Abstract
Determination of the optimal dose of renal replacement therapy in critically ill patients with acute kidney injury has been controversial. Questions have recently been raised regarding the design and execution of the US Department of Veterans Affairs/National Institutes of Health Acute Renal Failure Trial Network (ATN) Study, which demonstrated no improvement in 60-day all-cause mortality with more intensive management of renal replacement therapy. In the present article we present our rationale for these aspects of the design and conduct of the study, including our use of both intermittent and continuous modalities of renal support, our approach to initiation of study therapy and the volume management during study therapy. In addition, the article presents data on hypotension during therapy and recovery of kidney function in the perspective of other studies of renal support in acute kidney injury. Finally, we address the implications of the ATN Study results for clinical practice from the perspective of the study investigators.
Introduction
The optimal intensity of renal replacement therapy (RRT) in acute kidney injury (AKI) remains controversial [1-4]. We recently published the results of the US Department of Veterans Affairs/National Institutes of Health Acute Renal Failure Trial Network (ATN) Study, which examined the effect of two strategies for the management of RRT on outcomes in critically ill patients with AKI [5]. Our study compared an intensive management strategy with a less-intensive (conventional) management strategy, with intensity defined based on clearance of low molecular weight solutes. No difference in survival or recovery of kidney function was found between the two management strategies. Since publication, several aspects of the study design and conduct have been criticized [6-11]. In the present commentary we provide the investigators' perspective on many of the issues that have been raised, the majority of which were carefully considered as the study was designed and conducted [12].
The combined use of intermittent and continuous RRT parallels clinical practice
We designed the ATN Study as a process-of-care study. As such, the use of both intermittent RRT and continuous RRT was intended to parallel usual clinical practice, in which hemodynamically unstable patients are commonly managed using continuous renal replacement therapy (CRRT) and hemodynamically stable patients are generally treated using intermittent hemodialysis (IHD) [13]. Approximately 40% of the study participants received both modalities over the course of their illness as their hemodynamic status changed (Table 1). To have restricted patients into a single modality or to have excluded individuals in which more than one modality was used, especially given that study therapy was provided for as long as 28 days, would have severely undermined the generalizability of the study results. To assure comparable management in both treatment arms, however, conversion between modalities was protocolized – which may have resulted in small differences as compared with clinical practice.
Table 1.
Modality of therapy during the study treatment
| Intensive management strategy (n = 563) | Less-intensive management strategy (n = 561) | ||||
|---|---|---|---|---|---|
| Initial modality of RRT | Number of modality switches | Frequencya | Mortality by day 60b | Frequencya | Mortality by day 60b |
| IHD | None | 108 (19.2) | 33 (30.6) | 138 (24.6) | 39 (28.3) |
| 1 | 18 (3.2) | 16 (88.9) | 8 (1.4) | 7 (87.5) | |
| ≥ 2 | 27 (4.8) | 16 (59.3) | 14 (2.5) | 4 (28.6) | |
| CRRT/SLED | None | 203 (36.1) | 165 (81.3) | 212 (37.8) | 166 (78.3) |
| 1 | 136 (24.2) | 33 (24.3) | 127 (22.6) | 40 (31.5) | |
| ≥ 2 | 58 (10.3) | 31 (53.4) | 47 (8.4) | 23 (48.9) | |
Data presented as n (%). IHD, intermittent hemodialysis; CRRT/SLED, continuous renal replacement therapy or sustained low-efficiency dialysis.
aCalculated as the percentage of participants in the treatment arm. bCalculated as the percentage of participants in the treatment arm treated with a specified initial modality of renal replacement therapy (RRT) and the number of switches in treatment modality.,
It has been suggested that the application of continuous and intermittent therapies in the same protocol precludes a valid interpretation of the ATN Study results. This criticism is based on the contention that the dose of intermittent therapy provided in the intensive arm was actually less than the dose of continuous therapy provided in the less-intensive arm [6]. This argument is predicated on one of several mathematical models proposed to establish equivalence of solute clearance when RRT is provided on different schedules [14-17]. Unfortunately, none of these models has been validated in clinical practice, particularly in the acute setting [18].
In designing the protocol, we recognized that combining continuous and intermittent modalities into a single treatment strategy would raise issues regarding the comparability of dose [12]. Given the absence of a reliable model for equivalence of therapies provided on different schedules, we selected doses of IHD and CRRT for the less-intensive treatment arm based on assessment of clinical practice: IHD generally being provided on a thrice-weekly or alternate-day schedule, and CRRT being provided at effluent flow rates of 20 ml/kg per hour or less [13]. In the intensive treatment arm, we set the dosing of IHD by doubling the frequency of treatment from three to six times per week and we increased the dose of CRRT slightly less than twofold, as previously published data from Ronco and colleagues showed no further improvement in outcomes with doses of CRRT beyond 35 ml/kg per hour [19].
An alternative (and less controversial) method for assessing equivalence of the treatment dose is to compare the time-averaged concentration of urea during each of the treatment modalities. While the study was not designed based on this approach, it is notable that the time-averaged blood urea nitrogen concentrations during IHD were remarkably similar to the mean daily concentration during CRRT in both treatment arms: 33 ± 17 mg/dl (12 ± 6 mmol/l) versus 33 ± 18 mg/dl (12 ± 6 mmol/l) in the intensive arm, and 48 ± 19 mg/dl (17 ± 7 mmol/l) versus 47 ± 23 mg/dl (17 ± 8 mmol/l) in the less-intensive treatment arm [5].
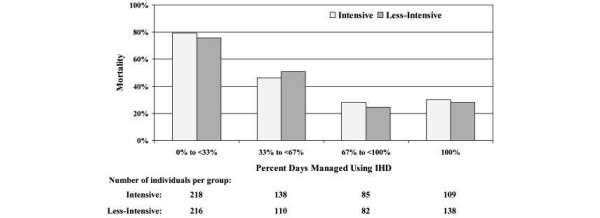
Finally, although the study was not designed to permit rigorous analysis of outcomes by treatment modality, in a post hoc analysis we examined 60-day all-cause mortality between treatment arms as a function of the percentage of time treated with IHD (Figure 1). Following the study protocol, the percentage of time eligible for treatment using IHD was a surrogate for hemodynamic stability. It is therefore not surprising that as the percentage of time eligible for IHD increased, the overall mortality was lower – ranging from more than 80% in persons with little to no time on IHD, to less than 30% among those who were treated predominantly with IHD. Similarly, as would be expected given that changes in the modality of RRT within each treatment arm were driven by hemodynamic status, participants who began treatment with intermittent therapy and were switched to CRRT had higher mortality than those who were converted from continuous therapy to IHD (Table 1). Regardless of the subgroup examined, there were no differences in survival as a function of the intensity of RRT.
Figure 1.
All-cause mortality at 60 days as a function of days managed using intermittent hemodialysis. The time in the intermittent hemodialysis (IHD) phase was defined as the number of days from the first IHD treatment or from the first day after continuous renal replacement therapy (CRRT) or sustained low-efficiency dialysis (SLED) was discontinued until the last day of IHD treatment, the last day before initiation of CRRT or SLED, or the discontinuation of study therapy. Days with IHD and with either CRRT or SLED were counted as in the IHD phase. The percentage of days managed using IHD was calculated by dividing the number of days in the IHD phase by the total number of days of study therapy.
The initiation of RRT was timely
Several commentaries have criticized the ATN Study for an unusually long interval between intensive care unit admission and initiation of RRT [6,10]. This criticism is based on a misconception regarding the relationship between onset of AKI and intensive care unit admission. Admission to the intensive care unit cannot be used as a surrogate for the timing of kidney injury. While the interval between intensive care unit admission and initiation of RRT was 6.7 ± 9.0 days, the interval between the clinically assessed onset of AKI and study randomization was only 3.2 ± 2.0 days. As there is no consensus in clinical practice regarding the optimal timing of RRT in AKI, we left the decision to start RRT to the treating bedside clinical team. Furthermore, the mean blood urea nitrogen at initiation of RRT was lower than that reported in other recent studies [20-22] and was not different between the two treatment arms. We therefore believe that the issue of timing of therapy has little or no impact on the generalizability of the study's results.
The permitted provision of up to 24 hours of CRRT or one IHD session prior to randomization has also been the subject of criticism [6,10]. We allowed this limited duration of pre-randomization RRT for ethical and safety reasons. As is common in the critical care setting, more than 90% of enrolled subjects lacked decision-making capacity at enrollment and therefore consent prior to participation had to be obtained from family or other surrogate decision-makers [23]. Since these surrogates were often not available within the hospital, allowing up to 24 hours of nonstudy RRT ensured that the enrollment process did not interfere with appropriate clinical care and delay the initiation of RRT. Although we felt that this brief period of nonstudy RRT would have little impact on study outcomes, we carefully monitored the provision of pre-randomization RRT, collected complete data on these treatments, and evaluated the impact of pre-randomization treatment on study outcomes. There were no differences in the use of pre-randomization RRT in the two treatment arms (Table 2). The use of pre-randomization RRT was not associated with 60-day all-cause mortality within the entire cohort (odds ratio = 1.04; 95% confidence interval = 0.79 to 1.36; P = 0.31) and there was no interaction between the use of pre-randomization RRT and the treatment group (P = 0.59).
Table 2.
Pre-randomization RRT and 60-day all-cause mortality
| Intensive management strategy (n = 563) | Less-intensive management strategy (n = 561) | Odds ratio (95% CI)a (between management strategies) | |
|---|---|---|---|
| Without pre-randomization RRT | 113/205 (55.1) | 106/194 (54.6) | 1.15 (0.85 to 1.53), P = 0.36 |
| With pre-randomization RRT | 189/358 (52.8) | 182/366 (49.7) | 1.00 (0.66 to 1.51), P >0.99 |
| Odds ratio (95% CI)a (within management strategy) | 0.90 (0.62 to 1.30), P = 0.58 | 1.04 (0.71 to 7.50), P = 0.85 |
Data presented as number died/number at risk (%) or odds ratio (95% confidence interval (CI)). RRT, renal replacement therapy. aOdds ratio calculated by conditional logistic regression modeling adjusted for randomization strata.
Convective or diffusive solute clearance?
The ATN Study design has also been criticized for an inadequate use of convective clearance during CRRT [6]. We believe that this criticism is not supported by rigorous evidence. While convective therapies provide greater clearance of higher molecular weight solutes, clearances of lower molecular weight solutes are similar when diffusive and convective therapies are provided at the same flow rates [24]. Furthermore, there is no evidence to support a benefit of convective therapy as compared with diffusive therapy in AKI [25], and one prior study demonstrated that the addition of diffusive clearance to a fixed dose of convection was associated with improved survival [20].
Volume management was similar in the two management strategies
Although intensity of therapy was defined in terms of low molecular weight solute clearance, the importance of volume removal was explicitly recognized in the study design. During the study, volume management remained under the control of the bedside clinical team. The impact of the study protocol on volume management should have been minimal during CRRT since volume management is independent of solute clearance during continuous therapy. In contrast, we were concerned that when intermittent therapies were employed, restricting the treatment frequency to an every-other-day schedule in the less-intensive arm could adversely influence volume management. We therefore allowed the use of isolated ultrafiltration on nondialysis days as required for volume management.
The use of ultrafiltration did not constitute a protocol deviation, as some have contended [6,10], and complete data on these treatments were collected. As expected, more ultrafiltration treatments were provided in the less-intensive management strategy, but even in this study arm there were fewer than 0.5 ultrafiltration treatments per participant during the course of RRT. More importantly, there were no differences in overall fluid balance between the two treatment arms. Over the first 14 days of study therapy, fluid balance was positive by a median of 1.9 l (interquartile range = -4.8 to 9.2 l) in the intensive arm as compared with 1.7 l (inter-quartile range = -4.0 to 8.8 l) in the less intensive arm (P = 0.94).
Documentation of treatment-associated hypotension
Critiques have intimated that the frequency of hypotension we reported was unusually high [6]. We previously reported hypotension based on standardized reporting of hypotension-associated adverse events, including discontinuation of treatment, initiation of vasopressor therapy and any other intervention in response to intradialytic hypotension during each IHD treatment [5]. We also, however, collected pre-dialysis and lowest (nadir) intradialytic blood pressures during each IHD session [5]. Using these data, the frequency of dialysis-associated hypotension in the ATN Study was actually similar to or lower than that reported in previously published trials. Using the same definition as in the French Hemodiafe Study, intradialytic hypotension occurred in 38.3% of ATN Study participants randomized to the intensive arm and in 36.8% of patients randomized to the less-intensive arm, as compared with 39% of the Hemodiafe IHD cohort [22]. Similarly, the requirement for initiation or escalation of vasopressor support in the ATN Study was lower than in a similar cohort described by Schortgen and colleagues [26].
Since changes in hemodynamic stability during continuous therapy were reflected by changes in vasopressor dose, we did not collect similar blood pressure data during CRRT. We observed escalations in vasopressor therapy sufficient to increase the cardiovascular component of the Sequential Organ Failure Assessment score during CRRT in 20.8% of participants on 3.8% of treatment days. These data suggest that hypotension is also a frequent occurrence during continuous therapy. While demonstrating that the rates of dialysis-associated hypotension in the ATN Study were not unusually high, these data also suggest that improved strategies are required to minimize hemodynamic instability during both IHD and CRRT.
Commentators have also questioned the difference in the rates of discontinuation of IHD and CRRT as a result of severe hypotension [6]. Once again these differences were intrinsically related to the relationship between hemodynamic status and treatment modality in the study design. The modality of RRT was changed after 35.3% of IHD treatments that were discontinued due to severe intradialytic hypotension, while RRT was permanently discontinued after only 11.8% of such episodes. In contrast, in no participants was the modality of RRT changed when CRRT was interrupted due to severe hypotension although the severe hypotension led to permanent discontinuation RRT after 42.3% of such episodes. Patient outcomes were also strikingly different; 53.8% of participants died or had life support withdrawn within 1 day of suspension of CRRT due to severe hypotension, as compared with only 12.8% following discontinuation of an IHD treatment because of severe intradialytic hypotension (P < 0.0001).
Defining recovery of kidney function
Several critiques of the ATN Study have speculated on the low rate of recovery of kidney function [6,11]. Unlike other studies that defined recovery of kidney function based on dialysis independence at hospital discharge, we used a more stringent criterion – a measured creatinine clearance >20 ml/minute by day 28. Using this definition, 41.2% of study participants in the intensive treatment arm alive at day 28 had recovered kidney function, as did 45.6% of patients in the less-intensive arm (P = 0.27). A substantial number of participants, however, achieved dialysis independence but did not meet the specified definition of recovery of kidney function.
Of the participants alive at day 28, 51.5% and 58.0% in the intensive and less-intensive strategies were dialysis independent (P = 0.10). These percentages increased to 74.6% and 76.2% (P = 0.67), respectively, among participants alive at day 60 [27]. Although some studies have reported recovery of kidney function in more than 90% of patients [19,22], the recovery rates we observed were comparable with those seen in other studies [20,28]. In the BEST Kidney Study – a prospective observational study of more than 1,000 critically ill patients with AKI requiring RRT – only 31.3% of patients were alive off dialysis at hospital discharge [29], as compared with 35.4% at day 60 in the ATN Study.
Erroneous suggestion of inconsistencies in reported data
Some authors have even questioned the reliability of our reported data with regard to the delivered dose of therapy, suggesting inconsistencies between the reported mean daily effluent volume during continuous therapy and the values they calculated from the mean daily duration of treatment and the mean values for dialysate, replacement fluid and net ultrafiltration rates [6]. This apparent inconsistency is actually the result of a repeated mathematical error: the product of mean values does not equal the mean of individual products.
It is therefore not surprising that the values these authors have attempted to calculate do not correspond to the actual measured values.
Conclusion
We designed the US Department of Veterans Affairs/National Institutes of Health ATN Study to test the hypothesis that more intensive RRT in critically ill patients with AKI is associated with improved outcomes. The study results do not support the contention that increasing intensity of therapy beyond a sufficient dose is associated with decreased mortality, improved recovery of kidney function or differences in the course of nonrenal organ failure. That is not to say that the study supports an approach of therapeutic nihilism, as suggested by Ronco and colleagues [6]. Rather, since the less-intensive strategy provided a level of renal support that often exceeds typical clinical practice, our results suggest there needs to be a greater emphasis on ensuring that an appropriate prescribed dose of therapy is actually delivered. For patients receiving intermittent therapy, this will require monitoring the delivered dose, with careful attention to ensure delivery of Kt/Vurea of at least 1.2 per treatment. For patients receiving continuous therapy, emphasis needs to be on ensuring that treatment times are maximized, since prior studies have suggested substantial underestimation of interruptions of treatment [30].
While it has been suggested that the use of a fixed dosed of therapy throughout the dynamic course of an episode of AKI may not be appropriate [9], we believe this hypothesis is untested and requires rigorous evaluation. We agree that treatment needs to be individualized and that more intensive therapy may be required in some situations. Although our study design used protocol-based criteria to guide switching between modalities of therapy, it also needs to be recognized that these criteria were empirically derived, using expert opinion and consensus, and remain untested as to whether they represent the best approach for every patient. For all modalities, new strategies to minimize complications of therapy – including hypotension and electrolyte disturbances – need to be implemented. In addition, the optimal timing of RRT and fluid management during therapy need to be rigorously evaluated.
While we need to optimize the care delivered, the results of the ATN Study also suggest that merely modifying the prescription and delivery of RRT is unlikely to result in substantial improvement in outcomes. We must recognize the limits of the treatment and shift our focus to other strategies for prevention and treatment of AKI.
Abbreviations
AKI: acute kidney injury; ATN: Acute Renal Failure Trial Network; CRRT: continuous renal replacement therapy; IHD: intermittent hemodialysis; RRT : renal replacement therapy.
Competing interests
The authors declare that they have no competing interests.
Contributor Information
Paul M Palevsky, Email: Palevsky@pitt.edu.
Theresa Z O'Connor, Email: Terry.O'Connor@va.gov.
Glenn M Chertow, Email: gchertow@stanford.edu.
Susan T Crowley, Email: susan.crowley@va.gov.
Jane Hongyuan Zhang, Email: jane.zhang@va.gov.
John A Kellum, Email: kellumja@ccm.upmc.edu.
Acknowledgements
Supported by the Cooperative Studies Program of the Department of Veterans Affairs Office of Research and Development and by the National Institute of Diabetes and Digestive and Kidney Diseases (interagency agreement Y1-DK-3508-01).
References
- Davenport A, Bouman C, Kirpalani A, Skippen P, Tolwani A, Mehta RL, Palevsky PM. Delivery of renal replacement therapy in acute kidney injury: what are the key issues? Clin J Am Soc Nephrol. 2008;3:869–875. doi: 10.2215/CJN.04821107. [DOI] [PubMed] [Google Scholar]
- Kellum JA, Mehta RL, Angus DC, Palevsky P, Ronco C. The first international consensus conference on continuous renal replacement therapy. Kidney Int. 2002;62:1855–1863. doi: 10.1046/j.1523-1755.2002.00613.x. [DOI] [PubMed] [Google Scholar]
- Palevsky PM. Clinical review: timing and dose of continuous renal replacement therapy in acute kidney injury. Crit Care. 2007;11:232. doi: 10.1186/cc6121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ronco C, Ricci Z, Bellomo R. Current worldwide practice of dialysis dose prescription in acute renal failure. Curr Opin Crit Care. 2006;12:551–556. doi: 10.1097/01.ccx.0000247447.17124.05. [DOI] [PubMed] [Google Scholar]
- Palevsky PM, Zhang JH, O'Connor TZ, Chertow GM, Crowley ST, Choudhury D, Finkel K, Kellum JA, Paganini E, Schein RM, Smith MW, Swanson KM, Thompson BT, Vijayan A, Watnick S, Star RA, Peduzzi P. for the VA/NIH Acute Renal Failure Trial Network. Intensity of renal support in critically ill patients with acute kidney injury. N Engl J Med. 2008;359:7–20. doi: 10.1056/NEJMoa0802639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ronco C, Cruz D, van Straaten HO, Honore P, House A, Bin D, Gibney N. Dialysis dose in acute kidney injury: no time for therapeutic nihilism – a critical appraisal of the Acute Renal Failure Trial Network study. Crit Care. 2008;12:308. doi: 10.1186/cc7016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ronco C, Honore P. Renal support in critically ill patients with acute kidney injury [letter] N Engl J Med. 2008;359:1959. doi: 10.1056/NEJMc081598. author reply 1961–1962. [DOI] [PubMed] [Google Scholar]
- Bouchard J, Macedo E, Mehta RL. Renal support in critically ill patients with acute kidney injury [letter] N Engl J Med. 2008;359:1959–1960. doi: 10.1056/NEJMc081598. author reply 1961–1962. [DOI] [PubMed] [Google Scholar]
- Maynar-Moliner J, Sanchez-Izquierdo-Riera JA, Herrera-Gutierrez M. Renal support in critically ill patients with acute kidney injury [letter] N Engl J Med. 2008;359:1960. author reply 1961–1962. [PubMed] [Google Scholar]
- Bagshaw SM, Gibney N. Renal support in critically ill patients with acute kidney injury [letter] N Engl J Med. 2008;359:1960–1961. author reply 1961–1962. [PubMed] [Google Scholar]
- Uchino S, Bell M, Bellomo R. Renal support in critically ill patients with acute kidney injury [letter] N Engl J Med. 2008;359:1961. author reply 1961–1962. [PubMed] [Google Scholar]
- Palevsky PM, O'Connor T, Zhang JH, Star RA, Smith MW. Design of the VA/NIH Acute Renal Failure Trial Network (ATN) Study: intensive versus conventional renal support in acute renal failure. Clin Trials. 2005;2:423–435. doi: 10.1191/1740774505cn116oa. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Overberger P, Pesacreta M, Palevsky PM. Management of renal replacement therapy in acute kidney injury: a survey of practitioner prescribing practices. Clin J Am Soc Nephrol. 2007;2:623–630. doi: 10.2215/CJN.00780207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keshaviah PR, Nolph KD, Van Stone JC. The peak concentration hypothesis: a urea kinetic approach to comparing the adequacy of continuous ambulatory peritoneal dialysis (CAPD) and hemodialysis. Perit Dial Int. 1989;9:257–260. [PubMed] [Google Scholar]
- Casino FG, Lopez T. The equivalent renal urea clearance: a new parameter to assess dialysis dose. Nephrol Dial Transplant. 1996;11:1574–1581. [PubMed] [Google Scholar]
- Clark WR, Mueller BA, Kraus MA, Macias WL. Dialysis prescription and kinetics in acute renal failure. Adv Ren Replace Ther. 1997;4(2 Suppl 1):64–71. [PubMed] [Google Scholar]
- Gotch FA. The current place of urea kinetic modelling with respect to different dialysis modalities. Nephrol Dial Transplant. 1998;13(Suppl 6):10–14. doi: 10.1093/ndt/13.suppl_6.10. [DOI] [PubMed] [Google Scholar]
- Gotch FA, Sargent JA, Keen ML. Whither goest Kt/V? Kidney Int Suppl. 2000;76:S3–S18. doi: 10.1046/j.1523-1755.2000.07602.x. [DOI] [PubMed] [Google Scholar]
- Ronco C, Bellomo R, Homel P, Brendolan A, Dan M, Piccinni P, La Greca G. Effects of different doses in continuous venovenous haemofiltration on outcomes of acute renal failure: a prospective randomised trial. Lancet. 2000;356:26–30. doi: 10.1016/S0140-6736(00)02430-2. [DOI] [PubMed] [Google Scholar]
- Saudan P, Niederberger M, De Seigneux S, Romand J, Pugin J, Perneger T, Martin PY. Adding a dialysis dose to continuous hemofiltration increases survival in patients with acute renal failure. Kidney Int. 2006;70:1312–1317. doi: 10.1038/sj.ki.5001705. [DOI] [PubMed] [Google Scholar]
- Liu KD, Himmelfarb J, Paganini E, Ikizler TA, Soroko SH, Mehta RL, Chertow GM. Timing of initiation of dialysis in critically ill patients with acute kidney injury. Clin J Am Soc Nephrol. 2006;1:915–919. doi: 10.2215/CJN.01430406. [DOI] [PubMed] [Google Scholar]
- Vinsonneau C, Camus C, Combes A, Costa de Beauregard MA, Klouche K, Boulain T, Pallot JL, Chiche JD, Taupin P, Landais P, Dhainaut J-F. for the Hemodiafe Study Group. Continuous venovenous haemodiafiltration versus intermittent haemodialysis for acute renal failure in patients with multiple-organ dysfunction syndrome: a multicentre randomised trial. Lancet. 2006;368:379–385. doi: 10.1016/S0140-6736(06)69111-3. [DOI] [PubMed] [Google Scholar]
- Crowley ST, Chertow GM, Vitale J, O'Connor T, Zhang J, Schein RM, Choudhury D, Finkel K, Vijayan A, Paganini E, Palevsky PM. for the VA/NIH Acute Renal Failure Trial Network Study Group. Lessons for successful study enrollment from the Veterans Affairs/National Institutes of Health Acute Renal Failure Trial Network Study. Clin J Am Soc Nephrol. 2008;3:955–961. doi: 10.2215/CJN.05621207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Troyanov S, Cardinal J, Geadah D, Parent D, Courteau S, Caron S, Leblanc M. Solute clearances during continuous venovenous haemofiltration at various ultrafiltration flow rates using Multiflow-100 and HF1000 filters. Nephrol Dial Transplant. 2003;18:961–966. doi: 10.1093/ndt/gfg055. [DOI] [PubMed] [Google Scholar]
- Pannu N, Klarenbach S, Wiebe N, Manns B, Tonelli M. Renal replacement therapy in patients with acute renal failure: a systematic review. JAMA. 2008;299:793–805. doi: 10.1001/jama.299.7.793. [DOI] [PubMed] [Google Scholar]
- Schortgen F, Soubrier N, Delclaux C, Thuong M, Girou E, Brun-Buisson C, Lemaire F, Brochard L. Hemodynamic tolerance of intermittent hemodialysis in critically ill patients: usefulness of practice guidelines. Am J Respir Crit Care Med. 2000;162:197–202. doi: 10.1164/ajrccm.162.1.9907098. [DOI] [PubMed] [Google Scholar]
- Palevsky PM, Franchini R, O'Connor TZ, Zhang JH. Recovery of kidney function in critically ill patients with acute kidney injury treated with intensive versus less intensive renal replacement therapy [abstract] J Am Soc Nephrol. 2008;19:790A. [Google Scholar]
- Tolwani AJ, Campbell RC, Stofan BS, Lai KR, Oster RA, Wille KM. Standard versus high-dose CVVHDF for ICU-related acute renal failure. J Am Soc Nephrol. 2008;19:1233–1238. doi: 10.1681/ASN.2007111173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uchino S, Bellomo R, Kellum JA, Morimatsu H, Morgera S, Schetz MR, Tan I, Bouman C, Macedo E, Gibney N, Tolwani A, Oudemans-Van Straaten HM, Ronco C. Beginning and Ending Supportive Therapy for the Kidney (B.E.S.T. Kidney) Investigators Writing Committee. Patient and kidney survival by dialysis modality in critically ill patients with acute kidney injury. Int J Artif Organs. 2007;30:281–292. doi: 10.1177/039139880703000402. [DOI] [PubMed] [Google Scholar]
- Venkataraman R, Kellum JA, Palevsky P. Dosing patterns for continuous renal replacement therapy at a large academic medical center in the United States. J Crit Care. 2002;17:246–250. doi: 10.1053/jcrc.2002.36757. [DOI] [PubMed] [Google Scholar]