The successful demonstration that insulin-producing β-cells can be isolated (in the form of cell clusters called islets containing β and other endocrine and nonendocrine cells) from a recently deceased donor's pancreas, then transplanted into subjects with type 1 diabetes, and thereby restore, at least temporarily, insulin-independent normoglycemia has firmly established the important “proof of concept.” Even so, worldwide efforts to advance the therapy for widespread applicability have served to focus attention on the hurdles yet to clear. This review will briefly describe the present state of the art and succinctly define the research problems being attacked along with some recent advances that demonstrate significant progress.
Since Paul Lacy's early rodent experiments in the 1960s established that pancreatic islets could be isolated from one animal and transplanted into a diabetic recipient to restore normoglycemia (1), investigators have pursued efforts to develop the therapy for clinical use. After years of development in various animal models and efforts to improve human islet isolation techniques (see [2–4] for reviews with a historical perspective), the first patient achieving short-term insulin independence was reported by the group at Washington University in St. Louis. That advance was based on new islet isolation technology utilizing islets pooled from several donors, intensive insulin treatment in the peritransplant period, and induction immunosuppression with antithymocyte globulin (ATG) to avoid glucocorticoid therapy (5). The development of new immunosuppressive drugs that allowed patients to remain off glucocorticoid therapy while awaiting subsequent islet infusions (because most recipients require islets from two or more donors) enabled the group in Edmonton to optimize the clinical islet transplantation procedure (6). The approach allowed the group to conclude that about 12,000 islet equivalents per recipient body weight (in kilograms) was required to restore insulin-independent normoglycemia (6) and sparked intense international interest and effort.
Current estimates are that ∼400 individuals have received allogeneic isolated islets since 1999 (7), with ∼40 centers actively engaged in further developing the therapy. The Edmonton case series remains the world's largest, and its data demonstrate that approximately two-thirds of the recipients enjoy insulin independence 1 year after receiving their final islet infusion (again, most recipients require islets from two or more donors). Unfortunately, islet function decreases over time such that by 5 years posttransplant, less than 10% remain insulin independent (8). On the other hand, the majority continues to display islet allograft function and, with it, decreased insulin requirements, less frequent hypoglycemia, and overall improved blood glucose control.
While other groups have reported incremental advances (i.e., predictable insulin independence using islets from a single donor, although typically from an ideal donor into a small recipient) (9), the Immune Tolerance Network multicenter trial results were as follows: less than half achieved insulin independence at 1 year and <15% remained insulin independent at 2 years (10). Further, recipients required (on average) islets from 2.1 donors, less than half of the pancreases donated for islet isolation yielded a product suitable for transplant, and for those recipients classified as insulin independent, blood glucose control was not normal for many using the American Diabetes Association criteria (10). Aside from the imperfect success, islet recipients experienced a small number of procedure-related complications (e.g., intraperitoneal bleeding, portal vein thrombosis, and gallbladder puncture).
Islets, though only small cell clusters, obey the same immunological laws that govern solid organ transplantation, i.e., allogeneic islets trigger immune-mediated rejection that must be controlled with immunosuppressive drugs, which are associated with an increased risk for declining kidney function, hyperlipidemia, infectious complications, and risk for malignancies. Also, if immunosuppression is stopped, recipients become immunologically sensitized against islet donor tissue antigens, and because islets from multiple donors are typically required, finding a suitable donor for subsequently required transplant therapy may prove difficult (e.g., should the patient develop kidney failure) (11,12). Although islets sharing HLA antigens with a recipient's previous kidney allotransplant may weaken the anti-islet donor immune response (13), repeatedly administering islet-associated alloantigens to recipients of previous allogeneic islets can jeopardize β-cell survival (14). Clearly, experience has identified problems to overcome (15), including the need to develop better assays for monitoring both anti-islet autoimmune and alloimmune responses (Table 1).
TABLE 1.
Immune correlates of type 1 diabetes and islet allograft function
| Immune marker | Correlate | Comment | References |
|---|---|---|---|
| Autoimmunity | |||
| Islet autoantibodies | Baseline prediction |
|
(27), (28), (92) |
|
(92), (95) | ||
|
(97) | ||
| Seroconversion |
|
(23) | |
|
(92), (95) | ||
| T-cell autoreactivity | Baseline prediction |
|
(23), (98) |
| Disease recurrence |
|
(22) | |
|
(13), (14) | ||
| Homeostatic expansion |
|
(25) | |
| Cytokines | γ-Interferon |
|
(94) |
| IL-10 |
|
(23) | |
| Alloreactivity | |||
| Alloantibodies | Baseline prediction |
|
(11) |
| Seroconversion |
|
(12), (95) | |
| Seroconversion after immunosuppression discontinued |
|
(11) | |
| T-cell alloreactivity | CTLp |
|
(22), (93) |
|
|||
|
(23) | ||
| Cytokines | γ-Interferon |
|
(23) |
| IL-10 |
|
(23), (96) | |
| Cytotoxic T-cell genes | |||
| Granzyme B | Loss of function |
|
(20), (95) |
| Perforin and Fas-L | Increased insulin needs |
|
(20), (95) |
Assays for immunological monitoring.
T-cell assays now can, with reasonable accuracy, identify anti–β-cell immune responses in individuals with type 1 diabetes compared with nondiabetic control subjects (16). The best validated assays measure T-cell proliferation in response to diabetes-related antigens. One such assay uses predefined diabetes-related peptide or protein antigens and includes exogenous interleukin (IL)-2 in the assay medium (17), whereas another group uses antigens eluted from gel-fractionated human islet cell proteins (18,19). Neither assay has been validated for its ability to monitor anti–β-cell immunoreactivity following an islet transplant, however.
Monitoring cellular-mediated immune reactivity using parameters like granzyme B, perforin, and Fas ligand can predict deteriorating islet allograft function. Indeed, studies correlating islet allograft recipients' C-peptide production with cytotoxic lymphocyte mRNA levels determined with real-time PCR have shown that the cytotoxic lymphocyte gene mRNA levels are increased 25–203 days before hyperglycemia and loss of insulin independence (20,21), with granzyme B most reliably indicating ongoing graft loss. Unfortunately, such transcriptional immune correlates do not specify whether the type of immune reaction against the islet allograft represents recurrent autoimmunity and/or alloimmunity. Experience from solid organ transplantation (kidney, liver, and heart) has taught that patient management is critically dependent on rapid and validated assays to monitor graft function and antigraft immune responses; yet at present, no such assays have been validated to monitor islet rejection.
Monitoring anti–β-cell autoimmunity.
Assays monitoring anti–β-cell autoimmunity after islet transplantation correlate with progressively deteriorating β-cell function, whereas the absence of both allo- and autoreactivity has been associated with successful islet allograft outcome (22). Further, assays that measure anti-islet cellular autoimmunity (performed before a patient receives an islet transplant) are associated with delayed insulin independence, less C-peptide production during the first year after transplantation, and more rapid return to insulin dependence (23). Notably, in this study islet allograft outcome did not appear to be influenced by either anti–β-cell autoantibody levels before or after islet implantation or assays measuring cellular alloreactivity. Recent HLA-A2insulin tetramer staining assays that focus on CD8+ (cytotoxic) T-cells (at least for the ∼50% of Caucasians carrying the HLA-A2 allele) have proved useful to detect insulin-specific T-cells correlating with recurrent autoimmunity and subsequent graft failure in islet transplant recipients (24). The importance of assays capable of monitoring the anti–β-cell immune response has recently been highlighted; the IL-2 receptor–blocking antibody strategy commonly used for induction immunotherapy before the islet allograft infusion has been associated with increased IL-7 and IL-15 serum concentrations and with the homeostatic proliferation of memory T-cells reactive against islet autoantigens, e.g., autoreactive GAD65-specific T-cell clones (25). Although recent studies enrolling kidney allograft recipients have found that anti–IL-2 receptor–based induction regimens are not as effective as ATG-based depletion strategies to prevent allograft rejection (26), it is not known whether such a depletion-based strategy would better protect allogeneic islets transplanted into a host with anti–β-cell autoimmunity. Clearly, such studies should be done.
Autoantibody titers and their relevance to graft function.
Although autoantibodies have proven most useful for predicting onset of type 1 diabetes, their predictive power in the islet transplantation setting is controversial. A correlation between increasing GAD65 and insulinoma-associated protein 2 (IA-2) autoantibody titers and graft loss as a result of recurrent autoimmunity has been reported in pancreas transplantation (27,28). With regard to islet transplantation, some have reported earlier islet graft failure in autoantibody-positive compared with autoantibody-negative recipients (29,30), whereas other investigators have found no such association (23,31). This may in part be attributed to different immune suppressive regimes and graft composition and transplantation procedures.
Alloimmune responses.
Most islet allograft recipients develop antidonor antibodies (11,12), typically after immunosuppressive medications are tapered due to either reduced islet allograft function or intolerable immunosuppressant agent toxicity, but islet allograft failure has also been correlated with increased alloantibody titers (32). Presence of specific antidonor alloantibodies should exclude patients from receiving islets from donors expressing the recognized HLA allodeterminants (i.e., those with a positive crossmatch) because they predict graft failure (11,23). Assays detecting recipient antidonor T-cell reactivity also correlate with graft failure in recipients of islet-alone allografts (22,23). Cytokine profiles also correlate with islet allograft fate (23) in that those skewed toward a regulatory phenotype were found in insulin-independent recipients, but not in insulin-requiring recipients. In particular, circulating IL-10 (a cytokine associated with regulatory T-cells) inversely correlated with proliferation in allo-mixed lymphocyte cultures and with alloreactive cytotoxic T-cell precursor frequency. These results imply that immune monitoring may provide surrogate markers to guide immunosuppressive agent dosing in the future.
Innate immune system effects on islet allograft survival.
As much as 50–60% of the transplanted islets may be lost in the early posttransplant period (33), thereby contributing to the need to transplant islets from multiple donors to achieve insulin independence. Islets express tissue factor (TF)—a 47 kDa transmembrane glycoprotein that initiates the extrinsic coagulation system and is pivotal for activation of the intrinsic pathway. Vascular injury exposes TF to soluble coagulation proteins and triggers clotting (34). In addition, TF binds to factor VIIa and thereby activates a number of intracellular signals that culminate in cell proliferation, diapedesis, and inflammation (35). The intravascular infusion of isolated islets results in TF-stimulated nonspecific inflammatory and coagulation pathways (36–40) promoting a so-called instant blood-mediated inflammatory reaction (IBMIR) that is detrimental to islet survival (41–44) and may delay islet revascularization and engraftment (45). IBMIR has been reported in pigs after intraportal islet transplantation (46) and in human islet allotransplantation (36,46,47). Administering a humanized anti–TF-specific monoclonal antibody (CNTO 859) (48) to nonhuman primate islet allograft recipients given a marginal islet mass significantly enhanced engraftment and function (49). The recent demonstration that potent inhibitors of inflammation, including α1-antitrypsin (50) and imatinib (a tyrosine kinase inhibitor) (51), can restore euglycemia in NOD mice with incipient diabetes further supports the critical importance of limiting innate inflammatory events in the early posttransplant period. The original Edmonton protocol has been modified in several ways; e.g., most centers now culture isolated islets to decrease tissue factor expression and administer anti-inflammatory tumor necrosis factor-α monoclonal antibody therapy peritransplant, recipients are now typically treated with heparin postislet infusion, and most teams now try to minimize sirolimus exposure. These and other changes may improve outcomes like those recently reported from the University of Minnesota (52), but objectively identifying which factor or group of factors that may have resulted in the improved outcome is still difficult due to the small number of subjects who are reported in such studies.
Safely targeting the anti–β-cell immune and inflammatory responses with either drug- or regulatory cell–based strategies has proved a major challenge. In addition to the toxicity associated with usual immune suppression discussed above, several agents appear to interfere with immune tolerance, and all drugs currently used clinically to prevent islet allograft loss adversely affect β-cell function and glycemia control (4). Specific issues with current regimens are as follows: sirolimus impairs engraftment (53), interferes with angiogenesis (54), induces insulin resistance (55), and inhibits β-cell replication (56), while it, as well as corticosteroids, tacrolimus (57), and mycofenolate mofetil (MMF), decreases insulin transcription and translation (rev. in 4). Lastly, a recent study suggests that MMF also inhibits β-cell neogenesis (58). The need for different strategies to prevent allograft rejection and/or recurrent islet autoimmunity is currently debated. In the most widely studied rodent models of type 1 diabetes (i.e., the NOD mouse and BB rat), immunosuppression that readily controls allograft rejection is unable to protect against recurrent autoimmunity. In contrast, after clinical pancreas transplantation both allo- and autoimmune responses are controlled by standard immunosuppression. The notion that autoimmunity in human type 1 diabetes can be controlled by a standard immunosuppression (e.g., low-dose cyclosporine A) is supported by clinical studies (59). Many novel immunotherapies are under development, yet most are directed at controlling alloimmune responses (60), whereas the anti–β-cell autoimmunity predating any therapeutic transplant efforts in subjects with type 1 diabetes may well pose particular impediments. For example, immunotherapies that prevent autoimmune diabetes in preclinical models have been less effective when tested in humans shortly following disease onset (61–63).
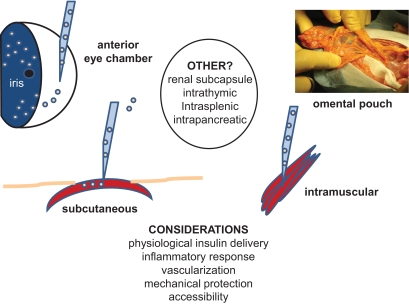
An additional consideration for enhancing outcomes in islet transplantation is identification of alternative implantation sites (Fig. 1). Infusing islets into the liver via the portal vein has been the site of choice for clinical islet transplantation and is the only site that has routinely demonstrated success in large animal models. The reasoning has been that the pancreas normally secretes insulin into the portal vein, intrahepatic islets avoid the systemic hyperinsulinemia observed in some pancreas allograft recipients, the portal blood is oxygenated (albeit at lower than arterial tensions) such that the isolated islets are exposed to oxygen until they can revascularize, and the portal vein can be accessed using a minimally invasive procedure. Disadvantages of the portal vein include the aforementioned IBMIR, higher levels of immune suppression in the portal circulation that may impair islet engraftment, vascularity, or function (4;53–58;60), periportal steatosis (64,65), and an inability to routinely biopsy the transplanted islets because they are dispersed within the liver. Isolated islets have been infused via the celiac artery into nonhuman primates, reasoning that the arterial tree could be more safely accessed and that intra-arterial islets might be more readily revascularized, but islet function was promptly lost (66). The highly vascular omental pouch of diabetic dogs has been successfully used as a site for autologous islet implants (67–69), and N.S.K. and colleagues (70) have demonstrated the feasibility of this site for allogeneic islet implant in cynomolgus monkeys. Experimental efforts testing the pancreas as an implantation site have also been reported in animal models (71). Recently, implanting a child's autologous islets into an intramuscular site has been reported to decrease her insulin requirements and help maintain euglycemia (72). Identification of scaffolds, gels, matrices, and devices that can enable exploitation of alternative sites is an active area of investigation.
FIG. 1.
Potential alternative islet implantation sites. Efforts directed to promote improved islet graft function and survival have led to studies testing alternative implantation sites, mostly in preclinical animal models, although the intramuscular route has shown some promise in the clinic (72). Recent pig model studies (73) have suggested promise for endoscopic transplant of the islets into the gastric submucosal space.
Cells capable of physiologically regulated insulin secretion.
Assuming techniques can be developed to safely protect insulin-producing cells once they are implanted into the diabetic individual, a widely applicable strategy will require a renewable β-like cell source. In the U.S. alone, over 22 million people have diabetes (type 1 diabetes or type 2 diabetes), and yet the country produces only about 8,000 organ donors each year. Because at present only approximately half of the isolation efforts yield islets suitable for transplant (10) and because recipients usually require islets from multiple donors (10), only ∼2,000 subjects in the U.S. could benefit from an islet transplant each year. Efforts to expand the pancreas donor pool (e.g., including non–heart-beating donors [74]) improve isolation techniques to more likely yield transplantable islets from each pancreas, and strategies to decrease a recipient's islet requirements, even when combined, will only marginally improve the current disparity between islet supply and potential recipients. Further, while recent promising efforts report the transplant of islets isolated from a living donor (75), other studies reporting long-term metabolic consequences for those donating half their pancreas considerably temper any optimism that living islet donors can fill the insulin-producing cell void (76).
Several groups are therefore pursuing strategies (Fig. 2) designed to use renewable sources for the insulin-producing cells; e.g., xenogeneic islets (predominantly from pigs), cells induced to differentiate from embryonic stem (ES) cells (or the related inducible pluripotent stem cells), or cells “reprogrammed” from their initial phenotype into β-like cells.
FIG. 2.
Recognizing the tremendous disparity between the islet number that can be isolated from cadaveric donors and the potential recipient population, many investigators are working to develop a renewable and cost-efficient source of islets or islet-like clusters. Each strategy has potential advantages but also unique problems to overcome.
Pig islets offer many advantages as a renewable islet source. Pigs have large litters, and the animals mature quickly; glucose set points for insulin release are similar in pigs and humans; pig insulin was used clinically for decades insuring its safety; and the widespread use of pigs for agricultural reasons minimizes animal rights concerns that may exist for other potential xenogeneic sources (15). Further, some investigators have transplanted isolated pig islets to diabetic nonhuman primates and thereby restored temporary near-normal glycemia to the immunosuppressed recipients (77,78). Factors limiting this xenogeneic islet source include particular species-specific difficulties associated with islet isolation and to-date only theoretical zoonotic infectious concerns; i.e., the species is known to harbor certain pig endogenous retroviruses (PERVs), and some have suggested that a large pig tissue inoculum, especially if placed in an immunosuppressed host, may support adaptation of the pig virus for human cells. More importantly, pig tissues express a cell surface moiety (galactose α1,3 galactose) against which humans have high-titer antibodies leading to accelerated and reinforced rejection. The latter problem is being attacked through the creation of genetically altered pigs (79,80).
Considerable excitement surrounds reports that human ES cells can be cultured in vitro under conditions that support differentiation into definitive pancreatic endoderm and even β-like cells, except that such in vitro–produced cells fail to secrete insulin in a glucose-regulated fashion (81). However, when definitive pancreatic endoderm is implanted into immunoincompetent mice, many of the cells differentiate into β-like cells that release insulin in response to glucose (82). Unfortunately, some of the implanted cells also display teratogenic potential, and it is not yet possible to select the desired cells from the undesired ones. Clearly, regulatory agencies such as the U.S. Food and Drug Administration would and should insist on strategies to overcome this shortfall. Lastly, unless ES cell lines can be established for all potential HLA haplotypes, the β-like cells produced from a particular ES line would face immune destruction from both antiallogeneic (unless the ES haplotype completely matched the recipient) and autoimmune processes. Recent progress with somatic cell nuclear transfer in the nonhuman primate (83) provides one potential solution for creating ES cells for any individual from a mature cell's nucleus taken from that individual, assuming moral/ethical issues can be worked out.
Another potential solution to overcome the alloimmune response has been offered by recent successes to create ES-like cells from fully differentiated somatic cells, so-called induced pluripotent stem (IPS) cells. This advance raises the possibility that each individual could serve as his or her own stem cell source to create new β-like cells. Unfortunately, at present the process of dedifferentiating such somatic cells requires transfection with potentially cell-transforming transcription factors like c-myc, and most strategies utilize viral vectors that integrate into the genome and thus further increase concerns that such cells may display malignant potential. Recent reports have shown that nonintegrating viral vectors can promote IPS cell generation, whereas others are conducting studies to avoid transcription factors altogether (84,85). By utilizing each individual's own cells to create new β-like cells, one anti–β-cell immune response (alloimmunity) is eliminated while another is quite possibly exacerbated (autoimmunity); i.e., multiple anti–β-cell T-cell clones exist in the individual with type 1 diabetes, and each T-cell recognizes major histocompatibility complex (MHC)-restricted β-cell antigenic peptides. For any given individual with type 1 diabetes, all such autoreactive T-cells would be able to recognize β-cells created from that same individual's IPS cells because the β-cells would express all the appropriate MHC restriction elements. Indeed, recurrent anti–β-cell–specific CD8 T-cell–mediated reactivity associated with loss of islet allograft function has been shown in cases where the donor shares HLA class 1 alleles with the recipient (86).
Lastly, transfecting rodent pancreatic acinar cells with an adenoviral vector mixture driving temporary expression of the transcription factors (Pdx-1, Ngn-3, and Maf A) appears to convert those mature pancreatic cells into β-like cells without an intermediate dedifferentiated state, so-called lineage reprogramming (87). Ongoing studies are exploring whether more readily accessible cells (e.g., cultured hepatocytes) might be similarly reprogrammed with these (or other) transcription factors. The facts that only transient vector-driven transcription factor expression is required to reprogram the cells and that the strategy avoids the dedifferentiated cell state may decrease the transformation potential, but recurrent autoimmune destruction would remain a problem.
DISCUSSION
Progress developing renewable cellular sources capable of physiologically regulated insulin secretion, assays for monitoring the immune response against those cells, and therapies to preserve those cells' function once transplanted have all converged to bring into clearer focus the long–dreamed of “finish line” (i.e., curing diabetes by correcting the afflicted individual's insulin deficiency). That said, prudence dictates that investigators begin planning for the end-game strategy to start clinically testing cell transplant–based strategies. The process will not be fast or trivial, and yet we argue that “fast-track” approaches should be considered with great caution, especially with regard to stem cell–based approaches. For instance, most would agree that the University of Pennsylvania's unfortunate gene therapy experience not only contributed to that study's first enrollee's premature death but that the entire gene therapy field was set back (88). We offer the following thoughts for forward progress.
In most developed countries, pancreas transplantation is the only accepted procedure to achieve normoglycemia. For pancreas transplantation, established techniques exist to procure the donated organ (or part of it) from both living and diseased donors and long-term graft function is similar to other whole-organ allografts. However, the pancreas transplant procedure is limited by additional risks related to the organ's exocrine enzyme production. While therapy using isolated human islets may be on the brink of becoming an accepted clinical therapy for a small type 1 diabetic subgroup with most severe hypoglycemia unawareness and while isolated islets enjoy an advantage over the intact pancreas in that the exocrine component is removed during the islet isolation process, those same isolation procedures impose ischemic and mechanical damages and thereby induce undesired cellular stress responses. Moreover, the injection of the cells into the blood stream is unique, and it is now generally accepted that only 10–20% of the islets transplanted survive the procedure and contribute to the recipient's metabolic control. And although some mechanisms underlying the substantial islet loss have been discussed, much remains unknown. Data obtained from rodent models suggest that these limitations can be overcome. Whether they can be successfully translated to larger animals and to humans is the focus of several ongoing studies.
Taking into consideration the enormous recent improvements in the type 1 diabetes treatment, the ultimate indications for islet transplantation could only be justified if there were almost no side effects related to the procedure and the immunosuppression/tolerance protocols applied. One could argue that there will be no need for xenogeneic or stem cell–derived β-like cells until robust immune tolerance or protection can be induced without severe side effects. The many forces conspiring to impair islet (or islet-like cell clusters) function or survival (Fig. 3) are all rich sources for study because most, if not all, will need be overcome.
FIG. 3.
Factors limiting islet graft function and survival. Present understanding is that transplanted islets (or islet-like clusters) face myriad overlapping forces all conspiring to limit graft function and/or survival. Effective strategies to develop islet transplantation for widespread clinical application will likely require effective countermeasures for each identified problem.
Testing therapies designed to restore anti–β-cell immune tolerance.
For therapies designed to generally weaken the anti–β-cell immune response or augment immunoregulatory processes, we point out that most therapies working in one T-cell–mediated disease process also generally work when applied to a different T-cell–mediated illness. Given that the prognosis for individuals with recent-onset type 1 diabetes is now outstanding (excess mortality of ∼0.1% per year [89]), the potential immunotherapy should have a safety profile known to not exceed that rate. For instance, the anti-VLA4 antibody (natalizumab) is estimated to carry with it 1 in 1,000 risk for progressive multifocal leukencephalopathy such that its use in subjects with recent-onset type 1 diabetes might be unwise. Even individuals with long-standing type 1 diabetes sufficiently severe to be listed for a solitary pancreas transplant have an annual mortality of 1–2% (90). Further, using islet transplantation as a model to test new immunomodulatory approaches is complicated by our present inability to reliably predict islet allograft rejection from either allo- or autoimmune processes and by our inability to reverse an early rejection episode. The current need for islets from two or more donors and the resulting allosensitization raise additional concerns. Lastly, many immunotherapeutic agents appear to directly influence β-cell function, vascularity, survival, and/or proliferative capacity (4;53–58;91). Consequently, one might argue that efforts directed to test therapies designed to promote immune tolerance should await the ability to promote long-term insulin independence to recipients receiving islets isolated from a single donor. In the meantime, novel immunomodulatory therapies could, in general, be first tested in another setting such as kidney transplantation where disease prognosis is worse, techniques for following the antigraft immune response exist, one donor's tissue suffices to restore the recipient's lost organ function, the immunological barrier can be determined (from the HLA mismatch score and by avoiding autoimmunity), and effective rescue therapies exist should the experimental immunotherapy fail. On the other hand, a transplanted kidney is a life-saving procedure, and some have questioned the ethics of testing new immunotherapies when effective ones exist. Further, acute rejection episodes are considered a surgical emergency and a potential threat for both the patient and the graft. Also, any immunomodulatory therapy will eventually need to be evaluated in individuals with autoimmune type 1 diabetes. These imponderable variables lead most to conclude that individual investigators should pursue specific protocol plans as guided by external peer review, their local institutional review board, and proper informed consent from the potential protocol participant.
Testing the cellular source for physiologically regulated insulin secretion.
In view of the potential adverse effects associated with xenogeneic cells (zoonotic infection) or cells engineered in vitro, most investigators agree that testing of the insulin-producing cells in a chronic large animal (ideally nonhuman primate) model should be performed if possible. One disadvantage of the nonhuman primate model is that anti–β-cell autoimmunity has not been reported in the species.
Lastly, as discussed throughout this review, because investigators are attempting to safely manipulate the immune system to prevent it from killing transplanted insulin-producing cells, it only makes sense for scientists to develop techniques to better measure the immune processes that affect the transplanted insulin-producing cells and to be able to quantify the insulin-producing cell mass in vivo. If successfully achieved, these techniques will become of immense importance not only for the development of replacement therapies for type 1 diabetes but also for early interventions (e.g., immune intervention for those at risk to prevent the disease or drugs to stimulate β-cell function and proliferation in those recently diagnosed) aiming to prevent clinical overt diabetes (type 1 or type 2).
Acknowledgments
D.M.H. is supported by the Intramural Research Program of the National Institute of Diabetes and Digestive and Kidney Diseases/National Institutes of Health of the Department of Health and Human Services.
No potential conflicts of interest relevant to this article were reported.
REFERENCES
- 1.Ballinger WF, Lacy PE: Transplantation of intact pancreatic islets in rats. Surgery 1972;72:175–186 [PubMed] [Google Scholar]
- 2.Pileggi A, Ricordi C, Kenyon NS, Froud T, Baidal DA, Kahn A, Selvaggi G, Alejandro R: Twenty years of clinical islet transplantation at the Diabetes Research Institute–University of Miami. Clin Transpl 2004;177–204 [PubMed] [Google Scholar]
- 3.Stock PG, Bluestone JA: Beta-cell replacement for type 1 diabetes. Annu Rev Med 2004;55:133–156 [DOI] [PubMed] [Google Scholar]
- 4.Robertson RP: Islet transplantation as a treatment for diabetes—a work in progress. N Engl J Med 2004;350:694–705 [DOI] [PubMed] [Google Scholar]
- 5.Scharp DW, Lacy PE, Santiago JV, McCullough CS, Weide LG, Falqui L, Marchetti P, Gingerich RL, Jaffe AS, Cryer PE: Insulin independence after islet transplantation into type 1 diabetic patient. Diabetes 1990;39:515–518 [DOI] [PubMed] [Google Scholar]
- 6.Shapiro AM, Lakey JR, Ryan EA, Korbutt GS, Toth E, Warnock GL, Kneteman NM, Rajotte RV: Islet transplantation in seven patients with type 1 diabetes mellitus using a glucocorticoid-free immunosuppressive regimen. N Engl J Med 2000;343:230–238 [DOI] [PubMed] [Google Scholar]
- 7.Alejandro R, Barton FB, Hering BJ, Wease S: 2008 Update from the Collaborative Islet Transplant Registry. Transplantation 2008;86:1783–1788 [DOI] [PubMed] [Google Scholar]
- 8.Ryan EA, Paty BW, Senior PA, Bigam D, Alfadhli E, Kneteman NM, Lakey JR, Shapiro AM: Five-year follow-up after clinical islet transplantation. Diabetes 2005;54:2060–2069 [DOI] [PubMed] [Google Scholar]
- 9.Hering BJ, Kandaswamy R, Ansite JD, Eckman PM, Nakano M, Sawada T, Matsumoto I, Ihm SH, Zhang HJ, Parkey J, Hunter DW, Sutherland DE: Single-donor, marginal-dose islet transplantation in patients with type 1 diabetes. JAMA 2005;293:830–835 [DOI] [PubMed] [Google Scholar]
- 10.Shapiro AM, Ricordi C, Hering BJ, Auchincloss H, Lindblad R, Robertson RP, Secchi A, Brendel MD, Berney T, Brennan DC, Cagliero E, Alejandro R, Ryan EA, DiMercurio B, Morel P, Polonsky KS, Reems JA, Bretzel RG, Bertuzzi F, Froud T, Kandaswamy R, Sutherland DE, Eisenbarth G, Segal M, Preiksaitis J, Korbutt GS, Barton FB, Viviano L, Seyfert-Margolis V, Bluestone J, Lakey JR: International trial of the Edmonton protocol for islet transplantation. N Engl J Med 2006;355:1318–1330 [DOI] [PubMed] [Google Scholar]
- 11.Campbell PM, Senior PA, Salam A, Labranche K, Bigam DL, Kneteman NM, Imes S, Halpin A, Ryan EA, Shapiro AM: High risk of sensitization after failed islet transplantation. Am J Transplant 2007;7:2311–2317 [DOI] [PubMed] [Google Scholar]
- 12.Cardani R, Pileggi A, Ricordi C, Gomez C, Baidal DA, Ponte GG, Mineo D, Faradji RN, Froud T, Ciancio G, Esquenazi V, Burke GW, 3rd, Selvaggi G, Miller J, Kenyon NS, Alejandro R: Allosensitization of islet allograft recipients. Transplantation 2007;84:1413–1427 [DOI] [PubMed] [Google Scholar]
- 13.Stobbe I, Duinkerken G, van Rood JJ, Lernmark A, Keymeulen B, Pipeleers D, De Vries RR, Glass FH, Roep BO: Tolerance to kidney allograft transplanted into type I diabetic patients persists after in vivo challenge with pancreatic islet allografts that express repeated mismatches. Diabetologia 1999;42:1379–1380 [DOI] [PubMed] [Google Scholar]
- 14.van Kampen CA, van de Linde P, Duinkerken G, van Schip JJ, Roelen DL, Keymeulen B, Pipeleers DG, Claas FH, Roep BO: Alloreactivity against repeated HLA mismatches of sequential islet grafts transplanted in non-uremic type 1 diabetes patients. Transplantation 2005;80:118–126 [DOI] [PubMed] [Google Scholar]
- 15.Rother KI, Harlan DM: Challenges facing islet transplantation for the treatment of type 1 diabetes mellitus. J Clin Invest 2004;114:877–883 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Seyfert-Margolis V, Gisler TD, Asare AL, Wang RS, Dosch HM, Brooks-Worrell B, Eisenbarth GS, Palmer JP, Greenbaum CJ, Gitelman SE, Nepom GT, Bluestone JA, Herold KC: Analysis of T-cell assays to measure autoimmune responses in subjects with type 1 diabetes: results of a blinded controlled study. Diabetes 2006;55:2588–2594 [DOI] [PubMed] [Google Scholar]
- 17.Dosch H, Cheung RK, Karges W, Pietropaolo M, Becker DJ: Persistent T cell anergy in human type 1 diabetes. J Immunol 1999;163:6933–6940 [PubMed] [Google Scholar]
- 18.Brooks-Worrell BM, Greenbaum CJ, Palmer JP, Pihoker C: Autoimmunity to islet proteins in children diagnosed with new-onset diabetes. J Clin Endocrinol Metab 2004;89:2222–2227 [DOI] [PubMed] [Google Scholar]
- 19.Brooks-Worrell BM, Starkebaum GA, Greenbaum C, Palmer JP: Peripheral blood mononuclear cells of insulin-dependent diabetic patients respond to multiple islet cell proteins. J Immunol 1996;157:5668–5674 [PubMed] [Google Scholar]
- 20.Han D, Xu X, Baidal D, Leith J, Ricordi C, Alejandro R, Kenyon NS: Assessment of cytotoxic lymphocyte gene expression in the peripheral blood of human islet allograft recipients: elevation precedes clinical evidence of rejection. Diabetes 2004;53:2281–2290 [DOI] [PubMed] [Google Scholar]
- 21.Froud T, Ricordi C, Baidal DA, Hafiz MM, Ponte G, Cure P, Pileggi A, Poggioli R, Ichii H, Khan A, Ferreira JV, Pugliese A, Esquenazi VV, Kenyon NS, Alejandro R: Islet transplantation in type 1 diabetes mellitus using cultured islets and steroid-free immunosuppression: Miami experience. Am J Transplant 2005;5:2037–2046 [DOI] [PubMed] [Google Scholar]
- 22.Roep BO, Stobbe I, Duinkerken G, van Rood JJ, Lernmark A, Keymeulen B, Pipeleers D, Claas FH, De Vries RR: Auto- and alloimmune reactivity to human islet allografts transplanted into type 1 diabetic patients. Diabetes 1999;48:484–490 [DOI] [PubMed] [Google Scholar]
- 23.Huurman VA, Hilbrands R, Pinkse GG, Gillard P, Duinkerken G, van de Linde P, van der Meer-Prins PM, Versteeg-van der Voort Maarschalk MF, Verbeeck K, Alizadeh BZ, Mathieu C, Gorus FK, Roelen DL, Claas FH, Keymeulen B, Pipeleers DG, Roep BO: Cellular islet autoimmunity associates with clinical outcome of islet cell transplantation. PLoS One 2008;3:e2435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pinkse GG, Boitard C, Tree TI, Peakman M, Roep BO: HLA class I epitope discovery in type 1 diabetes: independent and reproducible identification of proinsulin epitopes of CD8 T cells–report of the IDS T Cell Workshop Committee. Ann N Y Acad Sci 2006;1079:19–23 [DOI] [PubMed] [Google Scholar]
- 25.Monti P, Scirpoli M, Maffi P, Ghidoli N, De Taddeo F, Bertuzzi F, Piemonti L, Falcone M, Secchi A, Bonifacio E: Islet transplantation in patients with autoimmune diabetes induces homeostatic cytokines that expand autoreactive memory T cells. J Clin Invest 2008;118:1806–1814 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Brennan DC, Daller JA, Lake KD, Cibrik D, Del Castillo D: Rabbit antithymocyte globulin versus basiliximab in renal transplantation. N Engl J Med 2006;355:1967–1977 [DOI] [PubMed] [Google Scholar]
- 27.Braghi S, Bonifacio E, Secchi A, Di Carlo V, Pozza G, Bosi E: Modulation of humoral islet autoimmunity by pancreas allotransplantation influences allograft outcome in patients with type 1 diabetes. Diabetes 2000;49:218–224 [DOI] [PubMed] [Google Scholar]
- 28.Bosi E, Bottazzo GF, Secchi A, Pozza G, Shattock M, Saunders A, Gelet A, Touraine JL, Traeger J, Dubernard JM: Islet cell autoimmunity in type I diabetic patients after HLA-mismatched pancreas transplantation. Diabetes 1989;38(Suppl. 1):82–84 [DOI] [PubMed] [Google Scholar]
- 29.Jaeger C, Brendel MD, Hering BJ, Eckhard M, Bretzel RG: Progressive islet graft failure occurs significantly earlier in autoantibody-positive than in autoantibody-negative IDDM recipients of intrahepatic islet allografts. Diabetes 1997;46:1907–1910 [DOI] [PubMed] [Google Scholar]
- 30.Jaeger C, Brendel MD, Eckhard M, Bretzel RG: Islet autoantibodies as potential markers for disease recurrence in clinical islet transplantation. Exp Clin Endocrinol Diabetes 2000;108:328–333 [DOI] [PubMed] [Google Scholar]
- 31.Keymeulen B, Ling Z, Gorus FK, Delvaux G, Bouwens L, Grupping A, Hendrieckx C, Pipeleers-Marichal M, Van Schravendijk C, Salmela K, Pipeleers DG: Implantation of standardized beta-cell grafts in a liver segment of IDDM patients: graft and recipients characteristics in two cases of insulin-independence under maintenance immunosuppression for prior kidney graft. Diabetologia 1998;41:452–459 [DOI] [PubMed] [Google Scholar]
- 32.Olack BJ, Swanson CJ, Flavin KS, Phelan D, Brennan DC, White NH, Lacy PE, Scharp DW, Poindexter N, Mohanakumar T: Sensitization to HLA antigens in islet recipients with failing transplants. Transplant Proc 1997;29:2268–2269 [DOI] [PubMed] [Google Scholar]
- 33.Biarnes M, Montolio M, Nacher V, Raurell M, Soler J, Montanya E: β-Cell death and mass in syngeneically transplanted islets exposed to short- and long-term hyperglycemia. Diabetes 2002;51:66–72 [DOI] [PubMed] [Google Scholar]
- 34.Dahlback B: Blood coagulation. Lancet 2000;355:1627–1632 [DOI] [PubMed] [Google Scholar]
- 35.Versteeg HH, Peppelenbosch MP, Spek CA: The pleiotropic effects of tissue factor: a possible role for factor VIIa-induced intracellular signalling? Thromb Haemost 2001;86:1353–1359 [PubMed] [Google Scholar]
- 36.Moberg L, Johansson H, Lukinius A, Berne C, Foss A, Kallen R, Ostraat O, Salmela K, Tibell A, Tufveson G, Elgue G, Nilsson EK, Korsgren O, Nilsson B: Production of tissue factor by pancreatic islet cells as a trigger of detrimental thrombotic reactions in clinical islet transplantation. Lancet 2002;360:2039–2045 [DOI] [PubMed] [Google Scholar]
- 37.Ozmen L, Ekdahl KN, Elgue G, Larsson R, Korsgren O, Nilsson B: Inhibition of thrombin abrogates the instant blood-mediated inflammatory reaction triggered by isolated human islets: possible application of the thrombin inhibitor melagatran in clinical islet transplantation. Diabetes 2002;51:1779–1784 [DOI] [PubMed] [Google Scholar]
- 38.Nagata M, Mullen Y, Matsuo S, Herrera M, Clare-Salzler M: Destruction of islet isografts by severe nonspecific inflammation. Transplant Proc 1990;22:855–856 [PubMed] [Google Scholar]
- 39.Kaufman DB, Platt JL, Rabe FL, Dunn DL, Bach FH, Sutherland DE: Differential roles of Mac-1+ cells, and CD4+ and CD8+ T lymphocytes in primary nonfunction and classic rejection of islet allografts. J Exp Med 1990;172:291–302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kaufman DB, Gores PF, Field MJ, Farney AC, Gruber SA, Stephanian E, Sutherland DE: Effect of 15-deoxyspergualin on immediate function and long-term survival of transplanted islets in murine recipients of a marginal islet mass. Diabetes 1994;43:778–783 [DOI] [PubMed] [Google Scholar]
- 41.Weyrich AS, Elstad MR, McEver RP, McIntyre TM, Moore KL, Morrissey JH, Prescott SM, Zimmerman GA: Activated platelets signal chemokine synthesis by human monocytes. J Clin Invest 1996;97:1525–1534 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mandrup-Poulsen T, Bendtzen K, Nielsen JH, Bendixen G, Nerup J: Cytokines cause functional and structural damage to isolated islets of Langerhans. Allergy 1985;40:424–429 [DOI] [PubMed] [Google Scholar]
- 43.Johansson U, Olsson A, Gabrielsson S, Nilsson B, Korsgren O: Inflammatory mediators expressed in human islets of Langerhans: implications for islet transplantation. Biochem Biophys Res Commun 2003;308:474–479 [DOI] [PubMed] [Google Scholar]
- 44.Bottino R, Balamurugan AN, Tse H, Thirunavukkarasu C, Ge X, Profozich J, Milton M, Ziegenfuss A, Trucco M, Piganelli JD: Response of human islets to isolation stress and the effect of antioxidant treatment. Diabetes 2004;53:2559–2568 [DOI] [PubMed] [Google Scholar]
- 45.Grotting JC, Rosai J, Matas AJ, Frenzel EM, Payne WD, Sutherland DE, Najarian JS: The fate of intraportally transplanted islets in diabetic rats. A morphologic and immunohistochemical study. Am J Pathol 1978;92:653–670 [PMC free article] [PubMed] [Google Scholar]
- 46.Bennet W, Sundberg B, Groth CG, Brendel MD, Brandhorst D, Brandhorst H, Bretzel RG, Elgue G, Larsson R, Nilsson B, Korsgren O: Incompatibility between human blood and isolated islets of Langerhans: a finding with implications for clinical intraportal islet transplantation? Diabetes 1999;48:1907–1914 [DOI] [PubMed] [Google Scholar]
- 47.Johansson H, Lukinius A, Moberg L, Lundgren T, Berne C, Foss A, Felldin M, Kallen R, Salmela K, Tibell A, Tufveson G, Ekdahl KN, Elgue G, Korsgren O, Nilsson B: Tissue factor produced by the endocrine cells of the islets of Langerhans is associated with a negative outcome of clinical islet transplantation. Diabetes 2005;54:1755–1762 [DOI] [PubMed] [Google Scholar]
- 48.Ruf W, Edgington TS: An anti-tissue factor monoclonal antibody which inhibits TF.VIIa complex is a potent anticoagulant in plasma. Thromb Haemost 1991;66:529–533 [PubMed] [Google Scholar]
- 49.Berman DM, Cabrera O, Kenyon NM, Miller J, Tam SH, Khandekar VS, Picha KM, Soderman AR, Jordan RE, Bugelski PJ, Horninger D, Lark M, Davis JE, Alejandro R, Berggren PO, Zimmerman M, O'Neil JJ, Ricordi C, Kenyon NS: Interference with tissue factor prolongs intrahepatic islet allograft survival in a nonhuman primate marginal mass model. Transplantation 2007;84:308–315 [DOI] [PubMed] [Google Scholar]
- 50.Koulmanda M, Bhasin M, Hoffman L, Fan Z, Qipo A, Shi H, Bonner-Weir S, Putheti P, Degauque N, Libermann TA, Auchincloss H, Jr, Flier JS, Strom TB: Curative and beta cell regenerative effects of alpha1-antitrypsin treatment in autoimmune diabetic NOD mice. Proc Natl Acad Sci U S A 2008;105:16242–16247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Louvet C, Szot GL, Lang J, Lee MR, Martinier N, Bollag G, Zhu S, Weiss A, Bluestone JA: Tyrosine kinase inhibitors reverse type 1 diabetes in nonobese diabetic mice. Proc Natl Acad Sci U S A 2008;105:18895–18900 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bellin MD, Kandaswamy R, Parkey J, Zhang HJ, Liu B, Ihm SH, Ansite JD, Witson J, Bansal-Pakala P, Balamurugan AN, Papas K, Sutherland DE, Moran A, Hering BJ: Prolonged insulin independence after islet allotransplants in recipients with type 1 diabetes. Am J Transplant 2008;8:2463–2470 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zhang N, Su D, Qu S, Tse T, Bottino R, Balamurugan AN, Xu J, Bromberg JS, Dong HH: Sirolimus is associated with reduced islet engraftment and impaired β-cell function. Diabetes 2006;55:2429–2436 [DOI] [PubMed] [Google Scholar]
- 54.Cantaluppi V, Biancone L, Romanazzi GM, Figliolini F, Beltramo S, Ninniri MS, Galimi F, Romagnoli R, Franchello A, Salizzoni M, Perin PC, Ricordi C, Segoloni GP, Camussi G: Antiangiogenic and immunomodulatory effects of rapamycin on islet endothelium: relevance for islet transplantation. Am J Transplant 2006;6:2601–2611 [DOI] [PubMed] [Google Scholar]
- 55.Fraenkel M, Ketzinel-Gilad M, Ariav Y, Pappo O, Karaca M, Castel J, Berthault MF, Magnan C, Cerasi E, Kaiser N, Leibowitz G: mTOR inhibition by rapamycin prevents β-cell adaptation to hyperglycemia and exacerbates the metabolic state in type 2 diabetes. Diabetes 2008;57:945–957 [DOI] [PubMed] [Google Scholar]
- 56.Zahr E, Molano RD, Pileggi A, Ichii H, Jose SS, Bocca N, An W, Gonzalez-Quintana J, Fraker C, Ricordi C, Inverardi L: Rapamycin impairs in vivo proliferation of islet beta-cells. Transplantation 2007;84:1576–1583 [DOI] [PubMed] [Google Scholar]
- 57.Nir T, Melton DA, Dor Y: Recovery from diabetes in mice by beta cell regeneration. J Clin Invest 2007;117:2553–2561 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Gao R, Ustinov J, Korsgren O, Otonkoski T: Effects of immunosuppressive drugs on in vitro neogenesis of human islets: mycophenolate mofetil inhibits the proliferation of ductal cells. Am J Transplant 2007;7:1021–1026 [DOI] [PubMed] [Google Scholar]
- 59.The Canadian-European Randomized Control Trial Group. Cyclosporin-induced remission of IDDM after early intervention. Association of 1 yr of cyclosporin treatment with enhanced insulin secretion. Diabetes 1988;37:1574–1582 [PubMed] [Google Scholar]
- 60.Liu EH, Siegel RM, Harlan DM, O'Shea JJ: T cell-directed therapies: lessons learned and future prospects. Nat Immunol 2007;8:25–30 [DOI] [PubMed] [Google Scholar]
- 61.Keymeulen B, Vandemeulebroucke E, Ziegler AG, Mathieu C, Kaufman L, Hale G, Gorus F, Goldman M, Walter M, Candon S, Schandene L, Crenier L, De Block C, Seigneurin JM, De Pauw P, Pierard D, Weets I, Rebello P, Bird P, Berrie E, Frewin M, Waldmann H, Bach JF, Pipeleers D, Chatenoud L: Insulin needs after CD3-antibody therapy in new-onset type 1 diabetes. N Engl J Med 2005;352:2598–2608 [DOI] [PubMed] [Google Scholar]
- 62.Herold KC, Hagopian W, Auger JA, Poumian-Ruiz E, Taylor L, Donaldson D, Gitelman SE, Harlan DM, Xu D, Zivin RA, Bluestone JA: Anti-CD3 monoclonal antibody in new-onset type 1 diabetes mellitus. N Engl J Med 2002;346:1692–1698 [DOI] [PubMed] [Google Scholar]
- 63.Ludvigsson J, Faresjo M, Hjorth M, Axelsson S, Cheramy M, Pihl M, Vaarala O, Forsander G, Ivarsson S, Johansson C, Lindh A, Nilsson NO, Aman J, Ortqvist E, Zerhouni P, Casas R: GAD treatment and insulin secretion in recent-onset type 1 diabetes. N Engl J Med 2008;359:1909–1920 [DOI] [PubMed] [Google Scholar]
- 64.Bhargava R, Senior PA, Ackerman TE, Ryan EA, Paty BW, Lakey JR, Shapiro AM: Prevalence of hepatic steatosis after islet transplantation and its relation to graft function. Diabetes 2004;53:1311–1317 [DOI] [PubMed] [Google Scholar]
- 65.Maffi P, Angeli E, Bertuzzi F, Paties C, Socci C, Fedeli C, De Taddeo F, Nano R, Di Carlo V, Del Maschio A, Secchi A: Minimal focal steatosis of liver after islet transplantation in humans: a long-term study. Cell Transplant 2005;14:727–733 [DOI] [PubMed] [Google Scholar]
- 66.Hirshberg B, Montgomery S, Wysoki MG, Xu H, Tadaki D, Lee J, Hines K, Gaglia J, Patterson N, Leconte J, Hale D, Chang R, Kirk AD, Harlan DM: Pancreatic islet transplantation using the nonhuman primate (rhesus) model predicts that the portal vein is superior to the celiac artery as the islet infusion site. Diabetes 2002;51:2135–2140 [DOI] [PubMed] [Google Scholar]
- 67.al-Abdullah IH, Anil Kumar MS, Kelly-Sullivan D, Abouna GM: Site for unpurified islet transplantation is an important parameter for determination of the outcome of graft survival and function. Cell Transplant 1995;4:297–305 [DOI] [PubMed] [Google Scholar]
- 68.Gustavson SM, Rajotte RV, Hunkeler D, Lakey JR, Edgerton DS, Neal DW, Snead WL, Penaloza AR, Cherrington AD: Islet auto-transplantation into an omental or splenic site results in a normal beta cell but abnormal alpha cell response to mild non-insulin-induced hypoglycemia. Am J Transplant 2005;5:2368–2377 [DOI] [PubMed] [Google Scholar]
- 69.Ao Z, Matayoshi K, Lakey JR, Rajotte RV, Warnock GL: Survival and function of purified islets in the omental pouch site of outbred dogs. Transplantation 1993;56:524–529 [DOI] [PubMed] [Google Scholar]
- 70.Berman DM, O'Neil JJ, Coffey LC, Chaffanjon PC, Kenyon NM, Ruiz P, Jr, Pileggi A, Ricordi C, Kenyon NS: Long-term survival of nonhuman primate islets implanted in an omental pouch on a biodegradable scaffold. Am J Transplant 2009;9:91–104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Stagner JI, Rilo HL, White KK: The pancreas as an islet transplantation site. Confirmation in a syngeneic rodent and canine autotransplant model. JOP 2007;8:628–636 [PubMed] [Google Scholar]
- 72.Rafael E, Tibell A, Ryden M, Lundgren T, Savendahl L, Borgstrom B, Arnelo U, Isaksson B, Nilsson B, Korsgren O, Permert J: Intramuscular autotransplantation of pancreatic islets in a 7-year-old child: a 2-year follow-up. Am J Transplant 2008;8:458–462 [DOI] [PubMed] [Google Scholar]
- 73.Echeverri G, Starzl T, McGrath K, et al. : Endoscopic Gastric Submucosal Transplantation of Islets (ENDO-STI): technique and initial results in diabetic pigs. Am J Transplant In press [DOI] [PubMed] [Google Scholar]
- 74.Zhao M, Muiesan P, Amiel SA, Srinivasan P, Asare-Anane H, Fairbanks L, Persaud S, Jones P, Jones J, Ashraf S, Littlejohn W, Rela M, Heaton N, Huang GC: Human islets derived from donors after cardiac death are fully biofunctional. Am J Transplant 2007;7:2318–2325 [DOI] [PubMed] [Google Scholar]
- 75.Matsumoto S, Okitsu T, Iwanaga Y, Noguchi H, Nagata H, Yonekawa Y, Liu X, Kamiya H, Ueda M, Hatanaka N, Kobayashi N, Yamada Y, Miyakawa S, Seino Y, Shapiro AM, Tanaka K: Follow-up study of the first successful living donor islet transplantation. Transplantation 2006;82:1629–1633 [DOI] [PubMed] [Google Scholar]
- 76.Menge BA, Tannapfel A, Belyaev O, Drescher R, Muller C, Uhl W, Schmidt WE, Meier JJ: Partial pancreatectomy in adult humans does not provoke β-cell regeneration. Diabetes 2008;57:142–149 [DOI] [PubMed] [Google Scholar]
- 77.Cardona K, Korbutt GS, Milas Z, Lyon J, Cano J, Jiang W, Bello-Laborn H, Hacquoil B, Strobert E, Gangappa S, Weber CJ, Pearson TC, Rajotte RV, Larsen CP: Long-term survival of neonatal porcine islets in nonhuman primates by targeting costimulation pathways. Nat Med 2006;12:304–306 [DOI] [PubMed] [Google Scholar]
- 78.Hering BJ, Wijkstrom M, Graham ML, Hardstedt M, Aasheim TC, Jie T, Ansite JD, Nakano M, Cheng J, Li W, Moran K, Christians U, Finnegan C, Mills CD, Sutherland DE, Bansal-Pakala P, Murtaugh MP, Kirchhof N, Schuurman HJ: Prolonged diabetes reversal after intraportal xenotransplantation of wild-type porcine islets in immunosuppressed nonhuman primates. Nat Med 2006;12:301–303 [DOI] [PubMed] [Google Scholar]
- 79.Kuwaki K, Tseng YL, Dor FJ, Shimizu A, Houser SL, Sanderson TM, Lancos CJ, Prabharasuth DD, Cheng J, Moran K, Hisashi Y, Mueller N, Yamada K, Greenstein JL, Hawley RJ, Patience C, Awwad M, Fishman JA, Robson SC, Schuurman HJ, Sachs DH, Cooper DK: Heart transplantation in baboons using alpha1,3-galactosyltransferase gene-knockout pigs as donors: initial experience. Nat Med 2005;11:29–31 [DOI] [PubMed] [Google Scholar]
- 80.Phelps CJ, Koike C, Vaught TD, Boone J, Wells KD, Chen SH, Ball S, Specht SM, Polejaeva IA, Monahan JA, Jobst PM, Sharma SB, Lamborn AE, Garst AS, Moore M, Demetris AJ, Rudert WA, Bottino R, Bertera S, Trucco M, Starzl TE, Dai Y, Ayares DL: Production of alpha 1,3-galactosyltransferase-deficient pigs. Science 2003;299:411–414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.D'Amour KA, Bang AG, Eliazer S, Kelly OG, Agulnick AD, Smart NG, Moorman MA, Kroon E, Carpenter MK, Baetge EE: Production of pancreatic hormone-expressing endocrine cells from human embryonic stem cells. Nat Biotechnol 2006;24:1392–1401 [DOI] [PubMed] [Google Scholar]
- 82.Kroon E, Martinson LA, Kadoya K, Bang AG, Kelly OG, Eliazer S, Young H, Richardson M, Smart NG, Cunningham J, Agulnick AD, D'Amour KA, Carpenter MK, Baetge EE: Pancreatic endoderm derived from human embryonic stem cells generates glucose-responsive insulin-secreting cells in vivo. Nat Biotechnol 2008;26:443–452 [DOI] [PubMed] [Google Scholar]
- 83.Byrne JA, Pedersen DA, Clepper LL, Nelson M, Sanger WG, Gokhale S, Wolf DP, Mitalipov SM: Producing primate embryonic stem cells by somatic cell nuclear transfer. Nature 2007;450:497–502 [DOI] [PubMed] [Google Scholar]
- 84.Aoi T, Yae K, Nakagawa M, Ichisaka T, Okita K, Takahashi K, Chiba T, Yamanaka S: Generation of pluripotent stem cells from adult mouse liver and stomach cells. Science 2008;321:699–702 [DOI] [PubMed] [Google Scholar]
- 85.Yu J, Hu K, Smuga-Otto K, Tian S, Stewart R, Slukvin, Thomson JA: Human induced pluripotent stem cells free of vector and transgene sequences. Science 2009;324:797–801 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Pinkse GG, Tysma OH, Bergen CA, Kester MG, Ossendorp F, van Veelen PA, Keymeulen B, Pipeleers D, Drijfhout JW, Roep BO: Autoreactive CD8 T cells associated with beta cell destruction in type 1 diabetes. Proc Natl Acad Sci U S A 2005;102:18425–18430 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Zhou Q, Brown J, Kanarek A, Rajagopal J, Melton DA: In vivo reprogramming of adult pancreatic exocrine cells to beta-cells. Nature 2008;455:627–632 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Wilson JM: Medicine. A history lesson for stem cells. Science 2009;324:727–728 [DOI] [PubMed] [Google Scholar]
- 89.Khan M, Harlan DM: Transplant-based treatments for the patient with long-standing type 1 diabetes. In Diabetes: Translating Research into Practice Greenbaum CJ, Harrison LC: Eds. New York, Informa Healthcare USA, 2008, p. 193–208 [Google Scholar]
- 90.Venstrom JM, McBride MA, Rother KI, Hirshberg B, Orchard TJ, Harlan D: Pancreas transplantation decreases survival for patients with diabetes and preserved kidney function. JAMA 2003;290:2817–2823 [DOI] [PubMed] [Google Scholar]
- 91.Heit JJ, Apelqvist AA, Gu X, Winslow MM, Neilson JR, Crabtree GR, Kim SK: Calcineurin/NFAT signalling regulates pancreatic beta-cell growth and function. Nature 2006;443:345–349 [DOI] [PubMed] [Google Scholar]
- 92.Bosi E, Braghi S, Maffi P, Scirpoli M, Bertuzzi F, Pozza G, Secchi A, Bonifacio E: Autoantibody response to islet transplantation in type 1 diabetes. Diabetes 2001;50:2464–2471 [DOI] [PubMed] [Google Scholar]
- 93.Roelen DL, Huurman VA, Hilbrands R, Gillard P, Duinkerken G, van der Meer-Prins PW, Versteeg-van der Voort Maarschalk MF, Mathieu C, Keymeulen B, Pipeleers DG, Roep BO, Claas FH: Relevance of cytotoxic alloreactivity under different immunosuppressive regimens in clinical islet cell transplantation. Clin Exp Immunol 2009;156:141–148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Arif S, Tree TI, Astill TP, Tremble JM, Bishop AJ, Dayan CM, Roep BO, Peakman M: Autoreactive T cell responses show proinflammatory polarization in diabetes but a regulatory phenotype in health. J Clin Invest 2004;113:451–463 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Han D, Leith J, Alejandro R, Bolton W, Ricordi C, Kenyon NS: Peripheral blood cytotoxic lymphocyte gene transcript levels differ in patients with long-term type 1 diabetes compared to normal controls. Cell Transplant 2005;14:403–409 [DOI] [PubMed] [Google Scholar]
- 96.Huurman VAL, Velthuis JHL, Hilbrands R, Tree TIM, Gillard P, Van der Meer-Prins PMJ, Duinkerken G, Pinkse GGM, Keymeulen B, Roelen DL, Claas FHJ, Pipeleers DG, Roep BO: Allograft-specific cytokine profiles associate with clinical outcome after islet cell transplantation. Am J Transpl 2009;9:382–388 [DOI] [PubMed] [Google Scholar]
- 97.Gillard P, Huurman V, Van der Auwera B, Decallonne B, Poppe K, Roep BO, Gorus F, Mathieu C, Pipeleers D, Keymeulen B: Graves' hyperthyroidism after stopping immune suppressive therapy in type 1 diabetic islet cell recipients with pretransplant TPO-autoantibodies. Diabetes Care In press [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Hilbrands R, Huurman VA, Gillard P, Velthuis JH, De Waele M, Mathieu C, Kaufman L, Pipeleers-Marichal M, Ling Z, Movahedi B, Jacobs-Tulleneers-Thevissen D, Monbaliu D, Ysebaert D, Gorus FK, Roep BO, Pipeleers DG, Keymeulen B: Differences in baseline lymphocyte counts and autoreactivity are associated with differences in outcome of islet cell transplantation in type 1 diabetic patients. Diabetes In press [DOI] [PMC free article] [PubMed] [Google Scholar]