Abstract
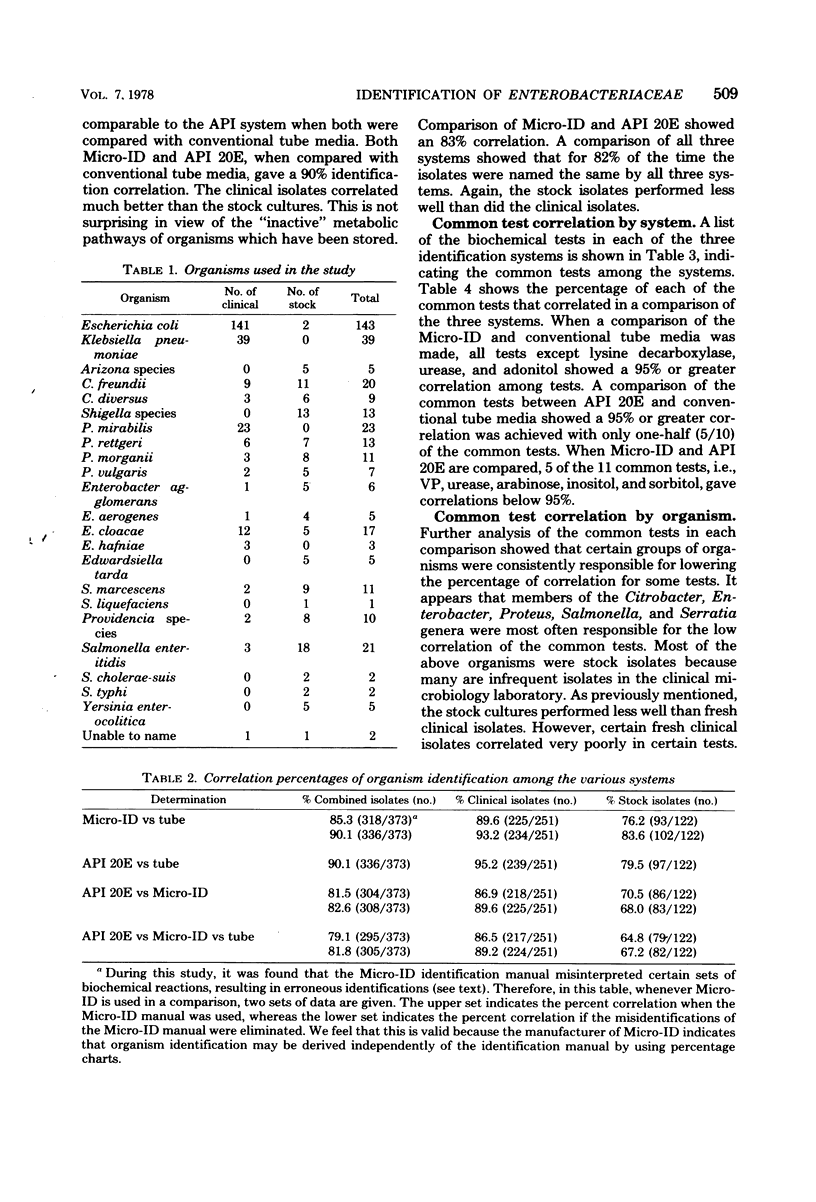
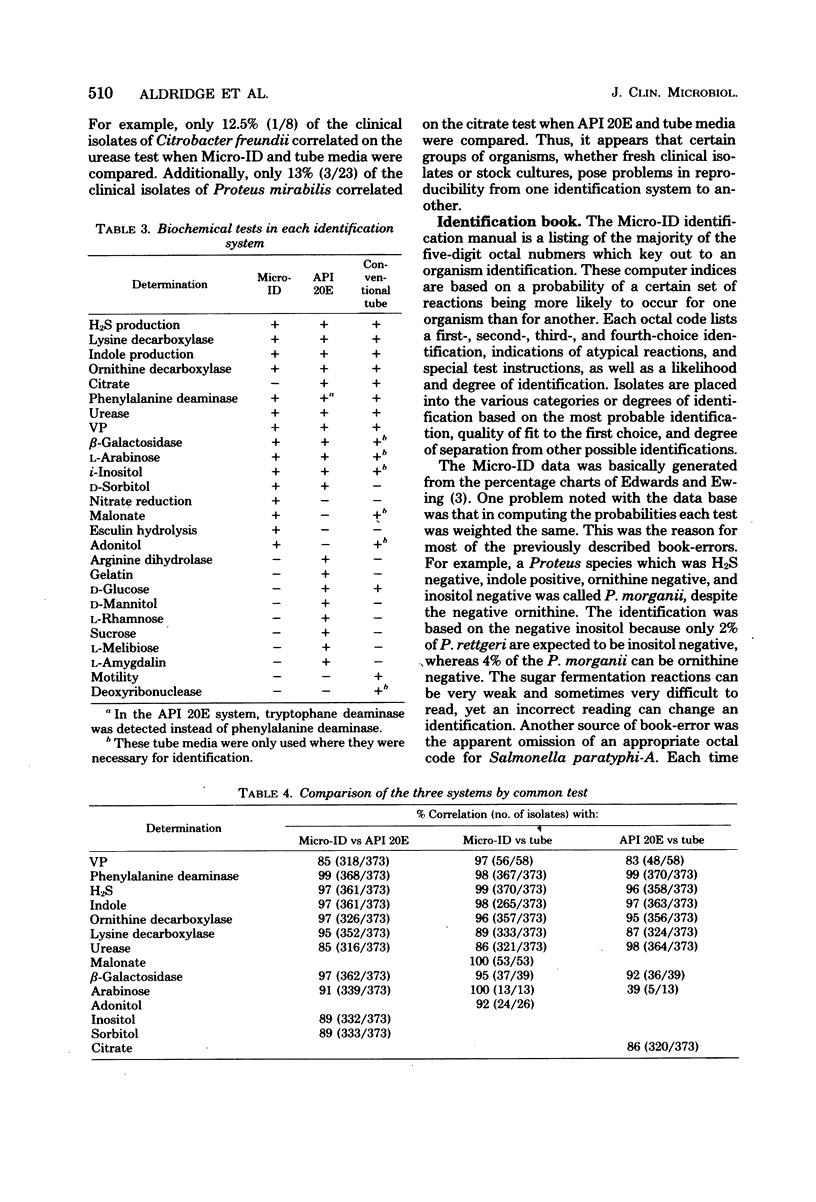
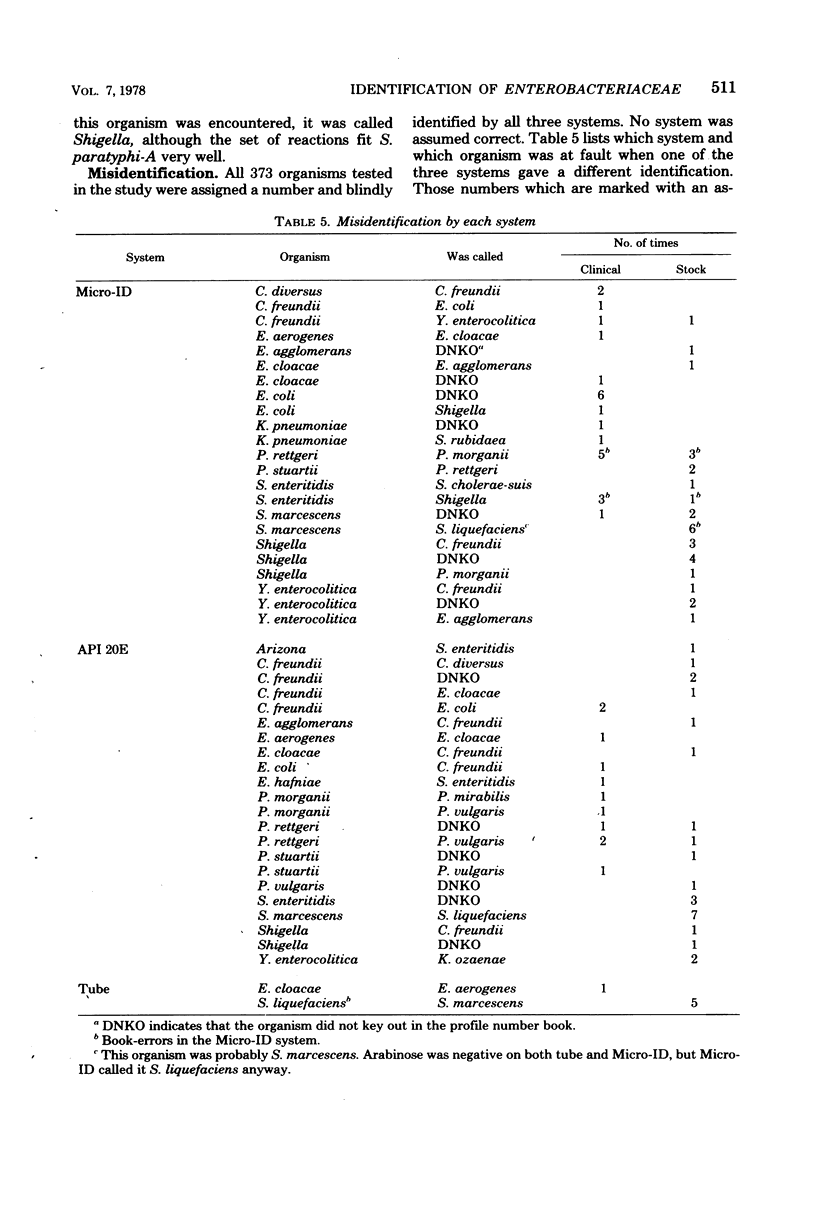
The Micro-ID, a new identification kit for Enterobacteriaceae, consists of 15 biochemical tests, with substrates and reagents impregnated in filter paper disks. A 0.2-ml amount of an organism suspension equal to a 0.5 McFarland standard is pipetted into each of the compartments. After 4 h of incubation and addition of potassium-hydroxide (KOH) to the Voges-Proskauer test, the color reactions are read according to the recommendations of the manufacturer. A five-digit octal code number is derived from each set of reactions from which an identification is derived by using a code book. In a single-blind, comparative study of the Micro-ID system with the API 20E system (Analytab Products Inc.) and conventional biochemical tube media, we found that the Micro-ID and the API 20E systems gave a 90% identification correlation when each was compared with the conventional tube media. A comparison of all three systems gave an 82% overall identification correlation. When the common tests of Micro-ID and API 20E were compared with conventional tube media, we found that the tests in the Micro-ID performed as well as or better than those of the API 20E. Certain groups of organisms, i.e., Citrobacter, Enterobacter, Proteus, Salmonella, and Serratia genera, were found to give low correlation on certain common tests. When using primary isolation MacConkey plates from the clinical laboratory, only 74% of the plates with Enterobacteriaceae had sufficient numbers of colonies of each enteric organism to produce the 0.5 McFarland inoculum density required. Problems concerning the misidentification of some organisms are discussed.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Butler D. A., Lobregat C. M., Gavan T. L. Reproducibility of the analytab (API 20E) system. J Clin Microbiol. 1975 Oct;2(4):322–326. doi: 10.1128/jcm.2.4.322-326.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coppel S. P., Coppel I. G. Comparison of the R-B system and the Enterotube for the identification of Enterobacteriaceae. Am J Clin Pathol. 1974 Feb;61(2):218–222. doi: 10.1093/ajcp/61.2.218. [DOI] [PubMed] [Google Scholar]
- Kiehn T. E., Brennan K., Ellner P. D. Evaluation of the Minitek system for identification of Enterobacteriaceae. Appl Microbiol. 1974 Oct;28(4):668–671. doi: 10.1128/am.28.4.668-671.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lachica R. V., Genigeorgis C., Hoeprich P. D. Metachromatic agar-diffusion methods for detecting staphylococcal nuclease activity. Appl Microbiol. 1971 Apr;21(4):585–587. doi: 10.1128/am.21.4.585-587.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsen J. M., Sherris J. C. Comparative study of the efficacy of seven paper-reagent strips and conventional biochemical tests in identifying gram-negative organisms. Appl Microbiol. 1969 Sep;18(3):452–457. doi: 10.1128/am.18.3.452-457.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nord C. E., Lindberg A. A., Dahlbäck A. Evaluation of five test-kits-API, AuxoTab, Enterotube, PathoTec and R-B-for identification of Enterobacteriaceae. Med Microbiol Immunol. 1974 Mar 22;159(3):211–220. doi: 10.1007/BF02121337. [DOI] [PubMed] [Google Scholar]