Abstract
OBJECTIVE
Common variants in FTO (the fat mass– and obesity-associated gene) associate with obesity and type 2 diabetes. The regulation and biological function of FTO mRNA expression in target tissue is unknown. We investigated the genetic and nongenetic regulation of FTO mRNA in skeletal muscle and adipose tissue and their influence on in vivo glucose and fat metabolism.
RESEARCH DESIGN AND METHODS
The FTO rs9939609 polymorphism was genotyped in two twin cohorts: 1) 298 elderly twins aged 62–83 years with glucose tolerance ranging from normal to type 2 diabetes and 2) 196 young (25–32 years) and elderly (58–66 years) nondiabetic twins examined by a hyperinsulinemic-euglycemic clamp including indirect calorimetry. FTO mRNA expression was determined in subcutaneous adipose tissue (n = 226) and skeletal muscle biopsies (n = 158).
RESULTS
Heritability of FTO expression in both tissues was low, and FTO expression was not influenced by FTO rs9939609 genotype. FTO mRNA expression in skeletal muscle was regulated by age and sex, whereas age and BMI were predictors of adipose tissue FTO mRNA expression. FTO mRNA expression in adipose tissue was associated with an atherogenic lipid profile. In skeletal muscle, FTO mRNA expression was negatively associated to fat and positively to glucose oxidation rates as well as positively correlated with expression of genes involved in oxidative phosphorylation including PGC1α.
CONCLUSIONS
The heritability of FTO expression in adipose tissue and skeletal muscle is low and not influenced by obesity-associated FTO genotype. The age-dependent decline in FTO expression is associated with peripheral defects of glucose and fat metabolism.
Obesity and type 2 diabetes are heterogenous disorders caused by both nongenetic and genetic components. Recent studies identified common variants in FTO (the fat mass– and obesity-associated gene) to be associated with obesity and type 2 diabetes in humans (1–5). The FTO genotype is suggested to be involved in the regulation of appetite and body weight (2,6–8) and may regulate transcription of genes involved in metabolism by nucleic acid demethylation (6). In addition, we demonstrated an association between the FTO genotype on one hand and increased energy efficiency and elevated blood glucose on the other, suggesting peripheral effects of FTO (9). A recent paper demonstrated that inactivation of the FTO gene protects from obesity in mice and supported the idea of FTO being involved in peripheral energy homeostasis, mitochondrial coupling, and/or substrate cycling (10). Thus, the association between FTO and obesity may not only involve control of appetite. Indeed, skeletal muscle and adipose tissue are important organs in the pathogenesis of obesity, insulin resistance, and type 2 diabetes.
Few studies have investigated the mRNA expression levels and biological function of FTO in human adipose tissue. In one study, expression levels of FTO mRNA was threefold higher in human subcutaneous adipose tissue (SAT) than in visceral adipose tissue (VAT) (11), whereas FTO expression decreased during adipocyte differentiation and was similar in SAT and VAT in another study (12). In our recent study, we were unable to document any impact of the FTO genotype on the expression of FTO in skeletal muscle (9). However, the number of subjects in this study was limited, and we are unaware of other studies that have investigated the FTO expression in human skeletal muscle and its putative peripheral role in glucose metabolism. The aim of the present study was to investigate the genetic versus nongenetic regulation of FTO mRNA expression in skeletal muscle and SAT and to study the impact of tissue FTO mRNA levels on obesity and insulin resistance in two twin cohorts.
RESEARCH DESIGN AND METHODS
The FTO rs9939609 polymorphism was genotyped in two cohorts of Danish twins: 1) a population-based cohort of 298 elderly twins (mean age 74 ± 5 years) and 2) a cohort of 110 young (age 28 ± 2 years) and 86 elderly (age 62 ± 2 years) twins without known type 2 diabetes. The subjects were identified through the Danish Twin Register, and all procedures were performed according to the Declaration of Helsinki and approved by the scientific ethics committees for the counties of Funen and Vejle. The recruitment and details of selection of cohorts 1 and 2 are previously described (13–16). In cohort 1, 57% were normal glucose tolerant (NGT), 27% had impaired glucose tolerance (IGT), and 16% had type 2 diabetes. In cohort 2, 74% had NGT, 22.0% had IGT, and 4% had previously unknown type 2 diabetes among elderly twins. Among the young twins, 98% had NGT and 2% had IGT.
Clinical examinations.
In cohort 1 the subjects were examined with a standardized 75-g oral glucose tolerance test (OGTT) and BMI and waist-to-hip ratio (WHR) were measured as previously described (15,16). In addition, a SAT biopsy was taken from the abdomen under local anesthesia using a Bergstrom needle, and the tissue was immediately frozen in liquid nitrogen and stored at −80°C. We were able to obtain adipose tissue biopsies and measure mRNA FTO expression in a subgroup of 226 twins.
In cohort 2 the subjects underwent a 2-day clinical examination as described previously (13,17). Day 1 included an OGTT, anthropometric measures, and a dual-energy X-ray absorptiometry (DEXA) scan to determine body composition, and physical fitness was estimated as VO2max calculated from the maximal load on ergometer bicycle (18). On day 2, a 2-h hyperinsulinemic-euglycemic clamp (40 mU/m2 per min) with tritiated glucose preceded by a 30-min intravenous glucose tolerance test was performed. Indirect calorimetry was performed during both the basal and insulin-stimulated steady-state period using a computerized flow-through canopy gas analyzer system (Deltarac; Datex, Helsinki, Finland). After an equilibrium period of 10 min, the average gas exchange rates were used to calculate the basal and insulin-stimulated fat and glucose oxidation rates (19,20). The respiratory coefficient, which is the quotient of CO2 production and O2 consumption, reflecting the macronutrient being oxidized, was obtained from the calorimetry data. Before and after the hyperinsulinemic-euglycemic clamp, skeletal muscle biopsies were excised from the vastus lateralis muscle under local anesthesia using a Bergstrom needle, and the tissue was immediately frozen in liquid nitrogen and stored at −80°C. We were able to measure FTO mRNA expression in 159 basal and 158 insulin-stimulated skeletal muscle biopsies.
Analytical methods and calculations.
Plasma glucose and insulin concentrations during the OGTT and the hyperinsulinemic-euglycemic clamp were analyzed as previously described (15,21). The fasting blood samples were analyzed for serum triglycerides, total cholesterol, HDL cholesterol, and LDL cholesterol as previously described (22). Homeostasis model assessment–insulin resistance was calculated as [(fasting plasma insulin × fasting plasma glucose)/22.5] × 0.144 (23). Calculation of hepatic glucose production, glucose disposal, and insulin secretion has been described previously (13,17). Glucose and fat oxidation rates were calculated with data obtained from the indirect calorimetry using the following equations: glucose; 4.55 VCO2 (L/min) – 3.21VO2 (L/min) – 2.87 n (g/min) and fat; 1.67 Vo2 (L/min) – 1.67 Vco2 (L/min) – 1.92 n (g/min), where n is nitrogen secreted in the urine (19). Glucose and lipid oxidation data are expressed as milligrams per kilograms of fat-free mass per minute.
Measurement of gene expression using real-time RT-PCR.
Total RNA was extracted from frozen skeletal muscle and adipose tissue samples using the Tri Reagent kit according to the manufacturer's instructions (Sigma-Aldrich). cDNA was synthesized using the QuantiTect Reverse Transcription kit (Qiagen). FTO mRNA levels were quantified using TaqMan Real-Time PCR with an ABI 7900 system (Applied Biosystems) and an Assay-on-demand (Hs01057145_m1) covering exon 8–9. All samples were run in duplicates and data were calculated using the standard curve method and normalized to the mRNA level of cyclophilin A (4326316E, Applied Biosystems). An additional endogenous control gene, hypoxanthine-guanine phosphoribosyltransferase (4333768E, Applied Biosystems), was initially analyzed in the skeletal muscle biopsies. The expression of cyclophilin A as well as the ratio between cyclophilin A and hypoxanthine-guanine phosphoribosyltransferase were compared, and there was no effect of a number of variables tested (i.e., age or insulin), which is why only one control gene (cyclophilin A) was subsequently used for the statistical analysis of genetic and q-traits. Expression of three OXPHOX genes, PGC1-α, and GLUT4 in vastus lateralis were previously performed with assays from Applied Biosystems: NDUFB6 (Hs00159583), UQCRB (Hs00559884), ATP50 (Hs00426889), PGC-1α (Hs00173304), and GLUT4 (Hs00168966) (24,25).
Genotyping.
The FTO polymorphism rs9939609 was genotyped using Taqman allelic discrimination (KBioscience, Hertz, U.K.) and obeyed Hardy-Weinberg equilibrium in both cohort 1 (P = 0.44) and cohort 2 (P = 0.99). The overall genotyping success rate was 97.4%. The minor allele frequency for FTO rs9939609 was 37.3% (33.3;41.3) and 39.1% (34.2;43.9) in cohorts 1 and 2, respectively.
Statistical methods.
Comparison of FTO mRNA expression between young and elderly twins and comparison between FTO genotype and FTO mRNA expression were done by a mixed-effects regression model. Correlation analyses were made using the Pearson's correlation coefficient test with ln-transformed data. Multiple regression analyses were performed to test the influence of potential factors on FTO mRNA expression in adipose tissue and skeletal muscle and to test the impact of FTO mRNA expression on in vivo metabolism. In all the multiple regression analyses, the outcome variable was ln transformed. We tested the influence of the following variables on FTO mRNA expression in skeletal muscle: age (elderly [1] or young [2]), sex (men [1] or women [2]), zygosity status (MZ [monozygotic] [1] or DZ [dizygotic] [2]), total fat percentages (continuous percent), and total body aerobic capacity Vo2max (continuous [ml · kg−1 · min−1]) and in adipose tissue: age (continuous years), sex (men [1] or women [2]), zygosity status (MZ [1] or DZ [2]), and BMI (continuous [kg/m2]). Both models took into account that the observations within twins cannot be assumed to be independent and that the dependency effects are different for MZ and DZ twin pairs. Thus, the full models include a random-effects term for twin pair membership and a fixed-effects term for zygosity. Because MZ twins have identical genotypes, any differences are theoretically because of environmental factors, whereas DZ twins, on average, share 50% of their genes. The extent to which MZ twins are more alike than DZ twins is presumed to reflect a genetic influence on the phenotype in question. Heritability (expressed as h2) gives the proportion of the total variation of a trait attributable to genetic variation and can be estimated by comparing the similarity (i.e., intraclass correlations) of a given phenotype between MZ and DZ twin pairs. Statistical comparisons of intraclass correlations were made after transformation using the Fisher z transformation. The heritability is expressed as twice the difference of the intraclass correlation of MZ and DZ twins h2 = 2(rMZ – rDZ) (26). The designation of a member in a twin pair is arbitrary; i.e., there is no consistency in which of the twins in a pair is assigned A and which is assigned B. The correlation coefficient may differ dependent on the assignment of the twins, and randomization is required. To avoid the randomization procedure, intrapair correlations were calculated using 2n, as previously recommended (27). All analyses were carried out in SAS (version 9.1; SAS Institute); P ≤ 0.05 was considered significant.
RESULTS
The clinical characteristics of the two cohorts are shown in Table 1. The A-allele of FTO rs9939609 was associated to measures of obesity, namely BMI (TT: 25.2 ± 0.3, AT: 26.1 ± 0.1, AA: 27.1 ± 0.6; P = 0.03) in cohort 1 and WHR in cohort 1 (TT: 0.87 ± 0.01, AT: 0.87 ± 0.01, AA: 0.91 ± 0.01; P = 0.004) and in the younger twins in cohort 2 (TT: 0.84 ± 0.02, AT: 0.81 ± 0.01, AA: 0.91 ± 0.02; P = 0.004). No associations between rs9939609 and either BMI (P = 0.93) or WHR (P = 0.92) were observed in the elderly twins in cohort 2. Furthermore, the A-allele of FTO rs9939609 was associated with reduced insulin-stimulated glucose uptake (TT: 12.0 ± 0.6, AT: 11.9 ± 0.5, AA: 9.9 ± 0.8; P = 0.07) and nonoxidative glucose metabolism (TT: 8.0 ± 0.6, AT: 7.3 ± 0.5, AA: 5.3 ± 0.7; P = 0.01) in the young (and not elderly) twins of cohort 2. However, these associations disappeared after adjustment for BMI. No association between rs9939609 and basal or insulin-stimulated glucose or fat oxidation was observed in either the young or elderly twins.
TABLE 1.
Subject characteristics
| Cohort 1 |
Cohort 2 |
||
|---|---|---|---|
| Elderly | Young | Elderly | |
| n (men/women) | 226 (103/123) | 110 (60/50) | 86 (38/48) |
| n (MZ/DZ) | 226 (92/134) | 110 (66/44) | 86 (42/44) |
| Age (years) | 73.4 ± 5.3 | 28 ± 2 | 62 ± 2 |
| BMI (kg/m2) | 26.1 ± 3.7 | 24.1 ± 3.1 | 26.1 ± 4.5 |
| WHR | 0.90 ± 0.1 | 0.84 ± 0.08 | 0.89 ± 0.1 |
| Total fat (%) | NA | 22.1 ± 7.0 | 27.9 ± 9.4 |
| Vo2max (ml · kg−1 · min−1) | NA | 39.6 ± 7.8 | 26.3 ± 7.0 |
| Basal GOX (mg · kg FFM−1 · min−1) | NA | 114 ± 55 | 90 ± 52 |
| Insulin-stimulated GOX (mg · kg FFM−1 · min−1) | NA | 246 ± 24 | 200 ± 28 |
| Basal FOX (mg · kg FFM−1 · min−1) | NA | 69 ± 26 | 70 ± 24 |
| Insulin-stimulated FOX (mg · kg FFM−1 · min−1) | NA | 20 ± 24 | 62 ± 24 |
| Insulin sensitivity (Rd) (mg · kg FFM−1 · min−1) | NA | 11.7 ± 1.9 | 9.9 ± 3.3 |
Data are expressed as means ± SD. NA, not appilicable; FFM, fat-free mass; FOX, fat oxidation; GOX, glucose oxidation.
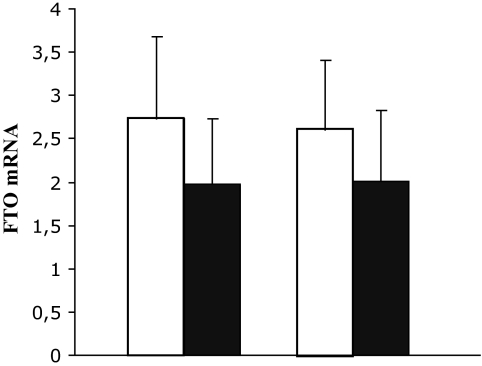
Young twins had significantly higher muscle FTO mRNA levels compared with elderly twins both in the basal state (2.73 ± 0.95 vs. 1.97 ± 0.77, P < 0.0001) and after insulin stimulation (2.61 ± 0.80 vs. 2.01 ± 0.82, P = 0.0004) (Fig. 1). The expression level of FTO mRNA did not change significantly by insulin stimulation in the group of all twins (P = 0.27) or in the subgroups of young (P = 0.12) and elderly (P = 0.86) twins, which is why only the mRNA expression levels during the basal state were used in the subsequent analyses.
FIG. 1.
FTO mRNA expression in skeletal muscle obtained before and after insulin stimulation in young (□) and elderly (■) twins. Results are expressed as means ± SD; mRNA levels were quantified with real-time PCR and normalized to the level of Cyclophilin A. After adjusting for intratwin pair relationship, P < 0.0001 for basal expression (left panel) and P = 0.0004 for post-clamp expression (right panel).
Genetic influence on FTO expression in skeletal muscle and adipose tissue.
We studied the association between FTO rs9939609 and FTO mRNA expression and found no significant association in either skeletal muscle or in adipose tissue (Table 2). Upon adjustment for age and sex in the analysis the association remained nonsignificant (P = 0.90 and P = 0.28, respectively). The intraclass correlations for FTO expression in skeletal muscle were similar in MZ (r = 0.72) and DZ twins (r = 0.57) (P = 0.28), with a heritability estimate of 0.30. In adipose tissue, the intraclass correlations for FTO expression in MZ were r = 0.30 and in DZ r = 0.25 (P = 0.83) with a heritability estimate of 0.10.
TABLE 2.
Association between FTO rs9939608 genotype and FTO mRNA expression in skeletal muscle and adipose tissue
| Skeletal muscle | n | TT | n | AT | n | AA | P |
|---|---|---|---|---|---|---|---|
| Basal FTO expression | 60 | 2.28 ± 0.81 | 76 | 2.26 ± 0.82 | 22 | 2.51 ± 1.31 | 0.46 |
| Basal adipose tissue FTO expression | 77 | 0.96 ± 0.38 | 108 | 0.89 ± 0.38 | 26 | 0.96 ± 0.39 | 0.37 |
Data are expressed as means ± SD.
Nongenetic predictors of FTO mRNA expression
Skeletal muscle.
This study aimed to identify nongenetic determinants of FTO mRNA expression including obesity and physical fitness, and correlation analyses were performed between FTO mRNA in skeletal muscle and total fat percentages (r = −0.35, P < 0.0001) and Vo2max (r = 0.41, P < 0.0001). To quantify the independent effect of these variables on FTO mRNA expression, multiple regression analyses with the additional inclusion of age, sex, and zygosity were performed. Age (exp[β] = 0.72, P < 0.0001) and sex (exp[β] = 1.27, P = 0.002) were the only significant independent predictors of FTO mRNA expression in skeletal muscle (Table 3). Thus, being an elderly twin is associated with a 28% decrease in FTO mRNA expression and being a male is associated with a 27% increase in FTO mRNA expression.
TABLE 3.
Variables with possible influence on FTO mRNA expression in vastus lateralis muscle
| Estimate | 95% CI | Percent change | Effect of 1 SD | Percent change | P | |
|---|---|---|---|---|---|---|
| Basal FTO mRNA level | ||||||
| Age (elderly vs. young) | 0.72 | (0.64–0.82) | ↓28% | — | — | <0.0001 |
| Sex (men vs. women) | 1.27 | (1,12–1.43) | ↑27% | — | — | 0.002 |
| Zygosity (MZ vs. DZ) | 1.08 | (0.98–1.20) | ↑8% | — | — | 0.11 |
| Total fat (%) | 1.00 | (0.99–1.01) | — | 0.99 | ↓1% | 0.82 |
| Vo2max (ml · kg−1 · min−1) | 0.99 | (0.99–1.00) | ↓1% | 0.97 | ↓3% | 0.42 |
Model: ln FTO = adjusted for age, sex, zygosity, total fat percentages, and Vo2max. SD: adjusted for total fat percentage = 8.65, Vo2max = 9.62. P values are adjusted for twin pair and zygosity status.
Adipose tissue.
Similarly, multiple regression analyses were performed to quantify the effect of age, sex, zygosity, and BMI on the level of FTO mRNA expression in adipose tissue. Age (exp[β] = 0.92, P = 0.002) and BMI (exp[β] = 1.13, P < 0.0001) were significant independent predictors of FTO mRNA expression in adipose tissue. A 1 SD increase in age (5.1 years) results in an 8% decline in FTO mRNA and a 1 SD increase in BMI (4.3 kg/m2) results in a 13% increase in FTO mRNA level (Table 4).
TABLE 4.
Variables with possible influence on FTO mRNA expression in adipose tissue
| Estimate | 95% CI | Percent change | Effect of 1 SD | Percent change | P | |
|---|---|---|---|---|---|---|
| Basal FTO mRNA level | ||||||
| Age (years) | 0.98 | (0.97–0.99) | ↓2% | 0.92 | ↓8% | 0.002 |
| Sex (men vs. women) | 1.09 | (0.97–1.21) | ↑9% | — | — | 0.13 |
| Zygosity (MZ vs. DZ) | 0.96 | (0.85–1.09) | ↓4% | — | — | 0.56 |
| BMI (kg/m2) | 1.03 | (1.02–1.04) | ↑3% | 1.13 | ↑13% | <0.0001 |
Model: ln FTO = adjusted for age, sex, zygosity, and BMI. SD: adjusted for age = 5.14, BMI = 4.26. P values are adjusted for twin pair and zygosity status.
Impact of FTO mRNA expression on in vivo metabolism
Skeletal muscle.
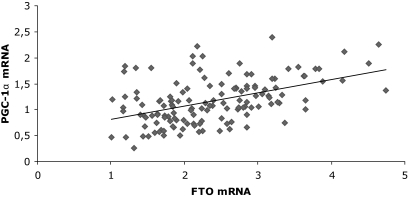
To investigate the relationships between FTO mRNA expression and in vivo metabolism, regression analyses were made with FTO mRNA expression levels as the explanatory variable. Based on the above findings the analyses were adjusted for age and sex. The expression of FTO mRNA had a significant impact on both the basal and insulin-stimulated fat oxidation rate. An increase in FTO mRNA level of 1 SD (0.78 AU) was associated with a 12% (P = 0.04) decrease in basal fat oxidation and 18% (P = 0.03) decrease in insulin-stimulated fat oxidation (Table 5). In addition, an increase of 1 SD in FTO expression was associated with a borderline significant increase of 10% (P = 0.07) in basal glucose oxidation and with a 1% increase in basal respiratory coefficient (P = 0.03) and insulin-stimulated respiratory coefficient (P = 0.03) (Table 5). The associations between FTO mRNA and fat oxidation remained significant after adjustment for total fat mass and percentage. No influence of skeletal muscle FTO mRNA expression on peripheral insulin sensitivity, hepatic glucose production, insulin secretion, energy expenditure, or Vo2max was observed (data not shown). Because of the putative effects on lipid and/or glucose oxidation, we investigated associations between FTO mRNA expression and expression of genes involved in mitochondrial function including OXPHOS genes and their key regulator PGC-1α. Significantly positive correlations between FTO expression and the OXPHOS genes NDUFB6 (r = 0.48, P < 0.0001), UQCRB (r = 0.48, P < 0.0001), ATP50 (r = 0.62, P < 0.0001), and the PPAR-γ coactivator 1-α (PGC-1α) (r = 0.44, P < 0.0001) (Fig. 2) in skeletal muscle were found. Despite no effect of FTO expression on whole-body glucose uptake, FTO mRNA was significantly and positively correlated to GLUT4 mRNA (r = 0.46, P < 0.0001).
TABLE 5.
The relationship between FTO mRNA expression in skeletal muscle and substrate oxidation
| Estimate | 95% CI | Percent change | Effect of 1 SD | Percent change | P | |
|---|---|---|---|---|---|---|
| Basal FOX (mg · kg FFM−1 · min−1) | 0.85 | (0.73–1.00) | ↓15% | 0.88 | ↓12% | 0.04 |
| Clamp FOX (mg · kg FFM−1 · min−1) | 0.77 | (0.61–0.97) | ↓23% | 0.82 | ↓18% | 0.03 |
| Basal GOX (mg · kg FFM−1 · min−1) | 1.12 | (0.99–1.28) | ↑12% | 1.10 | ↑10% | 0.07 |
| Clamp GOX (mg · kg FFM−1 · min−1) | 1.05 | (0.97–1.13) | ↑5% | 1.04 | ↑4% | 0.21 |
| Basal respiratory coefficient | 1.02 | (1.00–1.03) | ↑2% | 1.01 | ↑1% | 0.03 |
| Clamp respiratory coefficient | 1.02 | (1.00–1.03) | ↑2% | 1.01 | ↑1% | 0.03 |
Model: Ln dependent variable = FTO mRNA adjusted for age and sex. SD: Basal FTO mRNA = 0.78. P values are adjusted for twin pair and zygosity status. FOX, fat oxidation; GOX, glucose oxidation.
FIG. 2.
Correlation between FTO mRNA and PGC-1α mRNA in skeletal muscle. r = 0.44, P < 0.0001.
Adipose tissue.
Using univariate analyses we found that FTO mRNA was correlated with fasting serum levels of total LDL (r = 0.17, P = 0.009) and HDL (r = −0.21, P = 0.001) cholesterol. When adjusted for age and BMI, the observed independent predictors of FTO mRNA in adipose tissue in the present study, the association with HDL and LDL cholesterol remained significant. A 1-SD increase in FTO mRNA (0.35 AU) was associated with a 12% (P = 0.02) decrease in HDL cholesterol levels and a 5% (P = 0.02) increase in LDL cholesterol levels. No impact of FTO expression in adipose tissue was seen on either glucose tolerance (2-h post-OGTT plasma glucose) or indirect measures of insulin sensitivity (homeostasis model assessment–insulin resistance) (data not shown). Likewise, FTO mRNA expression did not differ among twins stratified according to glucose tolerance: NGT (n = 125) 0.94 ± 0.36; IGT (n = 62) 0.89 ± 0.38; type 2 diabetes (n = 37) 0.94 ± 0.30.
DISCUSSION
Variations in FTO have been associated with obesity and type 2 diabetes, but the biological functions and regulation of FTO mRNA expression in human tissues still remain to be clarified. Contributing to the sparse knowledge of human FTO mRNA expression, our key findings of the present study were that 1) the obesity-associated FTO rs9939609 does not influence tissue FTO mRNA expression in human skeletal muscle and adipose tissue in the basal state; 2) heritability of FTO mRNA expression is low; 3) accordingly, FTO mRNA expression in the basal state is mainly regulated by nongenetic factors in both skeletal muscle (age and sex) and adipose tissue (age and BMI); and 4) FTO mRNA expression in skeletal muscle correlates with whole-body substrate oxidation rates.
We found no associations between the examined FTO genotype and tissue FTO mRNA expression, indicating that the FTO genotype has no major or direct role in the regulation of FTO mRNA in either adipose tissue or skeletal muscle in the present study samples. The lack of association between the FTO polymorphism and level of mRNA expression is supported by our observation of a low heritability estimate of FTO mRNA expression in both adipose and skeletal muscle tissue. These findings do not, however, exclude that FTO genotype may influence the biological function of FTO in terms of activity. Previous findings (11,12) are in line with the present, although a recent study indeed has shown an association between FTO genotype and mRNA expression in SAT in very obese (BMI ≥40 kg/m2) subjects (28). Therefore, it cannot be excluded that a potential effect of genotype on tissue FTO mRNA expression levels may be unmasked in extreme states of metabolic disease such as severe obesity or upon certain challenges including exercise or dietary interventions. In fact, this was the case in a recent study demonstrating that insulin resistance per se was associated with a markedly reduced expression of PGC-1α in skeletal muscle only after an acute exercise bout (29). Moreover, it is possible that the polymorphism analyzed in this study, located in intron 1 of the FTO gene, may affect the expression pattern of the splice variants of FTO, which may vary in different tissues. The assay used in this study covers exon 8–9 and can detect three different splice variants. However, it does not distinguish between these variants. This question should be addressed in future ad hoc studies.
We showed that FTO expression in SAT decreased by 8% for every 5 years increase in age. Opposed to this finding, a study has shown that FTO expression in subcutaneous and visceral fat increased with age (11); this association disappeared, however, after adjustment for BMI. This discrepancy may be because of the narrow age range within the cohort of elderly twins in our study that in addition may limit generalization to younger individuals. Nevertheless, the findings are in line with those in skeletal muscle FTO expression, providing some support to a decrease in adipose tissue FTO expression with age. The few human studies of FTO expression in adipose tissue performed so far have been inconsistent concerning the relationship between obesity and FTO tissue expression. We found that the expression of FTO in adipose tissue was positively associated with obesity as measured by BMI, supporting some (12,28,30) but not all studies (11). A study in mice showed that FTO mRNA expression was 50% lower in adipocytes from db/db mice (a genetic model of obesity and type 2 diabetes) compared with wild-type mice (31). Independent of obesity, we demonstrated that increased FTO mRNA expression in adipose tissue was associated with an atherogenic serum lipid profile including an increase in circulating levels of LDL cholesterol and a decrease in HDL cholesterol levels. A previous study has demonstrated a borderline correlation between SAT FTO mRNA abundance and plasma glycerol, although no association to lipolysis was evident (12). FTO expression in adipose tissue was not influenced by glucose tolerance status and was not related to OGTT-derived measures of insulin sensitivity or insulin release. The lack of association between adipose tissue FTO expression and glucose metabolism is in accordance with a previous study of 55 Europeans (11).
FTO mRNA abundance in skeletal muscle was reduced by 28% in elderly compared with younger twins and significantly associated with in vivo measures of basal and insulin-stimulated fat oxidation and respiratory coefficient, in addition to a borderline significant association with glucose oxidation rate. These metabolic associations were independent of total fat mass and percentage, and collectively they point toward a shift from glucose to fat oxidation with an age-related decrease in FTO muscle expression. Because we previously demonstrated an age-related reduction in the mRNA expression of genes involved in oxidative phosphorylation (24), present expression data were adjusted for PGC-1α expression levels. Interestingly, the association between FTO mRNA expression in skeletal muscle and fat oxidation was persistent and independent of PGC-1α expression levels during both the basal (P = 0.08) state and after insulin stimulation (P = 0.05). Therefore, the age-related decline in skeletal muscle FTO expression may at least to some extent explain the higher fat oxidation rate among elderly twins.
The association between fat mass and fat oxidation is complex, with low fat oxidation potentially being causally related to risk of accumulating fat on one side and with fat accumulation in obesity potentially leading to increased fat oxidation as a consequence of excess tissue release of fatty acids on the other side. Thus, caution is warranted in the interpretation of cause and effect in these cross-sectional associations. We have recently reported that the obesity-associated A-allele of FTO rs9939609 was associated with increased mitochondrial energy efficiency in skeletal muscle from young healthy men (9). Furthermore, in accordance with our previous study, we here demonstrate in a large cohort of young and elderly twins that the FTO mRNA transcript was associated with the expression of PGC-1α and mitochondrial OXPHOS genes (9). The molecular mechanisms and sequences of events linking mitochondrial (dys)function and lipid oxidation to insulin resistance, obesity, and type 2 diabetes are incompletely understood and may involve both accumulation of lipid per se in muscle and liver as well as accumulation of by-products because of incomplete fat oxidation (32,33). Lipid intermediates such as fatty acyl CoA and diacylglycerol can inhibit insulin signaling and reduce glucose uptake, leading to insulin resistance (32,34).
In conclusion, the age-dependent decline in FTO expression in adipose tissue and skeletal muscle is associated with an atherogenic serum lipid profile, as well as with a shift in in vivo fat and glucose oxidation independent of obesity and gene expression levels of key metabolic regulators including PGC-1α. FTO expression in adipose tissue and skeletal muscle may therefore play a role in the development of some components of the metabolic syndrome, including dyslipidemia and peripheral metabolic regulation.
Acknowledgments
This study was funded by the Danish Diabetes Association, the 6th FP EU EXGENESIS consortium, and The Danish Strategic Research Council. L.G.G. was granted a PhD scholarship from the Faculty of International Health at the University of Copenhagen.
L.G. has been a consultant for and served on advisory boards for sanofi-aventis, GlaxoSmithKline, Novartis, Merck, Tethys Bioscience, and XOMA and received lecture fees from Lilly and Novartis. No other potential conflicts of interest relevant to this article were reported.
Marianne Modest provided assistance with the RNA extraction. We thank all the twins who participated in this study.
Footnotes
The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.Al-Attar S, Pollex R, Ban M, Young TK, Bjerregaard P, Anand S, Yusuf S, Zinman B, Harris S, Hanley A, Connelly P, Huff M, Hegele R: Association between the FTO rs9939609 polymorphism and the metabolic syndrome in a non-Caucasian multi-ethnic sample. Cardiovasc Diabetol 2008;7:5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dina C, Meyre D, Gallina S, Durand E, Korner A, Jacobson P, Carlsson LMS, Kiess W, Vatin V, Lecoeur C, Delplanque J, Vaillant E, Pattou F, Ruiz J, Weill J, Levy-Marchal C, Horber F, Potoczna N, Hercberg S, Le Stunff C, Bougneres P, Kovacs P, Marre M, Balkau B, Cauchi S, Chevre JC, Froguel P: Variation in FTO contributes to childhood obesity and severe adult obesity. Nat Genet 2007;39:724–726 [DOI] [PubMed] [Google Scholar]
- 3.Frayling TM: Genome-wide association studies provide new insights into type 2 diabetes aetiology. Nat Rev Genet 2007;8:657–662 [DOI] [PubMed] [Google Scholar]
- 4.Frayling TM, Timpson NJ, Weedon MN, Zeggini E, Freathy RM, Lindgren CM, Perry JRB, Elliott KS, Lango H, Rayner NW, Shields B, Harries LW, Barrett JC, Ellard S, Groves CJ, Knight B, Patch AM, Ness AR, Ebrahim S, Lawlor DA, Ring SM, Ben-Shlomo Y, Jarvelin MR, Sovio U, Bennett AJ, Melzer D, Ferrucci L, Loos RJF, Barroso I, Wareham NJ, Karpe F, Owen KR, Cardon LR, Walker M, Hitman GA, Palmer CNA, Doney ASF, Morris AD, Smith GD, The Wellcome Trust Case Control Consortium. Hattersley AT, McCarthy MI: A common variant in the FTO gene is associated with body mass index and predisposes to childhood and adult obesity. Science 2007;316:889–894 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Scuteri A, Sanna S, Chen WM, Uda M, Albai G, Strait J, Najjar S, Nagaraja R, Orru M, Usala G, Dei M, Lai S, Maschio A, Busonero F, Mulas A, Ehret GB, Fink AA, Weder AB, Cooper RS, Galan P, Chakravarti A, Schlessinger D, Cao A, Lakatta E, Abecasis GR: Genome-wide association scan shows genetic variants in the FTO gene are associated with obesity-related traits. PLOS GENET 2007;3:1200–1210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gerken T, Girard CA, Tung YC, Webby CJ, Saudek V, Hewitson KS, Yeo GS, McDonough MA, Cunliffe S, McNeill LA, Galvanovskis J, Rorsman P, Robins P, Prieur X, Coll AP, Ma M, Jovanovic Z, Farooqi IS, Sedgwick B, Barroso I, Lindahl T, Ponting CP, Ashcroft FM, O'Rahilly S, Schofield CJ: The obesity-associated FTO gene encodes a 2-oxoglutarate-dependent nucleic acid demethylase. Science 2007;318:1469–1472 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tschritter O, Preissl H, Yokoyama Y, Machicao F, Häring H-U, Fritsche A: Variation in the FTO gene locus is associated with cerebrocortical insulin resistance in humans. Diabetologia 2007;50:2602–2603 [DOI] [PubMed] [Google Scholar]
- 8.Wardle J, Carnell S, Haworth CMA, Farooqi IS, O'Rahilly S, Plomin R: Obesity-associated genetic variation in FTO is associated with diminished satiety. J Clin Endocrinol Metabolism 2008;93:3640–3643 [DOI] [PubMed] [Google Scholar]
- 9.Grunnet LG, Brons C, Jacobsen S, Nilsson E, Astrup A, Hansen T, Pedersen O, Poulsen P, Quistorff B, Vaag A: Increased recovery rates of phosphocreatine and inorganic phosphate after isometric contraction in oxidative muscle fibres and elevated hepatic insulin resistance in homozygous carriers of the A-allele of FTO rs9939609. J Clin Endocrinol Metabolism 2008;94:596–602 [DOI] [PubMed] [Google Scholar]
- 10.Fischer J, Koch L, Emmerling C, Vierkotten J, Peters T, Bruning JC, Ruther U: Inactivation of the FTO gene protects from obesity. Nature 2009;458:894. [DOI] [PubMed] [Google Scholar]
- 11.Klöting N, Schleinitz D, Ruschke K, Berndt J, Fasshauer M, Tönjes A, Schön R, Kovacs P, Stumvoll M, Blüher M: Inverse relationship between obesity and FTO gene expression in visceral adipose tissue in humans. Diabetologia 2008;51:641–647 [DOI] [PubMed] [Google Scholar]
- 12.Wahlen K, Sjolin E, Hoffstedt J: The common rs9939609 gene variant of the fat mass- and obesity-associated gene FTO is related to fat cell lipolysis. J Lipid Res 2008;49:607–611 [DOI] [PubMed] [Google Scholar]
- 13.Poulsen P, Levin K, Beck-Nielsen H, Vaag A: Age-dependent impact of zygosity and birth weight on insulin secretion and insulin action in twins. Diabetologia 2002;45:1649–1657 [DOI] [PubMed] [Google Scholar]
- 14.Poulsen P, Levin K, Petersen I, Christensen K, Beck-Nielsen H, Vaag A: Heritability of insulin secretion, peripheral and hepatic insulin action, and intracellular glucose partitioning in young and old Danish twins. Diabetes 2005;54:275–283 [DOI] [PubMed] [Google Scholar]
- 15.Poulsen P, Kyvik KO, Vaag A, Beck-Nielsen H: Heritability of type II (non-insulin-dependent) diabetes mellitus and abnormal glucose tolerance–a population-based twin study. Diabetologia 199942139–145. [DOI] [PubMed] [Google Scholar]
- 16.Poulsen P, Vaag A, Beck-Nielsen H: Does zygosity influence the metabolic profile of twins? A population based cross sectional study. BMJ 1999;319:151–154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Poulsen P, Vaag A: The intrauterine environment as reflected by birth size and twin and zygosity status influences insulin action and intracellular glucose metabolism in an age- or time-dependent manner. Diabetes 2006;55:1819–1825 [DOI] [PubMed] [Google Scholar]
- 18.Clausen JP, Oxhøj H, Hansen JF, Pedersen-Bjergaard O, Henningsen P, Uhrenholdt A: Exercise test and exercise ECG in the diagnosis and treatment of ischemic heart disease. Ugeskr Laeger 1980;142:1743–1749 [PubMed] [Google Scholar]
- 19.Frayn KN: Calculation of substrate oxidation rates in vivo from gaseous exchange. J Applied Physiology 1983;55:628–634 [DOI] [PubMed] [Google Scholar]
- 20.Vaag A, Alford F, Henriksen FL, Christopher M, Beck-Nielsen H: Multiple defects of both hepatic and peripheral intracellular glucose processing contribute to the hyperglycemia of NIDDM. Diabetologia 1995;38:326–336 [DOI] [PubMed] [Google Scholar]
- 21.Hother-Nielsen O, Beck-Nielsen H: On the determination of basal glucose production rate in patients with type 2 (non-insulin-dependent) diabetes mellitus using primed-continuous 3–3H-glucose infusion. Diabetologia 1990;33:603–610 [DOI] [PubMed] [Google Scholar]
- 22.Poulsen P, Vaag AA, Kyvik KO, Moller JD, Beck-Nielsen H: Low birth weight is associated with NIDDM in discordant monozygotic and dizygotic twin pairs. Diabetologia 1997;40:439–446 [DOI] [PubMed] [Google Scholar]
- 23.Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC: Homeostasis model assessment: insulin resistance and β-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 1985;28:412–419 [DOI] [PubMed] [Google Scholar]
- 24.Ling C: Multiple environmental and genetic factors influence skeletal muscle PGC-1α and PGC-1β gene expression in twins. J Clin Invest 2004;114:1518–1526 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ling C, Poulsen P, Simonsson S, Ronn T, Holmkvist J, Almgren P, Hagert P, Nilsson E, Mabey AG, Nilsson P, Vaag A, Groop L: Genetic and epigenetic factors are associated with expression of respiratory chain component NDUFB6 in human skeletal muscle. J Clin Invest 2007;117:3427–3435 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Neale MC: In methodology for genetic studies of twins and families. Psychol Med 1992;20:35–53 [Google Scholar]
- 27.Bring J, Wernroth L: Inefficient analysis of twin data: is there an association between diabetes and birth weight? Diabetologia 1999;42:898–899 [DOI] [PubMed] [Google Scholar]
- 28.Villalobos-Comparán M, Teresa Flores-Dorantes M, Teresa Villarreal-Molina M, Rodríguez-Cruz M, García-Ulloa AC, Robles L, Huertas-Vázquez A, Saucedo-Villarreal N, López-Alarcón M, Sánchez-Munoz F, Domínguez-López A, Gutiérrez-Aguilar R, Menjivar M, Coral-Vázquez R, Hernández-Stengele G, Vital-Reyes VS, Acuna-Alonzo V, Romero-Hidalgo S, Ruiz-Gómez DG, Riano-Barros D, Herrera MF, Gómez-Pérez FJ, Froguel P, García-García E, Teresa Tusié-Luna M, Guilar-Salinas CA, Canizales-Quinteros S: The FTO gene is associated with adulthood obesity in the Mexican population. Obesity 2008;16:2296–2301 [DOI] [PubMed] [Google Scholar]
- 29.De Filippis E, Alvarez G, Berria R, Cusi K, Everman S, Meyer C, Mandarino LJ: Insulin-resistant muscle is exercise resistant: evidence for reduced response of nuclear-encoded mitochondrial genes to exercise. Endocrinol Metabol 2008;294:E607–E614 [DOI] [PubMed] [Google Scholar]
- 30.Zabena C, Gonzáílez-Sáínchez JL, Martínez-Larrad MT, Torres-García A, Alvarez-Fernáíndez-Represa J, Corbatón-Anchuelo A, Pérez-Barba M, Serrano-Ríos M: The FTO obesity gene. Genotyping and gene expression analysis in morbidly obese patients. Obesity Surg 2009;19:87–95 [DOI] [PubMed] [Google Scholar]
- 31.Qi L, Kang K, Zhang C, van Dam RM, Kraft P, Hunter D, Lee CH, Hu FB: Fat mass-and obesity-associated (FTO) gene variant is associated with obesity: longitudinal analyses in two cohort studies and functional test. Diabetes 2008;57:3145–3151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Koves TR, Ussher JR, Noland RC, Slentz D, Mosedale M, Ilkayeva O, Bain J, Stevens R, Dyck JRB, Newgard CB, Lopaschuk GD, Muoio DM: Mitochondrial overload and incomplete fatty acid oxidation contribute to skeletal muscle insulin resistance. Cell Metab 2008;7:45–56 [DOI] [PubMed] [Google Scholar]
- 33.Muoio DM, Newgard CB: Obesity-related derangements in metabolic regulation. Annu Rev Biochem 2006;75:367–401 [DOI] [PubMed] [Google Scholar]
- 34.Muoio DM, Newgard CB: Mechanisms of disease: molecular and metabolic mechanisms of insulin resistance and β-cell failure in type 2 diabetes. Nat Rev Mol Cell Biol 2008;9:193–205 [DOI] [PubMed] [Google Scholar]