with the modeled crossmatch test was lower compared to T AHG & B in a particular sample An aHR >1.0 indicates that risk of graft failure in association with the modeled crossmatch test was higher compared to T AHG &B in a particular sample. P-values reflect the significance of difference in graft failure risk associated with the indicated crossmatch test compared to T AHG &B within a specified sample
aHR: adjusted hazards ratio
Stratification of the study sample based on recipient/transplant characteristics reduced sample sizes, limiting statistical power. Nonetheless, patients older than 60 years (aHR 0.85, 95% CI 0.73−0.99) and recipients of kidneys from deceased donors (aHR 0.92, 95% CI 0.86−0.99) transplanted with a negative T&B FC cross-match had modestly lower risk of graft failure than those transplanted with negative T AHG & B crossmatch. Caucasians and recipients with panel reactive antibodies between 10−50% showed an increased risk of graft failure when a B-cell crossmatch was not performed, whereas omission of B-cell crossmatch was not associated with improved graft survival in any subgroup.
Discussion
In histocompatibility laboratories, many different crossmatch strategies have been applied. We found that the utilization of T&B FC among crossmatch-negative transplants has approximately doubled since 1999, varies according to recipient and transplant characteristics, and is associated with decreased risk of acute rejection and a modest reduction in unadjusted, 5-year graft survival. Numerous centers have described superior results associated with the use of T&B FC crossmatch, particularly in the first month post transplantation,2,7,12 while other reports did not find such association.15,16 Our analysis did not detect an early graft survival benefit with the use of T&B FC crossmatch. However, the utilization of T&B FC crossmatch was strongly associated with decreased risk of first-year acute rejection.
Most laboratories using FC technology do so selectively, choosing the transplants that they feel will most benefit. In order to describe recent national practice patterns, we examined associations of recipient and transplant characteristics with the most sensitive negative cross-match modality used prior to transplantation. Non-Caucasians, sensitized recipients, recipients from living donors and of kidneys with cold ischemia time > 12 hours showed the largest proportional utilization of T&B FC crossmatch. Although multiple subgroups showed decreased risk of acute rejection when transplanted with negative T&B FC crossmatch, of these groups only Hispanics and patients with panel reactive antibodies > 50% had increased utilization of T&B FC crossmatch. Recipients of kidneys from deceased donors and patients > 60 years also showed superior graft survival when transplanted after negative T&B FC crossmatch than after negative T AHG & B crossmatch. Notably, both groups also had decreased utilization of T&B FC crossmatch. Our results suggest that current practice patterns have not aligned utilization of T&B FC crossmatch with associated benefits.
Previous studies have shown benefit in utilizing T&B FC crossmatch in patients who undergo re-transplantation.5 Although we were not able to confirm this observation with our graft survival data, re-transplanted recipients with negative T&B FC crossmatch had reduced risk of acute rejection. Notably, we did not demonstrate increased utilization of T&B FC crossmatch among re-transplant recipients.
We observed an association of T&B FC crossmatch utilization with increasing duration of cold ischemia time. Our data do not have the ability to identify causes for this association. Minimization of cold ischemia is particularly important for kidneys from expanded criteria donors, donated after cardiac death, and shipped across the country. We also found that T&B FC crossmatch was associated with lower risk of acute rejection and better graft survival among deceased donor transplants. Transplant centers have to balance the potential advantages of T&B FC crossmatch with speed of crossmatch performance, expected ischemia time, and donor quality in individual cases.
We were surprised to find that approximately a quarter of the crossmatches reported omission of B-cell targets. Multiple single-center reportsas well as recent registry data suggest that recipients with T-cell negative, B-cell positive crossmatches have poorer outcomes than those with totally negative crossmatches.7,9,15 Of note, our analysis did not evaluate B-cell positivity, rather it compared outcomes after negative T AHG crossmatch with and without negative B-cell crossmatch. Although rejection risk was inconsistent across subgroups, we found significantly increased risk of graft loss with omission of B-cell targets among subgroups of Caucasians and those with panel reactive antibodies, but we did not detect improved graft survival with B-cell target omission in any subgroup. Based on previous published studies, the findings of our analysis, and the knowledge that B-cell target inclusion does not significantly alter the time or the cost of the crossmatch, we feel that B-cell crossmatch should be routinely included as standard practice.
While most centers using FC technology apply it selectively, some laboratories use it exclusively. Commonly held deterrents to the exclusive use of FC technology include concerns of impracticality because of the cost of the equipment and maintenance, personnel training, quality control and assurance, and the large amount of sample required.8 However, it is the opinion of some with experience using FC crossmatch exclusively that difficulties primarily occur during the transition to this technique. Once equipment has been purchased, staff has been trained and protocols become routine, the use of FC crossmatch may be no more expensive and may not significantly prolong ischemia time when compared to other techniques (personal communication, R. Bray, PhD).
Our study has not addressed important topics relevant to the utilization of crossmatch techniques in renal transplantation that must be further investigated. The significance of weak IgM titers must be determined.23 Our analysis does not distinguish between crossmatches positive because of reactivity to HLA or non-HLA antigens. Most often, these non-HLA reactivities complicate the B-cell crossmatch, which may be one of the reasons some laboratories still resist performing B-cell crossmatch routinely. Although solid phase antibody testing identifies anti HLA antibodies exclusively, data have not yet been published to clearly substantiate the role of non-IgG non-HLA antibodies. Antibody reduction studies indicate that titers correlate with outcome,14 an issue not addressed in our analysis. Further, although routine pre-transplant crossmatch is still considered standard, some investigators have suggested that the result of a crossmatch test can be predicted with reasonable accuracy and replaced by antibody screening,10,13 particularly with solid phase techniques using solubilized HLA antigen adhered to a solid matrix or microparticles.18,27
The retrospective nature of this analysis and inability to randomize patients to testing modalities poses an inherent risk for selection bias. To control for this limitation, we performed multivariate regression, stratified sub-sampling according to clinical characteristics, and further adjusted our models with propensity scores for T&B FC and T AHG crossmatch. However, residual confounding from unobserved and uncontrolled factors, such as immunosuppressive agents choice, may occur. All studies evaluating lab results are at risk for false positive and false negative results. Despite its limitations, this study is strengthened by basis in a large sample that allows examination of national practice patterns and associations of crossmatch modalities with rejection and graft loss on a scale not possible within single center data. This study illustrates the importance of continuous investigation until we definitively establish national standards and proper indications for different methods of crossmatch testing in relation to recipient and transplant factors.2,8
In conclusion, our data show increased utilization of T&B FC in recent decades as the most sensitive crossmatch modality among crossmatch-negative kidney transplants. Overall, negative T&B FC crossmatch was associated with an approximate 15% reduction in the relative risk of acute rejection compared to negative T AHG &B crossmatch. T&B FC crossmatch was also associated with modestly improved 5-year graft survival among older recipients and recipients from deceased donors. In current practice, the apparently selective use of T&B FC crossmatch is not being targeted to groups that derive the most benefit. Increased use of T&B FC crossmatch, particularly in groups with indications of greatest benefit, may improve transplant outcomes. Prospective evaluation of this practice is warranted.
Acknowledgements
We thank Arline Webb (HLA Laboratory, Saint Louis University, St. Louis, MO) and Robert Bray, Ph.D. (HLA Laboratory, Emory University, Atlanta, GA) for the important suggestions for the manuscript. Dr. Lentine is supported by a grant from the NIDDK, K08-0730306. An abstract describing portions of this work was presented at the 2007 American Transplant Congress on May 8, 2007, San Francisco, CA.
The data reported here have been supplied by United Network for Organ Sharing as the contractor for the OPTN. The interpretation and reporting of these data are the responsibility of the authors and should in no way be seen as representing official policy of or interpretation by the OPTN or the U.S. Government.
References
- 1.Amos B, Bashir H, Boyle W, et al. A simple microcitoxicity test. Transplantation. 1969;7:220–3. doi: 10.1097/00007890-196903000-00023. [DOI] [PubMed] [Google Scholar]
- 2.Cho YW, Cecka JM. Crossmatch tests-an analysis of UNOS data from 1991−2000. Clin Transpl. 2001:237–46. [PubMed] [Google Scholar]
- 3.Cooper JT, Chin LT, Krieger NR, et al. Donation after cardiac death: the University of Wisconsin experience with renal transplantation. Am J Transplant. 2004;4:1490–4. doi: 10.1111/j.1600-6143.2004.00531.x. [DOI] [PubMed] [Google Scholar]
- 4.Fuller TC, Cosimi AB, Russell PS. Use of an antiglobulin-ATG reagent for detection of low levels of alloantibody-improvement of allograft survival in presensitized recipients. Transplant Proc. 1978;10:463–6. [PubMed] [Google Scholar]
- 5.Gallichio MH, Hudson S, Young CJ, et al. Renal retransplantation at the University of Alabama at Birmingham: incidence and outcome. Clin Transpl. 1998:169–75. [PubMed] [Google Scholar]
- 6.Garovoy MR, Rheinschmidt MA, Bigos M, et al. Flow cytometry analysis: a high technology crossmatch technique facilitating transplantation. Transplant Proc. 1983;15:1939–44. [Google Scholar]
- 7.Gebel HM, Bray RA, Nickerson P. Pre-transplant assessment of donor-reactive, HLA-specific antibodies in renal transplantation: contraindication vs. risk. Am J Transplant. 2003;3:1488–500. doi: 10.1046/j.1600-6135.2003.00273.x. [DOI] [PubMed] [Google Scholar]
- 8.Howden AJ, Sayer DC, Bennett G, et al. A quality control program for cross-matching procedures for solid organ transplantation. The participating laboratories of the Australasian and South Asian Tissue Typing Association. Hum Immunol. 2000;61:419–24. doi: 10.1016/s0198-8859(00)00098-7. [DOI] [PubMed] [Google Scholar]
- 9.Kerman R, Schakowsky S, Knight R, et al. Clinical relevance of cross-match results, HLA and non HLA antibodies for renal allograft recipients. Hum Immunol. 2006;67(Suppl 1):S40. [Google Scholar]
- 10.Kerman RH, Susskind B, Ruth J, et al. Can an immunologically, non-reactive potential allograft recipient undergo transplantation without a donor-specific cross-match? Transplantation. 1998;66:1833–4. doi: 10.1097/00007890-199812270-00044. [DOI] [PubMed] [Google Scholar]
- 11.Kiss Meyer-Nielsen FO, Petersen VP, Feldberg O. Hyper acute rejection of kidney allograft, associated with pre-existing humeral antibodies against donor cells. Lancet. 1966;2:662–5. doi: 10.1016/s0140-6736(66)92829-7. [DOI] [PubMed] [Google Scholar]
- 12.Mahoney RJ, Ault KA, Given SR, et al. The flow cytometric cross-match and early renal transplant loss. Transplantation. 1990;49:527–35. doi: 10.1097/00007890-199003000-00011. [DOI] [PubMed] [Google Scholar]
- 13.Matas AJ, Humar A, Kandaswamy R, et al. Kidney and pancreas transplantation without a cross-match in select circumstances-it can be done. Clin Transplant. 2001;15:236–9. doi: 10.1034/j.1399-0012.2001.150403.x. [DOI] [PubMed] [Google Scholar]
- 14.Mizutani K, Terasaki P, Hamdani E, et al. The importance of anti-HLA-specific antibody strength in monitoring kidney transplant patients. Am J Transplant. 2007;7:1027–31. doi: 10.1111/j.1600-6143.2006.01721.x. [DOI] [PubMed] [Google Scholar]
- 15.O'Malley KJ, Cook DJ, Roeske L, et al. Acute rejection and the flow cytometry cross-match. Transplant Proc. 1999;31:1216–7. doi: 10.1016/s0041-1345(98)01969-1. [DOI] [PubMed] [Google Scholar]
- 16.O'Rourke RW, Osorio RW, Freise CE, et al. Flow cytometry cross-matching as a predictor of acute rejection in sensitized recipients of cadaveric renal transplants. Clin Transplant. 2000;14:167–73. doi: 10.1034/j.1399-0012.2000.140212.x. [DOI] [PubMed] [Google Scholar]
- 17.Patel R, Terasaki PI. Significance of the positive cross-match test in kidney transplantation. N Engl J Med. 1969;280:735–9. doi: 10.1056/NEJM196904032801401. [DOI] [PubMed] [Google Scholar]
- 18.Pei R, Lee JH, Shih NJ, et al. Single human leukocyte antigen flow cytometry beads for accurate identification of human leukocyte antigen antibody specificities. Transplantation. 2003;75:43–9. doi: 10.1097/00007890-200301150-00008. [DOI] [PubMed] [Google Scholar]
- 19.Port FK, Bragg-Gresham JL, Metzger RA, et al. Donor characteristics associated with reduced graft survival: an approach to expanding the pool of kidney donors. Transplantation. 2002;74:1281–6. doi: 10.1097/00007890-200211150-00014. [DOI] [PubMed] [Google Scholar]
- 20.Salz P, Blanck C. HLA Cytotoxicity testing. ASHI Laboratory Manual. (4th edition) 2000:Gic10.1. [Google Scholar]
- 21.Starzl TE, Lerner RA, Dixon FJ, et al. Schwartzman reaction after human renal homotransplantation. N Engl J Med. 1968;278:642–8. doi: 10.1056/NEJM196803212781202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Talbot D, Givan AL, Shenton BK, et al. Value of the flow cytometric cross-match in renal transplantation. Transplant Proc. 1987;19:4315–6. [PubMed] [Google Scholar]
- 23.ten Hoor GM, Coopmans M, Allebes WA. Specificity and Ig class of preformed alloantibodies causing a positive cross-match in renal transplantation. The implications for graft survival. Transplantation. 1993;56:298–304. doi: 10.1097/00007890-199308000-00008. [DOI] [PubMed] [Google Scholar]
- 24.Terasaki PI, JD M. Antibody response to homografts. VIII. Relation of mouse hemagglutinins and cytotoxins. J Exp Med. 1963;117:675–90. doi: 10.1084/jem.117.4.675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Williams G, Hume DM, Hudson R, Jr., Morris PJ, Kano K, Milgrom F. “Hyperacute”renal-homograft rejection in man. N Engl J Med. 1968;279:611–8. doi: 10.1056/NEJM196809192791201. [DOI] [PubMed] [Google Scholar]
- 26.Wilmonton-Hosey L, Chapman P, Holcomb J, et al. Flow cytometry cross-match and antibody detection. ASHI Laboratory Manual. 2000:B.1. [Google Scholar]
- 27.Zachary AA, Ratner LE, Graziani JA, et al. Characterization of HLA class I specific antibodies by ELISA using solubilized antigen targets: II. Clinical relevance. Hum Immunol. 2001;62:236–46. doi: 10.1016/s0198-8859(00)00253-6. [DOI] [PubMed] [Google Scholar]