Abstract
OBJECTIVE
The global epidemic of metabolic syndrome and its complications demands rapid evaluation of new and accessible interventions. Insulin resistance is the central biochemical disturbance in the metabolic syndrome. The citrus-derived flavonoid, naringenin, has lipid-lowering properties and inhibits VLDL secretion from cultured hepatocytes in a manner resembling insulin. We evaluated whether naringenin regulates lipoprotein production and insulin sensitivity in the context of insulin resistance in vivo.
RESEARCH DESIGN AND METHODS
LDL receptor–null (Ldlr−/−) mice fed a high-fat (Western) diet (42% calories from fat and 0.05% cholesterol) become dyslipidemic, insulin and glucose intolerant, and obese. Four groups of mice (standard diet, Western, and Western plus 1% or 3% wt/wt naringenin) were fed ad libitum for 4 weeks. VLDL production and parameters of insulin and glucose tolerance were determined.
RESULTS
We report that naringenin treatment of Ldlr−/− mice fed a Western diet corrected VLDL overproduction, ameliorated hepatic steatosis, and attenuated dyslipidemia without affecting caloric intake or fat absorption. Naringenin 1) increased hepatic fatty acid oxidation through a peroxisome proliferator–activated receptor (PPAR) γ coactivator 1α/PPARα-mediated transcription program; 2) prevented sterol regulatory element–binding protein 1c–mediated lipogenesis in both liver and muscle by reducing fasting hyperinsulinemia; 3) decreased hepatic cholesterol and cholesterol ester synthesis; 4) reduced both VLDL-derived and endogenously synthesized fatty acids, preventing muscle triglyceride accumulation; and 5) improved overall insulin sensitivity and glucose tolerance.
CONCLUSIONS
Thus, naringenin, through its correction of many of the metabolic disturbances linked to insulin resistance, represents a promising therapeutic approach for metabolic syndrome.
The metabolic syndrome is a burgeoning epidemic and represents an important predisposing factor for diabetes and atherosclerosis. It is defined as a cluster of abnormalities including abdominal obesity, hypertension, glucose intolerance, and dyslipidemia characterized by hepatic overproduction of VLDLs (1,2). Insulin resistance is central to the pathophysiology of the metabolic syndrome and results from the inability of insulin to normally signal to its receptor kinase and/or downstream targets (3,4). Although defective insulin signaling has been causally linked to these metabolic abnormalities, few available therapeutic strategies effectively correct insulin resistance with normalization of glucose tolerance and dyslipidemia (5).
Hepatic VLDL secretion is regulated through triglyceride and cholesterol availability and the transfer of lipid onto the apolipoprotein (apo) B100 (apoB) backbone via the rate-limiting microsomal triglyceride transfer protein (MTP) (6). Under normal conditions, insulin targets apoB for intracellular degradation, resulting in acute inhibition of VLDL-apoB secretion (7,8). Insulin also inhibits the secretion of apoB from cultured hepatocytes through activation of both the phosphatidylinositol 3-kinase (PI3K) and mitogen-activated protein kinase–extracellular regulated kinase (MAPKerk) signal transduction pathways, upregulating the LDL receptor (LDLR) (9,10) while suppressing expression of MTP (11,12).
Several mechanisms have been proposed to account for the increased VLDL secretion observed in type 2 diabetes and the metabolic syndrome. Peripheral insulin resistance gives rise to increased free fatty acid flux to the liver, enhanced triglyceride synthesis, decreased apoB degradation, and increased VLDL secretion (13,14). Furthermore, with insulin resistance, hyperinsulinemia drives hepatic hyperstimulation of sterol regulatory element–binding protein (SREBP) 1c–induced lipogenesis, leading to increased fatty acid synthesis and triglyceride accumulation (15). ApoB becomes resistant to degradation (16), and MTP fails to be downregulated (16), resulting in increased VLDL-apoB secretion (16,17). Thus, in states of hyperinsulinemia, availability of lipid is a key factor regulating VLDL-apoB secretion. In mice with complete deficiency of liver insulin receptors (LIRKO mice) (18) or very few hepatic insulin receptors (L1B6Ldlr−/−) (19), the hyperinsulinemia failed to stimulate SREBP1c-induced lipogenesis, resulting in greatly diminished VLDL-triglyceride secretion. Studies in humans support this concept, as patients with mild hyperinsulinemia harboring a defect in protein kinase B (PKB)-β (AKT2), but not patients with insulin receptor mutations, manifest increased lipogenesis, elevated liver fat content, triglyceride-enriched plasma VLDL, and hypertriglyceridemia (20).
The citrus-derived flavonoid, naringenin, has both lipid-lowering and insulin-like properties. In streptozotocin-induced diabetic rats, a diet supplement of naringenin 7-O-d-glucoside reduces blood glucose and improves plasma lipids (21). In cholesterol-fed rats, naringenin lowers plasma cholesterol by inhibiting hepatic cholesterol synthesis and esterification (22). In HepG2 hepatoma cells, naringenin, like insulin, inhibits apoB100 secretion resulting from both enhanced intracellular degradation of apoB100 and increased LDLR-mediated uptake of mature particles (23,24). The effect of naringenin does not require insulin receptor activation (7,9). Furthermore, naringenin potentiates intracellular signaling responses to low insulin doses, suggesting that naringenin sensitizes hepatocytes to insulin (7). Therefore, we hypothesize that naringenin lowers plasma lipids in vivo through inhibition of VLDL-apoB100 secretion and regulates insulin sensitivity in the setting of insulin resistance.
In the present study, we use C57BL/6J Ldlr−/− mice fed a Western diet, a model of diet-induced insulin resistance. These mice display many characteristics of the metabolic syndrome including dyslipidemia, obesity, and insulin resistance (25). Addition of naringenin to a high-fat diet corrects a wide range of metabolic disturbances associated with insulin resistance independent of caloric intake or dietary lipid absorption. Naringenin prevents hyperinsulinemia and, subsequently, SREBP1c-stimulated lipogenesis; activates hepatic fatty acid oxidation resulting in prevention of hepatic triglyceride accumulation; and leads to normalization of VLDL overproduction and amelioration of dyslipidemia. Finally, in muscle, naringenin prevents lipid accumulation, leading to improved glucose utilization and increased insulin sensitivity.
RESEARCH DESIGN AND METHODS
Male C57BL/6J and Ldlr−/− mice on the C57BL/6J (Jackson Laboratory) background were housed in pairs and maintained at 23°C on a 12-h light/dark cycle. Experiments were approved by the animal care committee of the University of Western Ontario. Eight- to 12-week-old mice were fed ad libitum a rodent standard diet (4% of calories from fat, TD8604; Harlan Teklad) or a high-fat diet (Western diet) containing 42% of calories from fat plus cholesterol (0.05% wt/wt) (TD96125; Harlan Teklad). Naringenin (Sigma, St. Louis) was added to the Western diet at 1 or 3% (wt/wt). Ldlr−/− mice were fed for 4 weeks and C57BL/6J mice for 30 weeks. Food intake was measured daily, and body weight was measured biweekly. Mice were fasted for 6 h before intervention.
Blood samples, tissue collection, and tissue histology.
See online supplement for details (available at http://diabetes.diabetesjournals.org/cgi/content/full/db09-0634/DC1).
Plasma and tissue lipids, blood glucose, insulin, and liver enzymes.
Plasma triglycerides and total cholesterol (Roche Diagnostics, Laval, Canada) and plasma nonesterified fatty acids (NEFAs) (Wako Chemicals, Richmond, VA) were determined enzymatically (26). Blood glucose was determined using an Ascensia Elite glucometer (Bayer Healthcare, Toronto, Canada). Plasma insulin and leptin were measured using ultrasensitive mouse-specific enzyme-linked immunosorbent assays (Alpco Diagnostics, Windham, NH). Liver enzymes (aspartate aminotransferase, alanine aminotransferase, and alkaline phosphatase) were measured using the kinetic rate method. Tissue lipids and oleate incorporation into triglycerides and cholesteryl ester were determined as previously described (26). Homeostatic model assessment for insulin resistance (HOMA-IR) was calculated as a surrogate for insulin resistance, as described previously for mice (27). Plasma cholesterol and triglyceride distributions were evaluated by size exclusion chromatography (26).
Lipid absorption, triglycerides, and apoB secretion.
Intestinal triglyceride and cholesterol absorption was determined using a modified fecal isotope ratio method, and the intraperitoneal Tyloxapol method was used to measure triglyceride and apoB secretion. See online supplement for details.
Glucose and insulin tolerance tests and glucose uptake.
Mice were administered glucose (intraperitoneally or gavage) or insulin (intraperitoneally). Blood glucose was measured up to 180 min postinjection. Glucose uptake was determined following intraperitoneal injection of 2-deoxy-d-[1-3H]-glucose. See online supplement for details.
Fatty acid and cholesterol synthesis, fatty acid oxidation, and lipoprotein lipase activity.
Fatty acid and cholesterol synthesis were measured following an intraperitoneal injection of [1-14C]-acetic acid. Fatty acid oxidation was determined by conversion of 3H-palmitate to 3H2O. Lipoprotein lipase (LPL) activity (LPLA) was assayed in postheparin plasma. See online supplement for details.
Energy expenditure.
Energy expenditure (EE) was determined using an indirect open-circuit calorimeter (Oxylet; Panlab, Cornella, Spain). See online supplement for details.
Gene expression and mtDNA measurement.
Tissue mRNA and mtDNA levels were determined by quantitative real-time RT-PCR. See online supplement for details.
Cold test.
Whole-body temperature was assessed rectally (Harvard Homeothermic Blanket Control Unit), initially at room temperature, then hourly (6 h) following transfer to a 4°C room.
Statistical analysis.
Data are presented as means ± SE. Analysis was performed using the Statistical Package for Social Science (SPSS version 14.0). Significant differences between groups were determined by a one-way ANOVA. Multiple comparisons were made using a post hoc Tukey's test, and differences were identified between groups at P < 0.05. Differences between groups for body weight, food consumption, glucose tolerance tests, and insulin tolerance tests were calculated using general linear modeling for repeated measures.
RESULTS
Naringenin does not affect food intake but suppresses diet-induced weight gain.
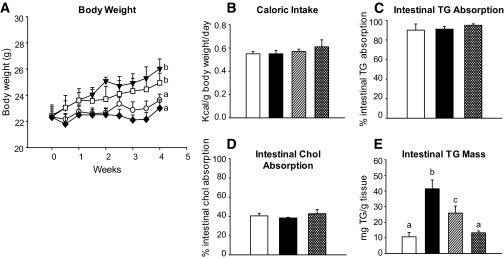
The metabolic effects of naringenin were initially evaluated in Ldlr−/− mice fed a Western diet containing 1 or 3% naringenin for 4 weeks. Western-fed mice gained significantly more weight than standard diet–fed mice. Naringenin dose-dependently attenuated weight gain so that the 3% naringenin group was not different from the standard diet group (Fig. 1A). Among groups, there were no significant differences in caloric intake (Fig. 1B) or liver enzyme levels (data not shown). Intestinal triglyceride and cholesterol absorption were >90 and 40%, respectively, and were unaffected by any diet (Fig. 1C and D). Intestinal total, free, and cholesteryl ester concentrations were unchanged (data not shown). Intestinal triglycerides were elevated fourfold in Western diet–fed mice and dose-dependently decreased by the addition of 1% (−50%) and 3% (−90%) naringenin (Fig. 1E).
FIG. 1.
Naringenin prevents diet-induced weight gain. Ldlr−/− mice (n = 12/group) were fed a standard diet or a high-fat diet alone or supplemented with naringenin (1 or 3%) for 4 weeks. A: Body weight gain. ○, standard diet; ▾, Western diet; □, Western diet and 1% naringenin; ♦, Western diet and 3% naringenin. B: Caloric intake expressed as kcal · g body wt−1 · day−1. Intestinal triglyceride (C) and cholesterol absorption (D) were determined in fasted mice using a fecal dual-isotope ratio method. Values are reported as percent absorption calculated from the isotopic ratio determined in feces collected over 48 h (n = 4/group). E: Intestinal triglyceride content determined in mice at the time they were killed. Samples of small intestine were cleaned, lipids extracted, and determined enzymatically. Values are the means ± SE, different letters are statistically different, P < 0.05. □, standard diet; ■, Western diet; ▨,, Western diet and 1% naringenin; ▩, Western diet and 3% naringenin.
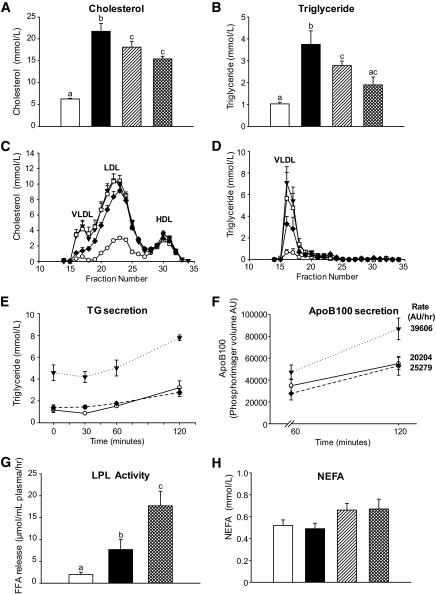
Naringenin attenuates dyslipidemia and corrects VLDL secretion.
The Western diet elevated fasting plasma cholesterol threefold compared with standard diet–fed mice (Fig. 2A). Naringenin at 1 and 3% decreased plasma cholesterol by 17 and 30%, respectively, compared with Western diet–fed mice (Fig. 2A). The Western diet increased peak levels of both VLDL cholesterol and LDL cholesterol relative to standard diet–fed mice, confirming the profile observed previously (25). Addition of 3% naringenin reduced VLDL cholesterol and LDL cholesterol, while HDL cholesterol was unaffected (Fig. 2C).
FIG. 2.
Plasma lipid metabolism and apoB secretion in naringenin-treated mice. Ldlr−/− mice (n = 12/group) were fed a standard diet or a high-fat diet alone or supplemented with naringenin (1 or 3%) for 4 weeks. A and B: Plasma cholesterol and triglyceride concentrations. □, standard diet; ■, Western diet; ▨, Western diet and 1% naringenin; ▩, Western diet and 3% naringenin. C and D: Plasma was subjected to fast protein liquid chromatography analysis and cholesterol and triglycerides were measured in the eluted fractions. ○, standard diet; ▾, Western diet; □, Western diet and 1% naringenin; ♦, Western diet and 3% naringenin. E: Mice were injected with tyloxapol and plasma triglycerides were measured at 0, 30, 60, and 120 min (n = 5–6/time point/group). F: Mice were injected with tyloxapol and [35S]methionine. Plasma was obtained at 60 and 120 min (n = 5–6/time point/group), and VLDL/IDL was isolated by ultracentrifugation. The rate of apoB secretion was measured as the difference in radiolabel in apoB and apoB48 between 60 and 120 min. G: Lipase activity was measured in plasma from mice (n = 6/group) obtained 30 min postinjection intraperitoneally with heparin. LPLA was determined as the difference between total and hepatic lipase activity. H: NEFA concentrations in plasma from fasted mice. Values are the means ± SE, different letters are statistically different, P < 0.05.
Plasma triglycerides were significantly elevated 3.8-fold in Western diet–fed mice compared with standard diet–fed mice. Naringenin at 1 and 3% decreased plasma triglycerides by 36 and 68%, respectively (Fig. 2B), which was primarily due to a reduction in VLDL-triglycerides (Fig. 2D). In metabolic studies, the Western diet significantly increased triglyceride secretion by 50%, which was completely prevented by 3% naringenin (Fig. 2E). Western diet–fed mice secreted twofold more radiolabeled apoB into plasma compared with standard diet–fed mice (Fig. 2F), whereas apoB secretion was reduced significantly by 36% in mice fed 3% naringenin. ApoB48 secretion was reduced similarly (data not shown). Postheparin LPLA increased 3.5-fold in Western diet–fed mice, which was increased a further twofold by 3% naringenin (Fig. 2G). Fasting plasma NEFAs were not different among the dietary groups (Fig. 2H).
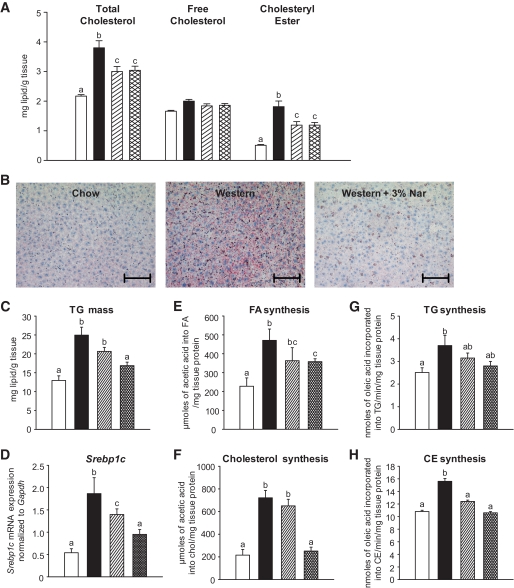
Naringenin prevents hepatic triglyceride accumulation.
Hepatic total cholesterol and cholesteryl ester concentrations were significantly elevated by 1.7- and 3.5-fold, respectively, in Western diet–fed compared with standard diet–fed mice. Total cholesterol and cholesteryl ester were decreased by 50% with 1 and 3% naringenin (Fig. 3A). Hepatic triglyceride levels were significantly elevated 1.9-fold in Western diet–fed mice and were markedly reduced by 1% naringenin (−40%) and by 3% naringenin (−67%) (Fig. 3B and C). The Western diet increased liver Srebp1c expression by 3.9-fold compared with standard diet–fed mice. Srebp1c was reduced dose dependently by 1% (−35%) and 3% naringenin (−65%) (Fig. 3D). Hepatic fatty acid and cholesterol synthesis increased twofold and threefold in Western diet–fed mice, which were attenuated by 3% naringenin (Fig. 3E and F). The 1.5-fold increases in hepatic triglyceride and cholesteryl ester synthesis in Western diet–fed mice were also reduced by 3% naringenin (Fig. 3G and H). No diet altered hepatic mRNA expression of Mttp, Acat1/2, or Dgat1 or protein expression of FoxO1 (data not shown).
FIG. 3.
Liver lipid metabolism and gene expression in naringenin-treated mice. Ldlr−/− mice (n = 12/group) were fed a standard diet or a high-fat diet alone or supplemented with naringenin (1 or 3%) for 4 weeks. A: Cholesterol concentrations in liver extracts. B: Oil Red O and hematoxylin-stained sections of liver. Representative photomicrographs. Scale bar = 100 μm. C: Triglyceride concentrations in liver extracts. D: Expression of Srebp1c relative to Gapdh in liver by qRT-PCR. E and F: Hepatic synthesis of fatty acid and cholesterol in liver (n = 5–7/group) obtained 60 min postinjection intraperitoneally with [14C]acetic acid. G and H: Hepatic synthesis of triglyceride and cholesteryl ester in liver homogenates using [14C]oleoyl-CoA (n = 6/group). □, standard diet; ■, Western diet; ▨, Western diet and 1% naringenin; ▩, Western diet and 3% naringenin. Values are means ± SE, different letters are statistically different, P < 0.05. (A high-quality digital representation of this figure is available in the online issue.)
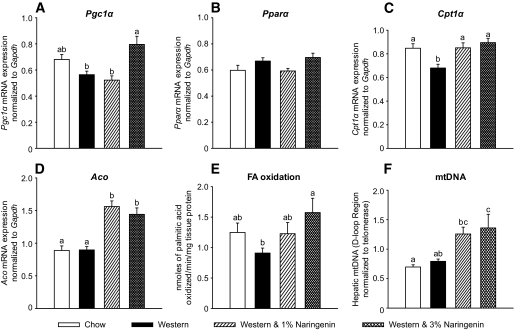
Naringenin increases hepatic fatty acid oxidation.
The marked reduction in hepatic triglyceride and VLDL-triglyceride and VLDL-apoB secretion suggested that naringenin also increased hepatic fatty acid oxidation. Peroxisome prolifertor–activated receptor (PPAR) γ coactivator 1α (PGC1α) activation can initiate mitochondrial biogenesis (28). Enzymes involved in mitochondrial and peroxisomal fatty acid oxidation, including Cpt1α and Aco, can be upregulated by PPARα (29). The Western diet decreased liver Pgc1α mRNA by 18% compared with standard diet–fed mice. However, 3% naringenin significantly increased Pgc1α expression by 30% compared with Western diet–fed mice (Fig. 4A). Pparα mRNA (Fig. 4B) and liver weight (data not shown) were unaffected by any diet. Naringenin increased Cpt1α expression by 25% compared with the Western diet alone (Fig. 4C). Aco expression was not affected in Western diet–fed mice, whereas naringenin significantly increased Aco mRNA by 30% (Fig. 4D). Consistent with these changes, 3% naringenin significantly increased mitochondrial DNA content (twofold) and fatty acid oxidation (twofold) compared with Western diet–fed mice (Fig. 4E and F). These data suggest that induction of hepatic fatty acid oxidation by naringenin contributes to the reduction of hepatic triglyceride availability for VLDL secretion.
FIG. 4.
Hepatic fatty acid oxidation and gene expression in naringenin-treated mice. Ldlr−/− mice (n = 12/group) were fed a standard diet or a high-fat diet alone or supplemented with naringenin (1 or 3%) for 4 weeks. A–D: Expression of Pgc1α (A), Pparα (B), Cpt1α (C), and Aco (D) relative to Gapdh in livers by qRT-PCR. E: Fatty acid oxidation in livers (n = 4–6/group) as determined as [3H]palmitate conversion to 3H2O. F: mtDNA copy number in liver (n = 10/group). Values are means ± SE, different letters are statistically different, P < 0.05.
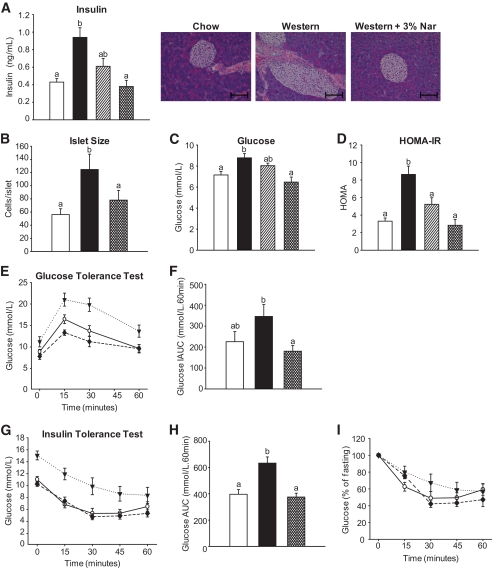
Naringenin improves glucose utilization and insulin sensitivity.
Fasting plasma insulin was significantly elevated 2.2-fold by the Western diet and completely prevented by 3% naringenin (Fig. 5A). Plasma insulin correlated with islet size, as significant hyperplasia was only identified in Western diet–fed mice (Fig. 5B). Despite hyperinsulinemia, mild hyperglycemia was observed in Western diet–fed mice, whereas naringenin at 3% normalized plasma glucose (Fig. 5C). HOMA-IR was significantly elevated 2.6-fold in Western diet–fed compared with standard diet–fed mice. HOMA-IR was completely normalized by 3% naringenin (Fig. 5D). The Western diet impaired glucose tolerance relative to standard diet–fed mice, whereas 3% naringenin completely normalized glucose utilization (Fig. 5E and F). Normalization by naringenin was maintained following correction for fasting glucose concentrations (Fig. 5F). Western diet–fed mice also showed a blunted response to exogenous insulin, resulting in a greater area under the curve compared with standard diet–fed mice. Naringenin completely normalized insulin sensitivity (Fig. 5G–I).
FIG. 5.
Glucose utilization and insulin sensitivity in naringenin-treated mice. Ldlr−/− mice (n = 12/group) were fed a standard diet or a high-fat diet alone or supplemented with naringenin (1 or 3%) for 4 weeks. A: Plasma insulin concentrations. B: Hematoxylin-eosin–stained sections of pancreas. Quantifications were performed on 100 islets/group. Scale bar = 100 μm. C: Blood glucose concentration. D: HOMA-IR. E: Glucose tolerance test was performed by intraperitoneal injection of 15% glucose (1 g/kg body wt) into mice and blood glucose was measured at 0, 15, 30, and 60 min postinjection (n = 7–8/group). ○, standard diet; ▾, Western diet; ♦, Western diet and 3% naringenin. F: Incremental area under the curve. G: Insulin tolerance test was performed by intraperitoneal injection of insulin (0.5 IU/kg body wt) into mice, and blood glucose was measured at 0, 15, 30, 45, and 60 min postinjection (n = 6/group). ○, standard diet; ▾, Western diet; ♦, Western diet and 3% naringenin. H: Insulin tolerance test area under the curve. I: Insulin tolerance test corrected for fasting glucose. □, standard diet; ■, Western diet; ▨, Western diet and 1% naringenin; ▩, Western diet and 3% naringenin. Values are means ± SE, different letters are statistically different, P < 0.05. (A high-quality digital representation of this figure is available in the online issue.)
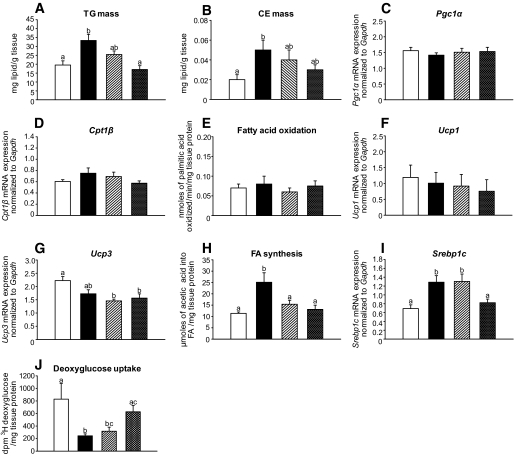
Intramyocellular lipids are associated with insulin resistance in vivo (30). Western diet–fed mice showed accumulation of both triglyceride and cholesteryl ester in muscle, whereas 3% naringenin completely prevented lipid deposition (Fig. 6A and B). In contrast to liver, there was no change in Pgc1α or Cpt1β expression or fatty acid oxidation (Fig. 6C–E). Furthermore, naringenin had no effect on uncoupled oxidation as both Ucp1 and Ucp3 mRNA were unchanged (Fig. 6F and G). However, both Srebp1c and fatty acid synthesis were significantly increased in Western diet–fed compared with standard diet–fed mice, and both were decreased by naringenin (Fig. 6H and I). Western diet–fed mice had impaired deoxyglucose uptake in muscle compared with standard diet–fed mice, whereas 3% naringenin significantly improved deoxyglucose uptake (Fig. 6J). These data suggest that naringenin decreases lipid accumulation in muscle, which may prevent peripheral insulin resistance.
FIG. 6.
Fatty acid oxidation, lipogenesis, and glucose uptake in quadriceps muscle in naringenin-treated mice. Ldlr−/− mice (n = 12/group) were fed a standard diet or a high-fat diet alone or supplemented with naringenin (1 or 3%) for 4 weeks. A and B: Triglyceride and cholesteryl ester concentrations in muscle lipid extracts. C and D: Expression of Pgc1α (C) and Cpt1β (D) relative to Gapdh in muscle by qRT-PCR. E: Fatty acid oxidation in muscle (n = 6/group) as determined as [3H]palmitate conversion to 3H2O. F and G: Expression of Ucp1 (F) and Ucp3 (G) relative to Gapdh in muscle by qRT-PCR. H: Synthesis of fatty acid in muscle (n = 6–8/group) obtained 60 min postinjection intraperitoneally with [14C]acetic acid. I: Expression of Srebp1c relative to Gapdh in muscle by qRT-PCR. J: Deoxyglucose uptake into muscle (n = 4–6/group) obtained 30 min postinjection intraperitoneally with [3H]deoxyglucose. Values are means ± SE, different letters are statistically different, P < 0.05. □, standard diet; ■, Western diet; ▨, Western diet and 1% naringenin; ▩, Western diet and 3% naringenin.
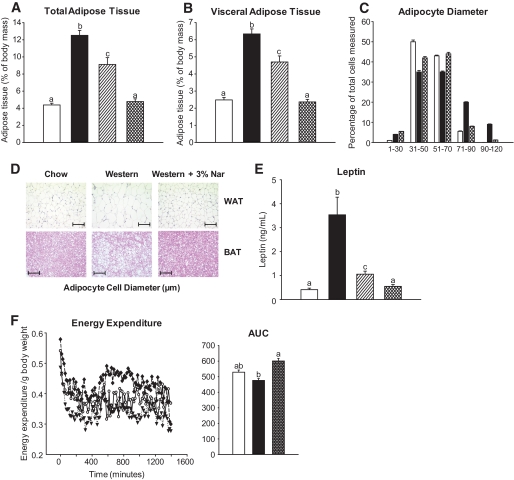
Naringenin decreases obesity.
Western diet–fed mice gained significantly more total and visceral adipose tissue compared with standard diet–fed mice (Fig. 7A and B); however, lean body mass was unchanged (data not shown). Naringenin dose-dependently attenuated adiposity so that 3% naringenin–fed mice were similar to standard diet–fed mice (Fig. 7A and B). Adipocytes in both epidydimal fat and intrascapular brown adipose tissue (BAT) of Western diet–fed mice shifted toward a hypertrophic phenotype compared with standard diet–fed mice and 3% naringenin–treated mice (Fig. 7C and D); however, macrophage infiltration into epididymal adipose stores was not observed with any diet (Fig. 7D). Plasma leptin levels correlated well with adipose tissue mass (Fig. 7E).
FIG. 7.
Naringenin-treated mice are resistant to diet-induced obesity. Ldlr−/− mice (n = 12/group) were fed a standard diet or a high-fat diet alone or supplemented with naringenin (1 or 3%) for 4 weeks. A: Total adiposity was determined as the weight of all adipose stores per gram body weight. B: Visceral adiposity was determined by dissection of mesenteric, epidydimal, and intraperitoneal pads from mice. C: Adipocyte size was determined by measuring the diameter of adipocytes from hematoxylin-eosin–stained sections of epidydimal adipose (n = 100 adipocytes/group). D: Representative photomicrographs of epidydimal white adipose tissue stained with MAC-1 antibody and intrascapular brown adipose tissue stained with hematoxylin-eosin. Scale bar = 100 μm. E: Plasma leptin concentrations. F: Energy expenditure was determined by changes in the O2 consumption and CO2 production via indirect calorimetry and area under the curve calculated (n = 6/group). ○, standard diet; ▾, Western diet; ♦, Western diet and 3% naringenin; □, standard diet; ■, Western diet; ▨, Western diet and 1% naringenin; ▩, Western diet and 3% naringenin. Values are means ± SE, different letters are statistically different, P < 0.05. (A high-quality digital representation of this figure is available in the online issue.)
Energy expenditure decreased with the Western diet compared with the standard diet. However, consistent with increased fatty acid oxidation, enhanced glucose uptake, and decreased lipogenesis, energy expenditure was significantly higher in mice fed 3% naringenin and was not different from standard diet (Fig. 7F). The respiratory quotient was 0.90 for standard diet–fed animals and 0.79 for Western diet–fed mice, whereas an intermediate value of 0.83 was observed with naringenin treatment, although differences were not significant. Intrascapular BAT is responsible for induction of thermogenesis; therefore, expression of Ucp1 and mtDNA were determined. Ucp1 mRNA was similar among the dietary groups (data not shown). While the Western diet modestly increased mtDNA, no further increase was observed in naringenin-treated mice (data not shown). Heat production was evaluated by a cold-challenge test. The ability of naringenin-treated mice to maintain body temperature at 4°C did not differ from standard diet– or Western diet–fed mice (data not shown), collectively suggesting that naringenin does not stimulate adaptive thermogenesis.
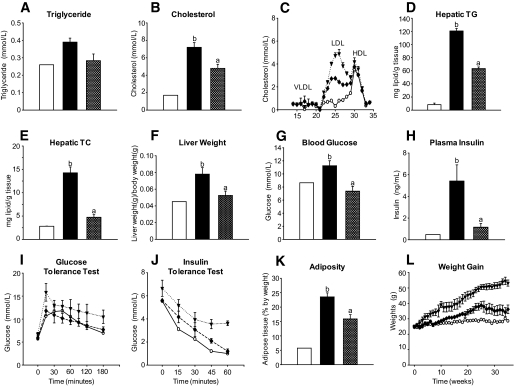
Naringenin improves dyslipidemia, hepatic steatosis, and insulin sensitivity in wild-type mice.
To confirm that the effect of naringenin was not restricted to Ldlr−/− mice, we performed similar studies in wild-type C57BL/6J mice fed the same diets for 30 weeks to establish insulin resistance. The Western diet increased plasma triglycerides and cholesterol compared with the standard diet, whereas 3% naringenin significantly improved plasma lipids (Fig. 8A–C). Naringenin prevented the significant increase in liver triglycerides and cholesterol observed in Western diet–fed mice (Fig. 8D–F). The hyperglycemia and hyperinsulinemia that developed in Western diet–fed mice were normalized by 3% naringenin. Glucose tolerance tests revealed that 3% naringenin corrected impaired glucose utilization and insulin insensitivity observed in Western diet–fed mice (Fig. 8G–J). The Western diet increased weight gain and adipose tissue accumulation, which was significantly attenuated by naringenin (Fig. 8K and L).
FIG. 8.
Liver and plasma lipid metabolism in naringenin-treated wild-type mice. Wild-type C57BL/6J mice were fed a standard diet (n = 2) or a high-fat Western diet alone (n = 4) or supplemented with 3% naringenin (n = 4) for 8 months. A and B: Triglyceride and cholesterol concentrations in plasma. C: Plasma was subjected to fast protein liquid chromatography analysis and cholesterol was measured in the eluted fractions. D and E: Triglyceride and cholesterol concentrations in liver lipid extracts. F: Liver weight/body weight (in grams) was determined at necropsy. G: Glucose concentrations in whole blood. H: Plasma insulin concentrations. I: Oral glucose tolerance test was performed by gavage of 20% glucose (1 g/kg body wt) into mice and blood glucose was measured up to 180 min postinjection. J: Insulin tolerance test was performed by intraperitoneal injection of insulin (0.5 IU/kg body wt) into mice, and blood glucose was measured up to 60 min postinjection. K: Total adiposity was determined by dissection of all adipose stores. L: Body weight gain. ○, standard diet; ▾, Western diet; ♦, Western diet and 3% naringenin; □, standard diet; ■, Western diet; ▨, Western diet and 1% naringenin; ▩, Western diet and 3% naringenin. Values are means ± SE, different letters are statistically different, P < 0.05.
DISCUSSION
Mice lacking the LDLR, when fed a Western-style diet, display many features of insulin resistance including VLDL overproduction, dyslipidemia, and obesity. Furthermore, hepatic lipids increase and fasting glucose and insulin become elevated, resulting from impaired glucose tolerance and reduced insulin sensitivity (25). The major findings of this study are that addition of naringenin to a high-fat diet 1) decreased plasma lipids, 2) reduced overproduction of total triglycerides and hepatic apoB, 3) decreased liver triglycerides and cholesterol, 4) inhibited stimulation of hepatic lipogenesis and prevented hepatic steatosis, 4) increased hepatic β-oxidation, 5) normalized blood glucose and plasma insulin, and 6) restored glucose tolerance. These results demonstrate a novel treatment to correct the metabolic abnormalities associated with diet-induced insulin resistance.
VLDL secretion is increased in the metabolic syndrome and contributes to the dyslipidemia associated with insulin resistance (31). Although regulation of hepatic VLDL secretion is complex, an important stimulus is lipid availability (6). In mouse models of diet-induced insulin resistance, the concept of selective hepatic insulin resistance has emerged (32). Hepatic lipogenesis remains sensitive to insulin. The hyperinsulinemia observed in Western diet–fed mice greatly increases hepatic SREBP1c, drives lipogenesis (15), and increases the contribution of de novo fatty acids to VLDL-triglycerides (33). In this study, naringenin prevents hyperinsulinemia, leading to a reduction in hepatic SREBP1c and hepatic lipogenesis in the fasted state. The reduction in hepatic triglyceride availability contributes to the significant decrease in VLDL-triglycerides and VLDL-apoB secretion and attenuation of dyslipidemia. These findings are consistent with recent studies in mice demonstrating that blocking the ability of insulin to activate hepatic SREBP1c–induced lipogenesis due to a complete absence (LIRKO) or very low expression of (L1B6Ldlr−/−) hepatic insulin receptors greatly diminished VLDL-triglyceride secretion (18,19). In some mouse models, however, hepatic apoB secretion is not tightly linked to SREBP1c-stimulated triglyceride availability. For instance, in ob/ob mice, increased hepatic Srebp1c and de novo lipogenesis were not associated with increased VLDL-apoB production (34). Liver-specific overexpression of DGAT1/2 greatly increased Srebp1c, Fas, and triglyceride accumulation; however, VLDL secretion was unchanged (35). Availability of newly synthesized cholesterol and cholesteryl ester, which have been shown to influence VLDL formation and secretion (36,37), were unaffected in ob/ob mice and hepatic DGAT1/2–overexpressing mice (34,35). In cultured HepG2 cells, naringenin inhibited ACAT2 expression and cholesterol esterification, leading to inhibition of apoB secretion (37). In the present study, naringenin also prevented the increase in hepatic cholesterol, and although Acat2 mRNA was unaffected, cholesteryl ester synthesis was significantly reduced. This demonstrates that naringenin may not only limit triglyceride availability for VLDL production but also cholesteryl ester.
The contribution of increased intestinal triglycerides in Western diet–fed mice to the overproduction of triglycerides into plasma cannot be discounted. In insulin-resistant hamsters, de novo intestinal lipogenesis results in overproduction of intestinally derived lipoproteins (38). Prevention of intestinal triglyceride accumulation by naringenin may result in reduced secretion of intestinally derived lipoproteins and contribute to the normalization of triglyceride secretion into plasma. Whether the mechanism in the intestine is similar to that observed in liver requires further investigation.
Under normal conditions, insulin targets apoB for intracellular degradation in cultured hepatocytes, leading to acute reduction in VLDL secretion (7,39,40). Whether increased intracellular apoB degradation is a direct effect of insulin or is a consequence of reduced apoB lipidation is not well understood. In insulin-resistant hamsters, apoB degradation is compromised in hepatocytes obtained ex vivo (16). Hyperinsulinemic LIRKO mice overproduce cholesterol-rich apoB, even in the absence of insulin-stimulated SREBP1c expression (18), suggesting that insulin-mediated degradation of apoB is an important determinant of VLDL secretion. In contrast, in hyperinsulinemic L1B6Ldlr−/− mice with very few hepatic insulin receptors, apoB production was markedly diminished compared with Ldlr−/− controls, implying that the loss of SREBP1c-stimulated triglyceride synthesis was the primary determinant for reduced apoB secretion (19). Pulse-chase experiments in HepG2 cells revealed that naringenin, like insulin, reduces apoB secretion through rapid intracellular apoB degradation (7). Enhanced apoB degradation by naringenin was observed in the presence or absence of oleate-stimulated triglyceride synthesis and occurred independent of insulin receptor activation (7,9). In the present study, increased hepatic apoB degradation may contribute to the ability of naringenin to decrease VLDL-apoB secretion in Western diet–fed mice. However, direct experimental evidence requires further investigation.
The Western diet significantly increased LPLA compared with the standard diet, suggesting that a lipolytic defect did not contribute to the hypertriglyceridemia. Naringenin increased LPLA over twofold compared with Western diet–fed mice. The mechanism for this is unclear, as LPL mRNA was not different between Western diet–fed or naringenin-fed animals in liver or muscle (data not shown). Nevertheless, increased LPLA likely contributed to the normalization of plasma triglycerides in naringenin-treated mice.
In mice with diet-induced insulin resistance, SREBP1c-stimulated lipogenesis contributes to hepatic triglyceride accumulation. Reduced rates of hepatic fatty acid oxidation may also be involved. Key regulators of hepatic β-oxidation, including CPT1α, are controlled by a complex of transcription factors and coactivators, including PGC1α and PPARα. PGC1α regulates many metabolic pathways in liver, including gluconeogenesis, mitochondrial expansion, and fatty acid oxidation (28). Fasted Pgc1α−/− mice accumulated significant amounts of neutral lipid within the liver due to decreased hepatic fatty acid oxidation and enhanced SREBP1c-stimulated triglyceride synthesis (41). Hepatic-specific overexpression of Cpt1α in fat-fed rats stimulates fatty acid oxidation and corrects liver triglyceride accumulation (42). Here, we demonstrate that the Western diet decreased hepatic Cpt1α and Pgc1α mRNA and reduced fatty acid oxidation. Naringenin significantly increased hepatic Pgc1α mRNA, which coincided with increased mitochondrial DNA and enhanced fatty acid oxidation. Furthermore, hepatic expression of PPARα-responsive genes, Cpt1α and Aco, were significantly increased.
In fat-fed Ldlr−/− mice, the PPARα agonist fenofibrate increased hepatic expression of Aco and Lpl, while Srebp1c, Fas, and Dgat2 were reduced (43). In contrast to a classic PPARα agonist, naringenin did not increase hepatic Pparα expression or liver weight. Naringin, the glucoside form of naringenin, decreases liver triglyceride and hepatic fatty acid synthesis in db/db mice, leading to decreased plasma lipids (44). Treatment of fat-fed ICR rats with 1% naringenin increased the hepatic activities of Cpt1α and Aco and increased peroxisomal fatty acid oxidation (45). These data indicate that naringenin activates PGC1α and PPARα target genes to shift the metabolic program in the liver, resulting in a diminished triglyceride burden compared with Western diet–fed mice. Furthermore, naringenin-stimulated fatty acid oxidation likely contributed to the decreased availability of triglyceride for VLDL secretion.
Ldlr−/− mice fed a Western diet become dyslipidemic and hyperinsulinemic, with a metabolic profile characterized by reduced glucose utilization and impaired insulin sensitivity (25). Intramyocellular triglyceride accumulation has been associated with peripheral insulin resistance in animals and humans (46,47). Accumulation of fatty acid derivatives in muscle, derived from both plasma NEFAs and triglyceride-rich lipoproteins, inactivates insulin receptor signaling (47). Naringenin prevented the marked accumulation of triglycerides and cholesterol in muscle induced by the Western diet. Naringenin also normalized insulin sensitivity and pancreatic islet morphology, leading to significantly improved glucose tolerance, including enhanced glucose uptake in muscle. Insulin has been shown to increase Srebp1c and Fas in cultured myotubes (48). In quadriceps of Western diet–fed mice, we demonstrate that Srebp1c mRNA and fatty acid synthesis were significantly increased, indicating that hyperinsulinemia stimulated endogenous lipid synthesis, which contributed to muscle lipid accumulation and diminished insulin sensitivity. Naringenin attenuated SREBP1c-induced fatty acid synthesis in muscle, similar to the effect observed in liver. In contrast to liver, naringenin did not increase Pgc1α expression, nor did it stimulate fatty acid oxidation. This suggests that naringenin may not act directly in muscle, and the reduction in muscle lipid accumulation and improved glucose utilization is instead due to reduced uptake of lipoprotein-derived lipid, a consequence of decreased VLDL secretion, as well as reduced de novo lipogenesis, secondary to normalization of hyperinsulinemia.
Obesity is a component of the metabolic syndrome. Naringenin treatment resulted in almost complete resistance to diet-induced obesity without affecting lean body mass. Increased energy expenditure resulted in enhanced hepatic fatty acid oxidation and/or increased peripheral glucose oxidation, leading to normalized weight gain. Increased energy expenditure prevented visceral adipocyte hypertrophy and normalized the size of visceral fat pads. Naringenin had no effect on lipid metabolism in BAT, indicating the prevention of obesity was unrelated to adaptive thermogenesis.
Treatment of fat-fed C57BL/6J mice with another polyphenolic compound, resveratrol, increased fatty acid oxidation in gastrocnemius muscle and BAT through mitochondrial expansion via activation of SIRT1 and deacetylation of PGC1α (49). In contrast to naringenin, resveratrol had little effect in the liver. Resveratrol improved insulin sensitivity and glucose tolerance; however, compared with naringenin, plasma cholesterol was only modestly reduced and plasma triglycerides were unaffected (49,50). These data suggest that flavonoids have diverse and distinctive tissue-specific effects likely based on structural differences and pharmacokinetic behavior.
The protective effect of naringenin was not restricted to mice with Ldlr deficiency. In wild-type mice, we found that naringenin significantly reduced plasma and hepatic lipids, normalized glucose tolerance and insulin sensitivity, and prevented obesity compared with Western diet–fed mice.
Collectively, these findings demonstrate that naringenin has marked lipid- and lipoprotein-lowering potential. Naringenin normalizes hepatic VLDL production, glucose tolerance, and insulin sensitivity and prevents hepatic steatosis and obesity associated with a high-fat diet. The ability of naringenin to modulate metabolic pathways linked to the metabolic syndrome suggests that these molecules represent valuable tools in the search for regulators of insulin signaling, lipid homeostasis, and energy balance.
Acknowledgments
This work was supported by grants (T-5603 and PRG-5967) from the Heart and Stroke Foundation of Ontario (HSFO) to M.W.H. E.M.A. was the recipient of a joint AstraZeneca/Heart and Stroke Foundation of Canada postdoctoral fellowship, and E.E.M. was the recipient of an HSFO Masters Award and currently holds a Canadian Institutes of Health Research Canada Graduate Scholarship Doctoral Award.
No potential conflicts of interest relevant to this article were reported.
We thank Dr. Stewart Whitman and Mirela Hasu for completing the lipoprotein profiles, Dr. Nica Borradaile for valuable insight, Dr. Fred Dick for use of metabolic cages, and Dr. Jian Wang for technical assistance.
Footnotes
The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.Eckel RH, Grundy SM, Zimmet PZ: The metabolic syndrome. Lancet 2005;365:1415–1428 [DOI] [PubMed] [Google Scholar]
- 2.Pollex RL, Hegele RA: Genetic determinants of the metabolic syndrome. Nat Clin Pract Cardiovasc Med 2006;3:482–489 [DOI] [PubMed] [Google Scholar]
- 3.Biddinger SB, Kahn CR: From mice to men: insights into the insulin resistance syndromes. Annu Rev Physiol 2006;68:123–158 [DOI] [PubMed] [Google Scholar]
- 4.Kerouz NJ, Horsch D, Pons S, Kahn CR: Differential regulation of insulin receptor substrates-1 and -2 (IRS-1 and IRS-2) and phosphatidylinositol 3-kinase isoforms in liver and muscle of the obese diabetic (ob/ob) mouse. J Clin Invest 1997;100:3164–3172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bjornholm M, Zierath JR: Insulin signal transduction in human skeletal muscle: identifying the defects in type II diabetes. Biochem Soc Trans 2005;33:354–357 [DOI] [PubMed] [Google Scholar]
- 6.Dixon JL, Ginsberg HN: Regulation of hepatic secretion of apolipoprotein B-containing lipoproteins: information obtained from cultured liver cells. J Lipid Res 1993;34:167–179 [PubMed] [Google Scholar]
- 7.Allister EM, Mulvihill EE, Barrett PH, Edwards JY, Carter LP, Huff MW: Inhibition of apoB secretion from HepG2 cells by insulin is amplified by naringenin, independent of the insulin receptor. J Lipid Res 2008;49:2218–2229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sparks JD, Sparks CE: Insulin regulation of triacylglycerol-rich lipoprotein synthesis and secretion. Biochim Biophys Acta 1994;1215:9–32 [DOI] [PubMed] [Google Scholar]
- 9.Borradaile NM, de Dreu LE, Huff MW: Inhibition of net HepG2 cell apolipoprotein B secretion by the citrus flavonoid naringenin involves activation of phosphatidylinositol 3-kinase, independent of insulin receptor substrate-1 phosphorylation. Diabetes 2003;52:2554–2561 [DOI] [PubMed] [Google Scholar]
- 10.Phung TL, Roncone A, Jensen KL, Sparks CE, Sparks JD: Phosphoinositide 3-kinase activity is necessary for insulin-dependent inhibition of apolipoprotein B secretion by rat hepatocytes and localizes to the endoplasmic reticulum. J Biol Chem 1997;272:30693–30702 [DOI] [PubMed] [Google Scholar]
- 11.Allister EM, Borradaile NM, Edwards JY, Huff MW: Inhibition of microsomal triglyceride transfer protein expression and apolipoprotein B100 secretion by the citrus flavonoid naringenin and by insulin involves activation of the mitogen-activated protein kinase pathway in hepatocytes. Diabetes 2005;54:1676–1683 [DOI] [PubMed] [Google Scholar]
- 12.Au WS, Kung HF, Lin MC: Regulation of microsomal triglyceride transfer protein gene by insulin in HepG2 cells: roles of MAPKerk and MAPKp38. Diabetes 2003;52:1073–1080 [DOI] [PubMed] [Google Scholar]
- 13.Boden G, Shulman GI: Free fatty acids in obesity and type 2 diabetes: defining their role in the development of insulin resistance and beta-cell dysfunction. Eur J Clin Invest 2002;32(Suppl. 3):14–23 [DOI] [PubMed] [Google Scholar]
- 14.Ginsberg HN: Insulin resistance and cardiovascular disease. J Clin Invest 2000;106:453–458 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shimomura I, Matsuda M, Hammer RE, Bashmakov Y, Brown MS, Goldstein JL: Decreased IRS-2 and increased SREBP-1c lead to mixed insulin resistance and sensitivity in livers of lipodystrophic and ob/ob mice. Mol Cell 2000;6:77–86 [PubMed] [Google Scholar]
- 16.Taghibiglou C, Carpentier A, Van Iderstine SC, Chen B, Rudy D, Aiton A, Lewis GF, Adeli K: Mechanisms of hepatic very low density lipoprotein overproduction in insulin resistance: evidence for enhanced lipoprotein assembly, reduced intracellular apoB degradation, and increased microsomal triglyceride transfer protein in a fructose-fed hamster model. J Biol Chem 2000;275:8416–8425 [DOI] [PubMed] [Google Scholar]
- 17.Lewis GF, Steiner G: Acute effects of insulin in the control of VLDL production in humans. Implications for the insulin-resistant state. Diabetes Care 1996;19:390–393 [DOI] [PubMed] [Google Scholar]
- 18.Biddinger SB, Hernandez-Ono A, Rask-Madsen C, Haas JT, Aleman JO, Suzuki R, Scapa EF, Agarwal C, Carey MC, Stephanopoulos G, Cohen DE, King GL, Ginsberg HN, Kahn CR: Hepatic insulin resistance is sufficient to produce dyslipidemia and susceptibility to atherosclerosis. Cell Metab 2008;7:125–134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Han S, Liang CP, Westerterp M, Senokuchi T, Welch CL, Wang Q, Matsumoto M, Accili D, Tall AR: Hepatic insulin signaling regulates VLDL secretion and atherogenesis in mice. J Clin Invest 2009;119:1029–1041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Semple RK, Sleigh A, Murgatroyd PR, Adams CA, Bluck L, Jackson S, Vottero A, Kanabar D, Charlton-Menys V, Durrington P, Soos MA, Carpenter TA, Lomas DJ, Cochran EK, Gorden P, O'Rahilly S, Savage DB: Postreceptor insulin resistance contributes to human dyslipidemia and hepatic steatosis. J Clin Invest 2009;119:315–322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Choi JS, Yokozawa T, Oura H: Improvement of hyperglycemia and hyperlipemia in streptozotocin-diabetic rats by a methanolic extract of Prunus davidiana stems and its main component, prunin. Planta Med 1991;57:208–211 [DOI] [PubMed] [Google Scholar]
- 22.Lee SH, Park YB, Bae KH, Bok SH, Kwon YK, Lee ES, Choi MS: Cholesterol-lowering activity of naringenin via inhibition of 3-hydroxy-3-methylglutaryl coenzyme A reductase and acyl coenzyme A: cholesterol acyltransferase in rats. Ann Nutr Metab 1999;43:173–180 [DOI] [PubMed] [Google Scholar]
- 23.Borradaile NM, de Dreu LE, Barrett PH, Behrsin CD, Huff MW: Hepatocyte apoB-containing lipoprotein secretion is decreased by the grapefruit flavonoid, naringenin, via inhibition of MTP-mediated microsomal triglyceride accumulation. Biochemistry 2003;42:1283–1291 [DOI] [PubMed] [Google Scholar]
- 24.Borradaile NM, de Dreu LE, Barrett PH, Huff MW: Inhibition of hepatocyte apoB secretion by naringenin: enhanced rapid intracellular degradation independent of reduced microsomal cholesteryl esters. J Lipid Res 2002;43:1544–1554 [DOI] [PubMed] [Google Scholar]
- 25.Merat S, Casanada F, Sutphin M, Palinski W, Reaven PD: Western-type diets induce insulin resistance and hyperinsulinemia in LDL receptor-deficient mice but do not increase aortic atherosclerosis compared with normoinsulinemic mice in which similar plasma cholesterol levels are achieved by a fructose-rich diet. Arterioscler Thromb Vasc Biol 1999;19:1223–1230 [DOI] [PubMed] [Google Scholar]
- 26.Telford DE, Sutherland BG, Edwards JY, Andrews JD, Barrett PH, Huff MW: The molecular mechanisms underlying the reduction of LDL apoB-100 by ezetimibe plus simvastatin. J Lipid Res 2007;48:699–708 [DOI] [PubMed] [Google Scholar]
- 27.Xia Z, Sniderman AD, Cianflone K: Acylation-stimulating protein (ASP) deficiency induces obesity resistance and increased energy expenditure in ob/ob mice. J Biol Chem 2002;277:45874–45879 [DOI] [PubMed] [Google Scholar]
- 28.Lin J, Handschin C, Spiegelman BM: Metabolic control through the PGC-1 family of transcription coactivators. Cell Metab 2005;1:361–370 [DOI] [PubMed] [Google Scholar]
- 29.Dreyer C, Keller H, Mahfoudi A, Laudet V, Krey G, Wahli W: Positive regulation of the peroxisomal beta-oxidation pathway by fatty acids through activation of peroxisome proliferator-activated receptors (PPAR). Biol Cell 1993;77:67–76 [DOI] [PubMed] [Google Scholar]
- 30.Petersen KF, Shulman GI: Etiology of insulin resistance. Am J Med 2006;119:S10–16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Avramoglu RK, Basciano H, Adeli K: Lipid and lipoprotein dysregulation in insulin resistant states. Clin Chim Acta 2006;368:1–19 [DOI] [PubMed] [Google Scholar]
- 32.Brown MS, Goldstein JL: Selective versus total insulin resistance: a pathogenic paradox. Cell Metab 2008;7:95–96 [DOI] [PubMed] [Google Scholar]
- 33.Grefhorst A, Elzinga BM, Voshol PJ, Plosch T, Kok T, Bloks VW, van der Sluijs FH, Havekes LM, Romijn JA, Verkade HJ, Kuipers F: Stimulation of lipogenesis by pharmacological activation of the liver X receptor leads to production of large, triglyceride-rich very low density lipoprotein particles. J Biol Chem 2002;277:34182–34190 [DOI] [PubMed] [Google Scholar]
- 34.Wiegman CH, Bandsma RH, Ouwens M, van der Sluijs FH, Havinga R, Boer T, Reijngoud DJ, Romijn JA, Kuipers F: Hepatic VLDL production in ob/ob mice is not stimulated by massive de novo lipogenesis but is less sensitive to the suppressive effects of insulin. Diabetes 2003;52:1081–1089 [DOI] [PubMed] [Google Scholar]
- 35.Millar JS, Stone SJ, Tietge UJ, Tow B, Billheimer JT, Wong JS, Hamilton RL, Farese RV, Jr, Rader DJ: Short-term overexpression of DGAT1 or DGAT2 increases hepatic triglyceride but not VLDL triglyceride or apoB production. J Lipid Res 2006;47:2297–2305 [DOI] [PubMed] [Google Scholar]
- 36.Burnett JR, Wilcox LJ, Huff MW: Acyl coenzyme A: cholesterol acyltransferase inhibition and hepatic apolipoprotein B secretion. Clin Chim Acta 1999;286:231–242 [DOI] [PubMed] [Google Scholar]
- 37.Wilcox LJ, Borradaile NM, de Dreu LE, Huff MW: Secretion of hepatocyte apoB is inhibited by the flavonoids, naringenin and hesperetin, via reduced activity and expression of ACAT2 and MTP. J Lipid Res 2001;42:725–734 [PubMed] [Google Scholar]
- 38.Haidari M, Leung N, Mahbub F, Uffelman KD, Kohen-Avramoglu R, Lewis GF, Adeli K: Fasting and postprandial overproduction of intestinally derived lipoproteins in an animal model of insulin resistance: evidence that chronic fructose feeding in the hamster is accompanied by enhanced intestinal de novo lipogenesis and ApoB48-containing lipoprotein overproduction. J Biol Chem 2002;277:31646–31655 [DOI] [PubMed] [Google Scholar]
- 39.Chirieac DV, Chirieac LR, Corsetti JP, Cianci J, Sparks CE, Sparks JD: Glucose-stimulated insulin secretion suppresses hepatic triglyceride-rich lipoprotein and apoB production. Am J Physiol Endocrinol Metab 2000;279:E1003–E1011 [DOI] [PubMed] [Google Scholar]
- 40.Pullinger CR, North JD, Teng BB, Rifici VA, Ronhild de Brito AE, Scott J: The apolipoprotein B gene is constitutively expressed in HepG2 cells: regulation of secretion by oleic acid, albumin, and insulin, and measurement of the mRNA half-life. J Lipid Res 1989;30:1065–1077 [PubMed] [Google Scholar]
- 41.Leone TC, Lehman JJ, Finck BN, Schaeffer PJ, Wende AR, Boudina S, Courtois M, Wozniak DF, Sambandam N, Bernal-Mizrachi C, Chen Z, Holloszy JO, Medeiros DM, Schmidt RE, Saffitz JE, Abel ED, Semenkovich CF, Kelly DP: PGC-1alpha deficiency causes multi-system energy metabolic derangements: muscle dysfunction, abnormal weight control and hepatic steatosis. PLoS Biol 2005;3:e101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Stefanovic-Racic M, Perdomo G, Mantell BS, Sipula IJ, Brown NF, O'Doherty RM: A moderate increase in carnitine palmitoyltransferase 1a activity is sufficient to substantially reduce hepatic triglyceride levels. Am J Physiol Endocrinol Metab 2008;294:E969–E977 [DOI] [PubMed] [Google Scholar]
- 43.Srivastava RA, Jahagirdar R, Azhar S, Sharma S, Bisgaier CL: Peroxisome proliferator-activated receptor-alpha selective ligand reduces adiposity, improves insulin sensitivity and inhibits atherosclerosis in LDL receptor-deficient mice. Mol Cell Biochem 2006;285:35–50 [DOI] [PubMed] [Google Scholar]
- 44.Jung UJ, Lee MK, Park YB, Kang MA, Choi MS: Effect of citrus flavonoids on lipid metabolism and glucose-regulating enzyme mRNA levels in type-2 diabetic mice. Int J Biochem Cell Biol 2006;38:1134–1145 [DOI] [PubMed] [Google Scholar]
- 45.Huong DT, Takahashi Y, Ide T: Activity and mRNA levels of enzymes involved in hepatic fatty acid oxidation in mice fed citrus flavonoids. Nutrition 2006;22:546–552 [DOI] [PubMed] [Google Scholar]
- 46.Lewis GF, Carpentier A, Adeli K, Giacca A: Disordered fat storage and mobilization in the pathogenesis of insulin resistance and type 2 diabetes. Endocr Rev 2002;23:201–229 [DOI] [PubMed] [Google Scholar]
- 47.Shulman GI: Unraveling the cellular mechanism of insulin resistance in humans: new insights from magnetic resonance spectroscopy. Physiology (Bethesda) 2004;19:183–190 [DOI] [PubMed] [Google Scholar]
- 48.Guillet-Deniau I, Mieulet V, Le Lay S, Achouri Y, Carre D, Girard J, Foufelle F, Ferre P: Sterol regulatory element binding protein-1c expression and action in rat muscles: insulin-like effects on the control of glycolytic and lipogenic enzymes and UCP3 gene expression. Diabetes 2002;51:1722–1728 [DOI] [PubMed] [Google Scholar]
- 49.Lagouge M, Argmann C, Gerhart-Hines Z, Meziane H, Lerin C, Daussin F, Messadeq N, Milne J, Lambert P, Elliott P, Geny B, Laakso M, Puigserver P, Auwerx J: Resveratrol improves mitochondrial function and protects against metabolic disease by activating SIRT1 and PGC-1alpha. Cell 2006;127:1109–1122 [DOI] [PubMed] [Google Scholar]
- 50.Baur JA, Pearson KJ, Price NL, Jamieson HA, Lerin C, Kalra A, Prabhu VV, Allard JS, Lopez-Lluch G, Lewis K, Pistell PJ, Poosala S, Becker KG, Boss O, Gwinn D, Wang M, Ramaswamy S, Fishbein KW, Spencer RG, Lakatta EG, Le Couteur D, Shaw RJ, Navas P, Puigserver P, Ingram DK, de Cabo R, Sinclair DA: Resveratrol improves health and survival of mice on a high-calorie diet. Nature 2006;444:337–342 [DOI] [PMC free article] [PubMed] [Google Scholar]