Abstract
The crystal structure of 2-isopropyl-5-methyl-1,4-benzoquinone (thymoquinone) and its thermal behavior—as necessary physical and chemical properties—were determined in order to enhance the current understanding of thymoquinone chemical action by using high resolution x-ray powder diffraction, Fourier transform infrared spectroscopy (FTIR), and 3 thermo-analytical techniques thermogravimetric analysis (TGA), differential thermal analysis (DTA), and differential scanning calorimetry (DSC). The findings obtained with high-resolution x-ray powder diffraction and molecular location methods based on a simulated annealing algorithm after Rietveld refinement showed that the triclinic unit cell was a=6.73728(8) Å, b=6.91560(8) Å, c=10.4988(2) Å, α=88.864(2)o, β=82.449(1)o, γ=77.0299(9)o; cell volume=472.52(1) Å3, Z=2, and space group 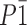 . In addition, FTIR spectrum revealed absorption bands corresponding to the carbonyl and C-H stretching of aliphatic and vinylic groups characteristically observed in such p-benzoquinones. Also, a chemical decomposition process starting at 65°C and ending at 213°C was noted when TGA was used. DSC allowed for the determination of onset at 43.55°C and a melting enthalpy value of ΔHm=110.6 J/g. The low value obtained for the fusion point displayed a van der Waals pattern for molecular binding, and the thermograms performed evidence that thymoquinone can only be found in crystalline triclinic form, as determined by DRX methods.
. In addition, FTIR spectrum revealed absorption bands corresponding to the carbonyl and C-H stretching of aliphatic and vinylic groups characteristically observed in such p-benzoquinones. Also, a chemical decomposition process starting at 65°C and ending at 213°C was noted when TGA was used. DSC allowed for the determination of onset at 43.55°C and a melting enthalpy value of ΔHm=110.6 J/g. The low value obtained for the fusion point displayed a van der Waals pattern for molecular binding, and the thermograms performed evidence that thymoquinone can only be found in crystalline triclinic form, as determined by DRX methods.
KeyWords: thymoquinone, x-ray powder diffraction, thermo-analytical techniques, FTIR spectrum
Full Text
The Full Text of this article is available as a PDF (404.1 KB).
References
- 1.Gilani AH, Aziz N, Khurram IM, Chaudhary KS, Iqbal A. Bronchodilator, Spasmolytic and Calcium Antagonist Activities of Nigella sativa seeds (Kalonji): a traditional herbal product with Multiple Medicinal Uses. J Pak Med Assoc. 2001;51:115–119. [PubMed] [Google Scholar]
- 2.Salomi N, Nair S, Jayawardhanan K, Vorghese C, Pankkar K. Antitumour Principles from Nigella sativa seeds. Cancer Lett. 1992;63:41–46. doi: 10.1016/0304-3835(92)90087-C. [DOI] [PubMed] [Google Scholar]
- 3.Worthen DR, Ghosheh OA, Crooks PA. The in vitro anti-tumor activity of some crude and purified components of blackseed, Nigella sativa Linn. Anticancer Res. 1998;18:1527–1532. [PubMed] [Google Scholar]
- 4.Houghton PJ, Zarka R, Delasheras B, Hoult JRS. Fixed oil of Nigella sativa and derived thymoquinone inhibit eicosanoid generation in leukocytes and membrane lipid-peroxidation. Planta Med. 1995;61:33–36. doi: 10.1055/s-2006-957994. [DOI] [PubMed] [Google Scholar]
- 5.Chakravarty N. Inhibition of Histamine release from mast cells by nigellone. Ann Allergy. 1993;70:237–242. [PubMed] [Google Scholar]
- 6.Abou Basha Alila I, Rashed Mohamed S, Aboul-Enein Asan Y. TLC assay of thymoquinone in black seed oil (Nigela sativa Linn) and identification of dithymoquinone and thymol. J Liquid Chromatogr. 1995;18:105–115. doi: 10.1080/10826079508009224. [DOI] [Google Scholar]
- 7.Ghosheh OA, Houdi AA, Crooks PA. High-performance liquid chromatographic analysis of the pharmacologically active quinines and related compounds in the oil of the black seed (Nigella sativa L) J Pharm Biomed Anal. 1999;19:757–762. doi: 10.1016/S0731-7085(98)00300-8. [DOI] [PubMed] [Google Scholar]
- 8.Badary OA. Thymoquinone attenuates ifosfamide-induced Fanoni syndrome in rats and enhances its antitumor activity in mice. J Etlmopharmacol. 1999;67:135–142. doi: 10.1016/S0378-8741(98)00242-6. [DOI] [PubMed] [Google Scholar]
- 9.Badary OA, Nagi MN, Al-Shabanah OA, Al-Sawaf HA, Al-Sohaibani MO, Al-Bekairi AM. Thymoquinone ameliorates the nephrotoxicity induced by cisplatin in rodents and potentiates its antitumor activity. Can J Physiol Pharmacol. 1997;75:1356–1361. doi: 10.1139/cjpp-75-12-1356. [DOI] [PubMed] [Google Scholar]
- 10.Milos M, Matelic J, Jerkovic I. Chemical composition and antioxidant effect of glycosidically bound volatile compounds from oregano (Origanum vulgare L ssp hirtum) Food Chem. 2000;71:79–83. doi: 10.1016/S0308-8146(00)00144-8. [DOI] [Google Scholar]
- 11.Badary O, Abdel-Naim A, Abdel-Wajab M, Hamada F. The influence of thymoquinone on doxorubicin-induced hyperlipidemic nephropathy in rats. Toxicology. 2000;143(3):219–226. doi: 10.1016/S0300-483X(99)00179-1. [DOI] [PubMed] [Google Scholar]
- 12.Al-Shabanah OA, Badary OA, Nagi MN, Al-Gharably NM, Al-Rikabi AC, Al-Bekairi AM. Thymoquinone protects against doxorubicin-induced cardiotoxicity without compromising its antitum or activity. J Exp Clin Cancer Res. 1998;17:193–198. [PubMed] [Google Scholar]
- 13.Pagola S, Stephens PW, Bohle DS, Kosar AD, Madsen SK. The structure of malaria pigment beta-haematin. Nature. 2000;404:307–310. doi: 10.1038/35005132. [DOI] [PubMed] [Google Scholar]
- 14.Shankland K, McBride L, David WIF, Shankland N, Steele G. Molecular, crystallographic and algorithmic factors in structure determination from powder diffraction data by simulated annealing. J Appl Crystallogr. 2002;35:443–454. doi: 10.1107/S0021889802007835. [DOI] [Google Scholar]
- 15.Engel GE, Wilke S, Konig O, Harris KDM, Leusen FJJ. Powder Solve—a complete package for crystal structure solution from powder diffraction patterns. J. Appl Crystallogr. 1999;32:1169–1179. doi: 10.1107/S0021889899009930. [DOI] [Google Scholar]
- 16.Le Bail A, Duroy H, Fourquet JL. Ab-initio structure determination of LiSbWO6 by X-ray powder diffraction. Mater Res Bull. 1988;23:447–452. doi: 10.1016/0025-5408(88)90019-0. [DOI] [Google Scholar]
- 17.Stephens PW, Pagola S. Powder Structure Solution Program. Available at; http://powder.physics.sunysb.edu.Accessed March 27, 2004.
- 18.Werner PE, Eriksson L, Westdahl M. TREOR, a semiexhaustive trial-and-error powder indexing program for all symmetries. J Appl Crystallogr. 1985;18:367–370. doi: 10.1107/S0021889885010512. [DOI] [Google Scholar]
- 19.Visser JW. A fully automatic program for finding the unit cell from powder data. J Appl Crystallogr. 1969;2:89–95. doi: 10.1107/S0021889869006649. [DOI] [Google Scholar]
- 20.Krivy I, Gruber B. A unified algorithm for determining, the reduced (Niggli) cell. Acta Crystallogr. 1976;32:297–298. doi: 10.1107/S0567739476000636. [DOI] [Google Scholar]
- 21.Rodriguez-Carvajal J. FULLPROF: A program for Rietveld refinement and pattern matching analysis. Abstracts of the Satellite Meeting on Powder Diffraction of the XV Congress of the IUCr. 1990; Toulouse, France; 127.
- 22.Larson AC, Von Dreele RB. GSAS—Generalized Crystal Structure Analysis System. Los Alamos, NM: Los Alamos National Laboratory; 1987. Report No. LA-UR-86-748.
- 23.Spek AL. PLATON-A Multipurpose Crystallographic Tool [computer program]. 1990; Available at: http://www.cryst.chem.uu.nl/platon. Accessed March 22, 2004.
- 24.HyperChem Molecular Modeling System [computer program]. Version 6.01. Gainesville, FL: Hypercube Inc; 2000.
- 25.Allen FH, Kennard O. 3D Search and Research using the Cambridge Structural Database. Chemical Design Automation News. 1998;8:31–37. [Google Scholar]
- 26.CS Chem 3D Pro Molecular Modeling and Analysis Program [computer program]. Cambridge, MA: CambridgeSoft Corp; 1996.
- 27.Scott HG. The estimation of standard deviation in powder diffraction Rietveld Refinements. J Appl Crystallogr. 1983;16:159–163. doi: 10.1107/S0021889883010195. [DOI] [Google Scholar]
- 28.Farrugia LJ. WinGX suite for small-molecule single-crystal crystallography. J Appl Crystallogr. 1999;32:837–838. doi: 10.1107/S0021889899006020. [DOI] [Google Scholar]
- 29.Dinnebier RE, Dollase WA, Helluv X, et al. Order-disorder phenomena determined by high-resolution powder diffraction: the structures of tetrakis (trimethylsilyl) methane C[Si(CH3)3]4 and tetrakis (trimethylsilyl) silane Si[Si(CH3)3]4. Acta Crystallogr. 1999;55:1014–1029. doi: 10.1107/S0108767399007114. [DOI] [PubMed] [Google Scholar]
- 30.Integrated Spectral Data Base System for Organic Compounds [database online]. Ibaraki, Japan: National Institute of Advanced Industrial Science and Technology; 2003. Updated September 30, 2003. Available at: http://www.aist.go.jp/RIODB/SDBS/menu-e.html.
- 31.Yamakita Y, Tasumi M. Vibrational analyses of p-benzoquinodimethane ethane and p-benzoquinone based on ab initio Hartree-Fock and second-order Moller-Plesset calculations. J. Phys Chem. 1995;99:8524–8534. doi: 10.1021/j100021a013. [DOI] [Google Scholar]


