Abstract
Introduction
Critically ill patients requiring intensive care uniformly develop insulin resistance. This is most pronounced in patients with sepsis. Recently, several hormones secreted by adipose tissue have been identified to be involved in overall insulin sensitivity in metabolic syndrome-related conditions. However, little is known about these adipokines in critical illness.
Methods
We studied circulating levels of the adipokines adiponectin, retinol-binding protein 4 (RBP4), and leptin during critical illness, and the impact of intensive insulin therapy, a therapy shown to affect insulin sensitivity, in serum samples from prolonged critically ill patients with a respiratory critical illness (n = 318). For comparison, we studied healthy subjects (n = 22) and acutely stressed patients (n = 22).
Results
During acute critical illness, circulating levels of adiponectin, RBP4, and leptin were low. Patients with sepsis had lower levels of leptin and RBP4 than did nonseptic patients. When critical illness was sustained, adipokine levels returned to normal reference values. Insulin therapy enhanced adiponectin, blunted the rise of RBP4, and did not alter leptin levels.
Conclusions
Acute critical illness is associated with immediate, but transiently low serum adipokine levels. Adiponectin and RBP4 are associated with altered insulin resistance in critical illness.
Introduction
Critically ill patients requiring intensive care uniformly develop hyperglycemia in the presence of hyperinsulinemia, suggesting insulin resistance. This is most pronounced in patients with sepsis [1].
Recently, several hormones secreted by adipose tissue have been identified to be involved in overall insulin sensitivity in metabolic syndrome-related conditions. Adiponectin appears to increase insulin sensitivity, with low levels observed in conditions of insulin resistance, such as obesity and type 2 diabetes, and with higher levels being associated with increased insulin sensitivity [2,3]. Leptin and retinol-binding protein-4 (RBP4), the only specific transporter for retinol in the circulation, appear to affect insulin action. Circulating levels of RBP4 are elevated in subjects with obesity and type 2 diabetes and lower with improved insulin sensitivity [4,5]. Low leptin levels are present with insulin resistance, and insulin infusion can induce leptin secretion [6].
In insulin resistance of the critically ill, little is known about these adipokines. Furthermore, the impact of insulin therapy during critical illness on these adipokines has not been studied. We hypothesized that the insulin resistance that is present in critical illness would affect circulating adiponectin, RBP4, and leptin levels and that the improved insulin sensitivity that we observed in intensive insulin therapy (IIT) patients [7] would change the adipokine levels.
Materials and methods
Study design
This study was a subanalysis of a large (n = 1,200) prospective, randomized controlled study on the effects of IIT on outcome of critical illness [8]. The detailed protocol of the study was previously published [8]. Written informed consent was obtained from the closest family member. The protocol and consent forms, including later analyses, were approved by the Institutional Ethical Board. Patients randomly assigned to conventional insulin therapy (CIT) received insulin only when glucose concentrations exceeded 215 mg/dl, resulting in mean blood glucose of 153 mg/dl (hyperglycemia). IIT maintained blood glucose levels between 80 and 110 mg/dl, resulting in mean blood glucose of 111 mg/dl (normoglycemia). Caloric intake was not different between the two therapy groups. To reduce the number of samples for the current study, we chose to work with the largest homogeneous subset (43%) of the 1,200 originally included patients, identifiable on admission, being the subset of 512 patients with a respiratory disease as main reason for admission to the ICU. From those, we studied the 318 patients requiring at least 5 days of intensive care to assess the impact of IIT within the time frame that was required to bring about clinical benefits (Table 1). In this selection of long-stay respiratory patients, 100% of the patients assigned to IIT received insulin during their ICU stay (mean daily insulin dose, 83.1 ± 4.9 IU (mean ± SEM)), whereas 86.2% of the patients assigned to CIT received insulin (mean daily insulin dose, 25.3 ± 3.0 IU). In this selection of long-stay respiratory patients, all baseline characteristics except age were comparable in the two treatment populations (Table 1). IIT significantly reduced maximal Sequential Organ Failure Assessment (SOFA) score (indicating reduced organ failure), length of ICU stay, and in-hospital mortality (Table 1); no difference was seen in the cause of death in the ICU. For comparison, we studied, after informed consent, 22 overnight-fasted healthy volunteers (mean ± SD; age, 69 ± 8 years; BMI, 26.8 ± 3.5; 15 males), and 22 matched, not critically ill patients who underwent elective abdominal surgery (mean ± SD; age, 69 ± 13 years; BMI, 25.1 ± 2.6; 14 males). Blood samples taken from patients under acute surgical stress were obtained at the end of the procedure, before skin closure. After sampling and centrifugation, serum was kept frozen at -80°C until analysis. The protocols for both control studies were approved by the Institutional Ethical Board.
Table 1.
Baseline and outcome characteristics of critically ill patients (ICU stay ≥5 days)
| CIT (n = 152) |
IIT (n = 166) |
P | |
|---|---|---|---|
| Sex (number [percentage] male) | 98 [64.5] | 107 [64.5] | >0.9 |
| Age (years; mean ± SD) | 69 ± 14 | 64 ± 15 | 0.008 |
| BMI (mean ± SD) | 24.2 ± 5.2 | 24.7 ± 5.1 | 0.5 |
| Adm. Apache II score (median [IQR]) | 23 [17.5–28.5] | 22 [17–28] | 0.4 |
| Adm. SOFA score (median [IQR]) | 7 [4–9] | 6 [4–8] | 0.3 |
| Kidney failure on admission (number [percentage]) | 23 [15.1] | 23 [13.9] | 0.7 |
| History of diabetes (number [percentage]) | 16 [10.5] | 23 [13.9] | 0.4 |
| Blood glucose on admission (mg/dl; mean ± SD) | 167 ± 68 | 165 ± 68 | 0.7 |
| Maximum SOFA score during ICU stay (mean ± SD) | 10.5 ± 4.5 | 9.7 ± 4.3 | 0.05 |
| Days in ICU (median [IQR]) | 14 [8–22] | 10 [16–17] | 0.008 |
| Death in ICU (number [percentage]) | 60 [39.5] | 50 [30.1] | 0.08 |
| In-hospital deaths (number [percentage]) | 90 [59.2] | 71 [42.8] | 0.003 |
| Newly acquired kidney injury (number [percentage]) | 23 [15.1] | 16 [9.6] | 0.1 |
Circulating adiponectin, RBP4, and leptin
Serum total adiponectin was measured with time-resolved immunofluorometric assay based on reagents from R&D Systems (Minneapolis, MN, USA). Serum RBP4 and serum leptin were determined with ELISA (Phoenix Pharmaceuticals, Burlingame, CA, USA) and RIA (Linco Research, Billerica, MA, USA), respectively. For all assays the intra- and interassay coefficients of variations (CVs) were less than 5% and 10%, respectively.
Statistical analysis
The results were compared with unpaired Student's t-tests and Mann-Whitney U tests. We used the χ2-test for comparison of proportions. The significance of correlations between parameters was assessed by the Pearson (R) correlation coefficient. Statistical significance was considered when two-sided P values were below or equal to 0.05. Stat View 5.0.1 was used.
Results
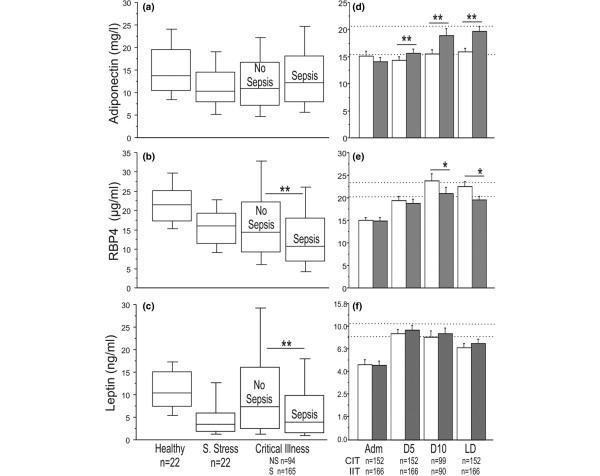
Circulating adiponectin, RBP4, and leptin levels in the critically ill patients were low on admission to ICU as compared with those in healthy subjects (Figure 1). For RBP4, 38%, and for leptin, 27% of the admission values were below the lower limit of the 95% confidence interval of the levels observed in healthy volunteers. Patients undergoing elective surgery demonstrated lower serum adiponectin, RBP4, and leptin than did healthy controls (Figure 1). For RBP4, 20%, and for leptin, 24% of the values were below the lower limit of the 95% confidence interval of the levels observed in healthy volunteers. Critically ill patients with sepsis on ICU admission had even lower circulating admission levels of RBP4 and leptin than did patients without sepsis (Figure 1). Baseline patient characteristics of septic versus nonseptic patients were not different, except for the admission SOFA score (Table 2).
Figure 1.
Circulating adipokines during critical illness. (a-c) Impact of acute illness: Results from healthy volunteers, patients not critically ill undergoing elective surgery, and critically ill patients on admission to the ICU are presented as box plots (boxes are medians and interquartile ranges; whiskers are 10th and 90th percentiles). (d-f) Critically ill patients who received CIT (white bars) or IIT (gray bars). Reference values (mean ± SEM) of healthy controls are indicated by two horizontal dotted lines. Adm = admission day; D5 = day 5; D10 = day 10; LD = the last day of ICU stay; ICU = intensive care unit. Data are presented as mean ± SEM. *P ≤ 0.05; **P ≤ 0.01. For statistical analysis, we subtracted corresponding admission-day values, and for leptin, we used log-transformed data as indicated on the figure.
Table 2.
Baseline and outcome characteristics of septic versus nonseptic critically ill patients (ICU stay ≥5 days)
| Proven sepsis (n = 165) | Proven no sepsis (n = 94) | P | |
|---|---|---|---|
| Sex (number [percentage] male]) | 111 [67.3] | 59 [62.8] | 0.5 |
| Age (years; mean ± SD) | 66.1 ± 12.3 | 67.0 ± 15.2 | 0.6 |
| BMI (mean ± SD) | 25.1 ± 5.1 | 24.1 ± 5.0 | 0.1 |
| Admission Apache II score (median [IQR]) | 23 [17–29] | 21 [17–28] | 0.6 |
| Admission SOFA score (median [IQR]) | 7 [4–9] | 4 [3–7] | < 0.0001 |
| Kidney failure on admission (number [percentage]) | 27 [16.3] | 12 [12.8] | 0.4 |
| History of diabetes (number [percentage]) | 20 [12.1] | 15 [16.0] | 0.4 |
| Blood glucose on admission (mg/dl; mean ± SD) | 164.5 ± 66.6 | 166.7 ± 70.3 | 0.8 |
| Maximum SOFA score during ICU stay (mean ± SD) | 9.4 ± 4.0 | 7.1 ± 3.7 | < 0.0001 |
| Days in ICU (median [IQR]) | 12 [7–20] | 12.5 [7–19] | 0.5 |
| Death in ICU (number [percentage]) | 54 [32.7] | 24 [25.5] | 0.2 |
| In-hospital deaths (number [percentage]) | 85 [51.5] | 41 [43.6] | 0.2 |
All subjects were screened for infection on admission and were defined retrospectively for this post hoc study as "proven sepsis" when infection was proven and the Bone criteria were fulfilled [29]. Patients proven not to have sepsis with this strict definition were classified as "proven no sepsis". Fifty-nine patients for whom treatment with antibiotics was started before admission to the ICU were categorized as unknown and therefore excluded from this subanalysis.
With time in ICU, circulating levels of all three adipokines increased, although leptin remained low in septic patients throughout their stay in the ICU (P = 0.007 for day 5; P = 0.06 for day 10; and P = 0.002 for the last day). Neither adiponectin, leptin, nor RBP4 correlated significantly with the cytokines IL-6, IL-8, IL-10, and TNF-α.
On all studied time points, adiponectin and leptin levels were significantly higher in female subjects than in male subjects (data not shown) and correlated with BMI (R = -0.194; P = 0.0002 for adiponectin; R = 0.456; P < 0.0001 for leptin). No correlation was present between any of the studied adipokines and age.
IIT further increased the serum levels of adiponectin, whereas it blunted the rise of serum RBP4, with no effect on serum leptin (Figure 1). Circulating RBP4 correlated positively with serum creatinine levels throughout ICU stay (admission R = 0.363; day 5 R = 0.406; day 10 R = 0.584; last-day R = 0.475; P < 0.0001 for all). Serum leptin levels correlated positively with the corresponding circulating insulin levels (admission R = 0.373; day 10 R = 0.330; last-day R = 0.292; P < 0.0001 for all).
Discussion
We observed low circulating levels of adiponectin, RBP4, and leptin in critically ill patients on admission to the ICU, with lowest values in patients with sepsis, but also in patients not critically ill under acute surgical stress. These observations would suggest an acute stress response. Low levels of circulating RBP4 and leptin were previously observed after burn injury or trauma or both [9,10], and low levels of adiponectin were reported in rats with sepsis [11]. It is unclear what mediates this acute lowering with stress. Theoretically, reduced synthesis or increased removal, or both, either by extravasation or by increased metabolic clearance, may play a role. The lower values in patients with sepsis would suggest the former, as this condition is characterized by capillary leakage, which could have removed the adipokines to the interstitial compartment. The low adipokine levels might in part be a consequence of the inactivity and malnourished status of the medical intensive care patients on admission to the ICU and of the overnight fasting of the patients undergoing elective surgery. Fasting reduces leptin levels [12], and severe calorie restriction with weight loss reduces circulating RBP4 [13]. However, an increase in circulating adiponectin levels would be expected [14], and thus, this cannot explain the changes in the ICU patients, particularly because the healthy volunteers were fasted overnight.
We found adiponectin and leptin levels to be higher in women than in men and correlating with the BMI. This gender difference has been related to the difference in sex steroids and to the higher ratio of subcutaneous to omental fat mass in women [15].
IIT targeted to normoglycemia further increased the rise of serum adiponectin with time in the ICU. This corresponds with the altered insulin sensitivity that has been associated with better glycemic control in patients with type 2 diabetes [16,17], but also in critically ill patients treated with IIT [2,7]. IIT blunted the rise in serum RBP4 that occurs with time in ICU. Decreasing levels of RBP4 were associated previously with altered insulin resistance [4,5]. Euglycemia with insulin therapy in severely burned children also decreased circulating RBP4 [18]. However, some contrasting results were reported regarding the association between circulating RBP4 and insulin resistance [19,20]. Furthermore, increased levels of circulating RBP4 have been described in patients with chronic renal failure and attributed to reduced glomerular filtration [21].
In our setting, IIT improved kidney function [8,22], and circulating RBP4 levels correlated with creatinine levels. Leptin levels are known to correlate with reduced insulin sensitivity, and insulin infusion can induce leptin secretion [6,23]. However, IIT failed to change leptin levels throughout the ICU stay. This can be explained by the observation that, although several-fold higher insulin doses were required to maintain normoglycemia with IIT, serum insulin levels were equal between the two therapy groups [24] and correlated well with leptin at all studied time points. Furthermore, it was reported that C-reactive protein, which reaches very high levels in critical illness, inhibits leptin binding to its receptor and blocks cellular signaling [25].
We must highlight two shortcomings in our study. As we studied only those patients with a respiratory disease as the main reason for admission to the ICU, extrapolation of our results to other diagnostic categories should be done with great caution. We studied adipokine levels only in prolonged critically ill patients, as our primary objective was to assess the role of the studied adipokines on the impact of IIT within the time frame that was required to bring about clinical benefits. Hence, an early effect may have been missed in this study. Earlier studies on leptin levels in the acute phase (first 24 hours) of sepsis describe a sepsis-induced elevation in leptin [26-28]. The elevation does not appear to be driven by the severity of illness, as survivors showed higher levels than did nonsurvivors [26-28]. These results and the observations that diet, insulin, BMI, gender, and cytokines may affect leptin levels make it clear that the role of leptin in critical illness remains incompletely understood.
Conclusions
Acute stress, caused by surgery or by critical illness of pulmonary origin, was associated with immediate but transiently low serum adiponectin, leptin, and RBP4 levels. Sepsis especially reduced RBP4 and leptin levels. IIT accentuated the rise of circulating adiponectin levels occurring with time in the ICU, blunted the rise of RBP4, and did not alter leptin levels. The effects of IIT on adiponectin and RBP4 are indicative of altered insulin sensitivy with IIT in the ICU patients.
Key messages
• Acute surgical or medical stress were associated with transiently lowered levels of the circulating adipokines adiponectin, RBP4, and leptin.
• Sepsis was associated with low circulating leptin levels throughout an intensive care stay.
• Intensive insulin therapy increased circulating adiponectin and blunted circulating RBP4, indicative of altered insulin sensitivity.
Abbreviations
CIT: conventional insulin therapy; IIT: intensive insulin therapy; RBP4: retinol-binding protein 4.
Competing interests
The authors declare that they have no competing interests.
Authors' contributions
LL, SVDP, JF, and AF contributed to acquisition of the data; LL, TKH, and GVDB participated in the design, coordination, and statistical analysis. LL and GVDB drafted the manuscript. All authors read and approved the final manuscript.
Contributor Information
Lies Langouche, Email: lies.langouche@med.kuleuven.be.
Sarah Vander Perre, Email: sarah.vanderperre@med.kuleuven.be.
Jan Frystyk, Email: jan@frystyk.dk.
Allan Flyvbjerg, Email: allan.flyvbjerg@dadlnet.dk.
Troels Krarup Hansen, Email: tkh@dadlnet.dk.
Greet Van den Berghe, Email: greet.vandenberghe@med.kuleuven.be.
Acknowledgements
We thank P. Wouters, I. Milants, H. Petersen, and E. Van Herck for excellent technical assistance. This work was supported by the Fund for Scientific Research Flanders, Belgium (F.W.O) and the Research Council of the Katholieke Universiteit Leuven. Lies Langouche is a Postdoctoral Fellow for the F.W.O. The original trial described in the M&M has been registered on ClinicalTrials.gov with the Identifier NCT00115479.
References
- Van Cromphaut SJ, Vanhorebeek I, Berghe G Van den. Glucose metabolism and insulin resistance in sepsis. Curr Pharm Des. 2008;14:1887–1899. doi: 10.2174/138161208784980563. [DOI] [PubMed] [Google Scholar]
- Okamoto Y, Kihara S, Funahashi T, Matsuzawa Y, Libby P. Adiponectin: a key adipocytokine in metabolic syndrome. Clin Sci (Lond) 2006;110:267–278. doi: 10.1042/CS20050182. [DOI] [PubMed] [Google Scholar]
- Wang C, Mao X, Wang L, Liu M, Wetzel MD, Guan KL, Dong LQ, Liu F. Adiponectin sensitizes insulin signaling by reducing p70 S6 kinase-mediated serine phosphorylation of IRS-1. J Biol Chem. 2007;282:7991–7996. doi: 10.1074/jbc.M700098200. [DOI] [PubMed] [Google Scholar]
- Graham TE, Yang Q, Bluher M, Hammarstedt A, Ciaraldi TP, Henry RR, Wason CJ, Oberbach A, Jansson PA, Smith U, Kahn BB. Retinol-binding protein 4 and insulin resistance in lean, obese, and diabetic subjects. N Engl J Med. 2006;354:2552–2563. doi: 10.1056/NEJMoa054862. [DOI] [PubMed] [Google Scholar]
- Yang Q, Graham TE, Mody N, Preitner F, Peroni OD, Zabolotny JM, Kotani K, Quadro L, Kahn BB. Serum retinol binding protein 4 contributes to insulin resistance in obesity and type 2 diabetes. Nature. 2005;436:356–362. doi: 10.1038/nature03711. [DOI] [PubMed] [Google Scholar]
- Kolaczynski JW, Nyce MR, Considine RV, Boden G, Nolan JJ, Henry R, Mudaliar SR, Olefsky J, Caro JF. Acute and chronic effects of insulin on leptin production in humans: studies in vivo and in vitro. Diabetes. 1996;45:699–701. doi: 10.2337/diabetes.45.5.699. [DOI] [PubMed] [Google Scholar]
- Langouche L, Perre S Vander, Wouters PJ, D'Hoore A, Hansen TK, Berghe G Van den. Effect of intensive insulin therapy on insulin sensitivity in the critically ill. J Clin Endocrinol Metab. 2007;92:3890–3897. doi: 10.1210/jc.2007-0813. [DOI] [PubMed] [Google Scholar]
- Berghe G Van den, Wilmer A, Hermans G, Meersseman W, Wouters PJ, Milants I, Van Wijngaerden E, Bobbaers H, Bouillon R. Intensive insulin therapy in the medical ICU. N Engl J Med. 2006;354:449–461. doi: 10.1056/NEJMoa052521. [DOI] [PubMed] [Google Scholar]
- Moody BJ. Changes in the serum concentrations of thyroxine-binding prealbumin and retinol-binding protein following burn injury. Clin Chim Acta. 1982;118:87–92. doi: 10.1016/0009-8981(82)90229-7. [DOI] [PubMed] [Google Scholar]
- Jeevanandam M, Begay CK, Petersen SR. Plasma leptin levels in trauma patients: effect of adjuvant recombinant human growth hormone in intravenously fed multiple trauma patients. JPEN J Parenter Enteral Nutr. 1998;22:340–346. doi: 10.1177/0148607198022006340. [DOI] [PubMed] [Google Scholar]
- Tsuchihashi H, Yamamoto H, Maeda K, Ugi S, Mori T, Shimizu T, Endo Y, Hanasawa K, Tani T. Circulating concentrations of adiponectin, an endogenous lipopolysaccharide neutralizing protein: decrease in rats with polymicrobial sepsis. J Surg Res. 2006;134:348–353. doi: 10.1016/j.jss.2006.01.001. [DOI] [PubMed] [Google Scholar]
- Boden G, Chen X, Mozzoli M, Ryan I. Effect of fasting on serum leptin in normal human subjects. J Clin Endocrinol Metab. 1996;81:3419–3423. doi: 10.1210/jc.81.9.3419. [DOI] [PubMed] [Google Scholar]
- Vitkova M, Klimcakova E, Kovacikova M, Valle C, Moro C, Polak J, Hanacek J, Capel F, Viguerie N, Richterova B, Bajzova M, Hejnova J, Stich V, Langin D. Plasma levels and adipose tissue messenger ribonucleic acid expression of retinol-binding protein 4 are reduced during calorie restriction in obese subjects but are not related to diet-induced changes in insulin sensitivity. J Clin Endocrinol Metab. 2007;92:2330–2335. doi: 10.1210/jc.2006-2668. [DOI] [PubMed] [Google Scholar]
- Yang WS, Lee WJ, Funahashi T, Tanaka S, Matsuzawa Y, Chao CL, Chen CL, Tai TY, Chuang LM. Weight reduction increases plasma levels of an adipose-derived anti-inflammatory protein, adiponectin. J Clin Endocrinol Metab. 2001;86:3815–3819. doi: 10.1210/jc.86.8.3815. [DOI] [PubMed] [Google Scholar]
- Wajchenberg BL. Subcutaneous and visceral adipose tissue: their relation to the metabolic syndrome. Endocr Rev. 2000;21:697–738. doi: 10.1210/er.21.6.697. [DOI] [PubMed] [Google Scholar]
- Schulze MB, Rimm EB, Shai I, Rifai N, Hu FB. Relationship between adiponectin and glycemic control, blood lipids, and inflammatory markers in men with type 2 diabetes. Diabetes Care. 2004;27:1680–1687. doi: 10.2337/diacare.27.7.1680. [DOI] [PubMed] [Google Scholar]
- Mantzoros CS, Li T, Manson JE, Meigs JB, Hu FB. Circulating adiponectin levels are associated with better glycemic control, more favorable lipid profile, and reduced inflammation in women with type 2 diabetes. J Clin Endocrinol Metab. 2005;90:4542–4548. doi: 10.1210/jc.2005-0372. [DOI] [PubMed] [Google Scholar]
- Wu X, Thomas SJ, Herndon DN, Sanford AP, Wolf SE. Insulin decreases hepatic acute phase protein levels in severely burned children. Surgery. 2004;135:196–202. doi: 10.1016/j.surg.2003.08.018. [DOI] [PubMed] [Google Scholar]
- Janke J, Engeli S, Boschmann M, Adams F, Bohnke J, Luft FC, Sharma AM, Jordan J. Retinol-binding protein 4 in human obesity. Diabetes. 2006;55:2805–2810. doi: 10.2337/db06-0616. [DOI] [PubMed] [Google Scholar]
- Promintzer M, Krebs M, Todoric J, Luger A, Bischof MG, Nowotny P, Wagner O, Esterbauer H, Anderwald C. Insulin resistance is unrelated to circulating retinol binding protein and protein C inhibitor. J Clin Endocrinol Metab. 2007;92:4306–4312. doi: 10.1210/jc.2006-2522. [DOI] [PubMed] [Google Scholar]
- Bernard AM, Vyskocil AA, Mahieu P, Lauwerys RR. Assessment of urinary retinol-binding protein as an index of proximal tubular injury. Clin Chem. 1987;33:775–779. [PubMed] [Google Scholar]
- Berghe G Van den, Wouters P, Weekers F, Verwaest C, Bruyninckx F, Schetz M, Vlasselaers D, Ferdinande P, Lauwers P, Bouillon R. Intensive insulin therapy in critically ill patients. N Engl J Med. 2001;345:1359–1367. doi: 10.1056/NEJMoa011300. [DOI] [PubMed] [Google Scholar]
- Carlson GL, Saeed M, Little RA, Irving MH. Serum leptin concentrations and their relation to metabolic abnormalities in human sepsis. Am J Physiol. 1999;276:E658–E662. doi: 10.1152/ajpendo.1999.276.4.E658. [DOI] [PubMed] [Google Scholar]
- Langouche L, Meersseman W, Perre S Vander, Milants I, Wouters PJ, Hermans G, Gjedsted J, Hansen TK, Arnout J, Wilmer A, Schetz M, Berghe G Van den. Effect of insulin therapy on coagulation and fibrinolysis in medical intensive care patients. Crit Care Med. 2008;36:1475–1480. doi: 10.1097/CCM.0b013e31816f7bae. [DOI] [PubMed] [Google Scholar]
- Chen K, Li F, Li J, Cai H, Strom S, Bisello A, Kelley DE, Friedman-Einat M, Skibinski GA, McCrory MA, Szalai AJ, Zhao AZ. Induction of leptin resistance through direct interaction of C-reactive protein with leptin. Nat Med. 2006;12:425–432. doi: 10.1038/nm1372. [DOI] [PubMed] [Google Scholar]
- Bornstein SR, Preas HL, Chrousos GP, Suffredini AF. Circulating leptin levels during acute experimental endotoxemia and antiinflammatory therapy in humans. J Infect Dis. 1998;178:887–890. doi: 10.1086/515349. [DOI] [PubMed] [Google Scholar]
- Tzanela M, Orfanos SE, Tsirantonaki M, Kotanidou A, Sotiropoulou C, Christophoraki M, Vassiliadi D, Thalassinos NC, Roussos C. Leptin alterations in the course of sepsis in humans. In Vivo. 2006;20:565–570. [PubMed] [Google Scholar]
- Arnalich F, Lopez J, Codoceo R, Jim M, Madero R, Montiel C. Relationship of plasma leptin to plasma cytokines and human survival in sepsis and septic shock. J Infect Dis. 1999;180:908–911. doi: 10.1086/314963. [DOI] [PubMed] [Google Scholar]
- Bone RC, Balk RA, Cerra FB, Dellinger RP, Fein AM, Knaus WA, Schein RM, Sibbald WJ. Definitions for sepsis and organ failure and guidelines for the use of innovative therapies in sepsis: the ACCP/SCCM Consensus Conference Committee: American College of Chest Physicians/Society of Critical Care Medicine. Chest. 1992;101:1644–1655. doi: 10.1378/chest.101.6.1644. [DOI] [PubMed] [Google Scholar]