Abstract
Introduction
Adiponectin plays an important role in the regulation of tissue inflammation and insulin sensitivity. Perturbations in adiponectin concentration have been associated with obesity and the metabolic syndrome. Data on adiponectin pathophysiology in critical illness are limited.
Methods
Twenty three critically ill patients (9 severe sepsis, 7 burns, 7 trauma). Adiponectin assays on Days 3 (D3) and 7 (D7). Simultaneous, cortisol, cortisone and CRP measurements. Data from 16 historical controls were used for comparison.
Results
The mean plasma adiponectin concentration for the ICU cohort on D3 and D7 were not significantly different (4.1 ± 1.8 and 5.0 ± 3.3 mcg/ml respectively, P = 0.38). However, these were significantly lower than the mean plasma adiponectin in the control population (8.78 ± 3.81 mcg/ml) at D3 (P < 0.0001) and D7 (P = 0.002). Plasma adiponectin showed a strong correlation with plasma cortisol in the ICU group on both D3 (R2 = 0.32, P < 0.01) and D7 (R2 = 0.64, 0.001). There was an inverse correlation between plasma adiponectin and CRP on D7, R = -0.35.
Conclusions
In this preliminary study, critical illness was associated with lower adiponectin concentrations as compared with controls. A significant relationship between plasma cortisol and adiponectin in critically ill patients was evident, both during the early and late phases. These data raise the possibility that adiponectin may play a part in the inflammatory response in patients with severe illness.
Introduction
Adiponectin, a hormone secreted exclusively by adipose tissue, plays an important role in the regulation of tissue inflammation and insulin sensitivity [1]. Perturbations in circulating adiponectin concentrations are associated with the metabolic syndrome, altered inflammatory response and insulin resistance [2]. Hypoadiponectaemia is also associated with impaired endothelium-dependent vasorelaxation [3]. Although several of the above features are also evident in human critical illness, the underlying mechanisms are not fully understood. Some of these manifestations have been attributed to changes in plasma cortisol profile.
Data in patients with viral infections and human experimental endotoxaemia suggest altered release patterns of adiponectin in these states [4,5]. However, there are no published data on circulating serum adiponectin concentrations in human septic shock and critical illness. We therefore utilised available samples from a previously undertaken study of critically ill patients to examine serial changes in serum adiponectin concentration in a heterogeneous cohort of critically ill patients (sepsis, trauma and burns), determine the relation between the inflammatory response and adiponectin concentrations, and evaluate the correlation between plasma cortisol and adiponectin concentrations.
Materials and methods
The plasma samples for this study were obtained from our previously published study investigating plasma cortisol-cortisone ratios in 52 critically ill patients comprising of three cohorts – burns, trauma and sepsis [6]. Residual plasma samples for adiponectin analysis were only available in 23 of these patients (nine sepsis, seven trauma, seven burns; age range 26 to 65 years; 21 males and 2 females), which were used in the present study. An independent ethics committee approval was obtained from the Royal Brisbane Hospital Ethics Committee for this study and reporting of data. The original samples were collected after informed consent from either patients or their next of kin. The other measurements on the same samples from these patients performed in the original study (cortisol, cortisone and C-reactive protein (CRP)) were used for correlative analysis.
A detailed description of inclusion and exclusion criteria was provided in the original paper. Briefly, patients with septic shock (as defined in Consensus Criteria), burns of more than 30%, and blunt or penetrating trauma of at least two body regions requiring admission to the critical care unit were enrolled in the study.
Patients younger than 16 years of age, those with a previous history of adrenal or pituitary disease, prolonged use of oral or inhaled glucocorticoids or current therapy with any such agents were excluded. The care of the patients was as per standard practice. No patient received intravenous or oral glucocorticoids.
In the original study, blood samples were collected for analysis of cortisone, cortisol and CRP daily for the first five days and on days 7, 10, 15 and 28. Residual sera were stored in a freezer at -20°C. As the predominant number of residual samples, was available only on day 3 (D3) and D7, these samples were used for the adiponectin assay.
Biochemical measurements
Total serum adiponectin was measured using human adiponectin radioimmunoassay (Linco Research, St Charles, MI, USA; coefficients of variation (CV) for the assay <10%). Cortisol and cortisone were measured by high performance liquid chromatography. The CV at cortisol levels of 23 nmol/l and 1006 nmol/l were 6.5% and 2.4%, respectively, and for cortisone at concentrations of 110 nmol/l and 1026 nmol/L were 10.9% and 9.2%, respectively. CRP (standard assay) was assayed using an immunoturbimetric assay (Roche Diagnostics, Sydney, Australia). Interassay CV at 13 mg/L was 4.5%.
Statistical analysis
The means and standard deviations for normals obtained from population values from our laboratory (see Results section) were used to compare the patient populations. Continuous, normally distributed variables were summarised as mean ± standard deviation. Changes in plasma adiponectin between D3 and D7 were compared using an unpaired T-test assuming unequal variances. The degree of association between variables (adiponectin and cortisol, cortisone, CRP and cortisol/cortisone ratio) and skewed or ordinal outcome measures (such as Acute Physiology and Chronic Health Evaluation (APACHE) and simplified acute physiology score (SAPS)) was assessed using Spearman's correlation coefficient (rs). Statistical significance was taken at a level of 5%.
Results
The demographic profile, the diagnostic categories and the plasma endocrine profile of the patients are presented in Table 1.
Table 1.
Demographic, diagnostic, sickness severity and endocrine profiles of the study group
| Category | APACHE | Hospital survival | D3 Adiponectin | D3 Cortisone | D3 Cortisol | D3 CRP | D7 Adiponectin | D7 Cortisone | D7 Cortisol | D7 CRP |
|---|---|---|---|---|---|---|---|---|---|---|
| Sepsis | 10 | Survived | 4.7 | 24 | 238 | 187 | 4.6 | 33 | 321 | 146 |
| Sepsis | 16 | Survived | 1.6 | 34 | 331 | 223 | 3.1 | 33 | 413 | 85 |
| Sepsis | 22 | Survived | 4.4 | 26 | 462 | 286 | 7.4 | 24 | 490 | 301 |
| Sepsis | 8 | Survived | 4.4 | 19 | 97 | 147 | 2.6 | 31 | 444 | 403 |
| Sepsis | 19 | Survived | 1.0 | 23 | 288 | 265 | 2.4 | 31 | 325 | 204 |
| Sepsis | 28 | Survived | 2.4 | N/A | N/A | 447 | 3.4 | 13 | 323 | 266 |
| Sepsis | 24 | Survived | 5.1 | 21 | 173 | 202 | 8.9 | 23 | 998 | 94 |
| Sepsis | 14 | Died | 4.4 | 14 | 451 | 264 | 3.3 | 13 | 230 | 166 |
| Sepsis | 13 | Survived | 8.3 | 93 | 2620 | 278 | 14.3 | 35 | 1770 | 166 |
| Trauma | 12 | Survived | 2.9 | 17 | 371 | 347 | 3.6 | 12 | 336 | 184 |
| Trauma | 19 | Survived | N/A | 32 | 159 | 109 | 3.1 | 28 | 352 | N/A |
| Trauma | 15 | Survived | 4.7 | 36 | 162 | 266 | N/A | 2 | 463 | 76 |
| Trauma | 12 | Survived | 3.5 | 50 | 379 | 224 | 5.6 | 4 | 83 | 178 |
| Trauma | 12 | Survived | 6.0 | 62 | 681 | N/A | 7.0 | 14 | 517 | 76 |
| Trauma | 10 | Survived | 7.4 | 11 | 264 | 324 | 2.3 | N/A | N/A | 564 |
| Trauma | 18 | Survived | 4.8 | 42 | 588 | 258 | N/A | N/A | N/A | N/A |
| Burns | 15 | Unknown | 4.2 | 22 | 180 | N/A | N/A | N/A | N/A | N/A |
| Burns | 7 | Survived | N/A | 13 | 364 | N/A | 9.1 | 36 | 279 | 154 |
| Burns | 16 | Survived | 2.8 | 36 | 368 | 218 | N/A | 25 | 372 | 410 |
| Burns | 19 | Unknown | 4.0 | 28 | 135 | 104 | 3.0 | 17 | 217 | 198 |
| Burns | 17 | Survived | 3.2 | 26 | 262 | 202 | 5.3 | 35 | 411 | 376 |
| Burns | 25 | Survived | N/A | 34 | 311 | 241 | 2.8 | 17 | 197 | 251 |
| Burns | 24 | Died | 2.8 | 27 | 322 | 144 | 2.7 | 16 | 283 | 228 |
Cortisol and Cortisone expressed in nmol/.
Adiponectin expressed in mcg/ml.
APACHE = Acute Physiology and Chronic Health Evaluation; CRP = C-reactive protein; N/A = not available.
Plasma adiponectin profiles
The mean plasma adiponectin concentration for the whole cohort on D3 and D7 were 4.1 ± 1.8 and 5.0 ± 3.3 mcg/ml, respectively (P = 0.38). The mean plasma total adiponectin in the control population (16 historical controls; 12 males, 4 females, mean age 38.9 ± 8.7 years) was 8.78 ± 3.81 mcg/ml, significantly higher than the total intensive care group at D3 (P < 0.0001) and D7 (P = 0.002).
Plasma cortisol and cortisone profile
There were no differences in plasma cortisol (418 ± 512 vs 441 ± 362 nmol/L, P = 0.91) or plasma cortisone (31 ± 18 vs 22 ± 11 nmol/L, P = 0.06) between D3 and D7, respectively.
Relation between plasma adiponectin vs inflammatory markers
There was a poor correlation between plasma adiponectin and CRP on D3; however, on D7 an inverse correlation was noted, R = -0.35 (Figure 1).
Figure 1.
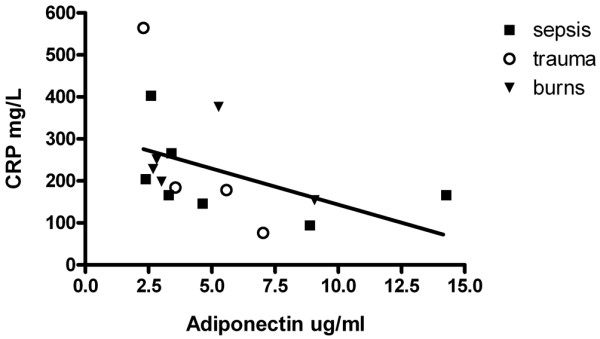
The relation between serum adiponectin and serum C-reactive protein (CRP) on day 7. A regression line is shown.
Relation between plasma adiponectin and sickness severity
There was a trend towards a relation between APACHE II scores and plasma adiponectin on D3 (R = 0.4, P = 0.07), but not on D7 (R = 0.17, P = 0.48).
Relation between plasma adiponectin and cortisol and cortisone
For correlative analysis, missing results were removed and only those with matching adiponectin and adrenal hormonal data were included. Plasma adiponectin showed a strong correlation with plasma cortisol in the group as a whole on D3 (R2 = 0.32, P = 0.01) and D7 (R2 = 0.64, P = 0.0001).
Discussion
To the best of our knowledge this is the first report of plasma adiponectin profiles in a heterogeneous cohort of critically ill patients. In this study, we have demonstrated a lower plasma adiponectin concentration as compared with historical controls and a strong association between plasma cortisol and adiponectin. This is notable because plasma cortisol concentrations vary widely in critically ill adults owing to the heterogeneity of the stress response. A trend towards an inverse relation between the inflammatory response and adiponectin, and a linear response between sickness severity and plasma adiponectin was also observed.
The mechanism behind the reduction in plasma adiponectin was not investigated in this study. However, it is well recognised that glucocorticoids, inflammation and oxidative stress (commonly associated with critical illness) are known to decrease adiponectin production [7]. This pattern is also consistent with what has been observed in rodent models of sepsis [8]. A significant positive relation between plasma cortisol and adiponectin has been previously shown in healthy volunteers [9,10], particularly in males [10]. In our cohort, more than 90% were males, and this strong association was evident even in critical illness in our study in both the early and late phases. A possible mechanism for the relation between cortisol and adiponectin could be that the promoter region for the adiponectin gene contains consensus sequences for glucocorticoid receptor binding [11]. The inverse association between adiponectin and CRP (R = -0.35) in our study is consistent with what has been reported in patients with coronary artery disease [12].
Significance in critical illness
As adiponectin plays an important role in tissue inflammation, endothelial function and vascular reactivity, this could represent a key pathway in determining steroid and inotrope responsiveness in septic shock. Adiponectin, through its negative feedback effects on TNF-alpha [13], may be a critical determinant of the severity of the inflammatory response and multiple organ dysfunction. In animal models of sepsis, adiponectin modulates inflammation and survival [14]. However, its role in human critical illness needs to be evaluated further.
Limitations
This is a preliminary study limited by a small sample size. Adiponectin analyses were performed on residual sera from our previous study [6] and historical controls were used. Comparisons between D3 and D7 are partly limited because paired samples were not available in every patient. As body mass index (BMI) is known to be associated with plasma adiponectin, a correlation between the BMI of patients and adiponectin would have provided additional useful information; however, as body weights are not routinely measured in critically ill patients, this analysis was not possible. Despite these limitations, the data from this study are in keeping with similar plasma profiles of adiponectin in human volunteers administered endotoxin [5]. Moreover, the results from D3 and D7 are relevant as patients would have completed their resuscitation phase and therefore large fluid shifts are less likely in this stage to have an impact on plasma concentrations.
Conclusions
In conclusion, in this preliminary study, we have demonstrated a significant relation between plasma cortisol and adiponectin in critically ill patients, both during the early and late phases. Overall, these data raise the possibility that adiponectin may play a part in the inflammatory response in patients with severe illness. These results are preliminary and hypothesis generating. The relation between adiponectin and the inflammatory response, organ dysfunction and outcome in critical illness should be the subject of future investigations.
Key messages
• Adiponectin a hormone secreted exclusively by adipose tissue has an important role in the regulation of tissue inflammation and insulin sensitivity.
• In this preliminary study of critically ill patients, serum adiponectin concentrations were reduced and were correlated with inflammatory markers such as CRP and plasma cortisol.
• Overall, these data raise the possibility that adiponectin may play a part in the inflammatory response in patients with severe illness.
Abbreviations
APACHE: Acute Physiology and Chronic Health Evaluation; BMI: body mass index; CRP: C-reactive protein; CV: coefficient of variation; D3: day 3; D7: day 7; SAPS: simplified acute physiology score; TNF: tumour necrosis factor.
Competing interests
The authors declare that they have no competing interests.
Authors' contributions
BV: Study design, analysis of data and manuscript preparation. IH: Adiponectin data analysis and manuscript review. JN: Adiponectin data analysis and manuscript review. JC: Cortisol and adiponectin analysis and manuscript review. JP: Study design, analysis of data and manuscript preparation.
See related commentary by Owecki, http://ccforum.com/content/13/4/174
Contributor Information
Bala Venkatesh, Email: bala_venkatesh@health.qld.gov.au.
Ingrid Hickman, Email: ihickman@cder.soms.uq.edu.au.
Janelle Nisbet, Email: janelle_nisbet@health.qld.gov.au.
Jeremy Cohen, Email: jeremy_cohen@health.qld.gov.au.
John Prins, Email: jprins@cder.soms.uq.edu.au.
References
- Whitehead JP, Richards AA, Hickman IJ, Macdonald GA, Prins JP. Adiponectin – a key adipokine in the metabolic syndrome. Diabetes Obes Metab. 2006;8:264–280. doi: 10.1111/j.1463-1326.2005.00510.x. [DOI] [PubMed] [Google Scholar]
- Hickman IJ, Whitehead JP, Prins JP, Macdonald GA. Raised alanine transaminase and decreased adiponectin are features of the metabolic syndrome in patients with type 2 diabetes. Diabetes Obes Metab. 2007;9:438–440. doi: 10.1111/j.1463-1326.2006.00604.x. [DOI] [PubMed] [Google Scholar]
- Ouchi N, Ohishi M, Kihara S, Funahashi T, Nakamura T, Nagaretani H, Kumada M, Ohashi K, Okamoto Y, Nishizawa H, Kishida K, Maeda N, Nagasawa A, Kobayashi H, Hiraoka H, Komai N, Kaibe M, Rakugi H, Ogihara T, Matsuzawa Y. Association of Hypoadiponectinemia With Impaired Vasoreactivity. Hypertension. 2003;42:231–234. doi: 10.1161/01.HYP.0000083488.67550.B8. [DOI] [PubMed] [Google Scholar]
- Palmer C, Hampartzoumian T, Lloyd A, Zekry A. A novel role for adiponectin in regulating the immune responses in chronic hepatitis C virus infection. Hepatology. 2008;48:374–384. doi: 10.1002/hep.22387. [DOI] [PubMed] [Google Scholar]
- Keller P, Møller K, Krabbe KS, Pedersen BK. Circulating adiponectin levels during human endotoxaemia. Clin Exp Immunol. 2003;134:107–110. doi: 10.1046/j.1365-2249.2003.02264.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venkatesh B, Cohen J, Hickman I, Nisbet J, Thomas P, Ward G, Hall J, Prins J. Evidence of altered cortisol metabolism in critically ill patients: a prospective study. Intensive Care Med. 2007;33:1746–1753. doi: 10.1007/s00134-007-0727-7. [DOI] [PubMed] [Google Scholar]
- Swarbrick MM, Havel PJ. Physiological, pharmacological, and nutritional regulation of circulating adiponectin concentrations in humans. Metab Syndr Relat Disord. 2008;6:87–102. doi: 10.1089/met.2007.0029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuchihashi H, Yamamoto H, Maeda K, Ugi S, Mori T, Shimizu T, Endo Y, Hanasawa K, Tani T. Circulating concentrations of adiponectin, an endogenous lipopolysaccharide neutralizing protein, decrease in rats with polymicrobial sepsis. J Surg Res. 2006;134:348–353. doi: 10.1016/j.jss.2006.01.001. [DOI] [PubMed] [Google Scholar]
- Gavrila A, Peng CK, Chan JL, Mietus JE, Goldberger AL, Mantzoros CS. Diurnal and ultradian dynamics of serum adiponectin in healthy men: comparison with leptin, circulating soluble leptin receptor, and cortisol patterns. J Clin Endocrinol Metab. 2003;88:2838–2343. doi: 10.1210/jc.2002-021721. [DOI] [PubMed] [Google Scholar]
- Fernandez-Real JM, Pugeat M, López-Bermejo A, Bornet H, Ricart W. Corticosteroid-binding globulin affects the relationship between circulating adiponectin and cortisol in men and women. Metabolism. 2005;54:584–589. doi: 10.1016/j.metabol.2004.11.015. [DOI] [PubMed] [Google Scholar]
- Comuzzie AG, Funahashi T, Sonnenberg G, Martin LJ, Jacob HJ, Black AE, Mass D, Takahashi M, Kihara S. The genetic basis of plasma variation in adiponectin, a global endophenotype for obesity and the metabolic syndrome. J Clin Endocrinol Metab. 2001;86:4321–4325. doi: 10.1210/jc.86.9.4321. [DOI] [PubMed] [Google Scholar]
- Ouchi N, Kihara S, Funahashi T, Nakamura T, Nishida M, Kumada M, Okamoto Y, Ohashi K, Nagaretani H. Reciprocal association of C-reactive protein with adiponectin in blood stream. Circulation. 2003;107:671–674. doi: 10.1161/01.CIR.0000055188.83694.B3. [DOI] [PubMed] [Google Scholar]
- Maeda N, Shimomura I, Kishida K, Nishizawa H, Matsuda M, Nagaretani H, Furuyama N, Kondo H, Takahashi M, Arita Y, Komuro R. Diet-induced insulin resistance in mice lacking adiponectin/ACRP30. Nat Med. 2002;8:731–737. doi: 10.1038/nm724. [DOI] [PubMed] [Google Scholar]
- Teoh H, Quan A, Bang KW, Wang G, Lovren F, Vu V, Haitsma JJ, Szmitko PE, Al-omran M, Wang CH, Gupta M, Peterson MD. Adiponectin deficiency promotes endothelial activation and profoundly exacerbates sepsis-related mortality. Am J Physiol Endocrinol Metab. 2008;295:E658–E664. doi: 10.1152/ajpendo.90384.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]


