Abstract
OBJECTIVE
We showed that 17β-estradiol (E2) favors pancreatic β-cell survival via the estrogen receptor-α (ERα) in mice. E2 activates nuclear estrogen receptors via an estrogen response element (ERE). E2 also activates nongenomic signals via an extranuclear form of ERα and the G protein–coupled estrogen receptor (GPER). We studied the contribution of estrogen receptors to islet survival.
RESEARCH DESIGN AND METHODS
We used mice and islets deficient in estrogen receptor-α (αERKO−/−), estrogen receptor-β (βERKO−/−), estrogen receptor-α and estrogen receptor-β (αβERKO−/−), and GPER (GPERKO−/−); a mouse lacking ERα binding to the ERE; and human islets. These mice and islets were studied in combination with receptor-specific pharmacological probes.
RESULTS
We show that ERα protection of islet survival is ERE independent and that E2 favors islet survival through extranuclear and membrane estrogen receptor signaling. We show that ERβ plays a minor cytoprotective role compared to ERα. Accordingly, βERKO−/− mice are mildly predisposed to streptozotocin-induced islet apoptosis. However, combined elimination of ERα and ERβ in mice does not synergize to provoke islet apoptosis. In αβERKO−/− mice and their islets, E2 partially prevents apoptosis suggesting that an alternative pathway compensates for ERα/ERβ deficiency. We find that E2 protection of islet survival is reproduced by a membrane-impermeant E2 formulation and a selective GPER agonist. Accordingly, GPERKO−/− mice are susceptible to streptozotocin-induced insulin deficiency.
CONCLUSIONS
E2 protects β-cell survival through ERα and ERβ via ERE-independent, extra-nuclear mechanisms, as well as GPER-dependent mechanisms. The present study adds a novel dimension to estrogen biology in β-cells and identifies GPER as a target to protect islet survival.
Preserving insulin secretion by the pancreatic β-cells is critical in both type 1 and the late stages of type 2 diabetes. In type 1 diabetes, the death of insulin-producing β-cells of the pancreas by apoptosis leads to insulin dependence. Insulin replacement therapy by pancreatic islet transplantation is a treatment that most closely replicates normal physiological conditions for treatment of type 1 diabetes (1), but its effectiveness is reduced by the loss of functional islet mass from apoptosis, impairing the survival of islet grafts. Similarly, in the late stages of type 2 diabetes, evidence of β-cell apoptosis is documented in animal models (2,3) and in humans (4). Thus, in the absence of novel immunotherapy and antiapoptotic drugs, novel strategies to protect insulin-producing cells in vivo represent a major opportunity for therapeutic intervention. One promising approach to protect β-cells from apoptosis involves the cytoprotective actions of estrogens. In addition to its reproductive functions, the female sex steroid 17β-estradiol (E2) is a neuroprotective hormone against multiple oxidative and proapoptotic insults in vivo and in vitro, acting via classic estrogen receptors (rev. in 5). Recently, we reported that E2 protects β-cells from streptozotocin (STZ)-induced apoptosis in mice of both sexes via the estrogen receptor (ER)-α (6). In cultured mouse and human islets, E2 has potent antiapoptotic properties against proinflammatory cytokines and reactive oxygen species (6,7). E2 acts via classic estrogen receptors, ERα and ERβ (8). In ERα-deficient female mice, E2 still partially protects β-cell survival via an alternative pathway (6), suggesting that ERβ may mediate the effects of E2 in the absence of ERα.
The G protein–coupled estrogen receptor (GPER), also known as GPR30, has been recognized as a membrane receptor for estrogens that mediates nongenomic signals (9). GPER is expressed in islets and has recently been suggested to mediate the estrogenic effect on islet insulin release (10). We analyzed the contribution of ERα, ERβ, and GPER to islet survival. We used mice individually deficient in ERα, ERβ, ERα and ERβ, and GPER; a mouse lacking ERα binding to the ERE; and human islets. These mutant mice and islets were exposed to oxidative stress using STZ or hydrogen peroxide, respectively, in combination with the use of specific pharmacological probes.
RESEARCH DESIGN AND METHODS
Generation of mutant mice.
The generation of αERKO−/−, βERKO−/−, and GPERKO−/− mice has previously been described (6,11). Mice were studied between 7–9 weeks of age. Mice with a mutation of the DNA-binding domain of ERα (AA allele) that eliminates ERα binding to the ERE (αERKOAA/+) were kindly provided by Larry Jameson (12). The αERKOAA/− mice were generated by crossing heterozygote male αERKOAA/+ with heterozygote female ERα null mice (αERKO+/−). Because female αERKOAA/+ are infertile, they cannot be crossed with male αERKOAA/+, and therefore αERKOAA/AA mice cannot be generated. All animal experiments were approved by Northwestern University Animal Care and Use Committee in accordance with the National Institutes of Health Guide for the Care and Use of Animals.
Metabolic studies.
Glucose tolerance tests (2 g/kg) and corresponding area under the curve for glucose (minus basal) and glucose-stimulated insulin secretion (3 g/kg) were performed as described (6). Serum insulin concentrations were measured by ELISA using mouse standards (Crystal Chem, Chicago, IL) (6).
Exogenous substance infusion and induction of experimental diabetes.
Diabetes was induced in 8-week-old female mice by a single intraperitoneal injection of 150 mg/kg of STZ as described (6). Blood glucose was measured every 48 h after STZ injection with a glucose monitor. At day 8 after STZ injection, mice were killed and pancreata collected.
Pancreas insulin concentration.
Whole pancreata were collected, weighed, and homogenized in acid/ethanol. Pancreas homogenates were centrifuged, and the supernatant was used to measure pancreas insulin concentration by radioimmunoassay (6).
Islet culture and compounds stimulation.
Islet isolation was performed as previously described (6). Islets were cultured in phenol red–free RPMI medium containing 11 mmol/l glucose, 10% charcoal stripped FBS, 1 mmol/l glutamine, 100 units/ml penicillin, 100 μg/ml streptomycin. Islets were incubated with E2 (10−8 M, steraloids), propyl-pyrazole-triol (PPT, 10−8 M) (13), methylpiperidino-pyrazole (MPP, 10−7 M) (14), diarylpropionitrile (DPN, 10−8 M) (15), tetrahydrochrysene (THC, 10−7 M) (16), estrogen-dendrimer conjugate (EDC) (10−8 M) (17) or G-1 (10−7 M) (18) for 48 h. Ethanol was used as the vehicle. After 48 h estrogen receptor ligands treatment, islets were exposed to H2O2 (100 μmol/l, Sigma) for the last 5 h before assessment of apoptosis. PPT, EDC, and DPN were a gift from John A. Katzenellenbogen, University of Illinois at Urbana.
Antibodies.
In immunohistochemical studies, the following primary antibodies were used: guinea pig anti-insulin (1:1,000, Linco Research), rabbit anti-glucagon (1:1,000, Linco Research), mouse anti-ERα (1:100, 1D5, Zymed Laboratories), and goat anti-ERβ (1:100, Y-19, Santa Cruz Biotechnology). Where tyramide signal amplification was performed, biotinylated secondary antibodies were used. Where signal was not amplified, FITC- or Cy3-conjugated secondary antibodies were used. Secondary antibodies were raised in donkey (Jackson ImmunoResearch) and used at a 1:200 dilution.
Immunohistochemistry.
Mouse pancreases were fixed and processed for immunohistochemistry as described (6). For staining with ERα and ERβ, Alexa 568 tyramide signal amplification kit (Molecular Probes) was used. Sections were counterstained with DAPI (1:50,000, 5 min) before confocal visualization (Zeiss LSM 510). For MIN6 immunolabeling, cells were seeded on coverslips and grown until ∼80% confluency. Twenty four hours before fixation, cells were maintained in phenol red–free DMEM media (Sigma). Cells were treated with E2 (10−8 M) or ethanol vehicle for 1 h, washed with warm PBS, and fixed in 10% neutral-buffered formalin for 20 min. For labeling of mitochondria, live cells were incubated for 45 min with 100 nmol/l MitoTracker reagent (Molecular Probes). Cells were permeabilized (0.1% Triton X-100/PBS), blocked (1 h, 5% normal donkey serum), labeled with antibodies, counterstained, and visualized with confocal or deconvolution (Applied Precision DeltaVision) microscopy.
Measurement of apoptosis by nuclear morphology.
Apoptosis was induced by H2O2 (100 μmol/l, 5 h) in groups of 100 islets per condition. After 5 h incubation with H2O2, islets were collected and washed with PBS. Single-cell suspensions were obtained by incubating the islets in 500 μl Accutase solution (Innovative Cell Technologies, San Diego, CA) at 37°C for 15 min, followed by gentle pipetting. To score apoptosis, cells were fixed with 1.25% glutaraldehyde for 15 min at room temperature and nuclei were stained with 10 μg/ml bisbenzimide (Hoechst 33258; Sigma) for 15 min at room temperature. The percentage of cells with apoptotic nuclei (condensed or fragmented) was determined by fluorescence microscopy (E400 Nikon Eclipse). For each condition, at least 200 cells were scored in duplicate. Results are representative of at least three independent experiments.
Assay for active caspase 3.
Caspase activity was measured in mouse islets using Caspase-Glo 3/7 assay kit (Promega). Briefly, after 5 h incubation with H2O2 (100 μmol/l, Sigma), islets were centrifuged for 5 min at 1,200 rpm and transferred to a 96-well plate in 100 μl medium. Islets were then lysed with 100 μl Caspase-Glo 3/7 reagent and incubated at room temperature for 30 min. Luciferase activity was measured using a Synergy 2 Multi-Mode Microplate Reader (BioTek). Values are reported as relative luciferase units corrected for total protein concentration.
Plasmid transfection and luciferase assay.
MIN6 cells were cultured in DMEM high medium and plated in 24-well plates (10 × 104 cells/well). Before the transfection, cells were cultured overnight in DMEM phenol red–free medium. Cells were incubated with the mixture of 2 μl Lipofectamine 2000 (Invitrogen) and 0.8 μg of a reporter construct containing an ERE (ERE-Luc) for 6 h (19). Cells were treated for 24 h with either E2 (10−8M), E2-BSA (10−8M), EDC (10−8M), or G1 (10−7M). For measurement of luciferase activity, cells were lysed with Cell Culture Lysis Reagent (Promega). After centrifugation, 2–6 μg of protein were used in a Luciferase Assay System (Promega). Values are reported as relative luciferase units corrected for total protein concentration.
Western blot analysis.
Cells were homogenized in lysis buffer as described (20). Thirty micrograms of protein was analyzed by SDS-PAGE. Cytoplasmic and nuclear fractions were extracted using a commercial kit (Pierce Biotechnology), and ERα expression was determined by immunoblotting with mouse anti-ERα antibody (1:100, MC-20, Santa Cruz Biotechnology). GPER expression was determined by immunoblotting with a polyclonal goat anti-GPR30 antibody (1:400, Santa Cruz Biotechnology).
Statistical analysis.
Results are presented as means ± SE unless otherwise stated. Data were analyzed using the unpaired Student's t test. Cumulative incidence of diabetes was determined by Kaplan-Meier estimates, and statistical analysis of differences was determined by log-rank test. A value of P < 0.05 was considered significant.
RESULTS
ERα protects islet survival independently of the classic estrogen response element.
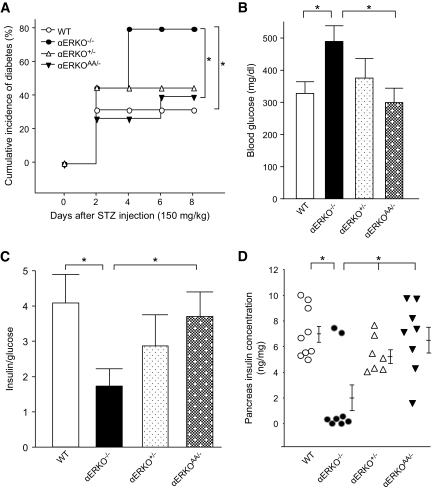
We previously reported that ERα-deficient mice lose cytoprotection from circulating E2 and are predisposed to β-cell apoptosis when their islets are exposed to oxidative stress in vivo (6). In the classic estrogen receptor signaling pathway, E2-activated ERα binds as a homodimer to either an ERE or a non-ERE tethered promoter to initiate gene transcription (21). To investigate whether an ERα-ERE or non-ERE signaling mechanism protects β-cell survival in vivo, we used an ERα knock-in mouse with a mutation of the DNA-binding domain of ERα that eliminates ERα binding to the ERE (αERKOAA/−) (12). To produce β-cell apoptosis in vivo, we used a single high-dose injection of STZ (150 mg/kg) in female mice. STZ provokes an increase in islet reactive oxygen species as can be encountered after exposure to hyperglycemia or cytokines in type 1 diabetes and type 2 diabetes (22,23). We compared the sensitivity to STZ in αERKO AA/− mice with one knock-in AA allele with that of αERKO+/− and αERKO−/− mice with one or no functional ERα allele. This enabled us to study the effect of the unique AA allele and non-ERE signaling on the predisposition to β-cell apoptosis in vivo. Compared with the null αERKO−/−, the αERKO+/− showed a minor predisposition to STZ-induced insulin-deficient diabetes, confirming a minor effect of ERα gene dosage (6). A single AA allele without ERE binding was sufficient to protect αERKO AA/− female mice from STZ-induced insulin-deficient diabetes to an extent similar to that observed in αERKO+/− mice (Fig. 1A–D), demonstrating that ERα protection of β-cell survival is independent of the classic ERE-dependent pathway.
FIG. 1.
ERα protects islet survival via ERE-independent pathway. A: Cumulative incidence of diabetes (random-fed blood glucose >250 mg/dl) in wild-type, αERKO−/−, αERKO+/−, and αERKOAA/− mice (n = 10–15) after STZ challenges (150 mg/kg). B: Random-fed blood glucose was measured after STZ injection (day 8). C: The ratio of random-fed insulin (pg/ml)/glucose (mg/dl) at day 8 was used as an index of insulin deficiency. Values represent the means ± SE. D: Pancreatic insulin concentration was measured after STZ injection (day 8). Values are represented as scatter plot. *P < 0.05. WT, wild type.
ERα prevents islet apoptosis via rapid, extranuclear actions.
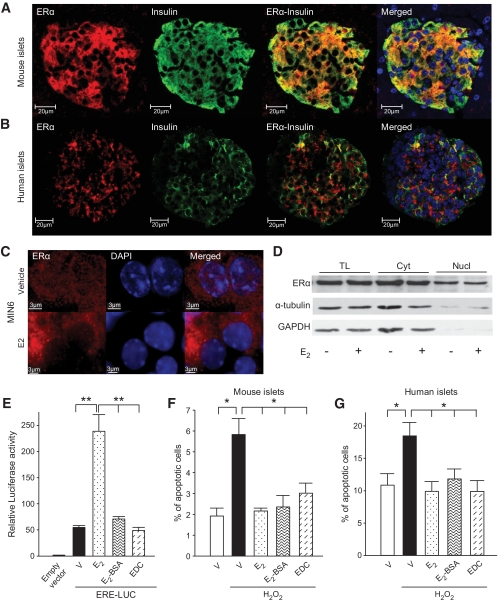
Because activation of ERα protects β-cell survival via an ERE-independent mechanism, we explored the possibility that ERα favors survival through rapid, extranuclear actions. We observed colocalization of ERα with insulin-producing β-cells in mouse and human pancreatic islets with a predominant cytosolic localization (Fig. 2A and B). In mouse MIN6 β-cells, we observed ERα predominantly in the cytosol, in close vicinity to the plasma membrane and mitochondria (Fig. S1A and B, available in an online appendix at http://diabetes.diabetesjournals.org/content/early/2009/07/08/db09-0257/suppl/DC3). The predominant cytosolic localization of ERα in β-cells, although it exhibits a predominant nuclear localization in reproductive tissues (supplemental Fig. S1C, available in an online appendix), suggested that ERα regulates β-cell biology through extranuclear signaling pathways. To explore that hypothesis, we first studied the subcellular localization of ERα by confocal microscopy in MIN6 β-cells. In the absence of E2, ERα showed a dual cytosolic and nuclear immunoreactivity (Fig. 2C). E2 stimulation for 1 h provoked a disappearance of nuclear ERα immunoreactivity and an increased density of ERα extranuclear signal (Fig. 2C). This pattern was similarly observed using two different ERα antibodies (supplemental Fig. S2, available in an online appendix). To explore the mechanism of alteration in ERα signal between cytosol and nucleus, we performed subcellular fractionation in MIN6 cells. After E2 stimulation, we observed no change in the amount of the classic 67kDa ERα isoform between the nucleus and cytosolic fractions (Fig. 2D), demonstrating that there is no transfer of ERα from the nucleus to the cytosol. The E2-induced alteration of ERα nuclear signal into irregular punctuate structures was interpreted as an organization of nuclear speckles symbolic of active transcription start sites in fluorescent microscopy (24). A similar appearance was observed after E2 stimulation in a classic model of ERα nuclear actions, the human breast cancer MCF7 cells (supplemental Fig. S1E, available in an online appendix). We hypothesized that the increased ERα extranuclear signal upon E2 stimulation was a sign of ERα extranuclear and antiapoptotic signaling that is dissociated from ERα nuclear presence. To address this latter possibility, we studied E2 protection from apoptosis in cultured islets after acute exposure to H2O2 (100 μmol/l) to mimic the oxidative injury observed in vivo after exposure of islets to a single high dose of STZ (25). We used two pharmacological probes specific for estrogen receptor nongenomic actions: 1) A membrane-impermeant preparation of E2, which is bound to a macromolecule, namely, BSA (E2–BSA). The response to the membrane-impermeant compounds is used as a gold standard to define membrane-initiated responses (26). 2) A novel EDC that activates cytosolic estrogen receptor pathways but remains outside the nucleus and is ineffective in stimulating transcription of estrogen target genes (17). Compared with E2, neither E2-BSA nor EDC showed transcriptional activity in MIN6 β-cells on a reporter construct containing an ERE (Fig. 2E). Exposure of mouse and human islets to E2, E2-BSA, or EDC produced a similar and robust protection against H2O2-induced apoptosis (Fig. 2F and G).
FIG. 2.
E2 prevents β-cell apoptosis via an extranuclear estrogen receptor. A: Female pancreas section showing a single islet with ERα immunofluorescent staining in β-cells (red). The insulin (green), nuclear (DAPI, blue), and triple staining (merge) are shown. B: Immunofluorescent staining of ERα in cultured human islets. C: Immunofluorescent staining of ERα (MC20) in MIN6 cells treated with vehicle or E2 (10−8M) for 1 h and imaged with deconvolution microscopy. D: Subcellular fractionation showing ERα expression by Western blotting from total lysates (TL), cytoplasmic (Cyt), and nuclear (Nucl) extracts of MIN6 cells after E2 treatment for 1 h. E: Relative luciferase activity in MIN6 cells transfected with an ERE reporter construct and treated with E2, EDC, or E2-BSA (10−8M). F: Percentage of apoptotic cells in cultured mouse islets. G: Percentage of apoptotic cells in cultured human islets. Islets from (F) and (G) were treated with E2, EDC, or E2-BSA (10−8M) for 48 h, followed by exposure to H2O2 (100 μmol/l) for the last 5 h. Apoptosis was assessed by nuclear fragmentation. Values represent the means ± SE of five independent experiments. (A high-quality digital representation of this figure is available in the online issue.)
Elimination of ERβ mildly predisposes to STZ-induced diabetes.
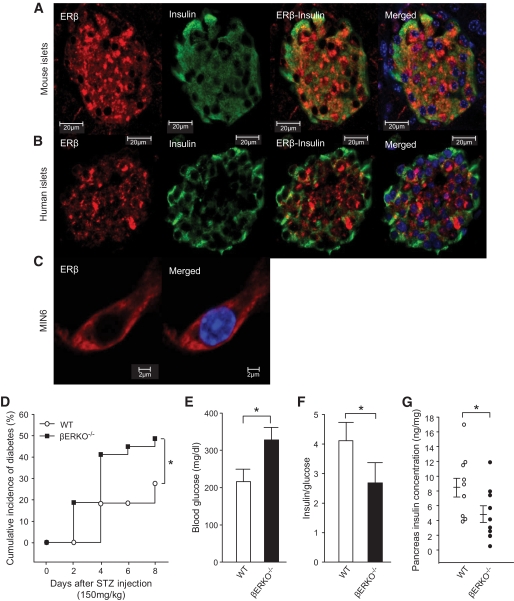
In αERKO−/− mice, E2 still partially protects β-cell survival via an alternative pathway (6), making ERβ a candidate for mediating the effects of E2 in the absence of ERα. We observed ERβ expression in mouse and human pancreatic β-cells and MIN6 cells with a cytosolic localization (Fig. 3A–C), although it exhibits a predominant nuclear localization in reproductive tissues (supplementary Fig. S1D, available in an online appendix). ERβ localization was not influenced by E2 treatment (supplementary Fig. S3, available in an online appendix). We sought to determine whether ERβ exerts E2 protection of β-cell survival using ERβ-deficient mice (βERKO−/− mice). In basal condition, female βERKO−/− mice showed no abnormality of fasting and fed blood glucose (supplemental Table 1, available at an online appendix). After exposure to STZ, as previously described (6), female wild-type mice were protected and retained normal blood glucose and pancreas insulin concentrations; conversely, female βERKO−/− mice were vulnerable to STZ and exhibited mild hyperglycemia and moderate insulin deficiency compared to wild type (Fig. 3 D–G). Male wild-type and βERKO−/− mice were exposed to a less diabetogenic single low dose of STZ (100 mg/kg). Such a low dose of STZ caused a moderate increase in diabetes incidence in male wild-type mice, while it predisposed to insulin-deficient diabetes in male βERKO−/− mice (supplementary Fig. S4, available in an online appendix).
FIG. 3.
βERKO–/– mice are mildly predisposed to STZ-induced diabetes. A: Female pancreas section showing a single mouse islet with ERβ immunofluorescent staining in β-cells (red). The insulin (green), nuclear (DAPI, blue), and triple staining (merge) are shown. B: Immunofluorescence staining of ERβ in cultured human islet. C: Immunofluorescence staining of ERβ in MIN6 cells. D: Cumulative incidence of diabetes in female βERKO–/– mice after STZ challenge (n = 8–14). E: Random-fed blood glucose (day 8). F: The ratio of random-fed of insulin and glucose (day 8). G: Pancreas insulin concentration (day 8). *P < 0.05. WT, wild type. (A high-quality digital representation of this figure is available in the online issue.)
E2 protects islet survival in the absence of ERα and ERβ in mice.
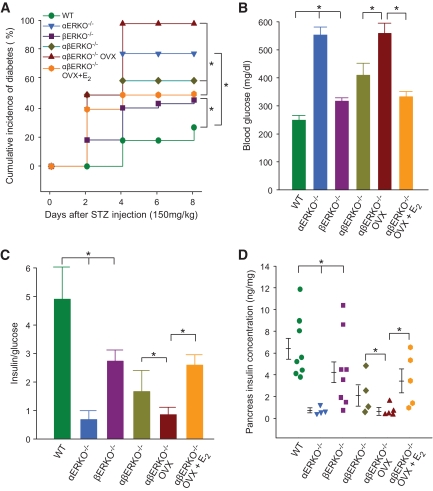
Because individual deletion of ERα and ERβ impairs islet resistance to STZ in mice, we hypothesized that combined elimination of both ERα and ERβ would synergize to abolish E2 protection of islet survival. We thus compared the role of ERα and ERβ in islet survival from STZ injury in the single αERKO−/−, βERKO−/−, and the double αβERKO−/− littermate female mice. We confirmed that exposure to STZ caused a more severe predisposition to insulin-deficient diabetes in female αERKO−/− mice compared to littermate βERKO−/− mice (Fig. 4A–D). However, after exposure to STZ, the dual absence of ERα and ERβ in the double αβERKO−/− did not aggravate the predisposition to insulin-deficient diabetes, suggesting that in the absence of both estrogen receptors, E2 still protects via an alternative pathway (Fig. 4A–D). Thus, to determine the extent to which, in the absence of ERα and ERβ, circulating E2 could still protect the αβERKO−/−, we suppressed endogenous E2 production by ovariectomy (OVX). OVX further aggravated the predisposition to STZ in female αβERKO−/− mice while, conversely, E2 treatment of αβERKO−/− OVX mice restored protection from STZ-induced insulin-deficient diabetes (Fig. 4A–D). Similar findings were observed in male mice. The double αβERKO−/− mice did not show a more severe predisposition to STZ compared to the single αERKO−/− and βERKO−/− male littermates (supplementary Fig. S5, available in an online appendix).
FIG. 4.
E2 protects female mice from STZ-induced diabetes in the absence of ERα and ERβ. A: Cumulative incidence of diabetes in female αERKO−/−, βERKO−/−, and double αβERKO−/− mice after STZ challenge. B: Random-fed blood glucose (day 8). C: The ratio of random-fed insulin and glucose (day 8). D: Pancreas insulin concentration (day 8). WT, wild type.
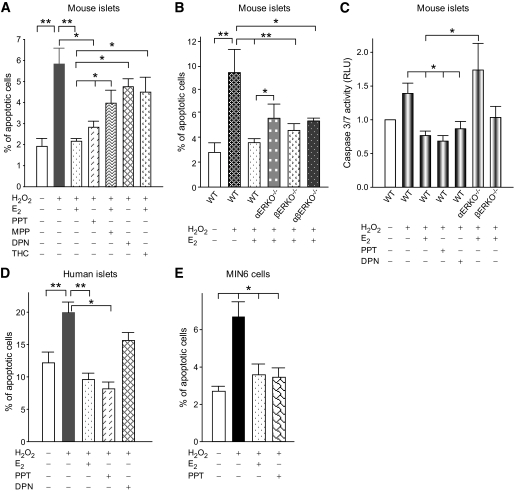
E2 protects islet survival in the absence of ERα and ERβ in vitro.
The individual contribution of ERα and ERβ to islet survival was next examined in cultured wild-type mouse islets using PPT and DPN, which are ERα- and ERβ- selective agonists, respectively (13,15). After induction of apoptosis with H2O2, we observed a similar prevention of apoptosis by E2 and PPT treatments (Fig. 5A); accordingly, E2 protection was impaired using the ERα-selective antagonist MPP (Fig. 5A) (14). DPN provided only minor islet protection, but E2 protection from islet apoptosis was impaired using the ERβ-selective antagonist THC (Fig. 5A). We next examined E2 protection of islet survival in ERα- and ERβ-deficient mouse islets. H2O2 provoked an increase in apoptotic cells from wild-type islets, which was prevented by E2 (Fig. 5B). E2 protection was impaired in αERKO−/− islets. Conversely, E2 protection was retained in βERKO−/− islets. In addition, consistent with the in vivo data, in the absence of both ERα and ERβ, E2 protection of apoptosis was retained in αβERKO−/− islets (Fig. 5B). E2 antiapoptotic action via ERα and ERβ was further investigated using a luminescent assay for activated caspase-3, the “executioner” of apoptosis. E2 prevented caspase-3 activation that was mimicked by PPT and DPN treatment. However, E2 prevention of caspase-3 activation was abolished in αERKO−/− islets but was retained in βERKO−/− islets (Fig. 5C). We also observed that E2, PPT, and to a lesser extent DPN protect human islets from H2O2-induced apoptosis (Fig. 5D). Lastly, the antiapoptotic protection of E2 and PPT was reproduced in MIN6 cells demonstrating a direct effect on β-cells (Fig. 5E). Thus, E2 protects β-cell survival in vivo and in cultured islets via ERα and ERβ, with a predominant ERα effect. However, in the absence of ERα and ERβ, E2 still protects β-cell survival.
FIG. 5.
E2 protects cultured islets from apoptosis in the absence of ERα and ERβ. A: Percentage of apoptotic cells in cultured mouse islets incubated with vehicle, E2 (10−8M), PPT (10−8M), MPP (10−7M), DPN (10−8M), or THC (10−7M) for 48 h, followed by exposure to H2O2 (100 μmol/l) for the last 5 h. B: Percentage of apoptotic cells in wild-type, αERKO−/−, βERKO−/−, and αβERKO−/− mouse islets. C: Caspase 3/7 activity was measured in mouse islets by luminescence. D: Percentage of apoptotic cells in cultured human islets and (E) in MIN6 cells. Values represent three independent replicate experiments. *P < 0.05, **P < 0.01. WT, wild type.
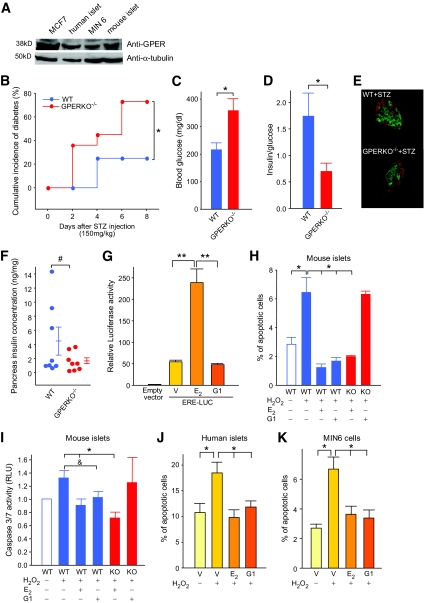
GPER is important for islet survival.
Recently, GPER, also known as GPR30, has been recognized as a membrane receptor for estrogens that mediates nongenomic, rapid signals (9,27). We observed that GPER protein is expressed in mouse islets, human islets, and MIN6 cells (Fig. 6A). Because E2 protects β-cell survival via a membrane-initiated mechanism (Fig. 2F and G) and in the absence of ERα and ERβ (Fig. 4 and 5), we investigated the possibility that E2 cytoprotection is mediated via GPER. We investigated the importance of GPER using mice deficient in the receptor (GPERKO−/−). When studied on a regular chow, female wild-type and GPERKO−/− littermate mice showed similar fasting and fed blood glucose and serum insulin concentrations (Table 1). We observed no difference between wild-type and female GPERKO−/− mice with regard to intraperitoneal glucose tolerance, glucose-stimulated insulin secretion, fed pancreas insulin concentrations (Table 1), and islet architecture (supplementary Fig S6, available in an online appendix). However, after exposure to STZ, compared to wild-type female mice, GPERKO −/− female mice lost the protection of endogenous E2 and were predisposed to insulin-deficient diabetes leading to loss of β-cells and decrease in pancreas insulin concentration (Fig. 6B–F). The predisposition to STZ-induced diabetes was not observed in male GPERKO−/− mice (supplementary Fig S7, available in an online appendix). We next studied the importance of GPER in cultured pancreatic islets from wild-type and GPERKO−/− mice. We used the GPER agonist G1, which selectively activates GPER in a cellular environment containing ERα and ERβ (18). G1 showed no transactivating activity on ERE-dependent gene expression in β-cells (Fig. 6G). We found that G1 prevents apoptosis as efficiently as E2 in wild-type mouse islets and human islets (Fig. 6H and J). We observed that E2 cytoprotection, measured either via nuclear morphology or caspase-3 activation, was retained in mouse GPERKO−/− islets compared to wild-type islets, demonstrating that ERα can compensate for the absence of GPER in GPERKO−/− islets (Fig. 6H and I). Conversely, G1 cytoprotection was abolished in GPERKO−/− islets, demonstrating its high selectivity toward GPER compared with ERα and ERβ (Fig. 6H and I). We confirmed that G1 protection from apoptosis was observed in human islets (Fig. 6J) and that G1 directly acts on β-cells (Fig. 6K). Thus, E2 protection is mediated via ERα, ERβ, and GPER with a direct effect on the islets and with a predominant ERα effect, via ERE-independent, extranuclear, and rapid mechanisms.
FIG. 6.
GPER is important to islet survival. A: Protein expression of GPER in MCF7 cells, MIN6 cells, mouse islets, and human islets was measured by Western blotting. B: Cumulative incidence of diabetes in female wild-type and GPERKO−/− mice (n = 8–11) after STZ challenge (150 mg/kg). C: Random-fed blood glucose (day 8). D: The ratio of random-fed of insulin and glucose (day 8). E: Representative pancreatic sections showing immunofluorescent staining for insulin (green) and glucagon (red) was performed in the wild-type and GPERKO−/− female mice after STZ injection (day 8). F: Pancreas insulin concentration (day 8). G: Relative luciferase activity in MIN6 cells transfected with an ERE reporter construct and treated with E2 (10−8M) or G1 (10−7M). H: Percentage of apoptotic cells in cultured wild-type and GPERKO−/− islets. I: Caspase 3/7 activity measured in cultured wild-type and GPERKO−/− mouse islets. J: Percentage of apoptotic cells in cultured human islets and (K) in MIN6 cells. Islets and cells were treated with E2, G1 for 48 h, followed by exposure to H2O2 (100 μmol/l) for the last 5 h. *P < 0.05, **P < 0.01, #P = 0.15 and P = 0.09. WT, wild type; KO, knockout. (A high-quality digital representation of this figure is available in the online issue.)
TABLE 1.
Metabolic parameters in wild-type and GPERKO−/− female mice
| Wild type | GPERKO−/− | P | |
|---|---|---|---|
| Body weight | 19.5 ± 0.7 | 19.1 ± 0.5 | n.s. |
| Fasting glucose (mg/dl) | 46.9 ± 2.8 | 50.7 ± 2.1 | n.s. |
| Fed glucose (mg/dl) | 148.9 ± 7.8 | 144.9 ± 5.1 | n.s. |
| Fasting insulin (ng/ml) | 0.6 ± 0.1 | 0.7 ± 0.1 | n.s. |
| Fed insulin (ng/ml) | 1.0 ± 0.2 | 1.1 ± 0.2 | n.s. |
| Pancreas insulin concentration (ng/mg) | 10.3 ± 1.2 | 10.7 ± 0.9 | n.s. |
| GSIS (30 min after glucose stimulation, ng/ml) | 1.05 ± 0.1 | 1.1 ± 0.05 | n.s. |
| Glucose tolerance test area under the curve (mg/dl × min/1,000) | 15.4 ± 2.3 | 13.6 ± 5.8 | n.s. |
Results represent the means ± SE. n.s., nonsignificant.
DISCUSSION
Recently, we reported that E2 protects β-cell survival in mice of both sexes via ERα (6). Many actions of E2, including the feminizing effects, are mediated via a classic and nuclear ERα-activating transcription through an ERE (21). Here, using a mouse model lacking ERE signaling, we show that ERα cytoprotection of islets in vivo is ERE independent. Both ERα and ERβ show cytosolic localization in β-cells, and we find that E2 favors β-cell survival via activation of extranuclear and perhaps membrane estrogen receptors with a predominant ERα effect. This finding extends the observation of Kousteni et al. (28), suggesting that the antiapoptotic actions of E2 in osteoblasts and fibroblasts are mediated via the ligand-binding domain of ERα and ERβ with similar efficiency, and can be dissociated from the transcriptional activity of the receptors. Thus, unlike in classic estrogen receptor genomic actions where E2-activated ERα and ERβ signal in opposite ways from an AP1 element (29), with regard to extranuclear, antiapoptotic actions, ERα and ERβ signal survival in similar direction. Indeed, the coexpression of both ERα and ERβ in β-cells does not demonstrate evidence of ERβ antagonism of ERα action because pharmacological inhibition or genetic elimination of ERβ in islets does not enhance E2 cytoprotection via ERα. However, despite the apparent antiapoptotic action of ERα and ERβ, the combined elimination of these receptors does not synergize to abolish E2 cytoprotection after exposure of islets to acute oxidative stress. This suggests that ERα and ERβ favor islet survival using nonredundant and distinct cellular pathways.
The second important finding is that the membrane G protein–coupled receptor, GPER, favors islet survival. GPER is a 7-transmembrane orphan G protein–coupled receptor that responds to E2 with rapid cellular signaling (9). GPER has been localized to either the plasma membrane (30) or the endoplasmic reticulum (9). The physiological function of GPER in vivo is still largely unknown. The existence of a membrane G protein–coupled receptor unrelated to ERα and ERβ and that may be GPER has been reported in β-cells (31,32). Recently, Martensson et al. (10) reported that GPER-deficient mice display altered E2-stimulated insulin release from isolated islets associated with impaired glucose-stimulated insulin secretion in vivo, suggesting that GPER is involved in islet biology. Because E2 protects β-cell from apoptosis (6,7) and recent studies have implicated GPER in cell survival (33,34), we hypothesized that GPER favors β-cell survival. We observe that GPER deficiency in female mice does not alter β-cell function or glucose homeostasis on a normal rodent chow. This different phenotype with the previous report (10) may be related to the different genetic background of the mice used in both studies and/or the greater ability of our GPERKO−/− mice to compensate for the loss of GPER via ERα. We find that elimination of GPER predisposes to STZ-induced islet apoptosis after exposure to acute oxidative stress in female mice. In addition, we show that pharmacological activation of GPER by G1 is efficient in protecting oxidative stress–induced apoptosis in cultured islets. The observation that G1 cytoprotection is lost in cultured GPER-deficient islets further supports the functional significance of GPER in islet survival. However, the maintenance of E2 cytoprotection from apoptosis in cultured GPER-deficient islets demonstrates that ERα and ERβ can compensate for GPER deficiency. Conversely, we confirm that E2 cytoprotection is impaired in ERα-deficient islets and can be compensated, only partially, by GPER or ERβ. Thus, ERα is the major E2 receptor to favor islet survival in mice.
A recent report has challenged the initial concept that GPER is indeed an estrogen receptor in vivo based on the observation that loss of GPER in mice does not alter estrogenic responses in reproductive organs that express GPER (35). However, other investigators reported that GPER mediates estrogen action in mice. For example, GPER is necessary for the normal estrogenic response on longitudinal bone growth (36). In addition, GPER-deficient mice lose E2-stimulated insulin release, arguing that GPER mediates E2 response in pancreatic islets (10). Thus, when referring to in vivo models, GPER appears to mediate E2 response in several tissues.
E2 binds a membrane-localized ERα, which signals through a large protein signalosome at the plasma membrane. This complex consists of scaffold proteins (caveolin-1, MNAR), linker proteins (Shc), nonreceptor tyrosine kinases (Src), threonine/serine kinases (p85 subunit of PI3 kinase, Akt), and growth factor receptors (EGFR, IGF1R) (37). It is possible that membrane-localized ERα signals by recruiting GPER from the endoplasmic reticulum or the plasma membrane (38). Our studies do not rule out this latter possibility because a membrane-impermeant formulation of E2 (E2-BSA) is as efficient as E2 or the selective ERα agonist PPT in protecting islet survival. Thus, E2 could either bind a membrane ERα, which would then signal through GPER, or both ERα and GPER, together utilizing the same signaling pathway. Studies are ongoing in our laboratory to dissect the signaling pathways of estrogen receptor cytoprotection in β-cells.
The protection by E2 described here in vivo in global estrogen receptor–deficient mice reflects the loss of estrogen receptor signaling in all tissues that express these receptors. Therefore, alteration in interorgan communication and metabolic regulation because of loss of estrogen receptors in a distant organ may also indirectly impair islet survival via circulating factors without a direct effect of E2 on β-cells. For example, the predisposition to STZ observed in vivo in estrogen receptor–deficient mice could be enhanced by subtle differences in glucose concentration or adipose production of proinflammatory cytokines (39). However, the therapeutic effects of estrogen receptor ligands obtained in cultured human islets have therapeutic implications in islet transplantation. The feminizing effects of estrogens limit their clinical application to protect islet survival in men and in some women. However, the observation that E2 utilizes extranuclear and membrane pathways that are sex nonspecific may help identify and develop new ligands that protect β-cells and allow retention of the beneficial effects of sex hormones in islets while lacking the mitogenic actions predisposing to hormone-dependent cancers.
In summary, E2 plays a major role in protecting β-cells from apoptosis that is mediated through ERα, ERβ, and GPER. The present study adds a novel dimension to estrogen biology in β-cells and identifies GPER as a new target to protect islet survival.
Acknowledgments
This work was supported by National Institutes of Health (NIH) Grants RO1 DK074970 and P50 HD044405, the Juvenile Diabetes Research Foundation, and the March of Dimes to F.M.-J. C.L.M. was the recipient of a Juvenile Diabetes Research Foundation Post-Doctoral Fellowship.
No potential conflicts of interest relevant to this article were reported.
We are grateful to John and Benita Katzenellenbogen for providing PPT, DPN, and EDC compounds. We acknowledge the Islet Cell Resource Consortium, funded by NIH and administered by the ABCC, for providing human islets.
Footnotes
The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.Shapiro AM, Lakey JR, Ryan EA, Korbutt GS, Toth E, Warnock GL, Kneteman NM, Rajotte RV: Islet transplantation in seven patients with type 1 diabetes mellitus using a glucocorticoid-free immunosuppressive regimen. N Engl J Med 2000;343:230–238 [DOI] [PubMed] [Google Scholar]
- 2.Pick A, Clark J, Kubstrup C, Levisetti M, Pugh W, Bonner-Weir S, Polonsky KS: Role of apoptosis in failure of β-cell mass compensation for insulin resistance and β-cell defects in the male Zucker diabetic fatty rat. Diabetes 1998;47:358–364 [DOI] [PubMed] [Google Scholar]
- 3.Donath MY, Gross DJ, Cerasi E, Kaiser N: Hyperglycemia-induced β-cell apoptosis in pancreatic islets of Psammomys obesus during development of diabetes. Diabetes 1999;48:738–744 [DOI] [PubMed] [Google Scholar]
- 4.Butler AE, Janson J, Bonner-Weir S, Ritzel R, Rizza RA, Butler PC: β-cell deficit and increased β-cell apoptosis in humans with type 2 diabetes. Diabetes 2003;52:102–110 [DOI] [PubMed] [Google Scholar]
- 5.Behl C: Oestrogen as a neuroprotective hormone. Nat Rev Neurosci 2002;3:433–442 [DOI] [PubMed] [Google Scholar]
- 6.Le May C, Chu K, Hu M, Ortega C, Simpson E, Korach K, Tsai M, Mauvais-Jarvis F: Estrogens protect pancreatic β-cells from apoptosis and prevent insulin-deficient diabetes mellitus in mice. Proc Natl Acad Sci U S A 2006;103:9232–9237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Contreras JL, Smyth CA, Bilbao G, Young CJ, Thompson JA, Eckhoff DE: 17β-Estradiol protects isolated human pancreatic islets against proinflammatory cytokine-induced cell death: molecular mechanisms and islet functionality. Transplantation 2002;74:1252–1259 [DOI] [PubMed] [Google Scholar]
- 8.Hewitt SC, Harrell JC, Korach KS: Lessons in estrogen biology from knockout and transgenic animals. Annu Rev Physiol 2005;67:285–308 [DOI] [PubMed] [Google Scholar]
- 9.Revankar CM, Cimino DF, Sklar LA, Arterburn JB, Prossnitz ER: A transmembrane intracellular estrogen receptor mediates rapid cell signaling. Science 2005;307:1625–1630 [DOI] [PubMed] [Google Scholar]
- 10.Martensson UE, Salehi SA, Windahl S, Gomez MF, Sward K, Daszkiewicz-Nilsson J, Wendt A, Andersson N, Hellstrand P, Grande PO, Owman C, Rosen CJ, Adamo ML, Lundquist I, Rorsman P, Nilsson BO, Ohlsson C, Olde B, Leeb-Lundberg LM: Deletion of the G protein-coupled receptor 30 impairs glucose tolerance, reduces bone growth, increases blood pressure, and eliminates estradiol-stimulated insulin release in female mice. Endocrinology 2009;150:687–698 [DOI] [PubMed] [Google Scholar]
- 11.Wang C, Dehghani B, Magrisso IJ, Rick EA, Bonhomme E, Cody DB, Elenich LA, Subramanian S, Murphy SJ, Kelly MJ, Rosenbaum JS, Vandenbark AA, Offner H: GPR30 contributes to estrogen-induced thymic atrophy. Mol Endocrinol 2008;22:636–648 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jakacka M, Ito M, Martinson F, Ishikawa T, Lee E, Jameson J: An estrogen receptor (ER)α deoxyribonucleic acid-binding domain knock-in mutation provides evidence for nonclassical ER pathway signaling in vivo. Mol Endocrinol 2002;16:2188–2201 [DOI] [PubMed] [Google Scholar]
- 13.Stauffer SR, Coletta CJ, Tedesco R, Nishiguchi G, Carlson K, Sun J, Katzenellenbogen BS, Katzenellenbogen JA: Pyrazole ligands: structure-affinity/activity relationships and estrogen receptor-α-selective agonists. J Med Chem 2000;43:4934–4947 [DOI] [PubMed] [Google Scholar]
- 14.Sun J, Huang YR, Harrington WR, Sheng S, Katzenellenbogen JA, Katzenellenbogen BS: Antagonists selective for estrogen receptor α. Endocrinology 2002;143:941–947 [DOI] [PubMed] [Google Scholar]
- 15.Meyers MJ, Sun J, Carlson KE, Marriner GA, Katzenellenbogen BS, Katzenellenbogen JA: Estrogen receptor-β potency-selective ligands: structure-activity relationship studies of diarylpropionitriles and their acetylene and polar analogues. J Med Chem 2001;44:4230–4251 [DOI] [PubMed] [Google Scholar]
- 16.Meyers MJ, Sun J, Carlson KE, Katzenellenbogen BS, Katzenellenbogen JA: Estrogen receptor subtype-selective ligands: asymmetric synthesis and biological evaluation of cis- and trans-5,11-dialkyl- 5,6,11, 12-tetrahydrochrysenes. J Med Chem 1999;42:2456–2468 [DOI] [PubMed] [Google Scholar]
- 17.Harrington WR, Kim SH, Funk CC, Madak-Erdogan Z, Schiff R, Katzenellenbogen JA, Katzenellenbogen BS: Estrogen dendrimer conjugates that preferentially activate extranuclear, nongenomic versus genomic pathways of estrogen action. Mol Endocrinol 2006;20:491–502 [DOI] [PubMed] [Google Scholar]
- 18.Bologa CG, Revankar CM, Young SM, Edwards BS, Arterburn JB, Kiselyov AS, Parker MA, Tkachenko SE, Savchuck NP, Sklar LA, Oprea TI, Prossnitz ER: Virtual and biomolecular screening converge on a selective agonist for GPR30. Nat Chem Biol 2006;2:207–212 [DOI] [PubMed] [Google Scholar]
- 19.Nawaz Z, Lonard DM, Smith CL, Lev-Lehman E, Tsai SY, Tsai MJ, O'Malley BW: The Angelman syndrome-associated protein, E6-AP, is a coactivator for the nuclear hormone receptor superfamily. Mol Cell Biol 1999;19:1182–1189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mauvais-Jarvis F, Ueki K, Fruman DA, Hirshman MF, Sakamoto K, Goodyear LJ, Iannacone M, Accili D, Cantley LC, Kahn CR: Reduced expression of the murine p85α subunit of phosphoinositide 3-kinase improves insulin signaling and ameliorates diabetes. J Clin Invest 2002;109:141–149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Beato M: Gene regulation by steroid hormones. Cell 1989;56:335–344 [DOI] [PubMed] [Google Scholar]
- 22.Mathis D, Vence L, Benoist C: β-Cell death during progression to diabetes. Nature 2001;414:792–798 [DOI] [PubMed] [Google Scholar]
- 23.Robertson RP, Harmon J, Tran PO, Tanaka Y, Takahashi H: Glucose toxicity in β-cells: type 2 diabetes, good radicals gone bad, and the glutathione connection. Diabetes 2003;52:581–587 [DOI] [PubMed] [Google Scholar]
- 24.Lamond AI, Spector DL: Nuclear speckles: a model for nuclear organelles. Nat Rev Mol Cell Biol 2003;4:605–612 [DOI] [PubMed] [Google Scholar]
- 25.Friesen NT, Buchau AS, Schott-Ohly P, Lgssiar A, Gleichmann H: Generation of hydrogen peroxide and failure of antioxidative responses in pancreatic islets of male C57BL/6 mice are associated with diabetes induced by multiple low doses of streptozotocin. Diabetologia 2004;47:676–685 [DOI] [PubMed] [Google Scholar]
- 26.Zheng J, Ali A, Ramirez VD: Steroids conjugated to bovine serum albumin as tools to demonstrate specific steroid neuronal membrane binding sites. J Psychiatry Neurosci 1996;21:187–197 [PMC free article] [PubMed] [Google Scholar]
- 27.Maggiolini M, Vivacqua A, Fasanella G, Recchia AG, Sisci D, Pezzi V, Montanaro D, Musti AM, Picard D, Ando S: The G protein-coupled receptor GPR30 mediates c-fos up-regulation by 17β-estradiol and phytoestrogens in breast cancer cells. J Biol Chem 2004;279:27008–27016 [DOI] [PubMed] [Google Scholar]
- 28.Kousteni S, Bellido T, Plotkin LI, O'Brien CA, Bodenner DL, Han L, Han K, DiGregorio GB, Katzenellenbogen JA, Katzenellenbogen BS, Roberson PK, Weinstein RS, Jilka RL, Manolagas SC: Nongenotropic, sex-nonspecific signaling through the estrogen or androgen receptors: dissociation from transcriptional activity. Cell 2001;104:719–730 [PubMed] [Google Scholar]
- 29.Paech K, Webb P, Kuiper GG, Nilsson S, Gustafsson J, Kushner PJ, Scanlan TS: Differential ligand activation of estrogen receptors ERα and ERβ at AP1 sites. Science 1997;277:1508–1510 [DOI] [PubMed] [Google Scholar]
- 30.Filardo E, Quinn J, Pang Y, Graeber C, Shaw S, Dong J, Thomas P: Activation of the novel estrogen receptor G protein-coupled receptor 30 (GPR30) at the plasma membrane. Endocrinology 2007;148:3236–3245 [DOI] [PubMed] [Google Scholar]
- 31.Nadal A, Rovira JM, Laribi O, Leon-quinto T, Andreu E, Ripoll C, Soria B: Rapid insulinotropic effect of 17β-estradiol via a plasma membrane receptor. Faseb J 1998;12:1341–1348 [DOI] [PubMed] [Google Scholar]
- 32.Nadal A, Ropero AB, Laribi O, Maillet M, Fuentes E, Soria B: Nongenomic actions of estrogens and xenoestrogens by binding at a plasma membrane receptor unrelated to estrogen receptor α and estrogen receptor β. Proc Natl Acad Sci U S A 2000;97:11603–11608 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kanda N, Watanabe S: 17β-estradiol inhibits oxidative stress-induced apoptosis in keratinocytes by promoting Bcl-2 expression. J Invest Dermatol 2003;121:1500–1509 [DOI] [PubMed] [Google Scholar]
- 34.Teng J, Wang ZY, Prossnitz ER, Bjorling DE: The G protein-coupled receptor GPR30 inhibits human urothelial cell proliferation. Endocrinology 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Otto C, Fuchs I, Kauselmann G, Kern H, Zevnik B, Andreasen P, Schwarz G, Altmann H, Klewer M, Schoor M, Vonk R, Fritzemeier KH: GPR30 does not mediate estrogenic responses in reproductive organs in mice. Biol Reprod 2009;80:34–41 [DOI] [PubMed] [Google Scholar]
- 36.Windahl SH, Andersson N, Chagin AS, Martensson UE, Carlsten H, Olde B, Swanson C, Moverare-Skrtic S, Savendahl L, Lagerquist MK, Leeb-Lundberg LM, Ohlsson C: The role of the G protein-coupled receptor GPR30 in the effects of estrogen in ovariectomized mice. Am J Physiol Endocrinol Metab 2008 [DOI] [PubMed] [Google Scholar]
- 37.Levin ER: Rapid signaling by steroid receptors. Am J Physiol Regul Integr Comp Physiol 2008;295:R1425–1430 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Levin E: GPR30: estrogen receptor or collaborator? Endocrinology 2009;150:1722–1730 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Riant E, Waget A, Cogo H, Arnal JF, Burcelin R, Gourdy P: Estrogens protect against high-fat diet-induced insulin resistance and glucose intolerance in mice. Endocrinology 2009;150:2109–2117 [DOI] [PubMed] [Google Scholar]