Abstract
High-speed countercurrent chromatography (HSCCC) was applied for separation and purification of flavonoids from the extract of belamcanda. High efficiency of HSCCC separation was achieved on a two-phase solvent system of n-hexane–ethyl acetate–methanol–water (4:5:5:5, v/v) by eluting the lower mobile phase at a flow-rate of 1.2mL/min and a revolution speed of 800 rpm. Three well-separated peaks were obtained in the HSCCC chromatogram and their purities were determined by HPLC-UV absorption spectrometry. These peaks were characterized by ESI-MSn and NMR, and the data compared with the reference standards where three peaks were identified as isorhamnetin, irigenin and hispidulin. The purities of each peak were 94, 95 and 90% respectively. In HSCCC experiment, 100 mg of the crude extract were separated yielding 10 mg of isorhamnetin, 8 mg of irigenin and 7 mg of hispidulin. HSCCC thus provides a cost-effective alternative to preparative scale HPLC for the semi-preparative scale separation and purification of flavonoids from Belamcanda.
Keywords: HSCCC, flavonoids, belamcanda, HPLC
Introduction
Belamcandae is a well known Chinese herbal medicine. Traditionally, it was used for the treatment of inflammation, asthma as well as throat disorders, e.g. cough, tonsillitis and pharyngitis[1]. It is a member of the family Iridacae, and widely distributed in eastern Asia including China, Korea, India and Japan. The major bioactive constituents in Belamcandae are isoflavonoids [2-7], stilbenes, xanthones, and iridal-type triterpeniods. In the recent years, the preparative separation and purification of the components from the Belamcanda by conventional methods such as column chromatography has been reported, and a number of flavonoids and isoflavonoids have been separated from Belamcandae by silica gel column chromatography. However, these conventional methods are tedious and require multiple chromatography steps.
High-speed countercurrent chromatography (HSCCC) is a form of liquid–liquid partition chromatography[8]. Solute separation is based on partitioning between the two immiscible liquid phases: the mobile phase and the support-free liquid stationary phase. Without solid matrix, the stationary phase is retained in the column with the aid of a centrifugal force, so that it eliminates irreversible adsorption of samples onto the solid support. Therefore, HSCCC is very suitable for separation of active components from traditional Chinese herbs and other natural products.
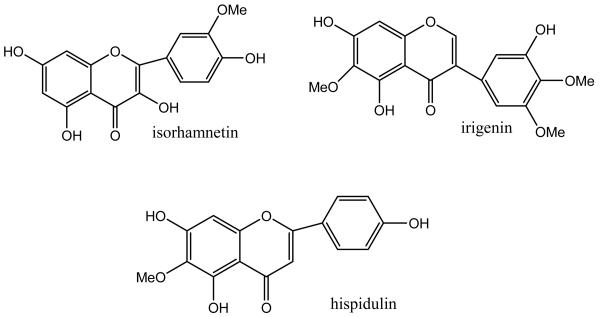
In this paper, the two-phase solvent system composed of n-hexane–ethyl acetate–methanol–water (4:5:5:5, v/v) was used for preparative separation and purification of isorhamnetin, irigenin and hispidulin from Belamcandae by HSCCC, and their structures (Fig. 1) were identified by ESI-MS.
Figure 1.
Chemical structures of target compounds from Belamcanda
Experimental
Apparatus
The HSCCC instrument employed in present study was GS10A high-speed countercurrent chromatograph (Beijing Institute of Technology Application, Beijing, China) with a multilayer coil separation column (i.d. of the tubing = 1.6mm, total volume = 230 ml) and a 20ml sample loop. The β value of the preparative column varied from 0.5 at the internal terminal to 0.7 at the external terminal (β = r/R, where r is the rotation radius or the distance from the coil to the holder shaft, and R is the revolution radius or the distances between the holder axis and central axis of the centrifuge). The revolution speed of the apparatus can be regulated with a speed controller in the range between 0 and 1000 rpm. The system was also equipped with one S-1007 constant flow pump, a Model 8823A-UV monitor operating at 254 nm, a Yokogawa 3057 recorder.
The analytical HPLC equipment was a Shimadzu LC-10ATvp consisting of a Shimadzu SPD-M10Avp UV detector, a sample injection valve (Model 7726) with a 20 ml loop, a system controller (SCL-10AVP) and a Shimadzu LC Solution Workstation (Shimadzu, Kyoto, Japan). The column applied in this work was a Diamonsil C18 column (250 mm × 4.6 mm i.d., 5 μm, Dikma Technologies China). ESI-MSn was LCQ Advantage (Thermo Finnigan, USA). Belamcandae was purchased from Guangzhou Traditional Chinese Medicine Company, Guangzhou, China.
Reagents
All organic solvents used for HSCCC were of analytical grade (Tianjing Damao Reagent Factory, Tianjing, China). Methanol and acetonitrile used for HPLC analysis was of chromatographic grade (DIKMA filiale in Guangzhou, China).
Preparation of Crude Sample
Belamcandae was obtained from a local drug store in our town, It was dried at a constant temperature of 60°C for 6 hours, then ground to powder (about 30-mesh) with a disintegrator. The powder (10 kg) was extracted with 30 L of 80% ethanol at 85°C for 3 hours, four times. The filtrate was then condensed by evaporating the solvent under a reduced pressure yielding 2605.5 g of crude extract. The extract was dissolved in water (2.5L) and water-insoluble matter was abandoned. Then it was extracted in turn by petroleum ether (boiling point: 60C°-90 C°), ethyl acetate and n-butyl alcohol. The ethyl acetate extract was evaporated to dryness and stored in a refrigerator at 4C° for further purification by HSCCC.
HSCCC Separation
In this work, n-hexane-ethyl acetate-methanol-water at a volume ratio of 4:5:4:5 was selected as a two-phase solvent system. The solvent system was prepared by adding all the solvents to a separatory funnel according to the volume ratios and thoroughly equilibrated by repetitive vigorous shaking, and the two phases were separated shortly before use. The sample solution was prepared by dissolving the crude extract in the lower phase and upper phase (1:1, v/v) of the solvent system used for HSCCC separation.
In each separation the multilayer coiled column was first entirely filled with the upper stationary phase. Then the apparatus was rotated at a revolution speed of 800 rpm. The lower phase was then pumped into the head end of the column inlet at a flow-rate of 1.2 mL·min−1. After the hydrodynamic equilibrium was reached, the sample solution (100 mg dissolved in 10mL of a 1:1 mixture of lower and upper phases of the solvent system) was injected through the sample port. The effluent from the tail end of the column was continuously monitored with a UV detector at the wavelength of 254 nm. Each peak fraction was manually collected according to the elution profile and finally analyzed by HPLC.
HPLC, ESI-MS and NMR analysis and identification of HSCCC peak fractions
The partially purified extract of Belamcandae and each peak fraction from HSCCC were analyzed by HPLC. The analyses were performed with a Diamonsil C18 column as follows: Methanol-acetonitrile-0.1 % acetic acid water was used as the mobile phase in gradient mode (methanol- acetonitrile: initial, 13%-13%; 20 min, 13%-13%; 27 min, 15%-15%; 28 min, 16%-27%; 50 min, 16%-27%; 53 min, 0%-80%; 57 min, stop). The flow-rate of the mobile phase was 0.8 mL·min−1. And the effluent was monitored by a dual-wavelength absorbance detector.
Identification of HSCCC peak fractions was carried out by ESI-MS and NMR. ESI-MS was carried out using a Finingan LCQ Deca ion trap mass XP MAX spectrometer equipped with and electrospray ionization source (Thermo Finnigan,San Jose, CA, USA).
Results and discussion
Optimization of HPLC Condition
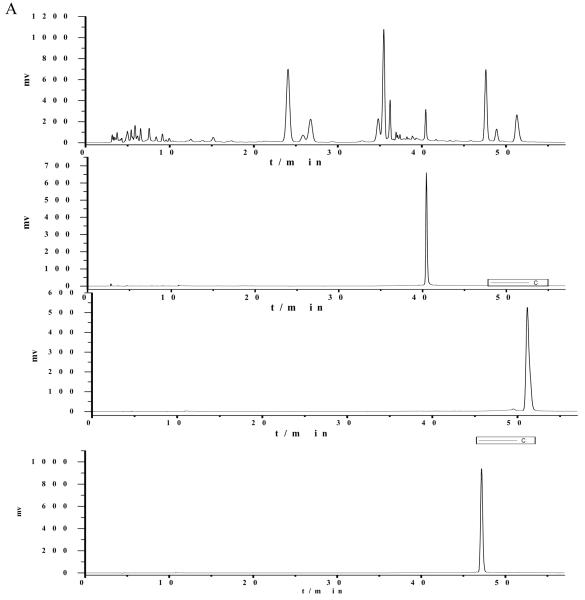
Several elution systems were tested in HPLC separation of the crude sample, such as methanol–acetic acid water, gradient elution of method-acetic acid water, gradient elution of acetonitrile-acetic acid water, etc. When methanol-acetonitrile-0.1 % acetic acid water was used as the mobile phase in gradient mode (methanol-acetonitrile: initial concentration, 13%-13%; 20 min, 13%-13%; 27 min, 15%-15%; 28 min, 16%-27%; 50 min, 16%-27%; 53 min, 0%-80%; 57min, stop), good results could be obtained. The crude extracts and peak fractions separated by HSCCC were analyzed by HPLC under this optimum condition. The chromatograms were shown in Fig. 2.
Figure 2.
HPLC chromatograms of crude extracts from Belamcandae (A) and HSCCC peak fractions (□-III). Column: Diamonsil C18 column (250×4.6 mm i.d., 5 μm); mobile phase: methanol-acetonitrile-0.1 % acetic acid water (methanol-acetonitrile: initial, 13%-13%; 20 min, 13%-13%; 27 min,15%-15%; 28 min, 16%-27%; 50 min, 16%-27%; 53 min, 0%-80%; 57 min, stop),; flowrate:0.8 ml·min−1; detection wavelength: 254 nm.
Selection of Two-Phase Solvent System and Other Conditions of HSCCC
In an HSCCC experiment, selection of the two-phase solvent system is the first and critical step; a good solvent system can provide an ideal partition coefficient (K) for the target compounds. At our present studies, HPLC was used to select suitable two-phase solvent system for HSCCC. This method is suited to the colored sample with moderate polarity. The search was started at ethyl acetate-water (1:1) where, isometric mixture of n-hexane and methanol was gradually added until the color of upper and lower phass became close. When n-hexane-ethyl acetate-methanol-water (4:5:4:5, v/v) was used as the two-phase solvent system, good separation results was obtained.
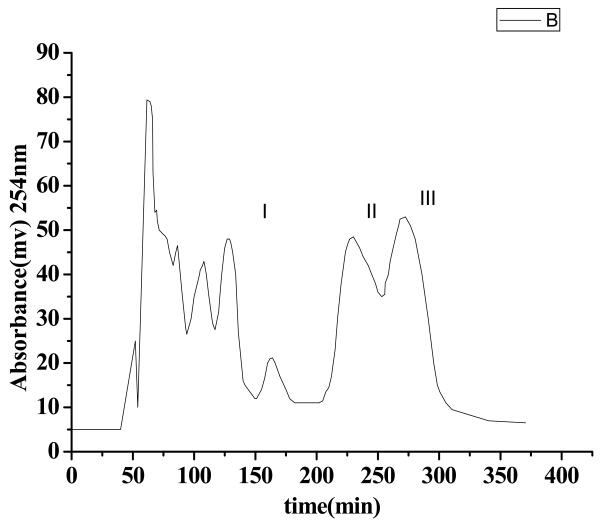
Also other conditions such as the revolution speed of the separation column, the flow rate of the mobile phase, the sample size were also investigated. The results indicated that reducing the flow rate was propitious to the separation, but at the same time the chromatogram peaks were broadend; the revolution speed of the separation coil tube has great influence on the retention of the stationary phase. Increasing the revolution speed gives higher retention of the stationary phase; reducing the sample size was also improved the separation Considering these aspects, in this experiment the flow rate of the mobile phase was set at 1.2 ml/min, revolution speed were performed at 800 rpm and the sample size was 100 mg crude extract dissolved in 10 mL isometric upper phase and lower phase. The crude samples from Belamcandae were separated and purified under the optimum HSCCC conditions. The typical HSCCC chromatogram was shown in Fig. 3. Three compounds were obtained in one-step separation and yielded 10 mg of isorhamnetin (Peak I), 8 mg of irigenin (Peak II) and 7 mg of hispidulin (Peak III). The purity of these compounds was 94%, 95% and 90%, respectively, as determined by HPLC.
Figure 3.
HSCCC chromatograms of crude extracts of Belamcandae, Peak I isorhamnetin, Peak II irigenin, Peak III hispidulin, solvent system: n-hexane-ethyl acetate-methanol-water (4:5:4:5, v/v); mobile phase: the lower phase; flow rate: 1.2 ml/min; revolution speed 800rpm; detection wavelength: 254 nm; sample size: 100mg of crude sample dissolved in 10 ml of mixture solution of lower phase and upper phase (1:1, v/v) of the solvent system
Identification of the Separated Peaks
The chemical structure of each peak fraction of HSCCC was identified according to its EIS-MS data.
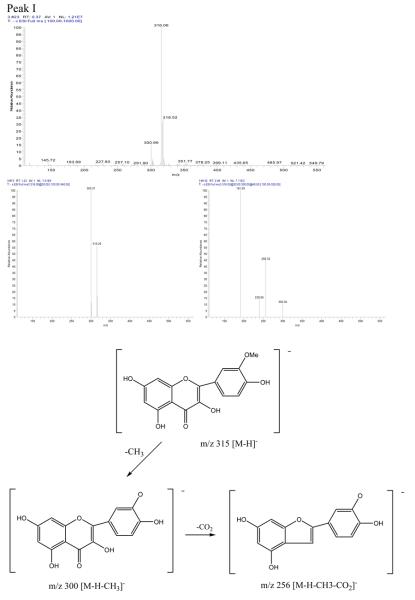
Peak I in Fig. 3: negative ESI-MS, m/z 315 [M-H]−,EIS-MS2: m/z300, EIS-MS3:256,239,191.
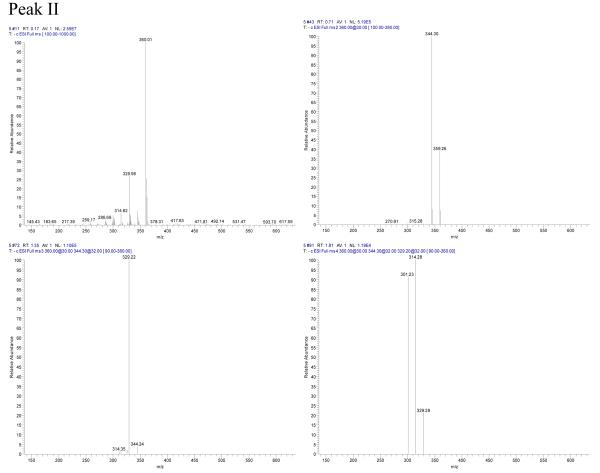
Peak II in Fig. 3: negative ESI-MS, m/z 359[M−], EIS-MS2: m/z344, EIS-MS3:329, EIS-MS4:314,301.
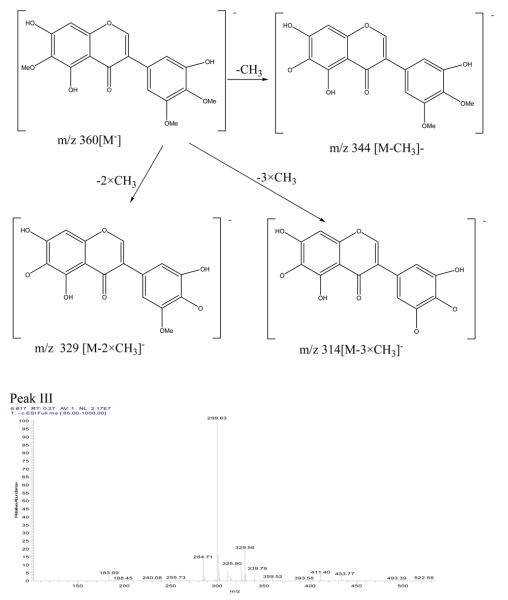
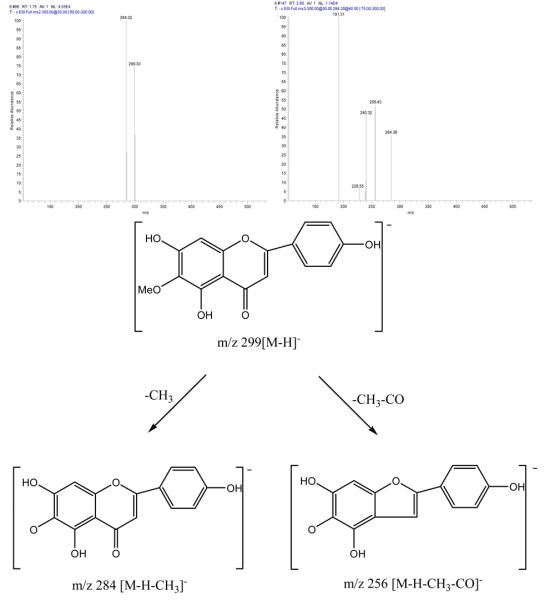
Peak III in Fig. 3: negative ESI-MS, m/z 299 [M-H]−, EIS-MS2: m/z, 284, EIS-MS3: m/z, 284,256,240,191.
The mass spectrogram of Peak I showed that it was isorhamnetin[2] with m/z values of 315 [M-H]−, EIS-MS2: m/z 300[M-H-CH3]−, EIS-MS3: m/z 256 [M-H-CH3-CO2]−, m/z 239 [M-H-CH3-CO2-OH]−; The mass spectrogram of Peak II showed that it was irigenin[2] with m/z values of 360[M−], EIS-MS2: m/z 344 [M-CH3]−, EIS-MS3: m/z 329 [M-2×CH3]−, EIS-MS4: m/z 314[M-3×CH3]−; The mass spectrogram of peak III showed that it was hispidulin[2] with its m/z of 299[M-H]−, EIS-MS2: m/z 284 [M-H-CH3]−, EIS-MS3: m/z 256 [M-H -CH3-CO]−. The mass spectrogram and the structure analysis were shown in Fig. 4.
Figure 4.
The mass spectrogram and the structure analysis of the compound separated from Belamcandae by HSCCC.
References
- 1.Chinese Pharmacopoeia Commission . Chinese Pharmacopoeia. 2005 ed I. Chemical and Industrial Publisher; 2005. p. 200. [Google Scholar]
- 2.Ito H, Onoue S, Yoshida T. Chem Pharm bulle. 2001;49:1229–1231. doi: 10.1248/cpb.49.1229. [DOI] [PubMed] [Google Scholar]
- 3.Monthakantirat O, De-Eknamkul W, Umehara K, Yoshinaga Y, Miyase T, Warashina H. J Nat Pro. 2005;68:361–364. doi: 10.1021/np040175c. [DOI] [PubMed] [Google Scholar]
- 4.Qin M-J, Li W-L, Wang Z-T, Ye W-C. J integr plant boil. 2005;47:1404–1408. [Google Scholar]
- 5.Moriyasu M, Igi Y, Ichimaru M, Iwasa K, Kobayakawa J. J.Nat Med. 2007;61:329–333. [Google Scholar]
- 6.Song ZJ, Luo F, Zhou Y, Bai B-R, Peng S-L, Ding L-S. Chin chem lett. 2007;18:694–696. [Google Scholar]
- 7.Li J, Li WZM, Huang W, Cheung AWH, Bi CWC, Ava R-D, Guo J-Y, Dong TTX, Tsim KWK. J. Chromatogr. 2008 doi:10.1016/j.chroma.2008.05.082. [Google Scholar]
- 8.Ito Y. J. Chromatogr. 1981;214:122. [Google Scholar]