Abstract
OBJECTIVE
Hyperglycemia induces reactive oxygen species (ROS) and apoptosis in cardiomyocytes, which contributes to diabetic cardiomyopathy. The present study was to investigate the role of Rac1 in ROS production and cardiomyocyte apoptosis during hyperglycemia.
RESEARCH DESIGN AND METHODS
Mice with cardiomyocyte-specific Rac1 knockout (Rac1-ko) were generated. Hyperglycemia was induced in Rac1-ko mice and their wild-type littermates by injection of streptozotocin (STZ). In cultured adult rat cardiomyocytes, apoptosis was induced by high glucose.
RESULTS
The results showed a mouse model of STZ-induced diabetes, 7 days of hyperglycemia-upregulated Rac1 and NADPH oxidase activation, elevated ROS production, and induced apoptosis in the heart. These effects of hyperglycemia were significantly decreased in Rac1-ko mice or wild-type mice treated with apocynin. Interestingly, deficiency of Rac1 or apocynin treatment significantly reduced hyperglycemia-induced mitochondrial ROS production in the heart. Deficiency of Rac1 also attenuated myocardial dysfunction after 2 months of STZ injection. In cultured cardiomyocytes, high glucose upregulated Rac1 and NADPH oxidase activity and induced apoptotic cell death, which were blocked by overexpression of a dominant negative mutant of Rac1, knockdown of gp91phox or p47phox, or NADPH oxidase inhibitor. In type 2 diabetic db/db mice, administration of Rac1 inhibitor, NSC23766, significantly inhibited NADPH oxidase activity and apoptosis and slightly improved myocardial function.
CONCLUSIONS
Rac1 is pivotal in hyperglycemia-induced apoptosis in cardiomyocytes. The role of Rac1 is mediated through NADPH oxidase activation and associated with mitochondrial ROS generation. Our study suggests that Rac1 may serve as a potential therapeutic target for cardiac complications of diabetes.
Diabetic cardiomyopathy has been defined as ventricular dysfunction that occurs in the absence of changes in blood pressure and coronary artery disease (1,2). Cell death by apoptosis is the predominant damage in diabetic cardiomyopathy (3,4). Diabetes increases cardiac apoptosis in animals and patients (3–7). Cardiomyocyte death causes a loss of contractile tissue, which initiates a cardiac remodeling (8). Loss of cardiomyocytes and hypertrophy of the remaining cells characterize the diabetic cardiomyopathy (9,10). Thus, suppression of cardiomyocyte apoptosis results in a significant prevention of the development of diabetic cardiomyopathy (4). However, the underlying mechanisms by which diabetes induce apoptosis remain not fully understood.
All forms of diabetes are characterized by chronic hyperglycemia. Hyperglycemia induces reactive oxygen species (ROS) production in cardiomyocytes (6,11), which plays a crucial role in cardiomyocyte apoptosis in diabetes because the administration of antioxidant agents are able to rescue hyperglycemia-induced cardiomyocytes (4,6). The mechanisms activated by hyperglycemia, leading to myocardial oxidative stress and apoptosis, are not completely clarified.
Although multiple sources of ROS have been demonstrated, NADPH oxidase is a critical determinant of the redox state of the myocardium (12–15). Higher myocardial NADPH oxidase activity has been detected in diabetes (16,17); more importantly, NADPH oxidase activity is markedly increased by high glucose levels (18). The NADPH oxidase is a multicomponent enzyme complex that consists of the membrane-bound cytochrome b558, which contains gp91phox and p22phox, the cytosolic regulatory subunits p47phox and p67phox, and the small guanosine triphosphate-binding protein Rac. An important step for the assembly and function of this multicomponent NADPH oxidase complex is the heterodimerization of gp91phox with p67phox, which is mediated by Rac (19). Three isoforms of Rac (Rac1, Rac2, and Rac3) have been identified (20), and Rac1 is the predominant isoform expressed in cardiomyocytes (21). Thus, Rac1 activation may lead to myocardial oxidative stress and apoptosis during hyperglycemia. A recent study showed that Rac1 contributes to vascular injury in diabetes (22). However, no direct evidence is available on Rac1 and NADPH oxidase activation in cardiomyocyte apoptosis in diabetes.
In this study, we generated cardiomyocyte-specific Rac1 knockout (Rac1-ko) mice; analyzed the impact of Rac1 on NADPH oxidase activation, mitochondrial ROS generation, and intracellular ROS production; and investigated the role of Rac1 and NADPH oxidase activation in cardiomyocyte apoptosis during hyperglycemia.
RESEARCH DESIGN AND METHODS
Animals and adult rat cardiomyocyte culture.
This investigation conforms with the guide for the care and use of laboratory animals published by the U.S. National Institutes of Health (NIH Publication No. 85–23). All experimental procedures were approved by the Animal Use Subcommittee at the University of Western Ontario, Canada. Breeding pairs of C57BL/6 mice, mice bearing the modified Rac1 gene containing loxP sites, and db/db mice were purchased from the Jackson Laboratory. Transgenic mice with cardiomyocyte-specific expression of Cre recombinase (Cre) under the control of α-myosin heavy chain (α-MHC) were generously provided by Dr. Dale Evan Abel (University of Utah, UT) (23). A breeding program for mice was implemented at our animal care facilities.
Adult male rats (Sprague Dawley, 200 g body weight) were purchased from Charles River Labs. Adult rat ventricle cardiomyocytes (ARVC) were isolated and cultured as described (24).
Streptozotocin hyperglycemic mice.
Adult male mice (2 months old) were intraperitoneally injected with a single dose of streptozotocin (STZ) at 150 mg/kg body weight, dissolved in 10 mmol/l sodium citrate buffer (pH 4.5). On day 3 after STZ treatment, whole blood was obtained from the mouse tail vein and random glucose levels were measured using OneTouch Ultra 2 blood glucose monitoring system (LifeScan, Mountainview, CA). Blood glucose content of 20 mmol/l or greater was chosen as hyperglycemia for the present study, whereas citrate buffer-treated mice were used as normoglycemia (blood glucose <12 mmol/l).
Adenoviral infection of cultured ARVC.
Cardiomyocytes were infected with adenoviral vectors containing a dominant-negative mutant Rac1 (Ad-RacN17, Vector Biolabs) or β-gal (Ad-gal, Vector Biolabs) as a control at a multiplicity of infection (MOI) of 100 PFU/cell. Adenovirus-mediated gene transfer was implemented as we previously described (24).
Gp91phox and p47phox knockdown using small interfering RNA.
To knock down gp91phox (Nox2) and p47phox expression, a small interfering RNA (siRNA) against rat Nox2 or p47phox was obtained (Santa Cruz Biotechnology, Santa Cruz, CA) and two different scramble siRNAs (Con1 and Con2) were employed as control. Transfection was performed using TransMessenger Transfection Reagent (Qiagen) according to manufacturer's protocol as described in our recent study (25).
Measurements of Rac1 activity.
Activated Rac1 was determined by p21-binding domain of p21-activated protein kinase 1 pull-down assay (Rac Activation Assay Kit; Cell Biolabs) according to the manufacturer's protocol.
NADPH oxidase activity assay.
NADPH oxidase activity was assessed in cell lysates by lucigenin-enhanced chemiluminescence (20 μg of protein, 100 μmol/l NADPH, 5 μmol/l lucigenin) with a multilabel counter (Victor3 Wallac) (25).
Intracellular ROS measurement.
The formation of ROS was measured by using the ROS-sensitive dye, 2,7-dichlorodihydro-fluorescein diacetate (DCF-DA, Invitrogen), as an indicator. The assay was performed on freshly dissected heart tissues. Samples (50 μg proteins) were incubated with 10 μl of DCF-DA (10 μmol/l) for 3 h at 37°C. The fluorescent product formed was quantified by spectrofluorometer at the 485/525 nm. Changes in fluorescence were expressed as arbitrary unit.
Active caspase-3 measurement.
As described in detail previously (26), caspase-3 activity in myocardial tissues and cardiomyocytes was measured by using a caspase-3 fluorescent assay kit (BIOMOL Research Laboratories).
In situ detection of apoptotic cells.
To localize cells undergoing nuclear DNA fragmentation in the myocardium, in situ terminal deoxynucleotidyl transferase–mediated dUTP nick-end labeling (TUNEL) was performed using an in situ apoptosis detection kit (Roche Biochemicals) as described previously (26).
Measurement of ROS in isolated mitochondria.
Mitochondria were isolated from the freshly harvested heart as previously described (27). Mitochondrial superoxide generation was measured by lucigenin-enhanced chemiluminescence with a multilabel counter (Victor3 Wallac) using NADH (250 μmol/l) or succinate (2 mmol/l) (27). The formation of ROS in freshly isolated mitochondria was measured on addition of pyruvate/malate by using DCF-DA as an indicator (28).
Heart function assessment.
Mouse hearts were isolated and perfused on a Langendorff-system. Myocardial function was then determined as described in our previous study (25). Maximal and minimal first derivatives of force (+dF/dtmax and −dF/dtmin) as the rate of contraction and relaxation were analyzed by PowerLab Chart program (ADInstruments).
Statistical analysis.
All data were given as means ± SD. Differences between two groups were compared by unpaired Student's t test. For multigroup comparisons, ANOVA followed by Student Newman-Keuls test was performed. A value of P < 0.05 was considered statistically significant.
RESULTS
Upregulation of Rac1 activity by hyperglycemia.
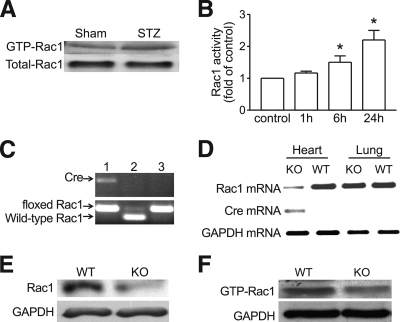
As shown in Fig. 1, hyperglycemia significantly increased Rac1 activity in STZ-treated compared with citrate buffer–treated hearts (Fig. 1A). In cultured ARVC exposed to normal (5.5 mmol/l) or high glucose (33 mmol/l) for 1, 6, and 24 h, Rac1 activity was significantly upregulated by high glucose compared with normal glucose (Fig. 1B).
FIG. 1.
A and B: Effect of hyperglycemia on Rac1 activity in the heart and cardiomyocytes. Hyperglycemia was induced in mice by STZ injection. Rac1 activity was determined in the heart. Hyperglycemia increased Rac1 activity in wild-type mice (A). B: Cultured ARVC were incubated with normal (5.5 mmol/l) or high glucose (33 mmol/l). Rac1 activity was measured at 1, 6, and 24 h after high glucose incubation. Data are means ± SD, n = 3. *P < 0.05 versus control. C–F: Characterization of mice with cardiomyocyte-specific Rac1-ko. C: Genotyping. Tail DNA was used to determine the presence of floxed Rac1 gene and the α-MHC-Cre transgene by PCR. Mouse with both homozygous loxP-Rac1-loxP and α-MHC-cre transgene was identified as knockout (lane 1). Lanes 2 and 3 show heterozygous and homozygous floxed Rac1 gene, respectively. D: Expressions of Cre, Rac1, and GAPDH mRNA were determined in the heart and lung by RT-PCR. Cre mRNA was detected in Rac1-ko heart only as shown in left lane. The levels of Rac1 mRNA were significantly reduced in Rac1-ko heart. The low level of Rac1 mRNA in Rac1-ko heart is most likely because of vascular Rac1. In contrast, the Rac1 mRNA was not decreased in Rac1-ko lung. E: Adult cardiomyocytes were isolated from Rac1-ko and wild-type mice. Rac1 protein expression was determined by Western blot analysis. The levels of Rac1 protein were significantly decreased in Rac1-ko cardiomyocytes. F: Rac1 activity was determined in Rac1-ko and wild-type hearts after 7 days hyperglycemia. A representative Western blot from three different hearts in each group shows downregulation of Rac1 activity in Rac1-ko hearts. WT, wild type; KO, knockout.
Generation of cardiomyocyte-specific Rac1-ko mice.
Mice with cardiomyocyte-specific Rac1-ko were generated by crossing the floxed Rac1 mice with mice overexpressing Cre under the control of α-MHC as we recently described (29). PCR analysis identified mice carrying both the homozygous loxP-Rac1-loxP gene and the α-MHC-cre transgene (Fig. 1C) as Rac1-ko mice. Deficiency of Rac1 decreased Rac1 mRNA expression in the heart (Fig. 1D). Isolated cardiomyocytes of Rac1-ko mice exhibited a significant reduction of Rac1 protein expression as compared with their wild-type littermates (Fig. 1E). These animals grow normally into adulthood without any health problems. Under normal condition, there were no differences in heart weight, body weight, ratio of heart weight over body weight, heart rate (data not shown), and cardiac contractility between Rac1-ko mice and their wild-type littermates. Histological analysis showed no pathological changes in Rac1-ko hearts. These observations suggest that cardiomyocyte-specific deletion of Rac1 does not affect basal myocardial function and morphology of the heart, thus excluding the possible effects of Cre expression on the heart of Rac1-ko mice.
Role of Rac1 in NADPH oxidase activation and ROS production in the hyperglycemic heart.
Hyperglycemia was induced in Rac1-ko mice and their corresponding wild-type littermates including wild-type, floxed Rac1, and Cre transgenic (Cre+) mice by STZ. The levels of blood glucose were monitored on day 0, 3, and 9 after STZ injection. To eliminate possible confounding effects of STZ, insulin was immediately given using a long-term insulin preparation (Lantus Insulin glargine injection; sanofi-aventis Canada, Laval, Canada) at a dose of 5–15 units per mouse per day to maintain the blood glucose levels between 5–12 mmol/l when hyperglycemia was greater than 20 mmol/l on day 3 after STZ treatment in wild-type mice. In sham- or STZ-injected mice, blood glucose was not different between Rac1-ko mice and their corresponding wild-type littermates including wild-type, Rac1 floxed, and Cre+ mice (data not shown).
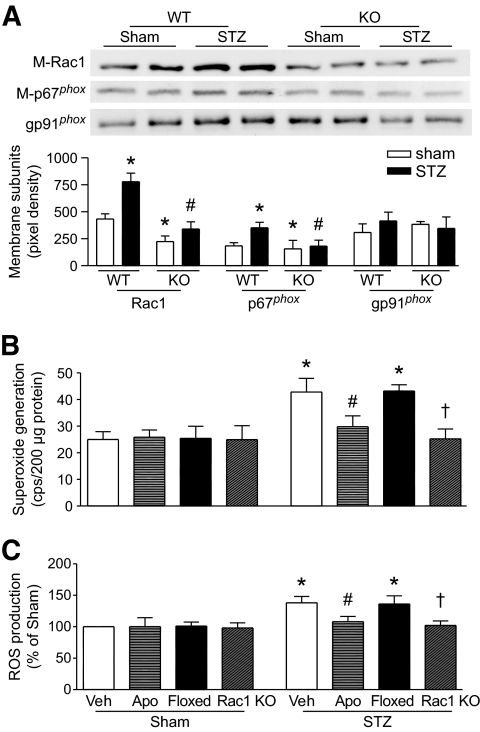
We first determined Rac1 activity in the heart. There was no difference of Rac1 activity in between Rac1-ko corresponding wild-type littermates under basal or diabetic conditions. Therefore, Rac1 floxed mice were presented as littermate wild-type controls for Rac1-ko mice. Rac1 activity was much lower in Rac1-ko compared with wild-type hearts during hyperglycemia (Fig. 1F). We then examined the membrane translocation of Rac1 and p67phox, as well as NADPH oxidase activation. As shown in Fig. 2A, the protein levels of Rac1 and p67phox in plasma membranes were significantly upregulated in hyperglycemic compared with normoglycemic wild-type hearts, which were correlated with the increases in NADPH oxidase activity and ROS production in hyperglycemic hearts (Fig. 2B and C). However, myocardial ROS production was not increased in insulin supplemented mice (data not shown). There were no changes in protein levels of NADPH oxidase subunits, gp91phox, p67phox, and Rac1 in hyperglycemic hearts (data not shown). Deletion of Rac1 decreased membrane Rac1 and p67phox, NADPH oxidase activation, and ROS production in the hyperglycemic heart (Fig. 2A–C). Finally, we analyzed the effect of NADPH oxidase activation on ROS production during hyperglycemia. Adult wild-type mice were injected with STZ. Two days later, apocynin (30 mg · kg−1 · day−1) or vehicle was given in drinking water for 7 days. The levels of blood glucose were similar between apocynin- and vehicle-treated mice on day 9 (data not shown). Administration of apocynin blocked NADPH oxidase activity and ROS production in hyperglycemic hearts (Fig. 2B and C). These results demonstrate that Rac1 is required for NADPH oxidase activation and ROS production during hyperglycemia.
FIG. 2.
Role of Rac1 in NADPH oxidase activation. Relocalization of Rac1 and p67phox to membrane (A), NADPH oxidase activity (B), and ROS production (C) were determined in the hyperglycemic heart. The protein levels of Rac1 (M-Rac1) and p67phox (M-p67phox) were decreased in the membrane fractions of Rac1-ko compared with wild-type hyperglycemic hearts (A). Deficiency of Rac1 or apocynin (Apo) treatment blocked the upregulation of NADPH oxidase activity (B) and elevation of ROS production in hyperglycemic hearts (C). Data are means ± SD, n = 4–6. *P < 0.05 versus sham or sham in wild type, #P < 0.05 versus STZ in wild type or vehicle (Veh) in STZ, †P < 0.05 versus floxed in STZ. M-Rac1 and M-p67phox indicate membrane Rac1 and p67phox, respectively. WT, wild type; KO, knockout.
Decreased apoptosis in the hyperglycemic Rac1-ko heart.
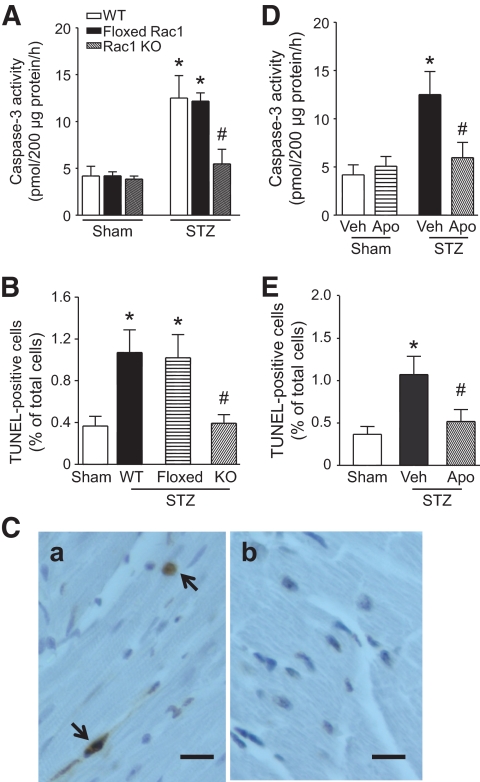
Myocardial caspase-3 activity was not different between Rac1-ko corresponding wild-type littermates including wild-type, Rac1 floxed, and Cre+ mice under either normal or diabetic conditions (data not shown). Therefore, Rac1 floxed mice were chosen as littermate wild-type controls for Rac1-ko mice in the following experiments. Compared with citrate buffer–treated mice, hyperglycemia significantly increased myocardial caspase-3 activity (Fig. 3A) and the number of TUNEL- positive cardiomyocytes in STZ-treated wild-type mice (Fig. 3B and C), whereas myocardial caspase-3 activity was not increased in insulin-supplemented mice (data not shown). These results exclude the direct possible effects of STZ on myocardial apoptosis, which is in agreement with a recent report (4). Deficiency of Rac1 decreased caspase-3 activity and the number of TUNEL-positive cells in the hyperglycemic heart (Fig. 3). Similarly, inhibition of NDAPH oxidase by apocynin blocked caspase-3 activation and reduced the number of TUNEL-positive cells in hyperglycemic hearts (Fig. 3D and E). These data demonstrate that Rac1-NADPH oxidase activation induces apoptosis in the hyperglycemic heart.
FIG. 3.
Role of Rac1 in apoptosis in the heart. Hyperglycemia was induced in Rac1-ko mice and wild-type littermates. A and D: Myocardial caspase-3 activity was increased in hyperglycemic wild-type littermates. Deficiency of Rac1 or apocynin treatment reduced caspase-3 activity in hyperglycemic hearts. B and E: Quantification of TUNEL staining–positive cardiomyocytes. Deficiency of Rac1 or apocynin decreased the number of TUNEL-staining cells. Data are means ± SD, n = 4–6. *P < 0.05 versus sham or wild type and #P < 0.05 versus STZ + wild-type littermates (floxed Rac1) or vehicle (Veh) in STZ. C: Representatives of TUNEL staining in cardiomyocytes (yellow-blown signal, arrows showing nuclear localization) from hyperglycemic wild-type hearts (a) and hyperglycemic Rac1-ko hearts (b). Magnification ×40; scale bar: 10 μm. WT, wild type; KO, knockout. (A high-quality digital representation of this figure is available in the online issue.)
Role of Rac1 and NADPH oxidase in mitochondrial ROS production.
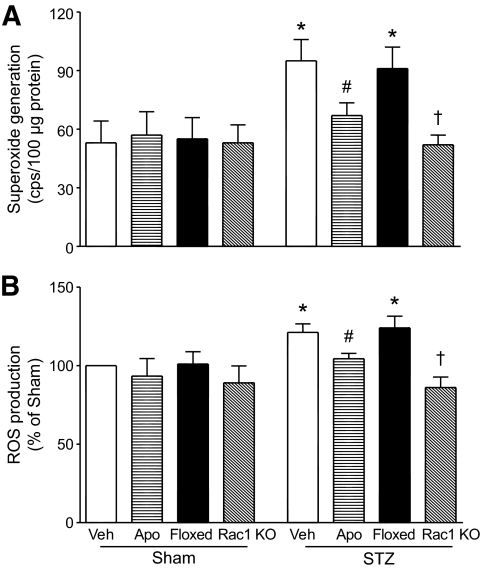
Mitochondria are known to constitutively generate superoxide radicals as a by-product of electron transport and are also important sources for intracellular ROS production in hyperglycemia (30,31). Inhibition of mitochondrial ROS production has been shown to prevent the development of diabetic cardiomyopathy (32,33). We explored the impact of Rac1-NADPH oxidase on mitochondria in producing ROS. Myocardial mitochondria were isolated from Rac1-ko mice, wild-type littermates, and apocynin-treated mice on day 9 after STZ treatment. We first determined superoxide generation in the isolated frozen-thawed mitochondria. In the normoglycemic hearts, addition of NADH but not NADPH slightly increased superoxide production and there was no difference between Rac1-ko mice and wild-type littermates. However, in the hyperglycemic hearts, mitochondria superoxide production was significantly increased (Fig. 4A). Deficiency of Rac1 or apocynin administration decreased mitochondrial superoxide production in the hyperglycemic heart (Fig. 4A). Addition of succinate did not elicit superoxide production in mitochondrial preparations (data not shown). This suggests that mitochondrial complex I and/or III but not complex II is involved in superoxide generation.
FIG. 4.
Impact of Rac1 and NADPH oxidase on ROS generation in isolated mitochondria. Myocardial mitochondria were isolated from wild-type and Rac1-ko mice under normoglycemia or hyperglycemia and were assayed for superoxide generation and ROS production (see details in Research Design and Methods). Hyperglycemia upregulated both superoxide generation (A) and ROS production (B). Deficiency of Rac1 or apocynin (Apo) treatment significantly decreased superoxide generation and ROS production in isolated mitochondria. Data are means ± SD, n = 5–6. *P < 0.05 versus sham, #P < 0.05 versus STZ + Veh and †P < 0.05 versus STZ + wild-type littermates (floxed). KO, knockout; Veh, vehicle.
To further confirm the role of Rac1 and NADPH oxidase in mitochondrial ROS production, we analyzed the release of ROS from mitochondria by using DCF-DA as an indicator. Similarly, ROS production was increased in intact mitochondria from the hyperglycemic compared with the normoglycemic heart on addition of pyruvate/malate (5/5 mmol/l). Deletion of Rac1 or apocynin administration blocked the increase of ROS production in mitochondria from the hyperglycemic heart (Fig. 4B). Thus, these data demonstrate that Rac1 and NADPH oxidase contribute to mitochondrial ROS generation in the hyperglycemic heart.
Effects of Rac1 and NADPH oxidase inhibition on apoptosis in cardiomyocytes.
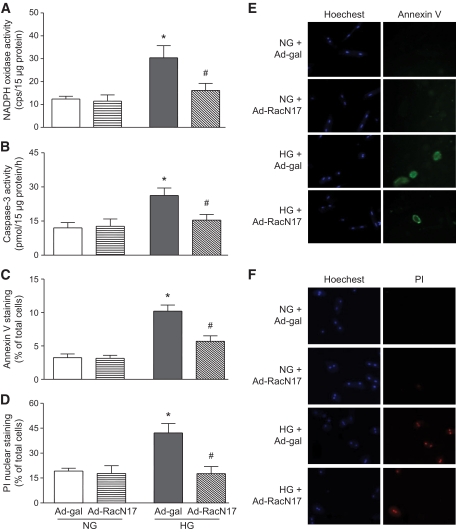
To determine whether Rac1 activation directly contributes to apoptosis in cultured ARVC exposed to high glucose, we used adenoviral vector Ad-RacN17 expressing a dominant negative mutant of Rac1, which specifically blocks Rac1 activation (22,34). ARVC were infected with Ad-RacN17 or Ad-gal and then incubated with normal (5.5 mmol/l) or high glucose (33 mmol/l) for 24 h. High glucose significantly induced NADPH oxidase activity in ARVC (Fig. 5A). In contrast, equal amount of mannitol did not affect NADPH oxidase activity (data not shown). Infection of Ad-RacN17 blocked NADPH oxidase activity in high glucose–treated ARVC (Fig. 5A). Similarly, high glucose significantly increased caspase-3 activity compared with normal glucose, whereas, as an osmotic control, equal amount of mannitol did not induce caspase-3 activity in ARVC (data not shown). Ad-RacN17 blocked high glucose–induced caspase-3 activity (Fig. 5B). In association with upregulation of caspase-3 activity, high glucose increased the percentage of annexin V positive cells, which was significantly reduced by infection of Ad-RacN17 (Fig. 5C and E). The increased percentage of annexin V positive cells was also significantly reduced by a selective caspase-3 inhibitor, AC-DEVD carbohydrate (up to 90% reduction). These results suggest that Rac1 is important in high glucose–induced apoptosis. Cardiomyocyte death was also measured by phosphatidylinositol nuclear staining. Compared with normal glucose, high glucose increased the percentage of phosphatidylinositol positive cells, which was significantly decreased by Ad-RacN17 (Fig. 5D and F). Although phosphatidylinositol is a viability exclusion dye and does not enter cells with intact membrane, in the late stage of apoptosis there is also a loss of membrane integrity as the cell dies (35). As such, the population of phosphatidylinositol-positive cells is a mixture of necrotic and apoptotic cells.
FIG. 5.
Effects of Rac1 inhibition on NADPH oxidase activity and apoptosis in cultured cardiomyocytes. Cultured cardiomyocytes were infected with Ad-RacN17 or Ad-gal for 24 h and followed by incubation with normal or high glucose for another 24 h. NADPH oxidase activity (A), caspase-3 activity (B), annexin V staining (C), and phosphatidylinositol staining (D) were determined. A representative staining for annexin V (E) or phosphatidylinositol staining (F) is shown from various groups. Data are means ± SD from at least three different cell cultures. *P < 0.05 versus Ad-gal in normal glucose and #P < 0.05 versus Ad-gal in high glucose. NG, normal glucose; HG, high glucose. (A high-quality digital representation of this figure is available in the online issue.)
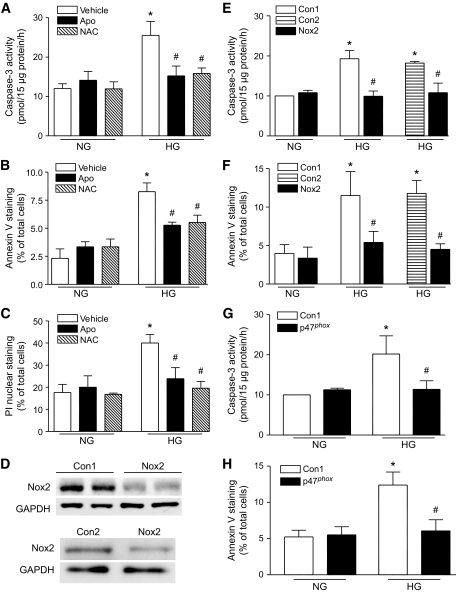
To examine if NADPH oxidase activation mediated apoptosis, we first incubated ARVC with normal or high glucose in the presence of apocynin (100 μmol/l), NAC (2 mmol/l), or vehicle. Twenty-four hours later, coincubation with apocynin or NAC inhibited caspase-3 activity and decreased the percentage of annexin-V positive and phosphatidylinositol-positive cells in high glucose–induced ARVC (Fig. 6A–C). To clarify the contribution of gp91phox-NADPH oxidase, we transfected ARVC with gp91phox-specific siRNA and then incubated these cells with normal or high glucose for another 24 h. Two different scramble siRNAs (Con1 and Con2) were used as controls. Transfection with gp91phox siRNA significantly decreased gp91phox protein compared with either Con1 or Con2 siRNA in ARVC (Fig. 6D). To confirm the specific knockdown by gp91phox siRNA, we also measured Nox4 expression because our recent study has shown that cardiomyocytes express both Nox2 and Nox4 under physiological conditions (25). Gp91phox siRNA did not alter Nox4 expression in ARVC (data not shown). In response to high glucose, gp91phox siRNA blocked caspase-3 activation and reduced annexin V–staining positive cells in ARVC (Fig. 6E and F), compared with either Con1 or Con2 scramble siRNA. These results demonstrate that gp91phox-NADPH oxidase activation induces apoptosis in high glucose–stimulated ARVC. The role of NADPH oxidase in high glucose–induced apoptosis was further demonstrated by using p47phox siRNA. Transfection of p47phox siRNA blocked NADPH oxidase activation in high glucose–stimulated ARVC (data not shown). Similarly, caspase-3 activation and annexin V–staining positive cells were significantly decreased in p47phox siRNA–transfected ARVC (Fig. 6G and H).
FIG. 6.
Roles of gp91phox-NADPH oxidase in cardiomyocyte apoptosis. Cultured cardiomyocytes were incubated with normal or high glucose in the presence of apocynin (Apo), NAC, or vehicle for 24 h. Caspase-3 activity and cell death were determined. Caspase-3 activity (A), the percentages of annexin V (B) and phosphatidylinositol staining–positive cells (C) were significantly inhibited by incubation with apocynin or NAC in high glucose–stimulated cardiomyocytes. D–F: Cardiomyocytes were transfected with gp91phox (Nox2) siRNA, p47phox siRNA, or scramble siRNAs (Con1 or Con2) as control. Twenty-four hours later, the cells were incubated with normal or high glucose for another 24 h. D: Representative Western blot for Nox2 protein in Nox2 siRNA and Con1- or Con2-siRNA–transfected cardiomyocytes. E and G: Caspase-3 activity. F and H: The percentages of annexin V. Data are means ± SD from at least three different cell cultures. *P < 0.05 versus Veh in normal glucose or Con1 in normal glucose, #P < 0.05 versus Veh in high glucose, Con1 in high glucose, or Con2 in high glucose. NG, normal glucose; HG, high glucose; Veh, vehicle.
Contribution of Rac1 to myocardial dysfunction in STZ-induced type 1 diabetic mice.
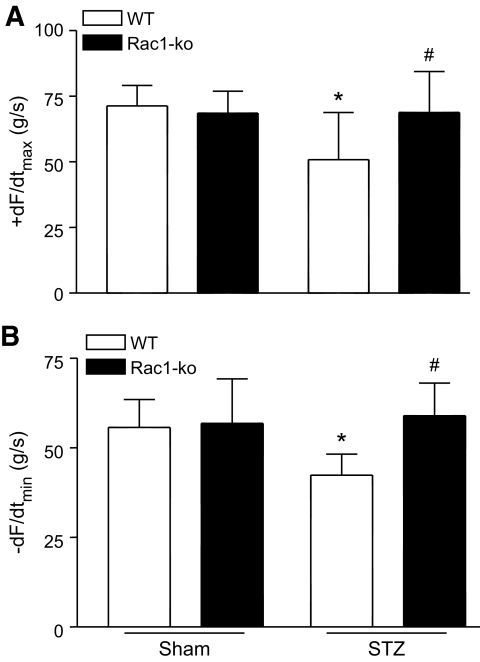
To investigate the functional significance of Rac1, we assessed myocardial function in Rac1-ko and wild-type mice after 8 weeks of STZ injection. Although there was no change in heart rate, rate of contraction/relaxation was significantly reduced in diabetic wild-type mice compared with sham animals (Fig. 7). Lack of Rac1 restored the rate of contraction and relaxation without affecting heart rate in diabetic Rac1-ko mice (Fig. 7). These results demonstrate that Rac1 contributes to myocardial dysfunction in diabetic mice.
FIG. 7.
Cardiac function in Rac1-ko and wild-type mice after 8 weeks of STZ injection. Mouse hearts were isolated and perfused in a Langendorff system. Contractile function of heart was determined. Changes in the rate of contraction (+dF/dtmax, A) and relaxation (−dF/dtmin, B) are presented. Data are means ± SD, n = 5–12 per group. *P < 0.05 versus wild type in sham; #P < 0.05 versus wild type in STZ. WT, wild type.
Role of Rac1 in cardiac apoptosis in db/db mice.
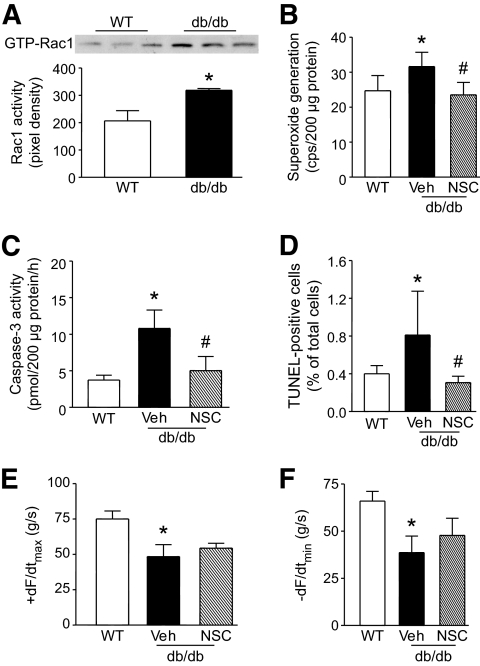
To further demonstrate the role of Rac1 in cardiac apoptosis in diabetes, we used type 2 diabetic model of db/db mice. A highly selective Rac1 inhibitor NSC23766 (36) was given to db/db mice starting at age of 12 weeks (2.5 mg · kg−1 day−1 i.p.) for 2 weeks. Rac1 activity, NADPH oxidase activity, caspase-3 activity, and TUNEL-positive cells were significantly increased in the heart of db/db mice compared with wild-type mice (Fig. 8A–D). Administration of NSC23766 inhibited NADPH oxidase activation, blocked caspase-3 activity, and reduced TUNEL-positive cells in db/db mice (Fig. 8B–D), whereas blood glucose levels were similar between NSC23766 and vehicle-treated db/db mice (20–28 mmol/l). Finally, myocardial dysfunction in db/db mice was slightly but not significantly (P = 0.1418 and 0.0648 for the rate of contraction and relaxation, respectively) attenuated by NSC23766 treatment (Fig. 8E and F).
FIG. 8.
Effects of NSC23766 (NCS) on NADPH oxidase, apoptosis, and myocardial function in db/db mice. Male db/db mice (12 weeks old) were given NSC (2.5 mg · kg−1 · day−1, i.p.) or Veh for 2 weeks. A: Rac1 activity was increased in db/db mouse hearts compared with wild-type hearts. Top panel is the representative blot for guanosine triphosphate-Rac1 from three hearts in each group and lower panel is the quantification of Rac1 activity. Myocardial NADPH oxidase activity (B), caspase-3 activity (C), and TUNEL staining–positive cardiomyocytes (D) were quantified in wild-type and db/db mice. Administration of NSC significantly attenuated NADPH oxidase and caspase-3 activity and decreased the number of TUNEL-staining cells in db/db mice. E and F: Cardiac function was measured. Changes in the rate of contraction (+dF/dtmax, E) and relaxation (−dF/dtmin, F) are presented. Data are means ± SD, n = 5–7. *P < 0.05 versus wild type and #P < 0.05 versus Veh in db/db. WT, wild type; Veh, vehicle.
DISCUSSION
The present study generated cardiomyocyte-specific Rac1-ko mice and investigated the role of Rac1 in ROS production and apoptosis in the hyperglycemic hearts. We demonstrated for the first time that Rac1, through gp91phox-NADPH oxidase activation and ROS production, induces apoptosis in both hyperglycemic hearts and high glucose–stimulated cardiomyocytes. Rac1 also plays a role in cardiac apoptosis in type 2 diabetic db/db mice. Furthermore, Rac1 and NADPH oxidase activation is associated with mitochondrial ROS production in the hyperglycemic hearts. Finally, Rac1 contributes to myocardial dysfunction in STZ-induced type 1 diabetes. Thus, Rac1/NADPH oxidase/mitochondrion axis is important in ROS production and cardiomyocyte apoptosis in hyperglycemia, which may contribute to the development of diabetic cardiomyopathy.
Accumulating evidence suggests that hyperglycemia induces ROS (6,11) and overproduction of ROS is associated with apoptosis in the diabetic heart (4,6). However, the pathophysiologically relevant source of increased myocardial ROS production in diabetes remains to be further characterized. Recent studies have demonstrated that myocardial NADPH oxidase activity is increased in diabetes (16,17). Hyperglycemia has been shown to activate NADPH oxidase in cardiomyocytes (18). These studies suggest the pathophysiological relevance of NADPH oxidase activation for cardiomyocyte apoptosis in hyperglycemia. An important step for the assembly of active NADPH oxidase is the interaction between cytosolic subunits (p67phox, p47phox, and Rac1) and membrane subunits (gp91phox and p22phox) (19). Rac1 is required to anchor cytosolic p67phox to the membrane for the assembly of active NADPH oxidase, leading to superoxide generation (21). In the present study, deficiency of Rac1 inhibited the relocalization of NADPH oxidase subunits to membrane and diminished NADPH oxidase activation and ROS production in the hyperglycemic heart. Either Rac1 deletion or inhibition of NADPH oxidase prevented myocardial apoptosis in hyperglycemia. These findings suggest that Rac1 contributes to apoptosis through NADPH oxidase activation in the hyperglycemic heart. To characterize whether the role of Rac1 in apoptosis could be reproduced by high glucose levels, we extended our analyses to cardiomyocytes. Direct exposure of RAVC to high glucose, through Rac1, promoted NADPH oxidase activation and induced apoptosis. Selective inhibition of Rac1, knockdown of gp91phox or p47phox, or scavenging ROS prevented apoptosis in high glucose–stimulated ARVC. These data support the notion that Rac1 is critical for gp91phox-NADPH oxidase activation, which is the major source of ROS production responsible for apoptosis in cardiomyocytes under hyperglycemic conditions. Rac1 was also reported to induce NADPH oxidase activation and ROS production in angiotensin-II stimulated cardiomyocytes (21). Thus, the results reported herein also suggest that Rac1 may be a universal mechanism for NADPH oxidase activation in the heart under various stresses, which may have potential therapeutic implications.
Mitochondrion is another important source of ROS production contributing to apoptosis (37,38). Increased mitochondrial ROS generation has been shown in diabetic hearts (4,33,39). However, relatively few studies have directly measured mitochondrial ROS production in the isolated mitochondria from diabetic hearts, and the cross talk of other ROS sources to mitochondrion has not been fully elucidated. In the present study, we measured ROS production directly from the isolated mitochondria and analyzed the role of Rac1-NADPH oxidase in mitochondrial ROS production. In agreement with a recent report of Boudina et al. (39), our data showed that ROS production was increased in the isolated mitochondria from the hyperglycemic hearts. The increased mitochondrial ROS was achieved by using mitochondrial complex I substrates, NADH or pyruvate/malate, but not succinate, a complex II substrate; this is different from the result of Boudina et al. (39), which demonstrated that mitochondrial ROS is induced on addition of succinate in the isolated mitochondria from the diabetic heart. The cause of this discrepancy is unknown but may result from different diabetic models used. Interestingly, cardiomyocyte-specific deletion of Rac1 or inhibition of NADPH oxidase significantly attenuated ROS production in isolated mitochondria from the hyperglycemic heart, suggesting that Rac1 and NADPH oxidase activation functions upstream of mitochondrion in generating mitochondrial ROS in cardiomyocytes exposed to hyperglycemia. This finding is supported by a recent observation demonstrating that NADPH oxidase–derived superoxide anion (O2-) directly activates the mitoKATP channels, presumably through a direct action on the sulfhydryl groups of this channel, or reacts with nitric oxide to form peroxynitrite in vascular endothelial cells in response to angiotensin-II (40). Both the increase in K+ influx through opening of the mitoKATP channels and the peroxynitrite formation can damage respiratory complexes, leading to mitochondrial ROS production (41). Alternatively, one-electron transfer and/or rapid dismutation of superoxide result in substantial H2O2 generation. H2O2 is a quantitatively important signaling species (42), which may mediate mitochondrial ROS production. Indeed, direct addition of H2O2 to isolated mitochondria significantly increased superoxide generation (E.S. and T.P., unpublished data). Whether these mechanisms operate in the diabetic heart is currently unknown but merits further investigations. On the other hand, H2O2 converted from superoxide in the mitochondria can diffuse to the cytoplasm, where it could activate NADPH oxidase producing ROS. Recent studies have suggested that mitochondrial ROS activate PI3K and PKC. Activation of PI3K in turn promotes Rac1 and subsequent NADPH oxidase activation (43,44), and PKC induces NADPH oxidase activation via p47phox phosphorylation (45). In the heart of type 1 diabetic mice, scavenging ROS inhibits NADPH oxidase expression and activation (46). Thus, a mutual functional relationship between NADPH oxidase and mitochondria in a feed-forward fashion may favor sustaining ROS production, which promotes apoptosis in the hyperglycemic heart. Future studies will be required to investigate this functional cross talk between Rac1-NADPH oxidase and mitochondrion.
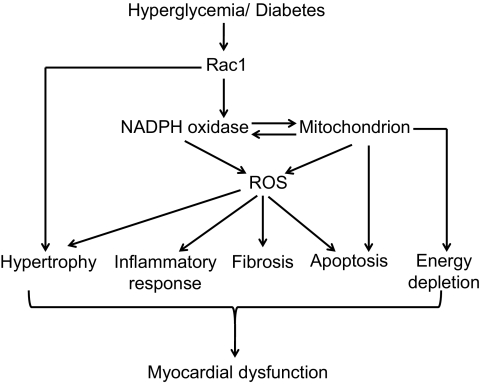
The present study also found that deficiency of Rac1 attenuated myocardial dysfunction in diabetic mice, suggesting a pathophysiological significance of Rac1 activation in diabetic hearts. This protection may result from inhibition of cardiomyocyte apoptosis by Rac1 blockade. Rac1-mediated ROS has been shown to induce cardiac hypertrophy in response to angiotensin-II stimulation (21). A recent study also indicated that Rac1 may be associated with cardiac inflammatory responses in hyperglycemia (47). Both cardiac hypertrophy and inflammation are important in the development of cardiac complications of diabetes. Thus, it is possible that Rac1 via NADPH oxidase activation and ROS production contributes to the diabetic cardiac hypertrophy, inflammatory responses, mitochondrial dysfunction, in addition to apoptosis, leading to myocardial dysfunction in diabetes (Fig. 9), which requires further investigations. However, Rac1 may also play a role in cardiac hypertrophy in diabetes through NADPH oxidase–independent pathways (48).
FIG. 9.
Proposed mechanisms for Rac1-induced cardiac damage in diabetes. Hyperglycemia/diabetes activates Rac1 and subsequent NADPH oxidase, producing ROS, which induce mitochondrial ROS production. ROS production induces cardiac apoptosis, hypertrophy, fibrosis, and inflammatory response, leading to myocardial dysfunction. ROS damages mitochondrial function resulting in apoptosis and energy depletion, which contributes to myocardial dysfunction. Rac1 activation may also induce cardiac hypertrophy through NADPH oxidase–independent mechanisms. ROS indicates reactive oxygen species.
It is worthwhile to mention that Rac1 activity was decreased by about 40% in the whole heart of Rac1-ko mice compared with their wild-type littermates. The exact deletion of Rac1 in cardiomyocytes from Rac1-ko mice is currently unknown. A recent study showed that the Cre-loxP system could achieve ∼70% deletion of Rac1 in cardiomyocytes in transgenic mice (21). Thus, the deletion of Rac1 in cardiomyocytes from our Rac1-ko mice is estimated to be around 40–70%, which may lead to some limitations of the interpretation of results from the present study. For example, it is unclear whether further down- regulation of Rac1 provides a better protection in diabetic hearts. However, complete deletion of Rac1 may have adverse effects since recent studies have suggested that Rac1 is also required for cardiovascular development and physiological functions (49,50).
In conclusion, Rac1 plays a critical role in hyperglycemia-induced apoptosis in cardiomyocytes through NADPH oxidase activation and ROS production, leading to myocardial dysfunction in diabetes. Furthermore, Rac1 and NADPH oxidase activation contributes to mitochondrial ROS production in the hyperglycemic heart. Given excessive ROS and cardiac apoptosis significantly contributes to diabetic cardiomyopathy (1–3,6,32,33,51), these findings suggest that myocardial Rac1 may serve as a potential target for the treatment of cardiac complications in diabetes.
Acknowledgments
This study was supported by grants from the Heart & Stroke Foundation of Ontario (NA 5940) and the Canadian Institutes of Health Research (MOP 93657) awarded to T.P. T.P. is a recipient of a New Investigator Award from Heart & Stroke Foundation of Canada and Rick Gallop Award for research excellence from Heart & Stroke Foundation of Ontario (2006).
No potential conflicts of interest relevant to this article were reported.
Footnotes
The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.Poornima IG, Parikh P, Shannon RP: Diabetic cardiomyopathy: the search for a unifying hypothesis. Circ Res 2006;98:596–605 [DOI] [PubMed] [Google Scholar]
- 2.Boudina S, Abel ED: Diabetic cardiomyopathy revisited. Circulation 2007;115:3213–3223 [DOI] [PubMed] [Google Scholar]
- 3.Frustaci A, Kajstura J, Chimenti C, Jakoniuk I, Leri A, Maseri A, Nadal-Ginard B, Anversa P: Myocardial cell death in human diabetes. Circ Res 2000;87:1123–1132 [DOI] [PubMed] [Google Scholar]
- 4.Cai L, Wang Y, Zhou G, Chen T, Song Y, Li X, Kang YJ: Attenuation by metallothionein of early cardiac cell death via suppression of mitochondrial oxidative stress results in a prevention of diabetic cardiomyopathy. J Am Coll Cardiol 2006;48:1688–1697 [DOI] [PubMed] [Google Scholar]
- 5.Fiordaliso F, Leri A, Cesselli D, Limana F, Safai B, Nadal-Ginard B, Anversa P, Kajstura J: Hyperglycemia activates p53 and p53-regulated genes leading to myocyte cell death. Diabetes 2001;50:2363–2375 [DOI] [PubMed] [Google Scholar]
- 6.Fiordaliso F, Bianchi R, Staszewsky L, Cuccovillo I, Doni M, Laragione T, Salio M, Savino C, Melucci S, Santangelo F, Scanziani E, Masson S, Ghezzi P, Latini R: Antioxidant treatment attenuates hyperglycemia-induced cardiomyocyte death in rats. J Mol Cell Cardiol 2004;37:959–968 [DOI] [PubMed] [Google Scholar]
- 7.Cai L, Li W, Wang G, Guo L, Jiang Y, Kang YJ: Hyperglycemia-induced apoptosis in mouse myocardium: mitochondrial cytochrome C-mediated caspase-3 activation pathway. Diabetes 2002;51:1938–1948 [DOI] [PubMed] [Google Scholar]
- 8.Kajstura J, Fiordaliso F, Andreoli AM, Li B, Chimenti S, Medow MS, Limana F, Nadal-Ginard B, Leri A, Anversa P: IGF-1 overexpression inhibits the development of diabetic cardiomyopathy and angiotensin II-mediated oxidative stress. Diabetes 2001;50:1414–1424 [DOI] [PubMed] [Google Scholar]
- 9.Adeghate E: Molecular and cellular basis of the aetiology and management of diabetic cardiomyopathy: a short review. Mol Cell Biochem 2004;261:187–191 [DOI] [PubMed] [Google Scholar]
- 10.Cai L, Kang YJ: Cell death and diabetic cardiomyopathy. Cardiovasc Toxicol 2003;3:219–228 [DOI] [PubMed] [Google Scholar]
- 11.Modesti A, Bertolozzi I, Gamberi T, Marchetta M, Lumachi C, Coppo M, Moroni F, Toscano T, Lucchese G, Gensini GF, Modesti PA: Hyperglycemia activates JAK2 signaling pathway in human failing myocytes via angiotensin II-mediated oxidative stress. Diabetes 2005;54:394–401 [DOI] [PubMed] [Google Scholar]
- 12.Isabelle M, Vergeade A, Moritz F, Dautreaux B, Henry JP, Lallemand F, Richard V, Mulder P, Thuillez C, Monteil C: NADPH oxidase inhibition prevents cocaine-induced up-regulation of xanthine oxidoreductase and cardiac dysfunction. J Mol Cell Cardiol 2007;42:326–332 [DOI] [PubMed] [Google Scholar]
- 13.Heymes C, Bendall JK, Ratajczak P, Cave AC, Samuel JL, Hasenfuss G, Shah AM: Increased myocardial NADPH oxidase activity in human heart failure. J Am Coll Cardiol 2003;41:2164–2171 [DOI] [PubMed] [Google Scholar]
- 14.Li JM, Gall NP, Grieve DJ, Chen M, Shah AM: Activation of NADPH oxidase during progression of cardiac hypertrophy to failure. Hypertension 2002;40:477–484 [DOI] [PubMed] [Google Scholar]
- 15.Xiao L, Pimentel DR, Wang J, Singh K, Colucci WS, Sawyer DB: Role of reactive oxygen species and NAD(P)H oxidase in α(1)-adrenoceptor signaling in adult rat cardiac myocytes. Am J Physiol Cell Physiol 2002;282:C926–C934 [DOI] [PubMed] [Google Scholar]
- 16.Wold LE, Ceylan-Isik AF, Fang CX, Yang X, Li SY, Sreejayan N, Privratsky JR, Ren J: Metallothionein alleviates cardiac dysfunction in streptozotocin-induced diabetes: role of Ca2+ cycling proteins, NADPH oxidase, poly(ADP-Ribose) polymerase and myosin heavy chain isozyme. Free Radic Biol Med 2006;40:1419–1429 [DOI] [PubMed] [Google Scholar]
- 17.Wendt MC, Daiber A, Kleschyov AL, Mulsch A, Sydow K, Schulz E, Chen K, Keaney JF, Jr, Lassegue B, Walter U, Griendling KK, Munzel T: Differential effects of diabetes on the expression of the gp91phox homologues nox1 and nox4. Free Radic Biol Med 2005;39:381–391 [DOI] [PubMed] [Google Scholar]
- 18.Privratsky JR, Wold LE, Sowers JR, Quinn MT, Ren J: AT1 blockade prevents glucose-induced cardiac dysfunction in ventricular myocytes: role of the AT1 receptor and NADPH oxidase. Hypertension 2003;42:206–212 [DOI] [PubMed] [Google Scholar]
- 19.Babior BM, Lambeth JD, Nauseef W: The neutrophil NADPH oxidase. Arch Biochem Biophys 2002;397:342–344 [DOI] [PubMed] [Google Scholar]
- 20.Haataja L, Groffen J, Heisterkamp N: Characterization of RAC3, a novel member of the ρ family. J Biol Chem 1997;272:20384–20388 [DOI] [PubMed] [Google Scholar]
- 21.Satoh M, Ogita H, Takeshita K, Mukai Y, Kwiatkowski DJ, Liao JK: Requirement of Rac1 in the development of cardiac hypertrophy. Proc Natl Acad Sci U S A 2006;103:7432–7437 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Vecchione C, Aretini A, Marino G, Bettarini U, Poulet R, Maffei A, Sbroggio M, Pastore L, Gentile MT, Notte A, Iorio L, Hirsch E, Tarone G, Lembo G: Selective Rac-1 inhibition protects from diabetes-induced vascular injury. Circ Res 2006;98:218–225 [DOI] [PubMed] [Google Scholar]
- 23.Abel ED, Kaulbach HC, Tian R, Hopkins JC, Duffy J, Doetschman T, Minnemann T, Boers ME, Hadro E, Oberste-Berghaus C, Quist W, Lowell BB, Ingwall JS, Kahn BB: Cardiac hypertrophy with preserved contractile function after selective deletion of GLUT4 from the heart. J Clin Invest 1999;104:1703–1714 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Li Y, Arnold JM, Pampillo M, Babwah AV, Peng T: Taurine prevents cardiomyocyte death by inhibiting NADPH oxidase-mediated calpain activation. Free Radic Biol Med 2009;46:51–61 [DOI] [PubMed] [Google Scholar]
- 25.Peng T, Lu X, Feng Q: Pivotal role of gp91phox-containing NADH oxidase in lipopolysaccharide-induced tumor necrosis factor-α expression and myocardial depression. Circulation 2005;111:1637–1644 [DOI] [PubMed] [Google Scholar]
- 26.Feng Q, Song W, Lu X, Hamilton JA, Lei M, Peng T, Yee SP: Development of heart failure and congenital septal defects in mice lacking endothelial nitric oxide synthase. Circulation 2002;106:873–879 [DOI] [PubMed] [Google Scholar]
- 27.Chen Q, Vazquez EJ, Moghaddas S, Hoppel CL, Lesnefsky EJ: Production of reactive oxygen species by mitochondria: central role of complex III. J Biol Chem 2003;278:36027–36031 [DOI] [PubMed] [Google Scholar]
- 28.Mattiasson G: Analysis of mitochondrial generation and release of reactive oxygen species. Cytometry A 2004;62:89–96 [DOI] [PubMed] [Google Scholar]
- 29.Rui T, Feng Q, Lei M, Peng T, Zhang J, Xu M, Abel ED, Xenocostas A, Kvietys PR: Erythropoietin prevents the acute myocardial inflammatory response induced by ischemia/reperfusion via induction of AP-1. Cardiovasc Res 2005;65:719–727 [DOI] [PubMed] [Google Scholar]
- 30.Yu T, Robotham JL, Yoon Y: Increased production of reactive oxygen species in hyperglycemic conditions requires dynamic change of mitochondrial morphology. Proc Natl Acad Sci U S A 2006;103:2653–2658 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lin Y, Berg AH, Iyengar P, Lam TK, Giacca A, Combs TP, Rajala MW, Du X, Rollman B, Li W, Hawkins M, Barzilai N, Rhodes CJ, Fantus IG, Brownlee M, Scherer PE: The hyperglycemia-induced inflammatory response in adipocytes: the role of reactive oxygen species. J Biol Chem 2005;280:4617–4626 [DOI] [PubMed] [Google Scholar]
- 32.Ye G, Metreveli NS, Donthi RV, Xia S, Xu M, Carlson EC, Epstein PN: Catalase protects cardiomyocyte function in models of type 1 and type 2 diabetes. Diabetes 2004;53:1336–1343 [DOI] [PubMed] [Google Scholar]
- 33.Shen X, Zheng S, Metreveli NS, Epstein PN: Protection of cardiac mitochondria by overexpression of MnSOD reduces diabetic cardiomyopathy. Diabetes 2006;55:798–805 [DOI] [PubMed] [Google Scholar]
- 34.Ito M, Adachi T, Pimentel DR, Ido Y, Colucci WS: Statins inhibit β-adrenergic receptor-stimulated apoptosis in adult rat ventricular myocytes via a Rac1-dependent mechanism. Circulation 2004;110:412–418 [DOI] [PubMed] [Google Scholar]
- 35.Trump BF, Berezesky IK, Chang SH, Phelps PC: The pathways of cell death: oncosis, apoptosis, and necrosis. Toxicol Pathol 1997;25:82–88 [DOI] [PubMed] [Google Scholar]
- 36.Gao Y, Dickerson JB, Guo F, Zheng J, Zheng Y: Rational design and characterization of a Rac GTPase-specific small molecule inhibitor. Proc Natl Acad Sci U S A 2004;101:7618–7623 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhao K, Zhao GM, Wu D, Soong Y, Birk AV, Schiller PW, Szeto HH: Cell-permeable peptide antioxidants targeted to inner mitochondrial membrane inhibit mitochondrial swelling, oxidative cell death, and reperfusion injury. J Biol Chem 2004;279:34682–34690 [DOI] [PubMed] [Google Scholar]
- 38.Nagy N, Malik G, Tosaki A, Ho YS, Maulik N, Das DK: Overexpression of glutaredoxin-2 reduces myocardial cell death by preventing both apoptosis and necrosis. J Mol Cell Cardiol 2008;44:252–260 [DOI] [PubMed] [Google Scholar]
- 39.Boudina S, Sena S, Theobald H, Sheng X, Wright JJ, Hu XX, Aziz S, Johnson JI, Bugger H, Zaha VG, Abel ED: Mitochondrial energetics in the heart in obesity-related diabetes: direct evidence for increased uncoupled respiration and activation of uncoupling proteins. Diabetes 2007;56:2457–2466 [DOI] [PubMed] [Google Scholar]
- 40.Doughan AK, Harrison DG, Dikalov SI: Molecular mechanisms of angiotensin II-mediated mitochondrial dysfunction: linking mitochondrial oxidative damage and vascular endothelial dysfunction. Circ Res 2008;102:488–496 [DOI] [PubMed] [Google Scholar]
- 41.Panov AV, Lund S, Greenamyre JT: Ca2+-induced permeability transition in human lymphoblastoid cell mitochondria from normal and Huntington's disease individuals. Mol Cell Biochem 2005;269:143–152 [DOI] [PubMed] [Google Scholar]
- 42.Jones DP: Radical-free biology of oxidative stress. Am J Physiol Cell Physiol 2008;295:C849–C868 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lee SB, Bae IH, Bae YS, Um HD: Link between mitochondria and NADPH oxidase 1 isozyme for the sustained production of reactive oxygen species and cell death. J Biol Chem 2006;281:36228–36235 [DOI] [PubMed] [Google Scholar]
- 44.Desouki MM, Kulawiec M, Bansal S, Das GM, Singh KK: Cross talk between mitochondria and superoxide generating NADPH oxidase in breast and ovarian tumors. Cancer Biol Ther 2005;4:1367–1373 [DOI] [PubMed] [Google Scholar]
- 45.Rathore R, Zheng YM, Niu CF, Liu QH, Korde A, Ho YS, Wang YX: Hypoxia activates NADPH oxidase to increase [ROS]i and [Ca2+]i through the mitochondrial ROS-PKCepsilon signaling axis in pulmonary artery smooth muscle cells. Free Radic Biol Med 2008;45:1223–1231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Guo Z, Xia Z, Jiang J, McNeill JH: Downregulation of NADPH oxidase, antioxidant enzymes, and inflammatory markers in the heart of streptozotocin-induced diabetic rats by N-acetyl-l-cysteine. Am J Physiol Heart Circ Physiol 2007;292:H1728–H1736 [DOI] [PubMed] [Google Scholar]
- 47.Van Linthout S, Riad A, Dhayat N, Spillmann F, Du J, Dhayat S, Westermann D, Hilfiker-Kleiner D, Noutsias M, Laufs U, Schultheiss HP, Tschope C: Anti-inflammatory effects of atorvastatin improve left ventricular function in experimental diabetic cardiomyopathy. Diabetologia 2007;50:1977–1986 [DOI] [PubMed] [Google Scholar]
- 48.Lezoualc'h F, Metrich M, Hmitou I, Duquesnes N, Morel E: Small GTP-binding proteins and their regulators in cardiac hypertrophy. J Mol Cell Cardiol 2008;44:623–632 [DOI] [PubMed] [Google Scholar]
- 49.Tan W, Palmby TR, Gavard J, Amornphimoltham P, Zheng Y, Gutkind JS: An essential role for Rac1 in endothelial cell function and vascular development. Faseb J 2008;22:1829–1838 [DOI] [PubMed] [Google Scholar]
- 50.Sawada N, Salomone S, Kim HH, Kwiatkowski DJ, Liao JK: Regulation of endothelial nitric oxide synthase and postnatal angiogenesis by Rac1. Circ Res 2008;103:360–368 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Cai L: Suppression of nitrative damage by metallothionein in diabetic heart contributes to the prevention of cardiomyopathy. Free Radic Biol Med 2006;41:851–861 [DOI] [PubMed] [Google Scholar]