Abstract
Objective
To summarise the effect of primary prevention with lipid lowering drugs on coronary heart disease events, coronary heart disease mortality, and all cause mortality.
Design
Meta-analysis.
Identification
Systematic search of the Medline database from January 1994 to June 1999 for English language studies examining drug treatment for lipid disorders (use of the MeSH terms “hyperlipidemia” and “anticholesteremic agents,” keyword searches for individual drug names, and a search strategy for identifying randomised trials to capture relevant articles); identification of older studies through systematic reviews and hand search of bibliographies.
Inclusion criteria
All randomised trials of at least one year's duration that examined drug treatment for patients with no known coronary heart disease, cerebrovascular disease, or peripheral vascular disease and that measured clinical end points, including all cause mortality, coronary heart disease mortality, and non-fatal myocardial infarctions.
Data extraction
Review of the articles and extracted relevant data by two authors separately, with disagreements resolved by consensus.
Results
Four studies met eligibility criteria. Drug treatment reduced the odds of a coronary heart disease event by 30% (summary odds ratio 0.70, 95% confidence interval 0.62 to 0.79) but not the odds of all cause mortality (0.94, 0.81 to 1.09). When statin drugs were considered alone, no substantial differences in results were found.
Conclusions
Treatment with lipid lowering drugs lasting five to seven years reduces coronary heart disease events but not all cause mortality in people with no known cardiovascular disease.
Introduction
The effectiveness of drug treatment for lipid disorders in patients with no history of coronary heart disease has been controversial.1–3 Although the effectiveness of lipid lowering agents for secondary prevention in people with lipid disorders is strongly supported, primary prevention trials and systematic reviews have reached mixed conclusions about the effect of lipid lowering on mortality from coronary heart disease and on all cause mortality. Earlier reviews cautioned against drug treatment in patients with low to moderate risk of death from coronary heart disease because of possible increases in all cause mortality with treatment.4 A more recent review of lipid lowering treatment with hydroxymethyl glutaryl coenzyme A reductase inhibitors (statins) found that coronary heart disease events and all cause mortality were decreased in primary prevention populations.5 Reviews, however, have not included data from the large air force/Texas coronary prevention study (AFCAPS/TexCAPS), which examined the effect of drug treatment in men and women with poor ratios of total cholesterol concentration to high density lipoprotein cholesterol concentration and a modest risk (0.5-1% a year) of coronary heart disease events.6
We performed an updated systematic review and quantitative meta-analysis of primary prevention trials to estimate the effect of lipid lowering drugs on the incidence of coronary heart disease events (defined as non-fatal myocardial infarction and deaths from coronary heart disease), on coronary heart disease mortality, and on all cause mortality.
Methods
We searched the Medline database for articles published from January 1994 to June 1999, using the MeSH subject headings “hyperlipidemia” and “anticholesteremic agents,” MeSH terms or keywords for individual drug names, and a combination of subject headings and key words designed to identify randomised trials. We also searched the clinical trials registry of the Cochrane Library (Oxford, UK: Update Software, 1999) to identify studies that were not included in Medline. We used the bibliographies of systematic reviews and clinical practice guidelines to identify older trials not found through our main search strategy.
Two authors (MP and CP) separately reviewed the abstracts produced by the literature search to identify studies that were randomised trials which lasted at least one year and which measured clinical end points, including coronary heart disease events, coronary heart disease mortality, and all cause mortality. We excluded non-randomised studies, trials lasting less than one year, and trials examining only the change in serum cholesterol concentrations or angiographic outcomes. We also excluded studies published in languages other than English, studies published in abstract form only, and studies of secondary prevention that enrolled primarily patients with known coronary heart disease, cerebrovascular disease, or peripheral vascular disease. Studies that included a mixture of primary and secondary prevention patients were excluded if the results could not be distinguished for each group.If the information in the abstract was insufficient to determine eligibility for this review or if the reviewers disagreed about eligibility, we carried the article forward to the next stage.
We then reviewed the full articles for eligibility. For articles meeting inclusion criteria, we extracted the relevant data and entered them into evidence tables. Meta-analysis was performed with the Peto method for fixed effects models and then the DerSimonian and Laird method for random effects models. Graphs of the outcomes for included trials were examined visually and by using the χ2 test to identify heterogeneity in the outcome variables across different studies. Because the results of our meta-analysis did not differ according to whether the fixed or random effects model was used, we present only the fixed effects results here. The results are displayed as summary odds ratios and 95% confidence intervals for the effect of drug treatment on total coronary heart disease events, coronary heart disease mortality alone, and all cause mortality.
We performed a sensitivity analysis on the effect of including or excluding certain trials by repeating the meta-analysis after adding four trials that were difficult to categorise as primary prevention or mixed primary and secondary prevention. These four studies included three in which the eligibility of participants and the primary outcome measures were based on the degree of atherosclerosis in the femoral or carotid arteries that had been determined with ultrasonography7–9 and one that used clofibrate,10 a drug that is not currently used for lipid lowering in the United States.
Finally, because a previous meta-analysis had examined the effect of statins alone,5 we also analysed the three trials that used statins and compared the result of this analysis with our overall result.
Results
Literature search
Our searches identified 516 articles, of which 448 were rejected after the abstract was reviewed. The remaining 68 articles included 34 that were rejected because they involved secondary prevention populations; 10 that were rejected because they had mixed primary and secondary prevention groups,11–15 were not randomised,16 or did not measure relevant end points17–20; 16 articles with supplemental information only; 4 articles that met all inclusion criteria; and 4 studies that were considered to be “possibly suitable for inclusion.”7–10
Trial characteristics
The four eligible studies were the Lipid Research Clinic primary prevention trial, the Helsinki heart study, the west of Scotland coronary prevention study, and the air force/Texas coronary prevention study.6,21–23 The table shows the basic study characteristics.
Main results
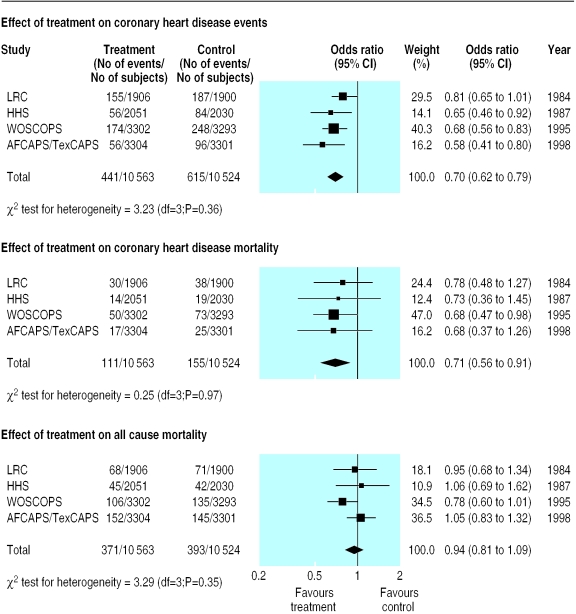
Figure 1 shows the effect of treatment with lipid lowering drugs on coronary heart disease events, coronary heart disease mortality, and all cause mortality. Treatment reduced the relative risk of coronary heart disease events by 30% compared with placebo (summary odds ratio 0.70, 95% confidence interval 0.62 to 0.79). The relative risk of coronary heart disease mortality was reduced by 29% (0.71, 0.56 to 0.91). There was either a small or no effect on all cause mortality (0.94, 0.81 to 1.09). In each of these analyses, the results of χ2 tests for heterogeneity were not significant (P>0.10).
Figure 1.
Effect of lipid lowering drugs (compared with placebo) on odds of coronary heart disease events, coronary heart disease mortality, and all cause mortality (fixed effects model). LRC=Lipid Research Clinic primary prevention trial; HHS=Helsinki heart study; WOSCOPS=west of Scotland coronary prevention study; AFCAPS/TexCAPS=air force/Texas coronary prevention study
Sensitivity analysis
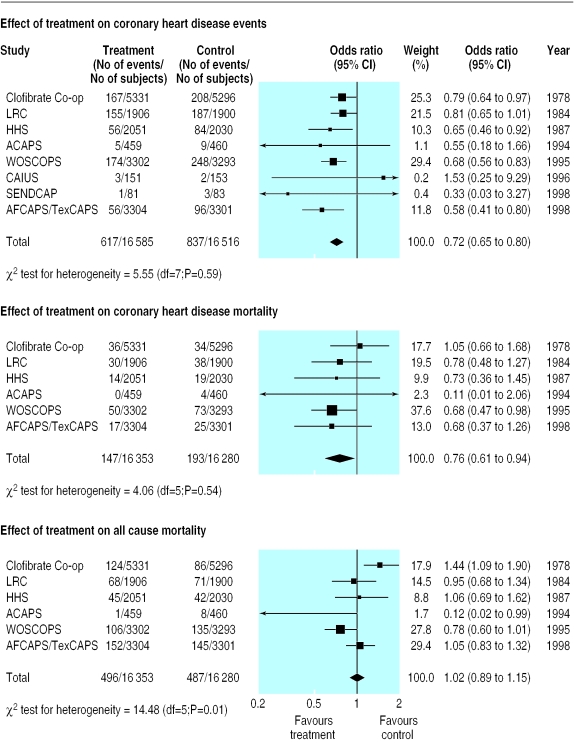
As figure 2 shows, the inclusion of the four studies that were considered possibly suitable for inclusion had little effect on the estimate of the reduction in coronary heart disease events (0.72, 0.65 to 0.80). The effect on coronary heart disease mortality was slightly attenuated for the six studies reporting this outcome (0.76, 0.61 to 0.94), and the effect on all cause mortality remained non-significant (1.02, 0.89 to 1.15). The χ2 test for heterogeneity was significant only for the outcome of all cause mortality, as two trials—the asymptomatic carotid artery progression study and the clofibrate cooperative study—had substantially different point estimates of effect size.
Figure 2.
Analysis as in figure 1 but with inclusion of four studies considered to be “possibly suitable for inclusion.” ACAPS=asymptomatic carotid artery progression study; Clofibrate Co-op=clofibrate cooperative study; CAIUS=carotid atherosclerosis Italian ultrasound study; SENDCAP=St Mary's, Ealing, Northwick Park diabetes cardiovascular disease prevention study (for full names of other studies see figure 1)
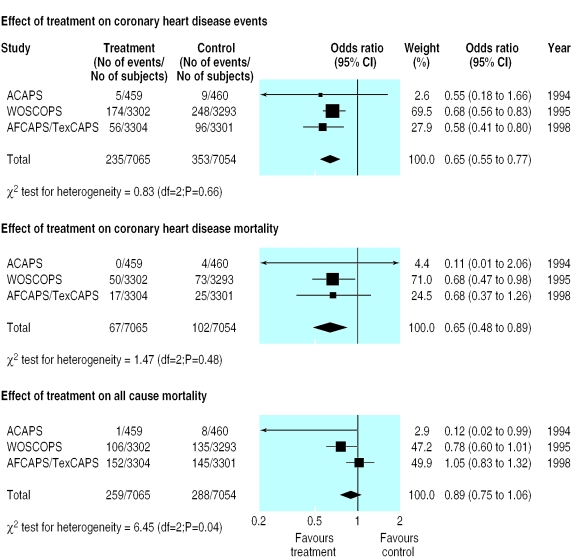
Figure 3 shows the results of the meta-analysis limited to the three trials using statins (two from the main analysis and one of the studies considered to be possibly suitable for inclusion). The summary effect on the incidence of coronary heart disease events was slightly greater than for the main analysis (0.65, 0.55 to 0.77), as was the effect on deaths from coronary heart disease (0.65, 0.48 to 0.89). The effect on all cause mortality remained non-significant (0.89, 0.75 to 1.06). Results of tests for heterogeneity were non-significant for total coronary heart disease events and coronary heart disease mortality but were significant (P=0.04) for all cause mortality, mainly because of the extreme value for the asymptomatic carotid artery progression study.
Figure 3.
Supplemental analysis of studies that used only statins (for full names of other studies see figures 1 and 2)
Discussion
Our meta-analysis of primary prevention trials shows that lipid lowering drugs reduce the relative odds of coronary heart disease events and coronary heart disease mortality by about 30% but that their effect on all cause mortality over five years is small and not significant. Limiting the analysis to trials that used statin drugs suggests a slightly stronger effect on all outcomes compared with analyses that used all trials, but it does not show a significant reduction in all cause mortality.
Our meta-analysis reaches a different conclusion from that of Hebert et al, who found that statin drugs reduced all cause mortality (0.74, 0.58 to 0.95).5 Unlike Hebert et al, we included the results of the large air force/Texas trial (which had not been published in 1997)6 and did not include the Kuopio atherosclerosis prevention study, a trial that included some subjects (10%) with histories of myocardial infarctions.24
The failure of drug treatment to reduce all cause mortality in primary prevention is most likely due to the generally low risk of mortality in the patient populations that were studied rather than some adverse effect of lipid lowering drugs or of lowering cholesterol concentrations. Treatment targeted specifically at primary prevention patients with higher levels of risk of coronary heart disease events might reduce all cause mortality. The trial with the participants at highest risk (west of Scotland coronary prevention study), for example, found a 22% reduction in the relative risk of all cause mortality, which was of borderline significance at five years (P=0.051).23 Lower risk populations might also achieve significant reductions in all cause mortality if they were treated for longer than those tested in the trials. We have insufficient data, however, on patients with low levels of risk, such as those enrolled in the air force/Texas trial, to estimate precisely the true effect on all cause mortality.
Because the absolute risk of all cause mortality in primary prevention patients is relatively low (the risk among control subjects in these trials was only 2-4% over five years), the absolute benefit in lives saved will also be low initially. If the true relative risk reduction for all cause mortality were 10%, the number needed to treat for five years to prevent one death would be 250 to 500. If it were 20%, it would be 125 to 250. Preventing non-fatal events may also improve all cause mortality over a longer span than the five to seven years observed in these trials, but data about the magnitude of that effect are not currently available.
What is already known on this topic
Randomised trials have found that drug treatment for lipid disorders reduces the incidence of coronary heart disease events in patients with no history of cardiovascular disease
Previous meta-analyses have reached conflicting conclusions about the effect of drug treatment on all cause mortality
What this study adds
An updated meta-analysis shows that treatment with lipid lowering drugs reduces the relative risk of coronary heart disease events and mortality from coronary heart disease by about 30%
Overall, all cause mortality does not seem to be affected, perhaps because the relatively short follow up periods in the trials (five to seven years) do not allow sufficient time for differences to emerge in relatively low risk patients
The decision about whether to use lipid lowering drugs for patients with no history of coronary heart disease is difficult and requires consideration of outcomes other than all cause mortality. The results of our meta-analysis suggest that treatment will reduce the relative risk of coronary heart disease events and coronary heart disease mortality by about 30%, independent of absolute risk. The absolute risk reduction from treatment, therefore, is proportional to the underlying risk in the person or populations being considered for treatment. The risk of coronary heart disease events and mortality, and hence the absolute risk reduction and number needed to treat, varies considerably in patients with no history of coronary heart disease and different combinations of coronary heart disease risk factors. Risk assessment tools can be used to determine the risk of individual patients and help providers and patients to decide about treatment.25,26
Generalising these results to other populations—such as people of non-European descent, women, and elderly people—is challenging because the included studies enrolled primarily middle aged men of European descent. The effect of treatment for women, elderly people, and men of non-European descent has not been directly studied, although there is little reason to believe that the effect would differ for non-Europeans or elderly people with similar baseline risks of coronary heart disease and similar lipid abnormalities. Also, the concomitant use of other drugs—such as chemoprophylaxis with aspirin or treatment with β blockers, which were not widely prescribed in these trials—may lower the absolute risk (and thus the potential absolute risk reductions) for large numbers of patients at moderate risk of coronary heart disease.
Future research should examine whether the effects of lipid lowering treatment are similar for women and for people of non-European origin, groups that were not well represented in the trials included here. The effect of longer treatment (5-10 years) should also be examined to determine if it produces greater reductions in coronary heart disease events and possibly all cause mortality.
Supplementary Material
Table.
Study characteristics
| LRC | HHS | WOSCOPS | AFCAPS/TexCAPS | |
|---|---|---|---|---|
| Drug (dose) | Colestyramine (24 g four times daily) | Gemfibrozil (600 mg twice daily) | Pravastatin (40 mg four times daily) | Lovastatin (20-40 mg four times daily) |
| Study duration (years) | 7 | 5 | 5 | 5 |
| No of subjects (intervention/control) | 1906/1900 | 2051/2030 | 3302/3293 | 3304/3301 |
| Mean age (years) | 48 | 47 | 55 | 58 |
| % of male subjects | 100 | 100 | 100 | 85 |
| Mean initial total cholesterol (mmol/l) | 7.5 | 7.4 | 7.0 | 5.7 |
| Mean reduction in total cholesterol (%) | 8.5† | 10‡ | 20‡ | 18§ |
LRC=Lipid Research Clinic primary prevention trial; HHS=Helsinki heart study; WOSCOPS=west of Scotland coronary prevention study; AFCAPS/TexCAPS=air force/Texas coronary prevention study.
Not intention to treat analysis. With intention to treat, the difference was 16% at five years.
At 7.4 years.
At 5 years.
At 1 year.
Acknowledgments
The authors of this article are responsible for its contents, including any clinical or treatment recommendations. No statement in this article should be construed as an official position of the Agency for Healthcare Research and Quality or the US Department of Health and Human Services.
Footnotes
Funding: This work was supported by funding made available to the Research Triangle Institute (for the RTI-UNC Evidence-based Practice Center) from the Agency for Healthcare Research and Quality (contract No 290-97-0011).
Competing interests: None declared.
This article is part of the BMJ's randomised controlled trial of open peer review. Documentation relating to the editorial decision making process is available on the BMJ's website
References
- 1.Howes LG, Simons LA. Efficacy of drug intervention for lipids in the prevention of coronary artery disease. Aust N Z J Med. 1994;24:107–112. doi: 10.1111/j.1445-5994.1994.tb04445.x. [DOI] [PubMed] [Google Scholar]
- 2.Pharoah PD, Hollingworth W. Cost effectiveness of lowering cholesterol concentration with statins in patients with and without pre-existing coronary heart disease: life table method applied to health authority population. BMJ. 1996;312:1443–1448. doi: 10.1136/bmj.312.7044.1443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Caro J, Klittich W, McGuire A, Ford I, Norrie J, Pettitt D, et al. The west of Scotland coronary prevention study: economic benefit analysis of primary prevention with pravastatin. BMJ. 1997;315:1577–1582. doi: 10.1136/bmj.315.7122.1577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Smith GD, Song F, Sheldon TA. Cholesterol lowering and mortality: the importance of considering initial level of risk [correction appears in BMJ 1993;306:1648] BMJ. 1993;306:1367–1373. doi: 10.1136/bmj.306.6889.1367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hebert PR, Gaziano JM, Chan KS, Hennekens CH. Cholesterol lowering with statin drugs, risk of stroke, and total mortality: An overview of randomized trials. JAMA. 1997;278:313–321. [PubMed] [Google Scholar]
- 6.Downs JR, Clearfield M, Weis S, Whitney E, Shapiro DR, Beere PA, et al. Primary prevention of acute coronary events with lovastatin in men and women with average cholesterol levels: results of AFCAPS/TexCAPS, Air Force/Texas coronary atherosclerosis prevention study. JAMA. 1998;279:1615–1622. doi: 10.1001/jama.279.20.1615. [DOI] [PubMed] [Google Scholar]
- 7.Furberg CD, Adams HP, Jr, Applegate WB, Byington RP, Espeland MA, Hartwell T, et al. Effect of lovastatin on early carotid atherosclerosis and cardiovascular events. Asymptomatic Carotid Artery Progression Study (ACAPS) Research Group. Circulation. 1994;90:1679–1687. doi: 10.1161/01.cir.90.4.1679. [DOI] [PubMed] [Google Scholar]
- 8.Mercuri M, Bond MG, Sirtori CR, Veglia F, Crepaldi G, Feruglio FS, et al. Pravastatin reduces carotid intima-media thickness progression in an asymptomatic hypercholesterolemic mediterranean population: the carotid atherosclerosis Italian ultrasound study. Am J Med. 1996;101:627–634. doi: 10.1016/s0002-9343(96)00333-6. [DOI] [PubMed] [Google Scholar]
- 9.Elkeles RS, Diamond JR, Poulter C, Dhanjil S, Nicolaides AN, Mahmood S, et al. Cardiovascular outcomes in type 2 diabetes: a double-blind placebo-controlled study of bezafibrate: the St Mary's, Ealing, Northwick Park diabetes cardiovascular disease prevention (SENDCAP) study. Diabetes Care. 1998;21:641–648. doi: 10.2337/diacare.21.4.641. [DOI] [PubMed] [Google Scholar]
- 10.A co-operative trial in the primary prevention of ischaemic heart disease using clofibrate: report from the Committee of Principal Investigators. Br Heart J. 1978;40:1069–1118. doi: 10.1136/hrt.40.10.1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dorr AE, Gundersen K, Schneider JCJ, Spencer TW, Martin WB. Colestipol hydrochloride in hypercholesterolemic patients—effect on serum cholesterol and mortality. J Chronic Dis. 1978;31(1):5–14. doi: 10.1016/0021-9681(78)90076-0. [DOI] [PubMed] [Google Scholar]
- 12.McCaughan D. The long-term effects of probucol on serum lipid levels. Arch Intern Med. 1981;141:1428–1432. [PubMed] [Google Scholar]
- 13.Bradford RH, Shear CL, Chremos AN, Dujovne C, Downton M, Franklin FA, et al. Expanded clinical evaluation of lovastatin (EXCEL) study results. I: Efficacy in modifying plasma lipoproteins and adverse event profile in 8245 patients with moderate hypercholesterolemia. Arch Intern Med. 1991;151:43–49. doi: 10.1001/archinte.151.1.43. [DOI] [PubMed] [Google Scholar]
- 14.Pravastatin Multinational Study Group for Cardiac Risk Patients. Effects of pravastatin in patients with serum total cholesterol levels from 5.2 to 7.8 mmol/liter (200 to 300 mg/dl) plus two additional atherosclerotic risk factors. Am J Cardiol. 1993;72:1031–1037. doi: 10.1016/0002-9149(93)90858-a. [DOI] [PubMed] [Google Scholar]
- 15.Athyros VG, Papageorgiou AA, Hatzikonstandinou HA, Didangelos TP, Carina MV, Kranitsas DF, et al. Safety and efficacy of long-term statin-fibrate combinations in patients with refractory familial combined hyperlipidemia. Am J Cardiol. 1997;80:608–613. doi: 10.1016/s0002-9149(97)00430-x. [DOI] [PubMed] [Google Scholar]
- 16.Kyushu Lipid Intervention Study Group. A coronary primary intervention study of Japanese men: study design, implementation and baseline data. Journal of Atherosclerosis, Thrombosis. 1996;3(2):95–104. doi: 10.5551/jat1994.3.95. [DOI] [PubMed] [Google Scholar]
- 17.Ives DG, Kuller LH, Traven ND. Use and outcomes of a cholesterol-lowering intervention for rural elderly subjects. Am J Preventive Med. 1993;9:274–281. [PubMed] [Google Scholar]
- 18.Lansberg PJ, Mitchel YB, Shapiro D, Kastelein JJ, Altman R, Jerums G, et al. Long-term efficacy and tolerability of simvastatin in a large cohort of elderly hypercholesterolemic patients. Atherosclerosis. 1995;116:153–162. doi: 10.1016/0021-9150(95)05523-y. [DOI] [PubMed] [Google Scholar]
- 19.Bredie SJ, Westerveld HT, Knipscheer HC, de Bruin T, Kastelein JJ, Stalenhoef AF. Effects of gemfibrozil or simvastatin on apolipoprotein-B-containing lipoproteins, apolipoprotein-CIII and lipoprotein(a) in familial combined hyperlipidaemia. Netherlands J Med. 1996;49(2):59–67. doi: 10.1016/0300-2977(96)00015-0. [DOI] [PubMed] [Google Scholar]
- 20.Eriksson M, Hadell K, Holme I, Walldius G, Kjellstrom T. Compliance with and efficacy of treatment with pravastatin and cholestyramine: a randomized study on lipid-lowering in primary care. J Intern Med. 1998;243:373–380. doi: 10.1046/j.1365-2796.1998.00294.x. [DOI] [PubMed] [Google Scholar]
- 21.Lipid research clinics coronary primary prevention trial results. II: The relationship of reduction in incidence of coronary heart disease to cholesterol lowering. JAMA. 1984;251:365–374. [PubMed] [Google Scholar]
- 22.Frick M, Elo O, Haapa K, Heinonen OP, Heinsalmi P, Helo P, et al. Helsinki heart study: primary-prevention trial with gemfibrozil in middle-aged men with dyslipidemia. Safety of treatment, changes in risk factors, and incidence of coronary heart disease. N Engl J Med. 1987;317:1237–1245. doi: 10.1056/NEJM198711123172001. [DOI] [PubMed] [Google Scholar]
- 23.Shepherd J, Cobbe SM, Ford I, Isles CG, Lorimer AR, MacFarlane PW, et al. Prevention of coronary heart disease with pravastatin in men with hypercholesterolemia: West of Scotland Coronary Prevention Study Group. N Engl J Med. 1995;333:1301–1307. doi: 10.1056/NEJM199511163332001. [DOI] [PubMed] [Google Scholar]
- 24.Salonen R, Nyyssonen K, Porkkala E, Rummukainen J, Belder R, Park JS, et al. Kuopio atherosclerosis prevention study (KAPS). A population-based primary preventive trial of the effect of LDL lowering on atherosclerotic progression in carotid and femoral arteries. Circulation. 1995;92:1758–1764. doi: 10.1161/01.cir.92.7.1758. [DOI] [PubMed] [Google Scholar]
- 25.Hingorani AD, Vallance P. A simple computer program for guiding management of cardiovascular risk factors and prescribing. BMJ. 1999;318:101–105. doi: 10.1136/bmj.318.7176.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ramsay LE, Haq IU, Jackson PR, Yeo WW, Pickin DM, Payne JN. Targeting lipid-lowering drug therapy for primary prevention of coronary disease: an updated Sheffield table. Lancet. 1996;348:387–388. doi: 10.1016/s0140-6736(96)05516-x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.