Abstract
OBJECTIVE
Oxyntomodulin (OXM) is a glucagon-like peptide 1 (GLP-1) receptor (GLP1R)/glucagon receptor (GCGR) dual agonist peptide that reduces body weight in obese subjects through increased energy expenditure and decreased energy intake. The metabolic effects of OXM have been attributed primarily to GLP1R agonism. We examined whether a long acting GLP1R/GCGR dual agonist peptide exerts metabolic effects in diet-induced obese mice that are distinct from those obtained with a GLP1R-selective agonist.
RESEARCH DESIGN AND METHODS
We developed a protease-resistant dual GLP1R/GCGR agonist, DualAG, and a corresponding GLP1R-selective agonist, GLPAG, matched for GLP1R agonist potency and pharmacokinetics. The metabolic effects of these two peptides with respect to weight loss, caloric reduction, glucose control, and lipid lowering, were compared upon chronic dosing in diet-induced obese (DIO) mice. Acute studies in DIO mice revealed metabolic pathways that were modulated independent of weight loss. Studies in Glp1r−/− and Gcgr−/− mice enabled delineation of the contribution of GLP1R versus GCGR activation to the pharmacology of DualAG.
RESULTS
Peptide DualAG exhibits superior weight loss, lipid-lowering activity, and antihyperglycemic efficacy comparable to GLPAG. Improvements in plasma metabolic parameters including insulin, leptin, and adiponectin were more pronounced upon chronic treatment with DualAG than with GLPAG. Dual receptor agonism also increased fatty acid oxidation and reduced hepatic steatosis in DIO mice. The antiobesity effects of DualAG require activation of both GLP1R and GCGR.
CONCLUSIONS
Sustained GLP1R/GCGR dual agonism reverses obesity in DIO mice and is a novel therapeutic approach to the treatment of obesity.
Obesity is an important risk factor for type 2 diabetes, and ∼90% of patients with type 2 diabetes are overweight or obese (1). Among new therapies for type 2 diabetes, peptidyl mimetics of the gut-derived incretin hormone glucagon-like peptide 1 (GLP-1) stimulate insulin biosynthesis and secretion in a glucose-dependent manner (2,3) and cause modest weight loss in type 2 diabetic patients. The glucose-lowering and antiobesity effects of incretin-based therapies for type 2 diabetes have prompted evaluation of the therapeutic potential of other glucagon-family peptides, in particular oxyntomodulin (OXM). The OXM peptide is generated by post-translational processing of preproglucagon in the gut and is secreted postprandially from l-cells of the jejuno-ileum together with other preproglucagon-derived peptides including GLP-1 (4,5). In rodents, OXM reduces food intake and body weight, increases energy expenditure, and improves glucose metabolism (6–8). A 4-week clinical study in obese subjects demonstrated that repeated subcutaneous administration of OXM was well tolerated and caused significant weight loss with a concomitant reduction in food intake (9). An increase in activity-related energy expenditure was also noted in a separate study involving short-term treatment with the peptide (10).
OXM activates both, the GLP-1 receptor (GLP1R) and glucagon receptor (GCGR) in vitro, albeit with 10- to 100-fold reduced potency compared with the cognate ligands GLP-1 and glucagon, respectively (11–13). It has been proposed that OXM modulates glucose and energy homeostasis solely by GLP1R agonism, because its acute metabolic effects in rodents are abolished by coadministration of the GLP1R antagonist exendin(9–39) and are not observed in Glp1r−/− mice (7,8,14,15). Other aspects of OXM pharmacology, however, such as protective effects on murine islets and inhibition of gastric acid secretion appear to be independent of GLP1R signaling (14). In addition, pharmacological activation of GCGR by glucagon, a master regulator of fasting metabolism (16), decreases food intake in rodents and humans (17–19), suggesting a potential role for GCGR signaling in the pharmacology of OXM. Because both OXM and GLP-1 are labile in vivo (T1/2 ∼12 min and 2–3 min, respectively) (20,21) and are substrates for the cell surface protease dipeptidyl peptidase 4 (DPP-4) (22), we developed two long-acting DPP-4–resistant OXM analogs as pharmacological agents to better investigate the differential pharmacology and therapeutic potential of dual GLP1R/GCGR agonism versus GLP1R-selective agonism. Peptide DualAG exhibits in vitro GLP1R and GCGR agonist potency comparable to that of native OXM and is conjugated to cholesterol via a Cys sidechain at the C-terminus for improved pharmacokinetics. Peptide GLPAG differs from DualAG by only one residue (Gln3→Glu) and is an equipotent GLP1R agonist, but has no significant GCGR agonist or antagonist activity in vitro. The objective of this study was to leverage the matched GLP1R agonist potencies and pharmacokinetics of peptides DualAG and GLPAG in comparing the metabolic effects and therapeutic potential of a dual GLP1R/GCGR agonist with a GLP1R-selective agonist in a mouse model of obesity.
RESEARCH DESIGN AND METHODS
Experiments were performed in lean and diet-induced obese (DIO) C57BL/6 mice and in weight- and sex-matched Gcgr−/− (23), Glp1r−/− (24), or lean C57BL/6 control mice. All mice were obtained from Taconic Farms (Germantown, NY) and were maintained on either standard rat diet (Teklad 7012; Harlan Teklad) or high-fat diet (D12492: 60% kcal from fat; Research Diets) in a 12-h light/12-h dark cycle (light: 3:00 a.m. to 3:00 p.m.). Animal protocols used in these studies were approved by the Merck Research Laboratories Institutional Animal Care and Use Committee (Rahway, NJ).
Peptides.
The free thiol-containing peptide precursors of DualAG and GLPAG were synthesized by standard solid-phase peptide synthesis using Fmoc/t-Bu chemistry. Peptides were synthesized by reverse-phase HPLC using water/acetonitrile (0.1% trifluoroacetic acid) gradients. Cholesterol-peptide conjugates were synthesized by reaction of the thiol-containing peptide precursors with cholest-5-en-3-yl-1-bromo-2-oxo-6,9,12,15-tetraoxa-3-azaoctadecan-18-oate, which was previously assembled by standard solution chemistry. The peptide conjugates were purified by reverse-phase chromatography using water/acetonitrile (0.2% acetic acid) gradients. Purified peptides were characterized by electrospray MS on a Micromass LCZ platform spectrometer.
Determination of murine GLP1R and GCGR agonist potency.
In vitro agonist potency of peptides was determined in Chinese hamster ovary (CHO) cells stably expressing murine GLP1R or murine GCGR using the Cisbio cAMP Dynamic 2 assay. Peptides were diluted in assay buffer and incubated with cells in the presence of 20% mouse plasma. The assay was terminated with the addition of the Cisbio detection reagents as per the manufacturer's instructions. cAMP was detected by a decrease in time-resolved fluorescence energy transfer (TR-FRET) using an EnVision platereader (PerkinElmer).
Ex vivo liver glycogenolysis assay.
The ability of DualAG and GLPAG to stimulate glycogen breakdown was evaluated ex vivo in perfused livers harvested from C57BL/6 mice using a 13C-nuclear magnetic resonance (NMR)-based assay as previously described (25).
Measurement of plasma peptide exposures at the end of the chronic study.
DIO mice, 23 weeks old and maintained for 16 weeks on a high-fat diet, were anesthetized with isofluorane, and blood was collected by cardiocentesis into EDTA-coated microtainer tubes containing DPP-4 inhibitor and aprotinin. The in vitro cell-based cAMP bio-assay for determining GLP1R agonist potency was used with CHO cells stably transfected with human GLP1R to determine peptide concentrations by comparing the degree of cAMP accumulation in plasma samples from treated animals against a cAMP standard curve generated by spiking peptide standards into mouse plasma.
Single-dose studies in DIO mice.
Weight- (∼45 g) and age-matched DIO mice (23 weeks old) were subcutaneously injected at 9:00 a.m. with vehicle, DualAG, or GLPAG at a dose of 191 nmol/kg. Food was removed, and 6 h later (3:00 p.m., ∼Tmax for GLPAG and DualAG) the animals were killed for collection of plasma and tissue samples, which were immediately stored at −80°C.
RNA preparation and quantitative RT-PCR.
RNA was isolated from tissues using Ultraspec Total RNA Isolation Reagent (Biotecx Laboratories). The resulting total RNA was subjected to DNAse treatment using RNAse-freeDNAse (Qiagen). After reverse transcription of the RNA to generate cDNA, quantitative RT-PCR was performed with TaqMan PCR Reagent using the ABI Prism 7700 Sequence Detection System (Applied Biosystems). Taqman probes were purchased from Applied Biosystems: ChREBP (Mm00498811_m1), Cpt1a (Mm00550438_m1), Fgf21 (Mm00840165_g1), Ldlr (Mm00440169_m1), Pck1 (Mm00440636_m1), Pgc-1α (Mm00447183_m1). Expression was normalized to the copy number for β-actin (Actb).
Quantitation of malonyl-CoA and acetyl-CoA.
Mouse liver samples (∼100 mg) were homogenized in 1 ml of 10% sulfosalicylic acid, 10 mmol/l dithiothreitol. The samples were spun at 15,000 × g, and the supernatant was analyzed by LC/MS after 10-fold dilution using an Agilent 1100 series capillary pump interfaced to an LTQ ion trap mass spectrometer (Thermo Scientific). The assay was adapted from Minkler et al. (26).
Chronic dosing of DualAG and GLPAG in DIO mice.
Male DIO mice (23 weeks old, n = 8/dosing group, maintained for 16 weeks on a high-fat diet), were acclimated to nonspecific stress for 10 days before the onset of the chronic dosing study. DualAG (1.9 μmol/kg), GLPAG (1.9 μmol/kg), or vehicle (water) was injected subcutaneously every other day for 2 weeks. Body weight and food intake were measured daily. An intraperitoneal glucose tolerance test (IPGTT, 1.5 g/kg dextrose challenge) was performed on day 13 of the chronic study at 10:00 a.m. Whole body composition analysis of conscious mice was conducted before (day 0) and at the conclusion of the study by EchoMRI (Echo Medical Systems). Plasma samples for measurement of terminal plasma concentration of active GLPAG and DualAG were obtained 18 h after the last injection by cardiocentesis.
Food intake and body weight studies in Glp1r−/− and Gcgr−/− mice.
Single-caged weight-matched (∼30 g) wild-type (n = 24), Glp1r−/− (n = 24), and Gcgr−/− (n = 21) mice were injected daily (subcutaneously) with vehicle, DualAG (1.9 μmol/kg), or GLPAG (1.9 μmol/kg), 30 min before the onset of the dark cycle for 5 days. Food intake and body weight were recorded daily for the duration of the study.
Histology.
Liver histology was performed as described elsewhere (27).
Biochemical analyses.
Insulin and leptin levels in plasma were measured by ELISA (Linco/Millipore). Plasma free fatty acids and ketone bodies were measured using commercially available enzyme-coupled spectrophotometric assays (Wako Chemicals). Plasma triglyceride and total cholesterol were determined using an Olympus AU400e Bioanalyzer. Adiponectin was measured using a mouse adiponectin RIA kit (Linco/Millipore). Blood glucose levels were measured using a OneTouch glucometer (Ultra LifeScan).
Statistics.
All data are presented as means ± SE. Comparisons among groups were made using ANOVA or unpaired Student's t test, as appropriate. P < 0.05 was regarded as statistically significant.
RESULTS
Development of long-acting GLP1R/GCGR DualAG and GLP1R-selective agonist (GLPAG) peptides.
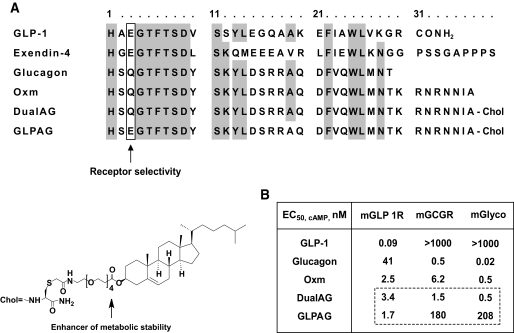
As summarized in Fig. 1A, glucagon and OXM, the only members of the glucagon superfamily that activate GCGR, incorporate a neutral polar residue at position three (glutamine, Q). In contrast, GLP-1 and the GLP-1 mimetic exendin-4 exhibit no significant GCGR agonist activity and incorporate an acidic residue at this position (glutamate, E), which decreases binding affinity to GCGR (28,29). Peptide DualAG is a long-acting analog of OXM with a Ser2→DSer substitution for resistance to DPP-4 cleavage. A Gln3→Glu substitution was introduced in DualAG to generate analog GLPAG. As illustrated in Fig. 1A, a cholesterol moiety was conjugated to the thiol side chain of a Cys residue appended to the C-terminus of each OXM analog to improve pharmacokinetics and enhance metabolic stability via plasma lipid and protein binding. The insertion of a short polyethylene glycol spacer between the cholesterol moiety and the peptides ameliorated a decrease in potency of the conjugates in the presence of plasma that occurs because of protein/lipid binding. A more complete description of the discovery and development of these peptides will be provided elsewhere (Bianchi et al., manuscript in preparation).
FIG. 1.
Sequence alignment and receptor agonist potencies of OXM and related peptides. A: Sequence alignment of OXM, exendin-4, and related glucagon superfamily peptides including the long acting OXM analogs DualAG and GLPAG. Conserved residues are highlighted. Gln3 is important for GCGR agonist activity. DualAG and GLPAG incorporate a DSer2 (S) substitution that confers resistance to DPP-4. The cholesterol moiety (chol) at the C-terminus of these peptides enhances metabolic stability and receptor affinity, and the intervening polyethylene glycol spacer minimizes the loss in agonist potency because of plasma protein/lipid binding. B: In vitro receptor agonist potencies (cAMP release) against mGLP1R and mGCGR and ED50 (nM) in the ex vivo mouse liver glycogenolysis assay (mGlyco). The Gln3→ Glu substitution in GLPAG reduces GCGR agonist activity ∼120- to 400-fold compared with DualAG.
Consistent with a previous report of DSer2-glucagon being resistant to DPP-4 cleavage (30), peptides DualAG and GLPAG exhibited no loss of in vitro potency after overnight incubation at 37°C with human recombinant soluble DPP-4 (31). Protease resistance of the peptides was further supported by pharmacokinetics data. The Tmax (time corresponding to peak plasma concentration postdose) for DualAG administered subcutaneously in lean C57Bl/6 mice at a dose of 3 mg/kg was 5 h, as determined using a bioassay for GLP1R agonism. The corresponding in vivo half-life (T1/2) for circulating active peptide was 1.7 h (compared with T1/2 ∼8–12 min for native OXM) (32). Peptide GLP1AG exhibited comparable in vivo stability to DualAG as reflected by comparable plasma concentrations of bioactive peptides measured at 24 h postdose in mice (Fig. 2B, inset).
FIG. 2.
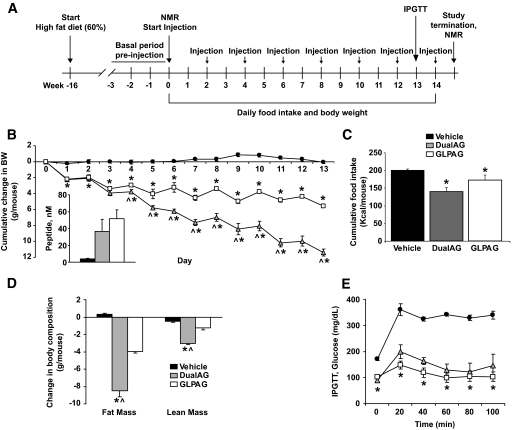
Superior efficacy of a long-acting dual GLP1/GCGR agonist in reducing body weight in DIO mice. A: Study protocol for chronic dosing of animals with vehicle or peptides DualAG and GLPAG (1.9 μmol/kg subcutaneously, every other day). B: Cumulative changes in body weight. Plasma exposures of each peptide measured at the end of the study were comparable (inset). C: Cumulative food intake and (D) body composition changes for each treatment group. E: IPGTT conducted on day 13 of the study. *P < 0.05, DualAG and GLPAG versus vehicle; ∧P < 0.05, DualAG versus GLPAG.
As summarized in Fig. 1B, DualAG is a full agonist of both, mGLP1R (EC50, cAMP = 3.4 nmol/l) and mGCGR (EC50, cAMP = 1.5 nmol/l) and is comparable in potency to OXM in vitro, using a cell-based assay that measures cAMP accumulation in CHO cells stably transfected with the respective recombinant murine receptor. GLPAG is an equipotent mGLP1R agonist (EC50, cAMP = 1.7 nmol/l), with at least 100-fold reduced mGCGR agonist potency compared with DualAG. The GCGR receptor selectivity of these long-acting peptides was further confirmed using an ex vivo assay that monitors glycogenesis and glycogenolysis in a perfused mouse liver (25). Briefly, a 13C-NMR visible pool of glycogen was created by perfusion of the gluconeogenic substrate [2-13C] pyruvate and liver glycogen was monitored in real time via the C1 resonance of the glucosyl units in the glycogen chain. DualAG or GLPAG was subsequently infused, and loss of label from glycogen (glycogenolysis) was monitored by 13C-NMR to assess GCGR activation (supplementary Fig. 1A and B, available in an online appendix at http://diabetes.diabetesjournals.org/cgi/content/full/db09-0278/DC1). Peptide DualAG induced full glycogenolysis at a perfusate concentration of ∼1 nmol/l (EC50, glyco ∼0.5 nmol/l), which compares favorably with its in vitro potency against mGCGR. In contrast, a much higher peptide perfusate concentration (∼300 nmol/l) was required for maximal stimulation of glycogenolysis by GLPAG (EC50, glyco ∼208 nmol/l), consistent with >100-fold reduced agonist potency against mGCGR. Peptides DualAG and GLPAG showed no antagonist activity at GCGR and were inactive at other receptors of the glucagon-secretin family (PAC1, VPAC1, VPAC2, and GIPR, EC50, cAMP >10 μmol/l).
DualAG reverses obesity and improves glucose metabolism in mice.
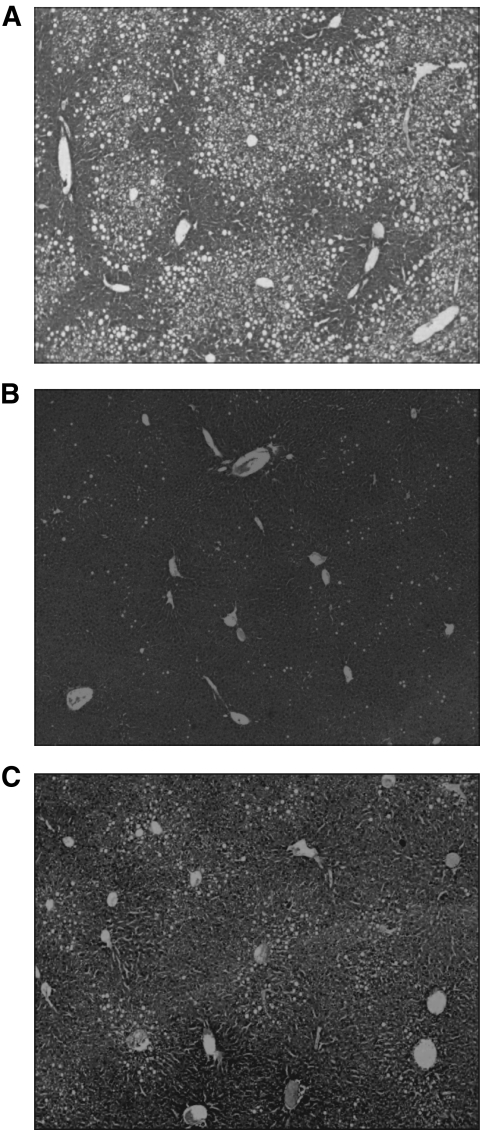
DIO mice were injected subcutaneously with DualAG or GLPAG (1.9 μmol/kg) in a 14-day chronic study (Fig. 2A). Although peak plasma peptide concentrations (Cmax) were achieved ∼5 h after subcutaneous injection and the plasma T1/2 suggests daily dosing as optimal, both peptides were injected every other day to minimize the stress caused by frequent injections. DualAG exhibited superior weight loss efficacy compared with GLPAG at day 14 (25 and 12% decrease in body weight, respectively, relative to vehicle-treated mice, Fig. 2B). As noted earlier, plasma peptide exposures measured at 24 h after the last injection were similar for DualAG and GLPAG (Fig. 2B, inset), confirming the matched pharmacokinetics of the two peptides. As depicted in Fig. 2C, cumulative food intake was reduced to a greater extent with the DualAG than with GLPAG (30 and 12% reduction, respectively, relative to vehicle). Body composition analysis confirmed that the decrease in body weight was primarily accounted for by a proportional decrease in fat mass (Fig. 2D). An IPGTT performed on day 13 (20 h after the last injection) revealed that glucose tolerance was significantly and comparably improved in both treatment groups (Fig. 2E). Furthermore, basal blood glucose levels were normalized by chronic treatment with either peptide (Fig. 2E, t = 0 measurement predextrose challenge in the IPGTT). Several other metabolic parameters in plasma were also improved by chronic treatment with the peptides (Table 1). Increases in adiponectin and decreases in leptin and insulin levels correlated with the decreased adiposity observed at the end of the study in each treatment group. Reduced cholesterol and triglyceride levels, increased ketone bodies, and decreased hepatic steatosis (Fig. 3) relative to vehicle treatment were also noted, especially for animals treated with DualAG.
TABLE 1.
Chronic treatment of DIO mice with DualAG and GLPAG: plasma parameters measured at the end of the 14-day study
| Vehicle | DualAG |
GLPAG |
|
|---|---|---|---|
| 1.9 μmol · kg−1 · day−1 | 1.9 μmol · kg−1 · day−1 | ||
| N | 6 | 7 | 7 |
| Insulin (ng/ml) | 13.2 ± 0.7 | 4.0 ± 0.2*† | 7.8 ± 1.1* |
| Leptin (ng/ml) | 32 ± 4 | 14 ± 1*† | 19 ± 1* |
| Adiponectin (μg/ml) | 15 ± 1 | 28 ± 2*† | 20 ± 1* |
| Fatty free acids (mM) | 0.2 ± 0.0 | 0.4 ± 0.1 | 0.3 ± 0.0 |
| Cholesterol | 153 ± 6 | 76 ± 7*† | 107 ± 5* |
| Triglycerides (mg/dl) | 68 ± 8 | 44 ± 5* | 47 ± 6* |
| β-hydroxybutyrate (mg/dl) | 4.1 ± 0.3 | 9.3 ± 0.9* | 7.2 ± 0.4* |
Data are means ± SE.
*P < 0.05 vs. vehicle;
†P < 0.05 DualAG vs. GLPAG.
FIG. 3.
Histological analysis (photomicrographs) of lipid accumulation in liver obtained from DIO mice treated chronically with DualAG and GLPAG. Hematoxylin-eosin stain of hepatic histological sections obtained from animals treated with vehicle (A), DualAG (B), and GLPAG (C). Magnification ×300.
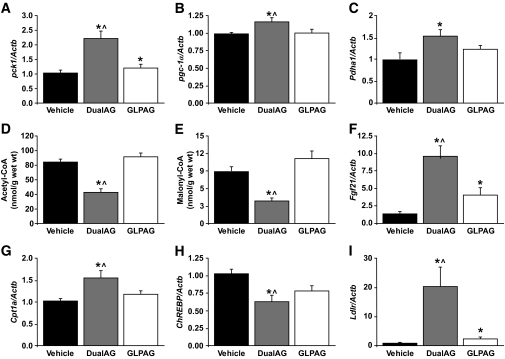
To identify metabolic pathways acutely altered by DualAG and GLPAG before any significant weight loss, age- and weight-matched DIO mice were injected with vehicle, DualAG, or GLPAG (191 nmol/kg subcutaneously each peptide) and killed 6 h later to collect plasma for metabolites and liver tissue samples for gene expression analysis. Despite the acute treatment, decreased plasma triglycerides and increased ketone bodies were detected albeit only in animals treated with the dual agonist (supplementary Fig. 2). Increased expression of the gluconeogenic genes Pck1, Pgc1α, and Pdha1 was observed with DualAG treatment, but not with GLPAG or vehicle (Fig. 4A–C). Liver pools of acetyl-CoA, the main product of pyruvate decarboxylation, and malonyl-CoA were decreased by DualAG (Fig. 4D and E). In addition, DualAG caused a significant upregulation of genes that induce fatty acid oxidation (FAO) in the liver, including Fgf21 and Cpt1a (Fig. 4F and G), and the downregulation of lipogenic genes such as ChREBP (Fig. 4H). A robust (∼15-fold) upregulation of Ldlr by DualAG (Fig. 4I) was also noted in the context of reduced cholesterol levels previously observed with chronic treatment (Table 1).
FIG. 4.
Gene expression and metabolite changes measured in liver tissue obtained from DIO mice treated with vehicle, DualAG, or GLPAG. A–C: Pck1 (A), Pgc-1α (B), and Pdha1 (C) mRNA. D and E: Acetyl-CoA (D) and malonyl-CoA (E) levels. F–I: Fgf21 (F), Cpt1a (G), ChREBP (H), and Ldlr mRNA (I). *P < 0.05 DualAG and GLPAG versus vehicle; ∧P < 0.05 DualAG versus GLPAG.
Metabolic effects of DualAG and GLPAG in Glp1r−/− and Gcgr−/− mice.
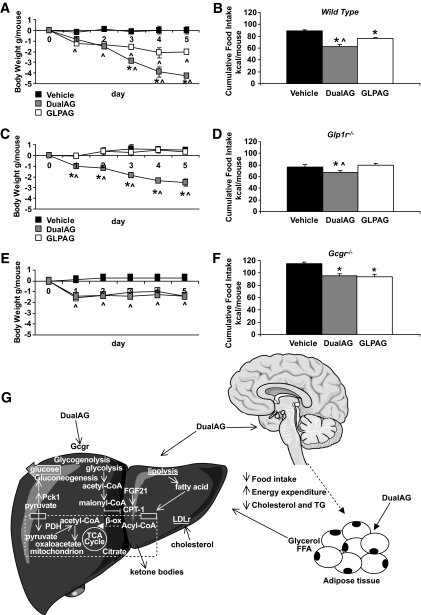
To provide mechanistic insight into the relative contributions of each target receptor to the metabolic effects of the long-acting peptides, we compared the effect of repeat administrations of DualAG and GLPAG (1.9 μmol/kg subcutaneously) on body weight and food intake in wild-type mice with that in animals lacking either GLP1R (Glp1r−/−) or GCGR (Gcgr−/−). Because both receptor knockout mouse lines are resistant to diet-induced obesity, weight-matched lean mice were used in the study. Both peptides significantly reduced cumulative food intake and body weight in wild-type mice. As observed previously, DualAG treatment effected superior body weight loss in wild-type mice compared with animals treated with GLPAG (Fig. 5). In mice lacking either GLP1R or GCGR, however, the efficacy of DualAG was sustained but partially attenuated compared with the weight loss achieved in wild-type mice. These results implicate both GLP1R and GCGR activation in the mechanism of action of DualAG. The weight loss efficacy of GLPAG in Gcgr−/− mice was comparable to the attenuated body weight effects of DualAG in these animals and was completely abolished in Glp1r−/− mice, confirming the GLP1R selectivity of this peptide.
FIG. 5.
DualAG lowers body weight and food intake via activation of GLP1R and GCGR. Effect of repeated injections of DualAG or GLPAG on cumulative food intake and body weight in wild-type (A and B), Glp1r−/− (C and D), and Gcgr−/− (E and F) mice. The antiobesity effects of DualAG are attenuated but not ablated in either receptor knockout mouse. G: Proposed mechanism of action of DualAG. In addition to the known effects associated with GLP1R activation, hepatic GCGR activation increases liver glucose production and stimulates FAO. The acetyl-CoA generated by β-oxidation challenges the processing capacity of the tricarboxylic acid (TCA) cycle and is used in the biosynthesis of ketone bodies. Consistent with the decrease in plasma cholesterol, animals treated with DualAG showed a robust upregulation of liver LDLr expression. In the adipose tissue, pharmacological activation of GCGR and GLP1R may stimulate hydrolysis of triglycerides (TG). Upregulation of liver Fgf21 in animals treated with DualAG may contribute to stimulation of FAO and ketogenesis. *P < 0.05 DualAG and GLPAG versus vehicle; ∧P < 0.05 DualAG versus GLPAG. FFA, free fatty acid.
DISCUSSION
Herein, we compare the antiobesity effects of a long-acting dual GLP-1/glucagon agonist (DualAG) with those of a long-acting GLP1R selective agonist (GLPAG) in a mouse model of obesity. To avoid confounding effects in these studies, a specific effort was made to match the pharmacokinetics, GLP1R agonist potencies, and plasma exposures of the two peptides during chronic dosing studies. Long-acting peptides DualAG and GLPAG lowered blood glucose, reduced food intake, and decreased body weight in DIO mice. We report for the first time that chronic treatment with a dual GLP1R/GCGR agonist compared with a GLP1R selective agonist causes superior weight loss and lipid lowering in DIO mice, without causing hyperglycemia. Instead, ambient glucose levels were normalized by both peptides, and glucose tolerance was comparably improved in both groups as measured in an IPGTT conducted at the end of the study. Of note, improvements in metabolic parameters such as plasma insulin, leptin, and adiponectin were typically more pronounced upon chronic treatment with DualAG than with GLPAG, consistent with the increased weight loss efficacy of the dual agonist.
To evaluate the contributions of GLP1R versus GCGR agonism to the observed pharmacology of the long-acting peptides, we evaluated the metabolic effects of repeat dosing with DualAG and GLPAG in Glp1r−/− and Gcgr−/− mice. Our studies clearly establish the importance of dual GLP1R/GCGR agonism in the increased weight loss efficacy of DualAG. Specifically, weight loss was observed with DualAG treatment in both Glp1r−/− and Gcgr−/− mice, although efficacy was reduced compared with body weight effects observed in weight-matched wild-type mice. In contrast, the metabolic effects of GLPAG were completely ablated in Glp1r−/− mice, confirming its GLP1R selective effects.
A unified hypothesis for the mechanism of action of peptide DualAG is illustrated in Fig. 5G. Under conditions of fasting metabolism, GCGR signaling pharmacologically accentuates the catabolic aspects of metabolism that favor weight loss. DualAG activates GCGR in the liver, rapidly upregulating key gluconeogenic genes. However, increased GLP1R signaling in the postprandial state is sufficient to improve glucose tolerance with a dual agonist peptide, as has been reported with native OXM in mice (6,14). Our studies also confirm the contribution of GLP1R agonism to the chronic antiobesity effect of DualAG, which is driven primarily by a reduction in adiposity. As illustrated in Fig. 5G, DualAG modulates metabolic pathways that decrease acetyl-CoA and malonyl-CoA pools in the liver, increase ketogenesis, and decrease plasma lipids. Hormone-sensitive lipase mRNA, which is downregulated in animal models of obesity (33) and in obese humans (34,35), is significantly upregulated in adipose tissue obtained from DIO mice treated with DualAG (data not shown). Although the role of glucagon in fat cell metabolism in humans is unclear, pharmacological activation of GCGR in adipose tissue may activate hormone-sensitive lipase, resulting in an increased free fatty acid pool available for β-oxidation (36). Our data suggest that FAO is acutely upregulated in rodents by GLP1R/GCGR dual agonism before any weight loss. The observed upregulation of liver Fgf21 by DualAG may also contribute to the action of a dual GLP1R/GCGR agonist because pharmacological levels of FGF21 stimulate hepatic FAO and increase energy expenditure (37,38).
The increased weight loss efficacy of DualAG compared with GLPAG is consistent with previous research on the pharmacology of glucagon. The hormone has been reported to decrease total cholesterol in rats and to cause a greater reduction in body weight compared with a pair-fed group of animals because of both reduced food intake and increased energy expenditure (19). Conversely, Langhans et al. (39) showed that intraperitoneal injections of antiglucagon antibodies in food-deprived rats increased meal size and duration. Furthermore, chronic administration of glucagon in humans has been reported to increase satiety and decrease hunger scores (17,18). Of note, however, GCGR-selective agonism is typically associated with the risk of hyperglycemia because elevation of endogenous glucagon levels and concomitant reduction in insulin levels/action are accepted as key players in the pathogenesis of diabetic hyperglycemia (40). According to this bihormonal hypothesis for diabetes, hyperglucagonemia results in excessive hepatic glucose production, which is not balanced by glucose utilization under conditions of hypoinsulinemia and insulin resistance. Indeed, the development of GCGR antagonists for the treatment of type 2 diabetes is being actively pursued (25,41–44) because these agents act toward restoring normal GCGR tone. Distinct from the demonstrated imbalance in endogenous hormonal action that characterizes the pathology of type 2 diabetes, however, we now report for the first time that concomitant activation of GLP1R in rodents mitigates the metabolic risks associated with GCGR activation while leveraging the beneficial pharmacological effects of activating each receptor, including enhanced weight loss efficacy, antihyperglycemic activity, and lipid-lowering effects. Our hypothesis is that pharmacological GLP1R agonism results in enhanced glucose-dependent insulin secretion, which enhances glucose disposal and provides sufficient anabolic tone to balance the glucoregulatory and catabolic effects of concomitant GCGR agonism. Hence, the GLP1R/GCGR dual agonist peptide DualAG mediates safe and effective weight loss and improves glucose tolerance in rodents without causing hyperglycemia or cachexia. Whether the observed rodent pharmacology is predictive of clinical effects with a long-acting dual agonist peptide remains to be determined, although the weight loss obtained with native OXM in overweight subjects is encouraging (9), given the rapid clearance of this peptide. In conclusion, we propose that long-acting GLP1R/GCGR dual agonists such as peptide DualAG represent novel pharmaceutical agents for the treatment of obesity.
Acknowledgments
No potential conflicts of interest relevant to this article were reported.
Parts of this study were presented in abstract form at the Keystone Symposia: Neuronal Control of Appetite, Metabolism and Weight, Banff, Alberta, Canada, 20–25 January 2009.
Footnotes
The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.Karnieli E: The growing prevalence of obesity worldwide is an increasing concern. Preface. Endocrinol Metab Clin North Am 2008;37:xvii–xviii [DOI] [PubMed] [Google Scholar]
- 2.Drucker DJ, Buse JB, Taylor K, Kendall DM, Trautmann M, Zhuang D, Porter L: Exenatide once weekly versus twice daily for the treatment of type 2 diabetes: a randomised, open-label, non-inferiority study. Lancet 2008;372:1240–1250 [DOI] [PubMed] [Google Scholar]
- 3.Knop FK, Vilsboll T, Larsen S, Madsbad S, Holst JJ, Krarup T: No hypoglycemia after subcutaneous administration of glucagon-like peptide-1 in lean type 2 diabetic patients and in patients with diabetes secondary to chronic pancreatitis. Diabetes Care 2003;26:2581–2587 [DOI] [PubMed] [Google Scholar]
- 4.Drucker DJ: Biologic actions and therapeutic potential of the proglucagon-derived peptides. Nat Clin Pract Endocrinol Metab 2005;1:22–31 [DOI] [PubMed] [Google Scholar]
- 5.Bloom SR, Kuhajda FP, Laher I, Pi-Sunyer X, Ronnett GV, Tan TM, Weigle DS: The obesity epidemic: pharmacological challenges. Mol Interv 2008;8:82–98 [DOI] [PubMed] [Google Scholar]
- 6.Parlevliet ET, Heijboer AC, Schroder-van der Elst JP, Havekes LM, Romijn JA, Pijl H, Corssmit EP: Oxyntomodulin ameliorates glucose intolerance in mice fed a high-fat diet. Am J Physiol Endocrinol Metab 2008;294:E142–E147 [DOI] [PubMed] [Google Scholar]
- 7.Dakin CL, Gunn I, Small CJ, Edwards CM, Hay DL, Smith DM, Ghatei MA, Bloom SR: Oxyntomodulin inhibits food intake in the rat. Endocrinology 2001;142:4244–4250 [DOI] [PubMed] [Google Scholar]
- 8.Baggio LL, Huang Q, Brown TJ, Drucker DJ: Oxyntomodulin and glucagon-like peptide-1 differentially regulate murine food intake and energy expenditure. Gastroenterology 2004;127:546–558 [DOI] [PubMed] [Google Scholar]
- 9.Wynne K, Park AJ, Small CJ, Patterson M, Ellis SM, Murphy KG, Wren AM, Frost GS, Meeran K, Ghatei MA, Bloom SR: Subcutaneous oxyntomodulin reduces body weight in overweight and obese subjects: a double-blind, randomized, controlled trial. Diabetes 2005;54:2390–2395 [DOI] [PubMed] [Google Scholar]
- 10.Wynne K, Park AJ, Small CJ, Meeran K, Ghatei MA, Frost GS, Bloom SR: Oxyntomodulin increases energy expenditure in addition to decreasing energy intake in overweight and obese humans: a randomised controlled trial. Int J Obes (Lond) 2006;30:1729–1736 [DOI] [PubMed] [Google Scholar]
- 11.Holst JJ: Enteroglucagon. Annu Rev Physiol 1997;59:257–271 [DOI] [PubMed] [Google Scholar]
- 12.Baldissera FG, Holst JJ, Knuhtsen S, Hilsted L, Nielsen OV: Oxyntomodulin (glicentin-(33–69)): pharmacokinetics, binding to liver cell membranes, effects on isolated perfused pig pancreas, and secretion from isolated perfused lower small intestine of pigs. Regul Pept 1988;21:151–166 [DOI] [PubMed] [Google Scholar]
- 13.Gros L, Thorens B, Bataille D, Kervran A: Glucagon-like peptide-1-(7–36) amide, oxyntomodulin, and glucagon interact with a common receptor in a somatostatin-secreting cell line. Endocrinology 1993;133:631–638 [DOI] [PubMed] [Google Scholar]
- 14.Maida A, Lovshin JA, Baggio LL, Drucker DJ: The glucagon-like peptide-1 receptor agonist oxyntomodulin enhances β-cell function but does not inhibit gastric emptying in mice. Endocrinology 2008;149:5670–5678 [DOI] [PubMed] [Google Scholar]
- 15.Sowden GL, Drucker DJ, Weinshenker D, Swoap SJ: Oxyntomodulin increases intrinsic heart rate in mice independent of the glucagon-like peptide-1 receptor. Am J Physiol Regul Integr Comp Physiol 2007;292:R962–R970 [DOI] [PubMed] [Google Scholar]
- 16.Longuet C, Sinclair EM, Maida A, Baggio LL, Maziarz M, Charron MJ, Drucker DJ: The glucagon receptor is required for the adaptive metabolic response to fasting. Cell Metabolism 2008;8:359–371 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schulman JL, Carleton JL, Whitney G, Whitehorn JC: Effect of glucagon on food intake and body weight in man. J Appl Physiol 1957;11:419–421 [DOI] [PubMed] [Google Scholar]
- 18.Penick SB, Hinkle LE, Jr: Depression of food intake induced in healthy subjects by glucagon. N Engl J Med 1961;264:893–897 [DOI] [PubMed] [Google Scholar]
- 19.Salter JM: Metabolic effects of glucagon in the Wistar rat. Am J Clin Nutr 1960;8:535–539 [Google Scholar]
- 20.Orskov C, Wettergren A, Holst JJ: Biological effects and metabolic rates of glucagon like peptide-1 7–36 amide and glucagon like peptide-1 7–37 in healthy subjects are indistinguishable. Diabetes 1993;42:658–661 [DOI] [PubMed] [Google Scholar]
- 21.Kervran A, Dubrasquet M, Blache P, Martinez J, Bataille D: Metabolic clearance rates of oxyntomodulin and glucagon in the rat: contribution of the kidney. Regul Pept 1990;31:41–52 [DOI] [PubMed] [Google Scholar]
- 22.Zhu L, Tamvakopoulos C, Xie D, Dragovic J, Shen X, Fenyk-Melody JE, Schmidt K, Bagchi A, Griffin PR, Thornberry NA, Sinha Roy R: The role of dipeptidyl peptidase IV in the cleavage of glucagon family peptides: in vivo metabolism of pituitary adenylate cyclase activating polypeptide-(1–38). J Biol Chem 2003;278:22418–22423 [DOI] [PubMed] [Google Scholar]
- 23.Gelling RW, Du XQ, Dichmann DS, Romer J, Huang H, Cui L, Obici S, Tang B, Holst JJ, Fledelius C, Johansen PB, Rossetti L, Jelicks LA, Serup P, Nishimura E, Charron MJ: Lower blood glucose, hyperglucagonemia, and pancreatic α-cell hyperplasia in glucagon receptor knockout mice. Proc Natl Acad Sci U S A 2003;100:1438–1443 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Scrocchi LA, Brown TJ, MaClusky N, Brubaker PL, Auerbach AB, Joyner AL, Drucker DJ: Glucose intolerance but normal satiety in mice with a null mutation in the glucagon-like peptide 1 receptor gene. Nat Med 1996;2:1254–1258 [DOI] [PubMed] [Google Scholar]
- 25.Qureshi SA, Candelore MR, Xie D, Yang X, Tota LM, Ding VD-H, Li Z, Bansal A, Miller C, Cohen SM, Jiang G, Brady E, Saperstein R, Duffy JL, Tata JR, Chapman KT, Moller DE, Zhang BB: A novel glucagon receptor antagonist inhibits glucagon-mediated biological effects. Diabetes 2004;53:3267–3273 [DOI] [PubMed] [Google Scholar]
- 26.Minkler PE, Kerner J, Kasumov T, Parland W, Hoppel CL: Quantification of malonyl-coenzyme A in tissue specimens by high-performance liquid chromatography/mass spectrometry. Anal Biochem 2006;352:24–32 [DOI] [PubMed] [Google Scholar]
- 27.Jiang G, Li Z, Liu F, Ellsworth K, Dallas-Yang Q, Wu M, Ronan J, Esau C, Murphy C, Szalkowski D, Bergeron R, Doebber T, Zhang BB: Prevention of obesity in mice by antisense oligonucleotide inhibitors of stearoyl-CoA desaturase-1. J Clin Invest 2005;115:1030–1038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Runge S, Wulff BS, Madsen K, Brauner-Osborne H, Knudsen LB: Different domains of the glucagon and glucagon-like peptide-1 receptors provide the critical determinants of ligand selectivity. Br J Pharmacol 2003;138:787–794 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Runge S, Gram C, Brauner-Osborne H, Madsen K, Knudsen LB, Wulff BS: Three distinct epitopes on the extracellular face of the glucagon receptor determine specificity for the glucagon amino terminus. J Biol Chem 2003;278:28005–28010 [DOI] [PubMed] [Google Scholar]
- 30.Hinke SA, Pospisilik JA, Demuth HU, Mannhart S, Kuhn-Wache K, Hoffmann T, Nishimura E, Pederson RA, McIntosh CH: Dipeptidyl peptidase IV (DPIV/CD26) degradation of glucagon. Characterization of glucagon degradation products and DPIV-resistant analogs. J Biol Chem 2000;275:3827–3834 [DOI] [PubMed] [Google Scholar]
- 31.Leiting B, Pryor KD, Wu JK, Marsilio F, Patel RA, Craik CS, Ellman JA, Cummings RT, Thornberry NA: Catalytic properties and inhibition of proline-specific dipeptidyl peptidases II, IV and VII. Biochem J 2003;371:525–532 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Schjoldager BT, Baldissera FG, Mortensen PE, Holst JJ, Christiansen J: Oxyntomodulin: a potential hormone from the distal gut. Pharmacokinetics and effects on gastric acid and insulin secretion in man. Eur J Clin Invest 1988;18:499–503 [DOI] [PubMed] [Google Scholar]
- 33.Zhang W, Della-Fera MA, Hartzell DL, Hausman D, Baile CA: Adipose tissue gene expression profiles in ob/ob mice treated with leptin. Life Sci 2008;83:35–42 [DOI] [PubMed] [Google Scholar]
- 34.Berndt J, Kralisch S, Kloting N, Ruschke K, Kern M, Fasshauer M, Schon MR, Stumvoll M, Bluher M: Adipose triglyceride lipase gene expression in human visceral obesity. Exp Clin Endocrinol Diabetes 2008;116:203–210 [DOI] [PubMed] [Google Scholar]
- 35.Jocken JW, Langin D, Smit E, Saris WH, Valle C, Hul GB, Holm C, Arner P, Blaak EE: Adipose triglyceride lipase and hormone-sensitive lipase protein expression is decreased in the obese insulin-resistant state. J Clin Endocrinol Metab 2007;92:2292–2299 [DOI] [PubMed] [Google Scholar]
- 36.Langin D: Adipose tissue lipolysis as a metabolic pathway to define pharmacological strategies against obesity and the metabolic syndrome. Pharmacol Res 2006;53:482–491 [DOI] [PubMed] [Google Scholar]
- 37.Xu J, Lloyd DJ, Hale C, Stanislaus S, Chen M, Sivits G, Vonderfecht S, Hecht R, Li YS, Lindberg RA, Chen JL, Jung DY, Zhang Z, Ko HJ, Kim JK, Véniant MM: Fibroblast growth factor 21 reverses hepatic steatosis, increases energy expenditure, and improves insulin sensitivity in diet-induced obese mice. Diabetes 2009;58:250–259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kharitonenkov A, Shanafelt AB: Fibroblast growth factor-21 as a therapeutic agent for metabolic diseases. BioDrugs 2008;22:37–44 [DOI] [PubMed] [Google Scholar]
- 39.Langhans W, Zeiger U, Scharrer E, Geary N: Stimulation of feeding in rats by intraperitoneal injection of antibodies to glucagon. Science 1982;218:894–896 [DOI] [PubMed] [Google Scholar]
- 40.Unger RH, Orci L: The role of glucagon in diabetes. Compr Ther 1982;8:53–59 [PubMed] [Google Scholar]
- 41.Winzell MS, Brand CL, Wierup N, Sidelmann UG, Sundler F, Nishimura E, Ahren B: Glucagon receptor antagonism improves islet function in mice with insulin resistance induced by a high-fat diet. Diabetologia 2007;50:1453–1462 [DOI] [PubMed] [Google Scholar]
- 42.Sloop KW, Cao JX, Siesky AM, Zhang HY, Bodenmiller DM, Cox AL, Jacobs SJ, Moyers JS, Owens RA, Showalter AD, Brenner MB, Raap A, Gromada J, Berridge BR, Monteith DK, Porksen N, McKay RA, Monia BP, Bhanot S, Watts LM, Michael MD: Hepatic and glucagon-like peptide-1-mediated reversal of diabetes by glucagon receptor antisense oligonucleotide inhibitors. J Clin Invest 2004;113:1571–1581 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Liang Y, Osborne MC, Monia BP, Bhanot S, Gaarde WA, Reed C, She P, Jetton TL, Demarest KT: Reduction in glucagon receptor expression by an antisense oligonucleotide ameliorates diabetic syndrome in db/db mice. Diabetes 2004;53:410–417 [DOI] [PubMed] [Google Scholar]
- 44.Petersen KF, Sullivan JT: Effects of a novel glucagon receptor antagonist (Bay 27–9955) on glucagon-stimulated glucose production in humans. Diabetologia 2001;44:2018–2024 [DOI] [PubMed] [Google Scholar]