Abstract
The physical association between the endoplasmic reticulum (ER) and mitochondria, which is known as the mitochondria-associated ER membrane (MAM), has important roles in various cellular ‘housekeeping’ functions including the non-vesicular transports of phospholipids. It has recently become clear that the MAM also enables highly efficient transmission of Ca2+ from the ER to mitochondria to stimulate oxidative metabolism and, conversely, might enable the metabolically energized mitochondria to regulate the ER Ca2+ homeostasis. Recent studies have shed light on molecular chaperones such as calnexin, calreticulin, ERp44, ERp57, grp75 and the sigma-1 receptor at the MAM, which regulate the association between the two organelles. The MAM thus integrates signal transduction with metabolic pathways to regulate the communication and functional interactions between the ER and mitochondrion.
Mitochondria and the ER physically interact
Intracellular organelles coordinate complex mechanisms of signal transduction metabolism and gene expression in the cell through their functional or physical interactions with one another. Physical interactions facilitate rapid and efficient pathways for signaling and metabolism, an example being the interaction between the endoplasmic reticulum (ER) and the plasma membrane, which ensures rapid transport of molecules from the extracellular space to intracellular organelles [1,2]. Extracellular Ca2+ can thus rapidly refill the intracellular Ca2+ reservoir at the ER upon completion of signaling events requiring Ca2+ efflux [2]. Like the ER, the mitochondrion, the powerhouse of the cell and a major Ca2+-buffering organelle, also associates with the plasma membrane. In neurons, peripherally distributed mitochondria not only provide energy to neurites but also proximally buffer Ca2+ that enters the cell through ion channels in close contact with the interface between mitochondrion and plasma membrane [3]. A similar function of mitochondria is also seen in non-excitable cells [4]. Great attention has recently been paid to the interaction between the ER and mitochondria. The physical interaction between the ER and mitochondria is referred to as the mitochondria-associated ER membrane (MAM). This association has pivotal roles in several cellular functions, including Ca2+ signaling, lipid transport, energy metabolism and cellular survival [5-8]. Recent studies have unveiled the structural configuration of the interface and identified its functions and the molecular entities involved in the interaction, including the chaperones calnexin, calreticulin, ERp44, ERp57, grp75 and the sigma-1 receptor.
The most important functions of the MAM include lipid transport and control of apoptosis but, here, we highlight Ca2+ signaling as being of particular current interest in light of the characterization of the chaperones now known to be crucial to the ER-mitochondrial interaction.
The mitochondria-associated ER membrane
Morphological evidence for the physical association or interaction between the ER and mitochondria emerged in the early 1990s, although the concept arose in the 1960s. Such contact has since been observed in mitochondria in many types of cell [9,10]. However, it is important to stress that only a small area of the outer mitochondrial membrane (OMM; approximately 12%) is estimated to associate with the ER [11]. The distance between the ER and the OMM was originally estimated to be approximately 100 nm [9,10]. However, a recent study using electron tomography showed that the minimum distance is even shorter (e.g. 10-25 nm) [11]. This distance thus enables ER proteins to associate directly with proteins and lipids of the OMM. This study also showed that the ER membrane and the mitochondrial membrane are tethered by trypsin-sensitive filaments seemingly composed of proteins [11]. Importantly, knockdown of inositol-1,4,5-trisphosphate (IP3) receptors did not prevent formation of the filament, indicating that other as yet unidentified proteins might constitute the bundle [11]. The tight association of membranes of the ER and mitochondria in cell homogenates further supports the existence of the tethering of the membranes of these two organelles [11,12]. Although the cytoskeleton is important for shaping and supporting organelles, the ER-mitochondria association is apparently stable even when the integrity of microtubules and intermediate filaments was disrupted [9,10]. The association is, however, sensitive to Ca2+ [11,13]. In living cells, some ER membranes are often seen migrating with highly mobile mitochondria [11,12,14].
Tethering of the two organelle membranes at an appropriate distance might affect mitochondrial function. For example, a close distance (<5 nm as revealed by electron tomography) between the ER and mitochondria promotes Ca2+ overloading of the mitochondria [11], leading to cellular damage. The distance is, however, heterogeneous in rough and smooth ERs and dynamically changes in response to the increase of cytosolic Ca2+ induced by IP3 [11]. Apparently, the ER-mitochondrion interface, by sensing cytosolic Ca2+ concentrations, might dynamically change shape and therein alter the interorganelle distance for proper cellular responses or signal transduction.
The MAM is vital for regulating Ca2+ levels in mitochondria. Mitochondrial Ca2+ has several roles, but Ca2+-regulated bioenergetics has received the greatest attention in the past decade. Mitochondrial Ca2+ levels are important for cellular bioenergetics because the enzymes in the tricarboxylic acid (TCA) cycle and the electron transport chain depend on Ca2+ to generate the high-energy compound ATP. Three key enzymes of the TCA cycle, which takes place in the lumen and the inner mitochondrion membrane (IMM), are Ca2+-dependent [15]. Pyruvate dehydrogenase is activated by Ca2+-dependent dephosphorylation, whereas α-ketoglutarate and isocitrate dehydrogenases are directly activated by Ca2+ [7,16]. The supply of Ca2+ to mitochondria is crucial for matching the ATP production by the TCA cycle with the ATP demand. Thus, upon the activation of a wide range of hormone and neurotransmitter receptors, the IP3 generated as the second messenger can activate the TCA cycle by increasing mitochondrial matrix Ca2+ concentration. An interesting characteristic of the Ca2+-regulated mitochondrial bioenergetics is the so-called ‘long-term metabolic priming’, in which the ATP production from pyruvate or lactate is greater in IP3-primed cells compared with controls [17]. In other words, the production of ATP from pyruvate or lactate is enhanced in cells previously exposed to a priming dose of IP3. Therefore, a transient but sufficient rise of mitochondrial matrix Ca2+ concentration via an activation of IP3 receptors has a role in the responsiveness of mitochondrial metabolism.
Several other proteins regulated by mitochondrial Ca2+ have been identified. For example, the accumulation of mitochondrial Ca2+ activates the manganese superoxide dismutase (MnSOD) by promoting its dephosphorylation [18]. Interestingly, metabolite transport is also regulated by Ca2+: aspartate or glutamate carriers have Ca2+-binding sites within loops protruding into the mitochondrial intermembrane space (IMS), which control the activity of the protein [19]. Thus, coordinated Ca2+-regulated steps occurring in the matrix and in the IMS can finely tune mitochondrial metabolism to cellular Ca2+ signals and the energy-consuming processes triggered by the extracellular stimuli.
The lipid transport function of the MAM has been well characterized (briefly described in Box 1). The MAM has since been shown to be enriched in functionally diverse enzymes involved not only in lipid metabolism but also glucose metabolism. Collectively, the enzymes include phosphatidylserine (PtdSer) synthase, phosphatidylethanolamine (PtdEtn) methyltransferase-2, acyl-CoA:cholesterol acyltransferase (ACAT), diacylglycerol acyltransferase (DGAT) and glucose-6-phosphatase (G6Pase) [20]. Growing evidence also indicates that the MAM might contain enzymes required for cholesterol and ceramide biosyntheses [20,21].
Box 1. Lipid transport at the MAM.
Concepts of membrane contacts between ER and mitochondria first emerged from lipid research back in the 1960s. The most important pathways of the de novo phospholipid biosynthesis are: (i) the synthesis of PtdSer from serine by the PtdSer synthase; (ii) decarboxylation of PtdSer by PtdSer decarboxylase for synthesis of PtdEtn; and (iii) methylation of PtdEtn by PtdEtn N-methyltransferase for synthesis of phosphatidylcholine (PtdChol) [6]. In early 1980s, it was shown that PtdEtn is synthesized in mitochondria. However, the intracellular localization of PtdSer synthase was controversial: the enzymatic activity was detected in both microsomal and crude mitochondrial membranes. The translocation and the subsequent decarboxylation of PtdSer can be achieved by simply adding microsomes to purified mitochondria [52]. Vance and co-workers partially purified the particular microsomal submembrane enriched in PtdSer synthase, which sediments with mitochondria in the differential centrifugation. The fraction was called Fraction X, but was later renamed the mitochondria-associated membrane (MAM) [53]. Since then, it was demonstrated that PtdSer synthase exclusively localizes at the MAM and that the rate-limiting step in PtdEtn synthesis is the transport of PtdSer from MAM to mitochondria [54]. The transport is not vesicular in nature [52] and does not require ATP. These findings identified unique routes of intermembrane transports for phospholipids in the cell.
The MAM, when visualized with MAM-specific protein sigma-1 receptors by using the immunodetection or GFP-labeling technique, which was further verified by the MAM-specific fractionation [12], is exceptionally enriched in cholesterol and neutral lipids [55,56]. Because the ACAT is highly enriched at the MAM, the MAM might serve as a site for cholesterol and neutral lipid synthesis. No data are available to indicate whether sterols or neutral lipids use the MAM as routes for transport.
Nevertheless, steroidogenesis substantially depends on sterols being shuttled between ER and mitochondria. For example, cholesterol, after its synthesis at the ER following >15 steps of enzymatic reactions, is transported to mitochondria for one single enzymatic reaction to become pregnenolone [57]. Pregnenolone is then back transported to the ER for the syntheses of several other steroids [57].
The MAM might also serve as transport routes for ceramides from the ER into mitochondria. It is interesting to note that an early study using fluorophore (NBD)-conjugated ceramide indicated the energy-independent transport of exogenously applied ceramides into mitochondria [58]. When NBD-ceramides were applied to living cells at 2 °C, under which vesicular transport mechanisms are shut down, the NBD fluorescence accumulated first at the ER and then at the mitochondria [58]. These findings illustrate the intermembrane transport of ceramides at both the ER-mitochondrion interface and the ER-plasma membrane interface.
The MAM seen in species from yeast to mammals is now accepted as a fundamental structural configuration insidethe cell. As described earlier, important functions of the MAM mainly involve local Ca2+ transfer from ER to mitochondria and lipid shuttling via non-vesicular transport. Though seemingly independent, Ca2+ signaling and lipid metabolism at the MAM are, however, functionally related. The metabolically energized mitochondria via supplies of pyruvate or malate can regulate the release of Ca2+ from the ER [22]. Whether the MAM is involved in this regard is unknown at present.
The MAM is also involved in apoptosis. Fas-signaling-induced apoptosis involves the increase of IP3 production, via the activation of phospholipase C-γ1, and the subsequent enhanced Ca2+ release from IP3 receptors [23]. Blocking of either segments of the signaling was cytoprotective [23].
Ca2+ signaling at the MAM
Growing evidence indicates that the Ca2+ uptake into mitochondria is controlled by specific proteins residing at the outer and inner mitochondrial membranes (OMM and IMM respectively), namely, the voltage-dependent anion channel (VDAC) and the Ca2+ uniporter [7,16].Ca2+ in mitochondria, however, is expelled by antiporters in a process exchanging for either Na+ or H+ [7,16]. As such, the uniporter and the exchanger are important for maintaining mitochondrial membrane potential and the Ca2+ concentration in mitochondria [7,16]. However, the amino acid sequence of either uniporter or exchanger has not been determined.
The importance of the MAM began to emerge when it was found that, after the agonist stimulation to the cell, mitochondria were able to uptake Ca2+ into their lumen directly from IP3 receptors at the MAM [24-26]. IP3 serves as the second messenger to the stimulation of agonists and is known to release Ca2+ from the ER by binding to IP3 receptors, which are Ca2+ channels on the ER membrane [26-29]. The most important Ca2+ transporters and channels at the MAM are depicted in Figure 1.
Figure 1.
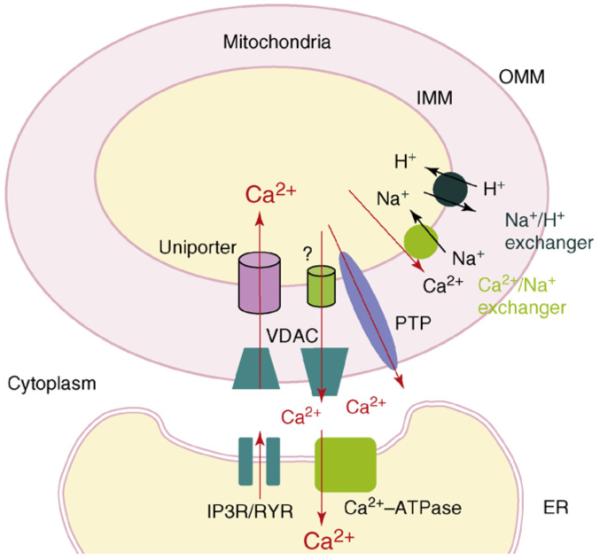
Major Ca2+ transporters and channels at the mitochondrion and ER. Ca2+ released upon the activation of IP3 receptors or ryanodine receptors at the ER is taken up into mitochondria via VDAC and Ca2+ uniporters at the OMM and IMM, respectively. Ca2+ extrusion from mitochondria is mediated via Na2+-dependent and Na2+-independent mechanisms. The Ca2+/Na+ exchanger responsible for the Na+-dependent mechanism has been extensively studied, whereas the characteristics of the molecule involved in Na2+-independent mechanism is elusive (indicated by?). The H+ gradient established by the Na+/H+ exchanger is thought to be important for the Na2+-dependent mechanism. PTP, which is often activated by pathological conditions, increases the permeability of IMM to small ions and molecules, leading to a collapse of the mitochondrial membrane potential as well as the membrane architecture. The ryanodine receptor is similar to the IP3 receptor in function except that the former is controlled under different mechanisms. Abbreviations: Ca2+-ATPase, ATP-dependent Ca2+ pump; IMM, inner mitochondrial membrane; IR3R, inositol 1,4,5-trisphosphate receptor; OMM, outer mitochondrial membrane; PTP, permeability transition pore; RYR, ryanodine receptor; VDAC, voltage-dependent anion channel.
Crosstalk between Ca2+ signaling and lipid metabolism at the MAM
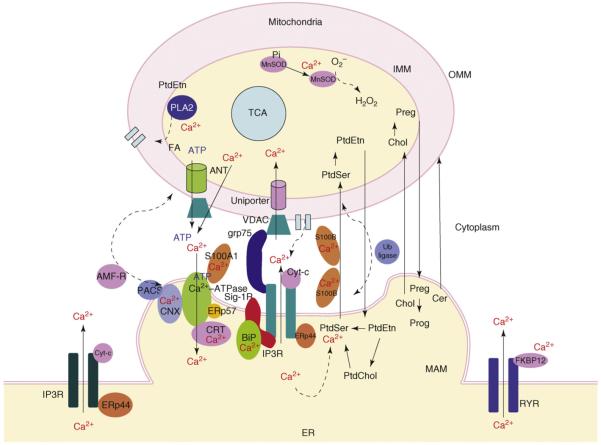
The crosstalk between Ca2+ signaling and lipid metabolism and transport might, in fact, take place within the surroundings of the MAM, namely, the ER lumen, the cytosolic interface and the mitochondrial matrix (Figure 2).
Figure 2.
Convergence of signal transduction and metabolism at the MAM. Ca2+-signaling molecules and lipid metabolizing enzymes are highly compartmentalized at the physical interface between the mitochondria-associated ER membrane (MAM) and the outer mitochondrial membrane (OMM). IP3 receptors (IP3R) are often seen at the MAM. In certain cells, such as CHO cells, type-3 IP3Rs are more enriched at MAM. IP3Rs at the MAM couple sigma-1 receptor chaperones (Sig-1Rs) at the ER lumen and grp75 in the cytosol. Sig-1Rs stabilize ligand-activated type-3 IP3Rs, whereas grp75 links IP3Rs to the voltage-dependent anion channel (VDAC) at the OMM. Cytochrome c (Cyt-c) released from mitochondria associates with IP3Rs, leading to the overloading of mitochondrial Ca2+. The MAM contains high levels of the Ca2+-binding chaperones calnexin (CNX), calreticulin (CRT) and BiP, thus, perhaps serving as a high-capacity Ca2+ reservoir at the ER-mitochondrion interface. Ca2+ released from IP3Rs at the MAM creates microdomains of high Ca2+ concentrations that, in turn, activate the Ca2+ uniporter for Ca2+ uptake into the mitochondrial matrix. IP3 receptors are also known to be regulated by another ER chaperone, ERp44. Ca2+ in the mitochondrial matrix activates certain enzymes in the TCA cycle, leading to an enhanced ATP production. ATP in mitochondria is released to the cytosol via the adenine nucleotide transporter (ANT) and VDAC, causing the activationof the Ca2+-ATPase at the ER. The activity of Ca2+-ATPase is also regulated by several Ca2+-binding proteins including calreticulin and ERp57. Mitochondrial Ca2+ also activates Mn2+-dependent superoxide dismutase (MnSOD) and phospholipase A2 (PLA2). Fatty acids (FAs) produced via the PLA2 activation facilitate the pore formation on the OMM. The ER-mitochondrion interface also serves to facilitate the intermembrane transport of phospholipids. The EF-hand (helix-loop-helix)-type Ca2+-binding protein S100B and ubiquitin (Ub) ligase at the interface might regulate phospholipid transport. Cholesterol (Chol) and ceramides (Cer) might also use the interface for their transport and metabolism. The vesicular sorting protein PACS and the ubiquitin ligase AMF-R are suggested to regulate the association of the MAM and the OMM. Abbreviations: Preg, pregnenolone; Prog, progesterone.
One line of evidence has indicated that the activity of PtdSer synthase in the ER lumen relies (at least in part) on the ability of Ca2+-ATPase at the MAM to uptake Ca2+ into the ER [30,31]. The Ca2+-ATPase is an energy-driven Ca2+-uptake complex at the ER membrane, which uses the hydrolysis of ATP as the energy source. The depletion of ATP thus inactivates PtdSer synthase. The synthase activity, however, can be restored by adding millimolar concentrations of Ca2+ to microsomes [30]. The activity of IP3 receptors at the MAM, which efflux ER Ca2+ into mitochondria, might thus also affect the enzymatic activity of the PtdSer synthase because of the ensued reduction of Ca2+ in the ER lumen.
As mentioned earlier, Ca2+ release from the MAM can cause rapid but reversible alterations in the distance between the MAM and the OMM [13]. The alterations that ensue can change the efficiency in transporting ER Ca2+ into mitochondria [11]. However, whether the Ca2+-dependent alteration of the distance might affect the transport of lipids has not been examined. Nevertheless, the possibility is supported by the description of a Ca2+-sensitive cytosolic S100B protein [32]. When added to a cell-free system containing both the MAM and mitochondria, S100B, which forms a homodimer exposing two protein-binding sites on each end in the presence of Ca2+, stimulates the transport of PtdSer from the MAM to mitochondria [32].
Thus, there could be multiple points of crosstalk between Ca2+ signaling and lipid metabolism, in part, because the lipid metabolism depends heavily on ATP and also because the enzymes involved are closely regulated by Ca2+ signaling.
Proteins at the ER-mitochondrion interface
MAM-specific proteins were identified serendipitously in cellular distribution studies, including the sigma-1 receptor chaperone [12] and autocrine motility factor receptor (AMF-R) [13]. The MAM-specific proteins identified thus far are listed in Table 1. Most of these proteins are ER proteins, with only a few belonging to mitochondria. Whether VDAC and uniporters (Figure 1) are more enriched at the MAM than at other areas of the mitochondrial membrane is not clear. However, these proteins were found to have a role in mitochondrial Ca2+ signaling at the MAM [31,33,34]. Cytochrome c, which is released from mitochondria upon activation of apoptotic pathways down-stream of Bcl-2, can bind IP3 receptors [35,36] found at the MAM, further activating the Ca2+ flux and enhancing apoptotic signaling [35,36].
Table 1. Proteins at the ER-mitochondrion interface (MAM).
| Protein class | Examples | Details |
|---|---|---|
| Metabolic enzymes | PtdSer synthase, PtdEtn methyltransferase-2, acyl-CoA:cholesterol acyltransferase, diacylglycerol acyltransferase (DGAT) and glucose-6-phosphatase [20] | See Box 1 |
| Ion channel or transporter | IP3 receptors, Ca2+-ATPase, VDAC, uniporter, antiporter | Type-3 IP3 receptors preferentially express at the MAM in certain cells [12,40] |
| Molecular chaperones | sigma-1 receptor, BiP, calnexin, calreticulin, ERp44, ERp57, FKBP12, Grp75, hsp60 [37,59] | There are also some heat-shock proteins associated with VDAC at OMM [59] |
| S-100 protein | S100B | S-100 is a protein with two calcium-binding sites of the EF-hand type helix-loop-helix conformation. There are >20 different types of S100 proteins. S100B is postulated to tether the MAM to OMM for phospholipid transport [32,60]. S100A1 associates with Ca2+-ATPase and regulates Ca2+ uptake in the ER [61] |
| Ubiquitin ligase | MET30 E3 ubiquitin ligase [62], autocrine motility factor receptor (AMF-R; i.e. gp78) [13] | AMF-R recruits the p97 (also known as valosin-containing protein) to the MAM and regulates mitochondrial association with the ER [63,64] |
| Vesicular-sorting protein | Phospho-acidic cluster sorting protein 2 (PACS-2) | Knockdown of the protein results in fragmentation of mitochondria and the uncoupling of mitochondria from the ER [51] |
| Electron transport chain proteins | Cytochrome c | Cytochrome c binding to IP3 receptors irreversibly activates the latter, leading to the overloading of mitochondrial Ca2+ and cell death [35,36] |
| Mitochondrial fusion protein | Mitofusin 2 | Normally required for mitochondrial fusions, mitofusin 2 also directly tethers ER to mitochondria, thus, facilitating efficient mitochondrial Ca2+ uptake [65] |
Details of the functions of many of the proteins found at the MAM, including ubiquitin ligases (e.g. AMF-R, Met30p) have been extensively described elsewhere [5-8]. The role of molecular chaperones in the ER-mitochondrion communication is only now becoming clear. Two recent studies demonstrate that the MAM uses chaperone machineries to regulate Ca2+ signaling [12,37].
Molecular chaperones at the MAM
Ca2+-binding and glucose-regulated chaperones are found abundantly on the membranes as well as in the lumens of both mitochondria and ER. In addition to serving as high-capacity Ca2+-binding depots for constitutive ER Ca2+ pools [38], these chaperones promote proper protein folding in a Ca2+-dependent manner. Furthermore, certain chaperones couple specific Ca2+ channels and regulate the channel activities. Physical, although weak, association of chaperones and their client proteins, such as the IP3 receptor, might stabilize cellular signaling and interorganellar networks such as the MAM [39]. Because IP3 receptors are vulnerable to ubiquitylation and proteasomal degradation upon the stimulation by IP3 [40], stabilizing IP3 receptors during signal transduction is of considerable importance in maintaining proper Ca2+ signaling not only in the cytosol but also in the mitochondrion.
Grp75 links IP3 receptors to VDAC1
Recent studies have shown that type-3 IP3 receptors are particularly enriched at the MAM, whereas type-1 IP3 receptors reside typically at the bulk ER membranes in neuronal cells [41]. Knockdown of type-3 IP3 receptors by the siRNA technique largely reduced the IP3-induced mitochondrial Ca2+ concentration in CHO cells, whereas knockdown of type-1 IP3 receptors reduced the Ca2+ concentration in the cytosol [41]. Type-1 IP3 receptors differ from type-3 IP3 receptors in minor sequence discrepancies and in their distributions in different types of cells. The N terminus of IP3 receptors comprises a long cytosolic domain containing the IP3-binding site, a Ca2+-binding site and glycosylation sites. Because the distance between the MAM and the OMM is estimated to be approximately 20 nm, the long cytosolic domains of the IP3 receptor should reside in close proximity to mitochondria. A recent study demonstrated that VDAC1 is physically linked to the type-1 IP3 receptor through the molecular chaperone grp75 [37].
Grp75 has been extensively examined for its role in protein transports through the IMM [42]. However, grp75 also exists at non-mitochondrial regions of the cell such as cytosol. The study found that the cytosolic grp75 tethers the ligand-binding domain of the IP3 receptors to VDAC1 [37]. The resulting association presumably enhances the Ca2+ accumulation in mitochondria by stabilizing conformations or the coupling of the two receptors. Importantly, the overexpression of the cytosolic but not mitochondrial grp75 selectively potentiates the IP3-induced mitochondrial Ca2+ accumulation [37], supporting a notion that grp75 is a key molecule constituting the link between the two proteins on each side of the two organelles.
Calnexin and calreticulin
ER chaperones, particularly the Ca2+-binding chaperones (calnexin, calreticulin and BiP), were also found to be compartmentalized at the MAM [12,43]. Under physiological conditions, these chaperones serve as high-capacity Ca2+-binding proteins at the ER [38]. Calreticulin indeed provides up to 45% of the Ca2+-buffering capacity for a pool of the IP3-sensitive Ca2+ inside the ER [44]. The compart-mentalized chaperones at the MAM therefore serve as high-capacity Ca2+ pools in the ER. In addition, independent of its Ca2+-buffering capacity in the ER, calreticulin inhibits IP3 receptor-mediated Ca2+ signaling by using its high-affinity-low-capacity Ca2+-binding domain [45]. Further, calreticulin regulates the activity of Ca2+-ATPase, providing dynamic control of ER Ca2+ homeostasis [46]. Calnexin can also regulate the activity of Ca2+-ATPase via a direct protein-protein interaction [47]. In addition, the activity and action of calnexin and calreticulin are regulated by other chaperones or proteins most likely occurring at the MAM of the ER.
PACS-2, ERp44 and ERp57
The cytosolic sorting protein PACS-2 regulates the distribution and activity of calnexin. Under control conditions, >80% of calnexin localizes to the ER, mainly at the MAM [43]. However, through a protein-protein interaction, PACS-2 causes calnexin to distribute between the ER and the plasma membrane [43]. PACS-2 thus affects the homeostasis of ER Ca2+.
The ERp44 chaperone has an important role in controlling the oxidative protein folding in the ER by interacting with Ero1-Lα which selectively oxidizes protein disulfide isomerase [48]. Ero1-Lα lacks the ER retention signal and is anchored to the ER by ERp44 via reversible mixed disulfides [48]. Thus, the ERp44 chaperone favors the maturation of disulfide-linked oligomeric proteins and ensures their quality control [48]. ERp44 is known to directly inhibit type-1 IP3 receptors in a planar lipid bilayer system, indicating that ERp44 senses the environment in the ER lumen and modulates the IP3 receptor signaling accordingly [49]. ER stress upregulates ERp44. Another ER chaperone, ERp57, can corroborate with calreticulin and facilitate the latter in regulating the activity of Ca2+-ATPase [46]. Thus, the Ca2+-binding chaperones and Ca2+ transport channels at the MAM are finely tuned by other ER chaperones including ERp44 and ERp57.
Sigma-1 receptor: a novel ER chaperone
A recent study identified an ER resident protein, the sigma-1 receptor, as a unique ligand-operated, Ca2+-sensitive ER chaperone [12] (Figure 3). The sigma-1 receptor was originally classified as a subtype of opioid receptor but was later found to be a non-opioid receptor [50]. The sigma-1 receptor exists not only in the central nervous system but also in the peripheral organs including the lung, liver, adrenal gland, spleen and pancreas [12]. Sigma-1 receptors are highly expressed in almost all types of cancer cells and have been implicated in many diseases including cancer, depression and neurodegenerative diseases [12]. The molecular action of the sigma-1 receptor was recently unveiled to be a ligand-regulated receptor chaperone at the ER [12].
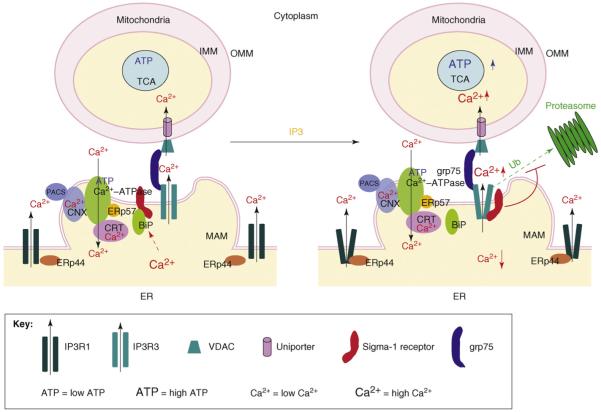
Figure 3.
The chaperone machinery regulating mitochondrial Ca2+ signaling and bioenergetics. Ca2+ signaling and its regulation at the MAM are crucial for the proper functioning of mitochondria specifically regarding their roles in the bioenergetics and apoptosis. Many chaperones are known to participate in the regulation of Ca2+ transporters and channels at the MAM particularly on the Ca2+-ATPase, which uptakes Ca2+ from the cytosol into the ER and the IP3 receptor, which transmits high concentration Ca2+ ‘puffs’ into mitochondria. ERp57 collaborates with another chaperone calreticulin (CRT) to attenuate the activity of Ca2+-ATPase. ERp57 does so by modulating the redox state of the Ca2+-ATPase and thus provides dynamic control of ER Ca2+ homeostasis. Another chaperone, ERp44, can sense the environment in the ER lumen and inhibits type-1 IP3 receptors, which reside mainly outside the MAM, for instance, in CHO cells. The chaperone grp75 serves to link the VDAC and IP3 receptor, thus, shortening the distance between the MAM and the mitochondria. The cytosolic sorting protein PACS-2 can cause the translocation of calnexin chaperone from the ER to the plasma membrane, thus, indirectly affecting the Ca2+ homeostasis in the ER lumen. Another chaperone player at the MAM is the newly identified receptor chaperone called the sigma-1 receptor. Under normal, resting conditions, the sigma-1 receptor (Sig-1R) chaperone, residing specifically at the MAM, forms a complex with BiP when the ER Ca2+ concentration is 0.5-1.0 mM. Ca2+ seems to facilitate the association between the Sig-1R and BiP. The Sig-1R in the complex is essentially in a dormant state with regard to chaperone activity. When IP3 receptors are activated, however, the subsequent drop of the ER Ca2+ concentration causes the dissociation of Sig-1Rs from BiP, unleashing the chaperone activity of the receptor. In the presence of high concentrations of cytosolic IP3, activated IP3 receptors are unstable and are readily ubiquitylated and degraded by proteasomes. The free form of Sig-1Rs associates with type-3 IP3 receptors (IP3R3) at the MAM, thus, preventing IP3R3 from being degraded by proteasomes. Sig-1Rs apparently do not chaperone type-1 IP3 receptors (IP3R1) at the bulk ER membrane. The stabilization of IP3R3 by Sig-1Rs therefore ensures the proper Ca2+ influx into mitochondria, presumably leading to the enhancement of ATP production in the TCA cycle or the electron transport chain. The refilling of the ER Ca2+ pool inactivates Sig-1R chaperones by promoting the re-association of the Sig-1R with BiP. Chaperone machinery on both sides of the ER and mitochondria thus works in concert, partly by sensing the ER Ca2+ concentration, to strengthen the interaction between the ER and mitochondrion, facilitating interorganelle signal transduction, metabolic regulation and the bioenergetics of the cell.
Under normal conditions in which the ER lumenal Ca2+ concentration is at 0.5-1.0 mM, sigma-1 receptors selectively reside at the MAM of the ER and form complexes with another ER chaperone, the glucose-regulated protein (GRP78, also known as BiP). Upon the activation of IP3 receptors, which causes the decrease of the Ca2+ concentration at the MAM, sigma-1 receptors dissociate from BiP to chaperone type-3 IP3 receptors [12], which would otherwise be degraded by proteasomes. The sigma-1 receptor thus ensures proper Ca2+ signaling from the ER into mitochondria. Inasmuch as the Ca2+ signaling from the ER into mitochondria is important for the generation of ATP from the TCA cycle and the electron transport chain, it is not unreasonable to speculate that sigma-1 receptors might have an important role in the bioenergetics of the cell by stabilizing IP3 receptors at the MAM. In this regard, cancer cells may use sigma-1 receptors for survival. However, degenerative neurons or tissues might benefit by sigma-1 receptor agonists that unleash the chaperone activity of sigma-1 receptors at the MAM [12].
Although the selection of chaperones discussed above might not be inclusive, the data indicate a new picture whereby molecular chaperones at the MAM might participate not only in the organization of interorganelle protein networks but also in sensing the ER environment and transmitting the message to mitochondria.
Concluding remarks and future perspectives
The physical interaction between ER and mitochondria is essential for functions of the two organelles including the control of cell death and survival. Available biochemical and genetic data implicate specific proteins and lipids as constituents in establishing the structural link and inter-organelle communication between these two organelles. Nevertheless, the number of molecules so far identified at the interface might represent only a small number of the total. Further elucidation and identification of other molecules at the interface will facilitate our understanding of the mechanisms involved in the interorganelle transport and communication. Urgent, as yet unanswered, questions include: what are the constituents of the bundles that tether the MAM to the mitochondrial membrane; what is the molecular nature of the uniporters; and what proteins are pertinent to lipid transports? It is interesting to see that, in response to various cellular stimuli, a small inter-membrane domain such as the ER-mitochondrion interface can regulate a spectrum of biological pathways. An ultimate question might be: what are the structural prerequisites for these multiple pathways to converge at the interface (the MAM) and where they are integrated in such a manner to be regulated by the dynamic ‘puffs’ of the second messenger Ca2+.
Finally, the recent discovery of chaperones and ubiquitin ligases at the ER-mitochondrion interface points to a new role for those two classes of stress proteins in the communication between the two organelles. The interactions between chaperones and client proteins are weak and are exchangeable depending on the conformation of the client proteins. The association occurs rapidly and reversibly without any need for covalent modifications of the client proteins. Ubiquitylation, however, targets proteins to degradation by proteasomes and thus promote a rapid elimination of proteins. Thus, the interplay and coordination of those two classes of stress proteins might impact the physical association between ER and mitochondria. The cellular machinery of stress proteins might also enable the organelles to respond promptly to changes in microenvironments, including for example the alteration of Ca2+ concentrations in the cytosol or lumens of intracellular organelles. Cellular stress might, therefore, affect the constitutive structure of the ER-mitochondrion interface and the crosstalk. As such, cellular stress might have an important role in cell death or survival by affecting the activity of critical molecules at the interorganelle interface known as the MAM. In this regard, the MAM might serve not only as an important cellular component that integrates Ca2+ signaling and metabolism but also as a cellular substrate that might respond properly to external stress. As such, it is appropriate to say that the MAM is more than just a housekeeper.
Update
A recent article [65] indicates that a mitochondrial fusion protein, mitofusin 2, can also tether ER to mitochondria, thus, facilitating mitochondrial Ca2+ uptake.
Acknowledgements
This work supported in part by the Intramural Research Program of the National Institute on Drug Abuse, the National Institutes of Health and the Department of Health and Human Services of the USA.
References
- 1.Blaustein MP, Golovina VA. Structural complexity and functional diversity of endoplasmic reticulum Ca2+ stores. Trends Neurosci. 2001;24:602–608. doi: 10.1016/s0166-2236(00)01891-9. [DOI] [PubMed] [Google Scholar]
- 2.Varnai P, et al. Visualization and manipulation of plasma membrane-endoplasmic reticulum contact sites indicates the presence of additional molecular components within the STIM1-Orai1 Complex. J. Biol. Chem. 2007;282:29678–29690. doi: 10.1074/jbc.M704339200. [DOI] [PubMed] [Google Scholar]
- 3.Lysakowski A, et al. Dense-cored vesicles, smooth endoplasmic reticulum, and mitochondria are closely associated with non-specialized parts of plasma membrane of nerve terminals: implications for exocytosis and calcium buffering by intraterminal organelles. J. Comp. Neurol. 1999;403:378–390. doi: 10.1002/(sici)1096-9861(19990118)403:3<378::aid-cne7>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- 4.Malli R, et al. Mitochondria efficiently buffer subplasmalemmal Ca2+ elevation during agonist stimulation. J. Biol. Chem. 2003;278:10807–10815. doi: 10.1074/jbc.M212971200. [DOI] [PubMed] [Google Scholar]
- 5.Vance JE. Molecular and cell biology of phosphatidylserine and phosphatidylethanolamine metabolism. Prog. Nucleic Acid Res. Mol. Biol. 2003;75:69–111. doi: 10.1016/s0079-6603(03)75003-x. [DOI] [PubMed] [Google Scholar]
- 6.Voelker DR. Bridging gaps in phospholipid transport. Trends Biochem. Sci. 2005;30:396–404. doi: 10.1016/j.tibs.2005.05.008. [DOI] [PubMed] [Google Scholar]
- 7.Rizzuto R, et al. Flirting in little space: the ER/mitochondria Ca2+ liaison. Sci. STKE. 2004;2004:re1. doi: 10.1126/stke.2152004re1. [DOI] [PubMed] [Google Scholar]
- 8.Hajnoczky G, et al. Calcium signaling and apoptosis. Biochem. Biophys. Res. Commun. 2003;304:445–454. doi: 10.1016/s0006-291x(03)00616-8. [DOI] [PubMed] [Google Scholar]
- 9.Soltys BJ, Gupta RS. Interrelationships of endoplasmic reticulum, mitochondria, intermediate filaments, and microtubules-a quadruple fluorescence labeling study. Biochem. Cell Biol. 1992;70:1174–1186. doi: 10.1139/o92-163. [DOI] [PubMed] [Google Scholar]
- 10.Mannella CA, et al. The internal compartmentation of rat-liver mitochondria: tomographic study using the high-voltage transmission electron microscope. Microsc. Res. Tech. 1994;27:278–283. doi: 10.1002/jemt.1070270403. [DOI] [PubMed] [Google Scholar]
- 11.Csordas G, et al. Structural and functional features and significance of the physical linkage between ER and mitochondria. J. Cell Biol. 2006;174:915–921. doi: 10.1083/jcb.200604016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hayashi T, Su TP. Sigma-1 receptor chaperones at the ER-mitochondrion interface regulate Ca2+ signaling and cell survival. Cell. 2007;131:596–610. doi: 10.1016/j.cell.2007.08.036. [DOI] [PubMed] [Google Scholar]
- 13.Wang HJ, et al. Calcium regulates the association between mitochondria and a smooth subdomain of the endoplasmic reticulum. J. Cell Biol. 2000;150:1489–1498. doi: 10.1083/jcb.150.6.1489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mironov SL, Symonchuk N. ER vesicles and mitochondria move and communicate at synapses. J. Cell Sci. 2006;119:4926–4934. doi: 10.1242/jcs.03254. [DOI] [PubMed] [Google Scholar]
- 15.Duchen MR. Ca2+-dependent changes in the mitochondrial energetics in single dissociated mouse sensory neurons. Biochem. J. 1992;283:41–50. doi: 10.1042/bj2830041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Szabadkai G, Duchen MR. Mitochondria: the hub of cellular Ca2+ signalling. Physiology (Bethesda) 2008;23:84–94. doi: 10.1152/physiol.00046.2007. [DOI] [PubMed] [Google Scholar]
- 17.Jouaville LS, et al. Regulation of mitochondrial ATP synthesis by calcium: evidence for a long-term metabolic priming. Proc. Natl. Acad. Sci. U. S. A. 1999;96:13807–13812. doi: 10.1073/pnas.96.24.13807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hopper RK, et al. Mitochondrial matrix phosphoproteome: effect of extra mitochondrial calcium. Biochemistry. 2006;45:2524–2536. doi: 10.1021/bi052475e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lasorsa FM, et al. Recombinant expression of the Ca2+-sensitive aspartate/glutamate carrier increases mitochondrial ATP production in agonist-stiumlated Chinese hamster ovary cells. J. Biol. Chem. 2003;278:38686–38692. doi: 10.1074/jbc.M304988200. [DOI] [PubMed] [Google Scholar]
- 20.Rusinol AE, et al. A unique mitochondria-associated membrane fraction from rat liver has a high capacity for lipid synthesis and contains pre-Golgi secretory proteins including nascent lipoproteins. J. Biol. Chem. 1994;269:27494–27502. [PubMed] [Google Scholar]
- 21.Bionda C, et al. Subcellular compartmentalization of ceramide metabolism: MAM (mitochondria-associated membrane) and/or mitochondria? Biochem. J. 2004;382:527–533. doi: 10.1042/BJ20031819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jouaville LS, et al. Synchronization of calcium waves by mitochondrial substrates in Xenopus laevis oocytes. Nature. 1995;377:438–441. doi: 10.1038/377438a0. [DOI] [PubMed] [Google Scholar]
- 23.Wozniak AL, et al. Requirement of biphasic calcium release from the endoplasmic reticulum for Fas-mediated apoptosis. J. Cell Biol. 2006;175:709–714. doi: 10.1083/jcb.200608035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kirichok Y, et al. The mitochondrial calcium uniporter is a highly selective ion channel. Nature. 2004;427:360–364. doi: 10.1038/nature02246. [DOI] [PubMed] [Google Scholar]
- 25.Nicholls DG, Brand MD. The nature of the calcium ion efflux induced in rat liver mitochondria by the oxidation of endogenous nicotinamide nucleotides. Biochem. J. 1980;188:113–118. doi: 10.1042/bj1880113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rizzuto R, et al. Microdomains with high Ca2+ close to IP3-sensitive channels that are sensed by neighboring mitochondria. Science. 1993;262:744–747. doi: 10.1126/science.8235595. [DOI] [PubMed] [Google Scholar]
- 27.Rizzuto R, et al. Close contacts with the endoplasmic reticulum as determinants of mitochondrial Ca2+ responses. Science. 1998;280:1763–1766. doi: 10.1126/science.280.5370.1763. [DOI] [PubMed] [Google Scholar]
- 28.Sharma VK, et al. Transport of Ca2+ from sarcoplasmic reticulum to mitochondria in rat ventricular myocytes. J. Bioenerg. Biomembr. 2000;32:97–104. doi: 10.1023/a:1005520714221. [DOI] [PubMed] [Google Scholar]
- 29.Hajnoczky G, et al. Decoding of cytosolic calcium oscillations in the mitochondria. Cell. 1995;82:415–424. doi: 10.1016/0092-8674(95)90430-1. [DOI] [PubMed] [Google Scholar]
- 30.Voelker DR. Characterization of phosphatidylserine synthesis and translocation in permeabilized animal cells. J. Biol. Chem. 1990;265:14340–14346. [PubMed] [Google Scholar]
- 31.Csordas G, Hajnoczky G. Sorting of calcium signalins at the junctions of endoplasmic reticulum and mitochondria. Cell Calcium. 2001;29:249–262. doi: 10.1054/ceca.2000.0191. [DOI] [PubMed] [Google Scholar]
- 32.Kuge O, et al. Enhancement of transport-dependent decarboxylation of phosphatidylserine by S100B protein in permeabilized Chinese hamster ovary cells. J. Biol. Chem. 2001;276:23700–23706. doi: 10.1074/jbc.M101911200. [DOI] [PubMed] [Google Scholar]
- 33.Pinton P, Rizzuto R. Bcl-2 and Ca2+ homeostasis in the endoplasmic reticulum. Cell Death Differ. 2006;13:1409–1418. doi: 10.1038/sj.cdd.4401960. [DOI] [PubMed] [Google Scholar]
- 34.Hajnoczky G, et al. Mitochondrial calcium signalling and cell death: approaches for assessing the role of mitochondrial Ca2+ uptake in apoptosis. Cell Calcium. 2006;40:553–560. doi: 10.1016/j.ceca.2006.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Boehning D, et al. Cytochrome c binds to inositol (1,4,5) trisphosphate receptors, amplifying calcium-dependent apoptosis. Nat. Cell Biol. 2003;5:1051–1061. doi: 10.1038/ncb1063. [DOI] [PubMed] [Google Scholar]
- 36.Sedlak TW, Snyder SH. Messenger molecules and cell death: therapeutic implications. J. Am. Med. Assoc. 2006;295:81–89. doi: 10.1001/jama.295.1.81. [DOI] [PubMed] [Google Scholar]
- 37.Szabadkai G, et al. Chaperone-mediated coupling of endoplasmic reticulum and mitochondrial Ca2+ channels. J. Cell Biol. 2006;175:901–911. doi: 10.1083/jcb.200608073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hendershot LM. The ER function BiP is a master regulator of ER function. Mt. Sinai J. Med. 2004;71:289–297. [PubMed] [Google Scholar]
- 39.Csermely P, et al. Chaperones as parts of cellular networks. Adv. Exp. Med. Biol. 2007;594:55–63. doi: 10.1007/978-0-387-39975-1_6. [DOI] [PubMed] [Google Scholar]
- 40.Bhanumathy CD, et al. Mechanism of proteasomal degradation of inositol trisphosphate receptors in CHO-K1 cells. J. Biol. Chem. 2006;281:3722–3730. doi: 10.1074/jbc.M509966200. [DOI] [PubMed] [Google Scholar]
- 41.Mendes CC, et al. The type III inositol 1,4. 5-trisphosphate receptor preferentially transmits apoptotic Ca2+signals into mitochondria. J. Biol. Chem. 2005;280:40892–40900. doi: 10.1074/jbc.M506623200. [DOI] [PubMed] [Google Scholar]
- 42.Zahedi RP, et al. Proteomic analysis of the yeast mitochondrial outer membrane reveals accumulation of a subclass of preproteins. Mol. Biol. Cell. 2006;17:1436–1450. doi: 10.1091/mbc.E05-08-0740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Myhill N, et al. The subcellular distribution of calnexin is mediated by PACS-2. Mol. Biol. Cell. 2008;19:2777–2788. doi: 10.1091/mbc.E07-10-0995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bastianutto C, et al. Overexpression of calreticulin increases the Ca2+ capacity of rapidly exchanging Ca2+ stores and reveals aspects of their lumenal microenvironment and function. J. Cell Biol. 1995;130:847–855. doi: 10.1083/jcb.130.4.847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Camacho P, Lechleiter JD. Calreticulin inhibits repetitive intracellular Ca2+ wave. Cell. 1995;82:765–771. doi: 10.1016/0092-8674(95)90473-5. [DOI] [PubMed] [Google Scholar]
- 46.Li Y, Camacho P. Ca2+-dependent redox modulation of SERCA 2b by ERp57. J. Cell Biol. 2004;164:35–46. doi: 10.1083/jcb.200307010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Roderick HL, et al. Cytosolic phosphorylation of calnexin controls intracellular Ca2+ oscillations via an interaction with SERCA2b. J. Cell Biol. 2000;149:1235–1248. doi: 10.1083/jcb.149.6.1235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Anelli T, et al. Thio-mediated protein retention in the endoplasmic reticulum: the role of ERp44. EMBO J. 2003;22:5015–5022. doi: 10.1093/emboj/cdg491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Higo T, et al. Subtype-specific and ER lumenal environment-dependent regulation of inositol 1,4,5-trisphosphate receptor type 1 by ERp44. Cell. 2005;120:85–98. doi: 10.1016/j.cell.2004.11.048. [DOI] [PubMed] [Google Scholar]
- 50.Su TP, et al. Steroid binding at sigma receptors suggests a link between endocrine, nervous, and immune systems. Science. 1988;240:219–221. doi: 10.1126/science.2832949. [DOI] [PubMed] [Google Scholar]
- 51.Simmen T, et al. PACS-2 controls endoplasmic reticulum-mitochondria communication and Bid-mediated apoptosis. EMBO J. 2005;24:717–729. doi: 10.1038/sj.emboj.7600559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Voelker DR. Phosphatidylserine translocation to the mitochondrion is an ATP-dependent process in permeabilized animal cells. Proc. Natl. Acad. Sci. U. S. A. 1989;86:9921–9925. doi: 10.1073/pnas.86.24.9921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Vance JE. Phospholipid synthesis in a membrane fraction associated with mitochondria. J. Biol. Chem. 1990;265:7248–7256. [PubMed] [Google Scholar]
- 54.Voelker DR. Reconstitution of phosphatidylserine import into rat liver mitochondria. J. Biol. Chem. 1989;264:8019–8025. [PubMed] [Google Scholar]
- 55.Hayashi T, Su TP. Intracellular dynamics of sigma-1 receptors (σ1 binding sites) in NG108-15 cells. J. Pharmacol. Exp. Ther. 2003;306:726–733. doi: 10.1124/jpet.103.051292. [DOI] [PubMed] [Google Scholar]
- 56.Hayashi T, Su TP. Sigma-1 receptors (σ1 binding sites) form raft-like microdomains and target lipid droplets on the endoplasmic reticulum: roles in endoplasmic reticulum lipid compartmentalization and export. J. Pharmacol. Exp. Ther. 2003;306:718–725. doi: 10.1124/jpet.103.051284. [DOI] [PubMed] [Google Scholar]
- 57.Baulieu EE, et al. Neurosteroids: beginning of the story. Int. Rev. Neurobiol. 2001;46:1–32. doi: 10.1016/s0074-7742(01)46057-0. [DOI] [PubMed] [Google Scholar]
- 58.Lipsky NG, Pagano RE. Sphingolipid metabolism in cultured fibroblasts: microscopic and biochemical studies employing a fluorescent ceramide analogue. Proc. Natl. Acad. Sci. U. S. A. 1983;80:2608–2612. doi: 10.1073/pnas.80.9.2608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Chelu MG, et al. Regulation of ryanodine receptors by FK506 binding proteins. Trends Cardiovasc. Med. 2004;14:227–234. doi: 10.1016/j.tcm.2004.06.003. [DOI] [PubMed] [Google Scholar]
- 60.Castagna A, et al. A proteomic approach to cisplatin resistance in the cervix squamous cell carcinoma cell line A431. Proteomics. 2004;4:3246–3267. doi: 10.1002/pmic.200400835. [DOI] [PubMed] [Google Scholar]
- 61.Remppis A, et al. The small EF-hand Ca2+ binding protein S100A1 increases contractility and Ca2+ cycling in rat cardiac myocytes. Basic Res. Cardiol. 2002;97(Suppl 1):I56–I62. doi: 10.1007/s003950200031. [DOI] [PubMed] [Google Scholar]
- 62.Voelker DR. Protein and lipid motifs regulate phosphatidylserine traffic in yeast. Biochem. Soc. Trans. 2005;33:1141–1145. doi: 10.1042/BST20051141. [DOI] [PubMed] [Google Scholar]
- 63.Goetz JG, Nabi IR. Interaction of the smooth endoplasmic reticulum and mitochondria. Biochem. Soc. Trans. 2006;34:370–373. doi: 10.1042/BST0340370. [DOI] [PubMed] [Google Scholar]
- 64.Li G, et al. The AAA ATPase p97 links peptide N-glycanase to the endoplasmic reticulum-associated E3 ligase autocrine motility factor receptor. Proc. Natl. Acad. Sci. U. S. A. 2006;103:8348–8353. doi: 10.1073/pnas.0602747103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.de Brito OM, Scorrano L. Mitofusin 2 tethers endoplasmic reticulum to mitochondria. Nature. 2008;456:605–610. doi: 10.1038/nature07534. [DOI] [PubMed] [Google Scholar]