Abstract
OBJECTIVE
Atherosclerosis is accelerated in subjects with type 2 diabetes by unknown mechanisms. We identified tissue inhibitor of metalloproteinase 3 (TIMP3), the endogenous inhibitor of A disintegrin and metalloprotease domain 17 (ADAM17) and other matrix metalloproteinases (MMPs), as a gene modifier for insulin resistance and vascular inflammation in mice. We tested its association with atherosclerosis in subjects with type 2 diabetes and identified Sirtuin 1 (SirT1) as a major regulator of TIMP3 expression.
RESEARCH DESIGN AND METHODS
We investigated ADAM10, ADAM17, MMP9, TIMP1, TIMP2, TIMP3, and TIMP4 expression levels in human carotid atherosclerotic plaques (n = 60) from subjects with and without diabetes. Human vascular smooth muscle cells exposed to several metabolic stimuli were used to identify regulators of TIMP3 expression. SirT1 small interference RNA, cDNA, and TIMP3 promoter gene reporter were used to study SirT1-dependent regulation of TIMP3.
RESULTS
Here, we show that in human carotid atherosclerotic plaques, TIMP3 was significantly reduced in subjects with type 2 diabetes, leading to ADAM17 and MMP9 overactivity. Reduced expression of TIMP3 was associated in vivo with SirT1 levels. In smooth muscle cells, inhibition of SirT1 activity and levels reduced TIMP3 expression, whereas SirT1 overexpression increased TIMP3 promoter activity.
CONCLUSIONS
In atherosclerotic plaques from subjects with type 2 diabetes, the deregulation of ADAM17 and MMP9 activities is related to inadequate expression of TIMP3 via SirT1. Studies in vascular cells confirmed the role of SirT1 in tuning TIMP3 expression.
Diabetes is characterized by accelerated atherosclerosis, although molecular mechanisms explaining this phenomenon are still undefined (1,2). We and others have shown that chronic hyperglycemia increases matrix metalloproteinase (MMP) and A disintegrin and metalloprotease domain (ADAM) activities providing a potential clue to atherosclerotic plaque progression, as confirmed by studies using vasculature from subjects with diabetes (3–5). Increased MMP and ADAM activities may be linked also to unbalanced expression of endogenous inhibitors called tissue inhibitor of metalloproteinases (TIMPs) 1–4 (4). We identified the deficiency of TIMP3 as a link between insulin resistance and vascular inflammation (6–8). Recently, Paigen and colleagues (9) found Timp3 gene among quantitative trait loci associated with diabetes and dyslipidemia, identifying a mutation causing lower gene expression in diabetic mice. Moreover, Timp3 is among the few genes downregulated in a microarray analysis of pericytes treated with glycated oxidized LDLs (10). Because TIMP3 uniquely among TIMPs retains the ability to inhibit shedding enzymes such as ADAM17, which are involved in inflammatory processes (11), we hypothesized downregulation of TIMP3 as a hallmark for atherosclerosis in diabetic subjects. We tested this hypothesis in atherosclerotic plaques from subjects with different degrees of glucose tolerance, linking TIMP3 expression to activity of deacetylase Sirtuin 1 (SirT1). SirT1 is a deacetylase localized at nuclear levels acting as transcriptional regulator either on histones or on transcription factors such as forkhead box class O1 (FoxO1), liver X receptor (LXR), p53, and transcriptional cofactors such as peroxisome proliferator–activated receptorγ coactivator 1α (12). Recently, it has been suggested that loss of SirT1 activity may be associated with metabolic diseases such as type 2 diabetes and atherosclerosis (13). Several laboratories have shown that SirT1 gain of function either by genetic manipulation or through ligand activation may protect from insulin resistance associated with obesity and from atherosclerosis in experimental disease models (14,15).
However, little is known about SirT1 activity in human subjects affected by atherosclerosis and diabetes. Our data reveal a new potential role for TIMP3 and SirT1 in the atherosclerosis process in subjects with diabetes.
RESEARCH DESIGN AND METHODS
This study included 60 atherosclerotic plaques from normal glucose tolerant (NGT; n = 37) or type 2 diabetic (n = 23; according to medical records or oral glucose tolerance test) subjects in whom carotid endarterectomy for symptomatic disease was performed at Policlinico Tor Vergata University Hospital, Rome, Italy. Subject characteristics and treatments are described in Table 1. The study was approved by the ethics committee, and subjects provided informed written consent for the use of atherosclerotic material for research use. All procedures were performed according to the Declaration of Helsinki.
TABLE 1.
Clinical data of patients subjected to carotid endarterectomy
| NGT | Type 2 diabetes | |
|---|---|---|
| n | 37 | 23 |
| Sex (men/women) | 25/12 | 14/9 |
| Age (years) | 71.0 ± 6.6 | 70.6 ± 8.9 |
| BMI (kg/m2) | 25.3 ± 3.9 | 24.1 ± 4.8 |
| Hypertension (yes/no) | 29/8 | 20/3 |
| Antihypertensive drugs (yes/no) | 23/14 | 15/8 |
| Antiaggregant drugs (yes/no) | 35/2 | 22/1 |
| Statins (yes/no) | 25/12 | 15/8 |
| Total cholesterol (mg/dl) | 188.9 ± 31.0 | 192.35 ± 36.86 |
| HDL cholesterol (mg/dl) | 45.32 ± 10.2 | 43.3 ± 9.6 |
| LDL cholesterol (mg/dl) | 115.7 ± 24.6 | 122.18 ± 28.1 |
| Triglycerides (mg/dl) | 125.69 ± 62.26 | 139.13 ± 72.04 |
| A1C (%) | 5.6 ± 0.2 | 7.2 ± 1.6* |
| Fasting plasma glucose (mg/dl) | 89.7 ± 12.0 | 126.9 ± 51† |
| Fasting plasma insulin (μU/ml) | 7.38 ± 5.59 | 12.96 ± 3.51 |
| Oral agents/insulin treatment | — | 15/8 |
Data are means ± SD and n.
*P < 0.001 by Student's t test.
†P < 0.001 by Student's t test.
Histological analysis.
Carotid plaques were removed en bloc during surgery to preserve plaque structure entirely. For histology, surgical samples were fixed for 24 h in 10% buffered formalin immediately upon removal. After decalcification, specimens were sectioned transversely every 5 mm and paraffin embedded. Hematoxylin-eosin was performed for morphologic study (4).
Immunohistochemistry was performed on serial 3-μm thick sections cut from paraffin blocks of carotid plaques using the following antibodies: 1) polyclonal rabbit anti-human TIMP3 antibody (Calbiochem, San Diego, CA), 2) monoclonal anti-CD68 (human macrophage) antibody (DAKO, Glostrup, Denmark), and 3) monoclonal anti-α smooth muscle actin antibody (Europa Ventana Medical System, Illkirch, France). The primary antibodies were detected with avidin-biotin-peroxidase complexes (DAKO). All antibodies were used with positive and negative controls.
Real-time quantitative RT-PCR analysis.
Frozen plaque samples were homogenized using a polytron homogenizer. Total RNA and single-strand cDNA were obtained as described (7). Real-time PCR RNA expression analysis of ADAM17, ADAM10, TIMP3, TIMP1, TIMP2, TIMP4, MMP9, and SirT1 (primers available upon request) was performed with ABI PRISM 7000 System (Applied Biosystems, Foster City, CA) and normalized to 18S rRNA. Each reaction was carried out in duplicate and analysis performed by 2ΔΔCt method as described previously (8).
ADAM17 and MMP9 activities.
Proteins were extracted as previously described (4,6–8). ADAM17 activity was determined by the SensoLyte 520 ADAM17 Activity Assay Kit Fluorimetric (AnaSpec, San Jose, CA). MMP9 activity was measured by the Amersham MMP-9 Biotrack Activity Assay System (GE Healthcare U.K.) according to manufacturer's instructions. Active MMP9 was detected through activation of the modified prodetection enzyme and subsequent cleavage of its chromogenic peptide substrate. The resultant color was read at 450 nm in a microplate spectrophotometer (Victor 1420).
LDL preparation, cell culture, and Western blots.
LDL preparation (16), cell culture, and Western blots are described in detail in the online appendix at http://diabetes.diabetesjournals.org/cgi/content/full/db09-0280/DC1.
Timp3 promoter regulation assay.
For Timp3 promoter regulation assay, coronary artery smooth muscle cells (CASMCs) were transfected with 2 μg Human SirT1 cDNA Clone (RG218134; Origene), 10 ng renilla, and Timp3 Gene Promoter Reporter Vector (LR1034; Panomics) or 2 μg TransLucent Control Vector using primary smooth muscle cells Nucleofector solution with program A-033 (AMAXA). The luciferase assay was performed with Dual-Luciferase Reporter Assay System (Promega) according to manufacturer's instructions.
Statistical analysis.
Statistical analysis was performed using Student's t test, one-way ANOVA, and Pearson's correlation coefficient (r) on SPSS software program v. 13.0 for Windows. Data are expressed as means ± SD. P < 0.05 was considered statistically significant.
RESULTS
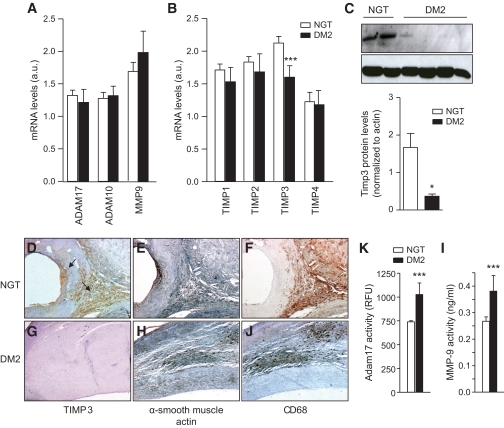
Our data show that in atherosclerotic plaques, among ADAM10, ADAM17, MMP9, TIMP1, TIMP2, TIMP3, and TIMP4, only TIMP3 expression was lower in those with type 2 diabetes than those with NGT (Fig. 1A and B). Western blot assay confirmed the significant decrease of TIMP3 in subjects with type 2 diabetes (Fig. 1C). Immunohistochemistry completed the link between TIMP3 downregulation and diabetes (Fig. 1D and G, for non–type 2 diabetic and type 2 diabetic subjects, respectively). Analysis of consecutive sections with anti-CD68 for macrophages and anti-α smooth muscle actin for smooth muscle cells suggested that both cell types are associated with TIMP3 expression in NGT subjects (Fig. 1E and F for NGT subjects and Fig. 1H and J for type 2 diabetic subjects; negative control for antibodies in supplemental Fig. 1). To verify that the reduction of TIMP3 in atherosclerotic plaques resulted in increased ADAM17 and MMP9 activity, we used a fluorimetric assay. We found that both ADAM17 and MMP9 activities were higher in those with type 2 diabetes than in those with NGT (Fig. 1K and I).
FIG. 1.
TIMP3 is reduced in atherosclerotic plaques of subjects with type 2 diabetes (DM2). ADAM10, ADAM17, and MMP9 (A) as well as TIMPs (B) expression in NGT (n = 37) and type 2 diabetes (n = 23) subjects; ***P < 0.001 by one-way ANOVA. C: Western blot using extracellular matrix extracts from representative NGT (n = 2) and type 2 diabetic (n = 4) subjects. *P < 0.05 NGT vs. type 2 diabetes by Student's t test. D–J: Immunohistochemistry confirmed that TIMP3 is reduced in type 2 diabetic (n = 8) versus NGT (n = 8) subjects; one representative image is shown for TIMP3, anti–α smooth muscle actin, and CD68 for NGT (D–F; 4× magnification) and type 2 diabetic (G–J; 4× magnification) subjects. K and I: ADAM17 activity measured by a fluorimetric assay (K) and MMP9 activity measured by a fluorimetric assay (I) are increased in type 2 diabetic (n = 23) compared with NGT (n = 37) subjects; ***P < 0.001 by Student's t test for both. (A high-quality digital representation of this figure is available in the online issue.)
Analysis of clinical characteristics (Table 1) showed that TIMP3 expression negatively correlates with LDL cholesterol (r = −0.29; P < 0.03) and A1C (r = −0.31; P < 0.02) but not with sex, age, or pharmacological treatment.
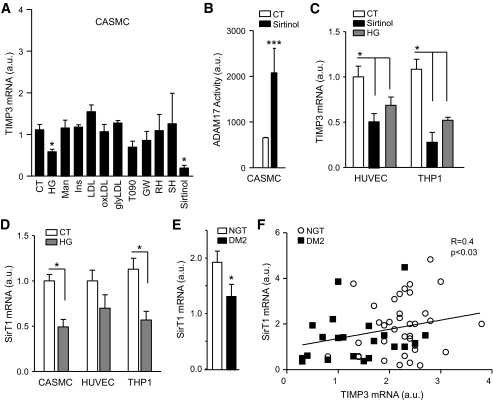
To identify metabolic factors reducing TIMP3 expression, CASMCs were treated with high glucose (20 mmol/l), mannitol (osmotic control, 20 mmol/l), hyperinsulinemia (10−7 M), LDL, oxidized LDL, and glycated LDL (100 ug/ml). Because previous data suggested that LXR regulates TIMP3 expression (17), we used LXR agonists such as T0901317, 22-s, and 22-r hydroxycholesterol as well as GW3965. Because LXR is regulated by SirT1 deacetylase (12), we also used SirT1 inhibitor Sirtinol. Interestingly, we found that among the various treatments, only high glucose and Sirtinol significantly reduced TIMP3 expression in CASMC (Fig. 2A).
FIG. 2.
Effects of diabetes on TIMP3 expression in vascular cells. A: TIMP3 expression in CASMC treated with various metabolic stimuli: high glucose (HG) 20 mmol/l; mannitol (Man) 20 mmol/l; insulin (Ins) 10−7 M; LDL 100 μg/ml; oxidized LDL (oxLDL) 100 μg/ml; glycated LDL (glyLDL) 100 μg/ml; LXR agonists (T0901317 [T090] 5 μmol/l; GW3965 [GW] 3 μmol/l; R-hydroxycholesterol [RH] 10 μmol/l; 22-S-hydroxycholesterol [SH] 10 μmol/l); SirT1 inhibitor (Sirtinol) 50 μmol/l. n = 4 for all experiments; *P < 0.05 by Student's t test versus control (CT). B: Sirtinol increased ADAM17 activity in CASMC. n = 4 for all experiments; ***P < 0.001 by Student's t test. C: TIMP3 expression in HUVEC and THP1 treated with Sirtinol and high glucose (20 mmol/l). n = 4 for all experiments; *P < 0.05 by Student's t test versus control. D: SirT1 expression is reduced in CASMC, HUVEC, and THP1 treated with high glucose (20 mmol/l) compared with control. n = 4 for all experiments; *P < 0.05 by Student's t test. E: SirT1 levels are decreased in type 2 diabetic compared with NGT subjects. *P < 0.05 by Student's t test. F: SirT1 correlates with TIMP3 in atherosclerotic plaques from NGT (n = 37) and type 2 diabetic (DM2) (n = 23) subjects.
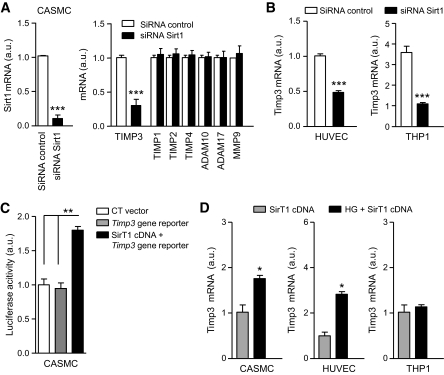
Treatment of CASMC with Sirtinol determined an increased metalloprotease activity as measured by ADAM17 activity assay (Fig. 2B). Sirtinol and high glucose significantly reduced TIMP3 expression also in human umbilical vein endothelial cells (HUVECs) and monocytic THP1 cells (Fig. 2C). Analysis of CASMC, HUVEC, and THP1 revealed that high glucose significantly reduced SirT1 expression (Fig. 2D) in CASMC and THP1 with a trend also in HUVEC. SirT1 expression was significantly reduced in atherosclerotic plaques from subjects with type 2 diabetes compared with those from NGT subjects (Fig. 2E), and we observed that TIMP3 expression positively correlated with SirT1 expression (r = 0.4, P < 0.03; Fig. 2F), although NGT and type 2 diabetic subjects were mixed and therefore diabetes may represent a confounding factor. To confirm a direct role of SirT1 in regulating TIMP3 expression, we used a small interference RNA approach. Knockdown of SirT1 in CASMC resulted in marked reduction of TIMP3 expression but not TIMP1, TIMP2, TIMP4, ADAM10, ADAM17, or MMP9 (Fig. 3A); a similar effect was observed in HUVEC and THP1 cells (Fig. 3B). To substantiate SirT1 effects on TIMP3, CASMC were cotransfected with human SirT1 cDNA and Timp3 promoter luciferase reporter vector, confirming that SirT1 positively modulates TIMP3 expression (Fig. 3C). Finally, in CASMC, HUVEC, and THP1 we found that SirT1 overexpression prevented the reduction of TIMP3 expression determined by high glucose (Fig. 3D).
FIG. 3.
Regulation of TIMP3 expression in CASMC. A: SirT1 knockdown decreased TIMP3 expression but not TIMP1, TIMP2, TIMP4, ADAM10, ADAM17, or MMP9 in CASMC. n = 4 for all experiments; ***P < 0.001 by Student's t test versus control. B: SirT1 knockdown decreased TIMP3 expression in HUVEC and THP1. n = 4 for all experiments; ***P < 0.001 by Student's t test. C: SirT1 cDNA overexpression increased Timp3 promoter activity. n = 4 for all experiments; **P < 0.01 by one-way ANOVA. D: SirT1 overexpression increased and prevented loss of TIMP3 expression caused by high glucose (HG; 20 mmol/l) in CASMC, HUVEC, and THP1. n = 4 for all experiments; *P < 0.05 by Student's t test.
DISCUSSION
We recently observed that TIMP3 deficiency is necessary to develop fatty streaks characterized by macrophage infiltrate using the insulin receptor heterozygous mouse model fed a diet rich in lipids (8). The relevance of TIMP3 is demonstrated by the reverse phenotype caused by TACE deficiency in the same mouse model, suggesting that loss of TIMP3 may favor the development of atherosclerotic lesions (8). Whereas other studies on models such as ApoE and LDL receptor knockout are necessary to fully characterize the role of TIMP3 in the progression of atherosclerosis under diabetic conditions, here we analyzed its role in human atherosclerosis accompanied by diabetes. Our results suggest that subjects with diabetes exhibit reduction of TIMP3, as well as increased activity of ADAM17 and MMP9, possibly because of the more intense oxidative stress caused by hyperglycemia, a known activator of both the enzymes in a protein kinase C–dependent manner (3,4,18,19). Therefore, our data suggest that metabolic-dependent reduction in TIMP3 expression may increase the activity of inflammatory and proteolytic enzymes, which play a role in atherothrombosis (20,21). Previous studies showed that TIMP3 expression was increased in extracts from atheroma compared with nonatherosclerotic tissue in nondiabetic subjects (22). In view of our results, TIMP3 reduction is emerging as a specific factor in the atherosclerosis process of subjects with diabetes. Loss of TIMP3 may lead to increased tumor necrosis factor-α and epidermal growth factor receptor signaling, potentially increasing the inflammatory burden inside the atherosclerotic plaque (6–8). Moreover, loss of TIMP3 increases MMP9 activity in atherosclerotic plaques, a feature known to be increased in vasculature from subjects with type 2 diabetes (5), and may potentially affect plaque stability in the long term.
The role of TIMPs in diabetic atherothrombosis is still undefined. Recent data in animal models supported a role for an imbalanced MMP-to-TIMP ratio favoring increased degradation of extracellular matrix that may promote progression of atherosclerosis (23).
Factors regulating TIMP expression in atherosclerotic plaques are undefined, although previous data suggested a role for growth factors such as transcription growth factor-β and platelet-derived growth factor (23). However, the role and regulation of TIMP3 in diabetic vascular disease has been thus far unexplored. Our data suggested that TIMP3 is negatively associated with A1C and LDL cholesterol levels. Exposure of CASMC to different stimuli linked to gluco- and lipotoxicity revealed that both high glucose and inhibition of the deacetylase SirT1 led to reduced TIMP3 expression and activity. SirT1 is emerging as a master of integrated metabolic response to nutrient availability (12–15,24). Data from Apolipoprotein E knockout mice suggest that overexpression of SirT1 from endothelial cells may defend against atherosclerosis progression, although the basic mechanisms remained undefined. Our results, via knockdown of SirT1 or its overexpression, confirmed a role for this deacetylase in the modulation of TIMP3 expression in vascular smooth muscle cells, and especially monocyte/macrophage. SirT1 controls gene expression through deacetylation of histones and transcription factors; one or both of the two mechanisms may be involved in regulating TIMP3 expression. Previous studies using T0901317 compound, an LXR agonist, suggested that LXR transcription factors regulate TIMP3 expression (17). In our cell systems we did not observe an effect of T0901317. However, this may depend on several factors including different experimental models and culture conditions. Moreover, SirT1 is a positive regulator of LXR as well as other transcription factors potentially involved in TIMP3 expression such as FoxO1 (25). Because SirT1 overexpression is able to rescue TIMP3 expression in the presence of glucotoxicity, it is possible that SirT1 affects events linked to de-repression of TIMP3 promoter via transcription factors such as FoxO1 or histone deacetylation.
To our knowledge, this is the first gene/mechanism linked to SirT1 identified in the context of diabetes and atherosclerosis diseases using human vascular specimens. Therefore, our results provide further support for the protective role played by SirT1 against metabolic diseases. In conclusion, we observed that atherosclerotic plaques from subjects with impaired glucose metabolism are characterized by TIMP3 deficiency and increased metalloproteases activity. In vitro studies and clinical correlations suggested that SirT1 regulates TIMP3 expression, which is emerging as a specific factor for the atherosclerosis process in diabetes.
Acknowledgments
This study was supported in part by SID Grant 2007, Ministry of Health RF2007, Telethon GGP08065, Juvenile Diabetes Research Foundation Grant 1-2007-665, a grant from Fondazione Roma 2008 (to M.F.), PRIN 2007 and ASI grant 2006 (to R.L.), and Start-up funds from the University of Texas Health Science Center (to F.F.).
No potential conflicts of interest relevant to this article were reported.
Footnotes
The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.Beckman JA, Creager MA, Libby P: Diabetes and atherosclerosis: epidemiology, pathophysiology, and management. JAMA 2002;287:2570–2581 [DOI] [PubMed] [Google Scholar]
- 2.Kanter JE, Johansson F, LeBoeuf RC, Bornfeldt KE: Do glucose and lipids exert independent effects on atherosclerotic lesion initiation or progression to advanced plaques? Circ Res 2007;100:769–781 [DOI] [PubMed] [Google Scholar]
- 3.Uemura S, Matsushita H, Li W, Glassford AJ, Asagami T, Lee KH, Harrison DG, Tsao PS: Diabetes mellitus enhances vascular matrix metalloproteinase activity: role of oxidative stress. Circ Res 2001;88:1291–1298 [DOI] [PubMed] [Google Scholar]
- 4.Federici M, Menghini R, Mauriello A, Hribal ML, Ferrelli F, Lauro D, Sbraccia P, Spagnoli LG, Sesti G, Lauro R: Insulin-dependent activation of endothelial nitric oxide synthase is impaired by O-linked glycosylation modification of signaling proteins in human coronary endothelial cells. Circulation 2002;106:466–472 [DOI] [PubMed] [Google Scholar]
- 5.Chung AW, Hsiang YN, Matzke LA, McManus BM, van Breemen C, Okon EB: Reduced expression of vascular endothelial growth factor paralleled with the increased angiostatin expression resulting from the upregulated activities of matrix metalloproteinase-2 and -9 in human type 2 diabetic arterial vasculature. Circ Res 2006;99:140–148 [DOI] [PubMed] [Google Scholar]
- 6.Federici M, Hribal ML, Menghini R, Kanno H, Marchetti V, Porzio O, Sunnarborg SW, Rizza S, Serino M, Cunsolo V, Lauro D, Mauriello A, Smookler DS, Sbraccia P, Sesti G, Lee DC, Khokha R, Accili D, Lauro R: TIMP3 deficiency in insulin receptor-haploinsufficient mice promotes diabetes and vascular inflammation via increased TNF-alpha. J Clin Invest 2005;115:3494–3505 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Serino M, Menghini R, Fiorentino L, Amoruso R, Mauriello A, Lauro D, Sbraccia P, Hribal ML, Lauro R, Federici M: Mice heterozygous for tumor necrosis factor-α converting enzyme are protected from obesity-induced insulin resistance and diabetes. Diabetes 2007;56:2541–2546 [DOI] [PubMed] [Google Scholar]
- 8.Menghini R, Menini S, Amoruso R, Fiorentino L, Casagrande V, Marzano V, Tornei F, Bertucci P, Iacobini C, Serino M, Porzio O, Hribal ML, Folli F, Khokha R, Urbani A, Lauro R, Pugliese G, Federici M: Tissue Inhibitor of Metalloproteinase 3 deficiency causes hepatic steatosis and adipose tissue inflammation in mice. Gastroenterology 2009;136:663–672 [DOI] [PubMed] [Google Scholar]
- 9.Su Z, Tsaih SW, Szatkiewicz J, Shen Y, Paigen B: Candidate genes for plasma triglyceride, free fatty acid, and glucose revealed from an intercross between inbred mouse strains NZB/B1NJ x NZW/LacJ. J Lipid Res 2008;49:1500–1510 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Barth JL, Yu Y, Song W, Lu K, Dashti A, Huang Y, Argraves WS, Lyons TJ: Oxidised, glycated LDL selectively influences tissue inhibitor of metalloproteinase-3 gene expression and protein production in human retinal capillary pericytes. Diabetologia 2007;50:2200–2208 [DOI] [PubMed] [Google Scholar]
- 11.Murphy G, Murthy A, Khokha R: Clipping, shedding and RIPping keep immunity on cue. Trends Immunol 2008;29:75–82 [DOI] [PubMed] [Google Scholar]
- 12.Feige JN, Auwerx J: DisSIRTing on LXR and cholesterol metabolism. Cell Metab 2007;6:343–345 [DOI] [PubMed] [Google Scholar]
- 13.Purushotham A, Schug TT, Xu Q, Surapureddi S, Guo X, Li X: Hepatocyte-specific deletion of SIRT1 alters fatty acid metabolism and results in hepatic steatosis and inflammation. Cell Metab 2009;9:327–338 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Banks AS, Kon N, Knight C, Matsumoto M, Gutiérrez-Juárez R, Rossetti L, Gu W, Accili D: SirT1 gain of function increases energy efficiency and prevents diabetes in mice. Cell Metab 2008;8:333–341 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhang QJ, Wang Z, Chen HZ, Zhou S, Zheng W, Liu G, Wei YS, Cai H, Liu DP, Liang CC: Endothelium-specific overexpression of class III deacetylase SIRT1 decreases atherosclerosis in apolipoprotein E-deficient mice. Cardiovasc Res 2008;80:191–199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Santini E, Lupi R, Baldi S, Madec S, Chimenti D, Ferrannini E, Solini A: Effects of different LDL particles on inflammatory molecules in human mesangial cells. Diabetologia 2008;51:2117–2125 [DOI] [PubMed] [Google Scholar]
- 17.Lehrke M, Lebherz C, Millington SC, Guan HP, Millar J, Rader DJ, Wilson JM, Lazar MA: Diet-dependent cardiovascular lipid metabolism controlled by hepatic LXRalpha. Cell Metab 2005;1:297–308 [DOI] [PubMed] [Google Scholar]
- 18.Chen CD, Podvin S, Gillespie E, Leeman SE, Abraham CR: Insulin stimulates the cleavage and release of the extracellular domain of Klotho by ADAM10 and ADAM17. Proc Natl Acad Sci U S A 2007;104:19796–19801 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Reddy AB, Ramana KV, Srivastava S, Bhatnagar A, Srivastava SK: Aldose reductase regulates high glucose-induced ectodomain shedding of tumor necrosis factor (TNF)-{alpha} via protein kinase C-{delta} and TNF-{alpha} converting enzyme in vascular smooth muscle cells. Endocrinology 2009;150:63–74 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Libby P: The molecular mechanisms of the thrombotic complications of atherosclerosis. J Intern Med 2008;263:517–527 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Garton KJ, Gough PJ, Raines EW: Emerging roles for ectodomain shedding in the regulation of inflammatory responses. J Leukoc Biol 2006;79:1105–1116 [DOI] [PubMed] [Google Scholar]
- 22.Fabunmi RP, Sukhova GK, Sugiyama S, Libby P: Expression of tissue inhibitor of metalloproteinases-3 in human atheroma and regulation in lesion-associated cells: a potential protective mechanism in plaque stability. Circ Res 1998;83:270–278 [DOI] [PubMed] [Google Scholar]
- 23.Boden G, Song W, Pashko L, Kresge K: In vivo effects of insulin and free fatty acids on matrix metalloproteinases in rat aorta. Diabetes 2008;57:476–483 [DOI] [PubMed] [Google Scholar]
- 24.Ungvari Z, Parrado-Fernandez C, Csiszar A, de Cabo R: Mechanisms underlying caloric restriction and lifespan regulation: implications for vascular aging. Circ Res 2008;102:519–528 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Buzzio OL, Lu Z, Miller CD, Unterman TG, Kim JJ: FOXO1A differentially regulates genes of decidualization. Endocrinology 2006;147:3870–3876 [DOI] [PubMed] [Google Scholar]