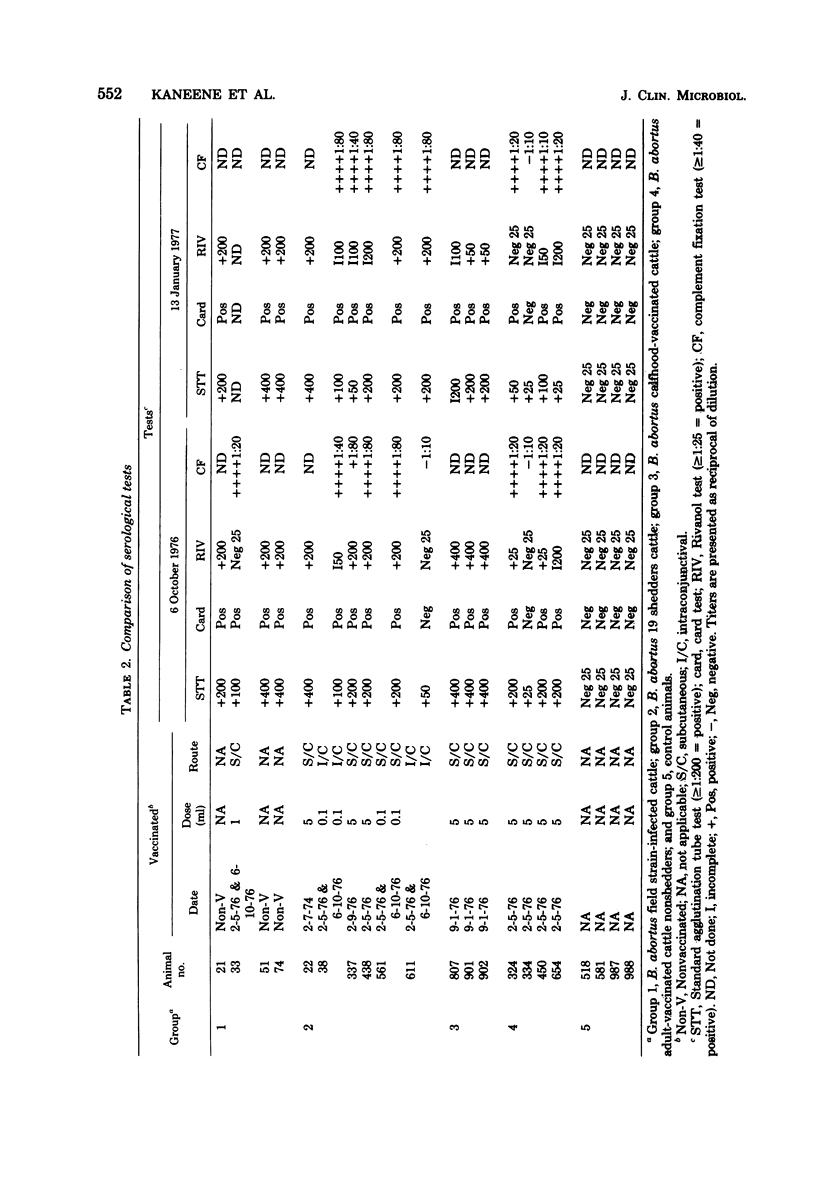
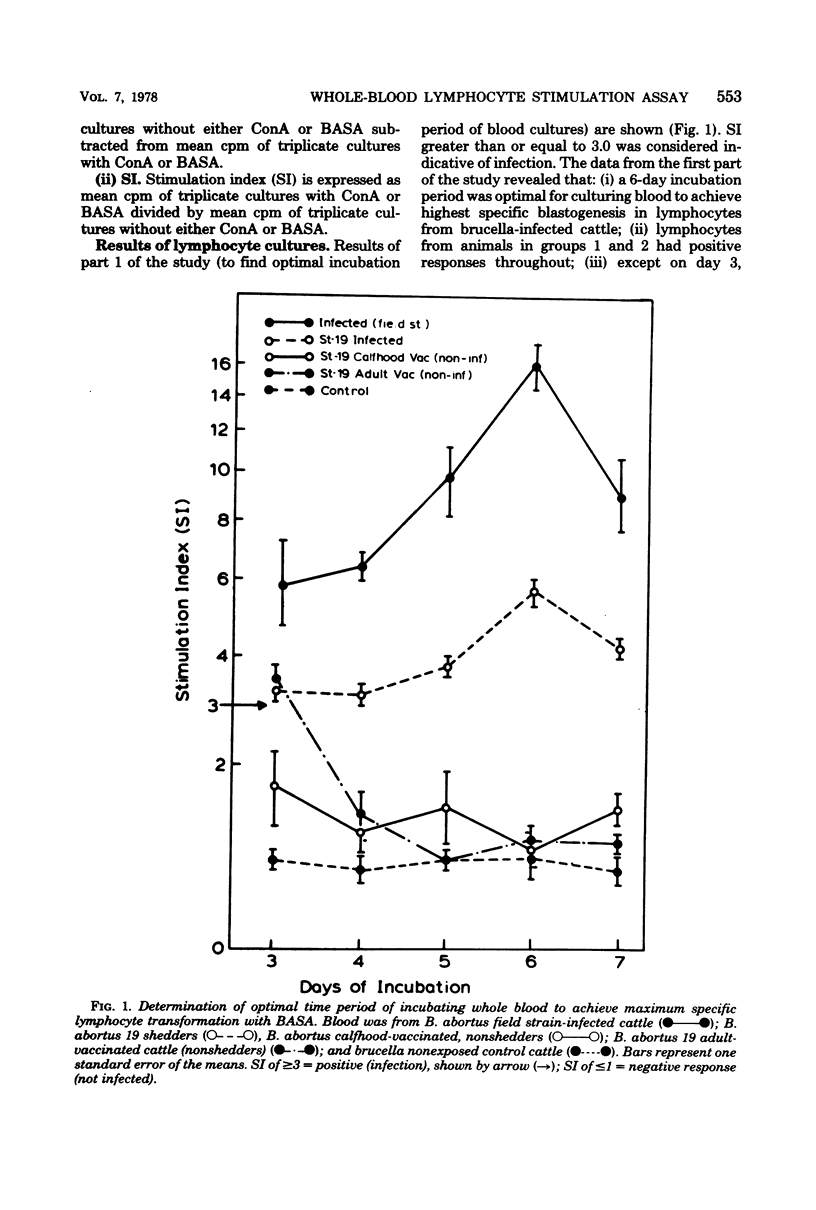
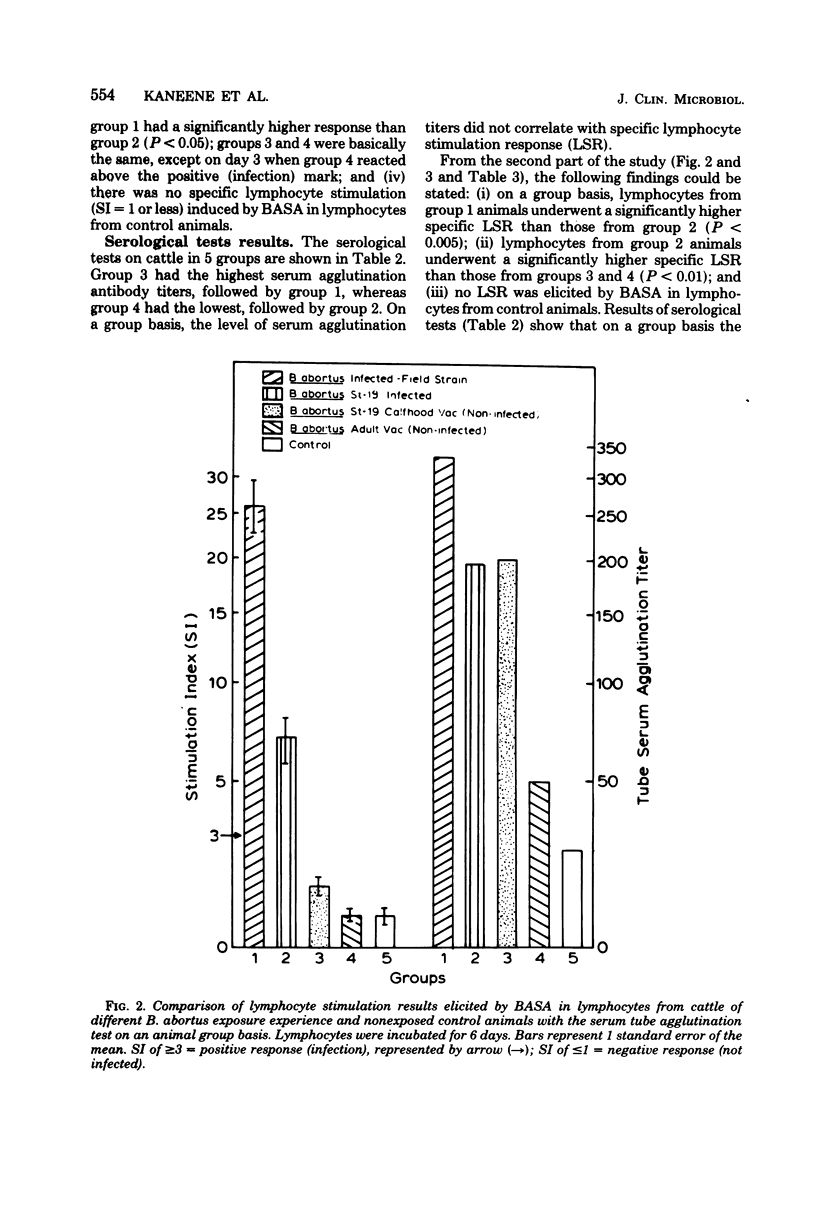
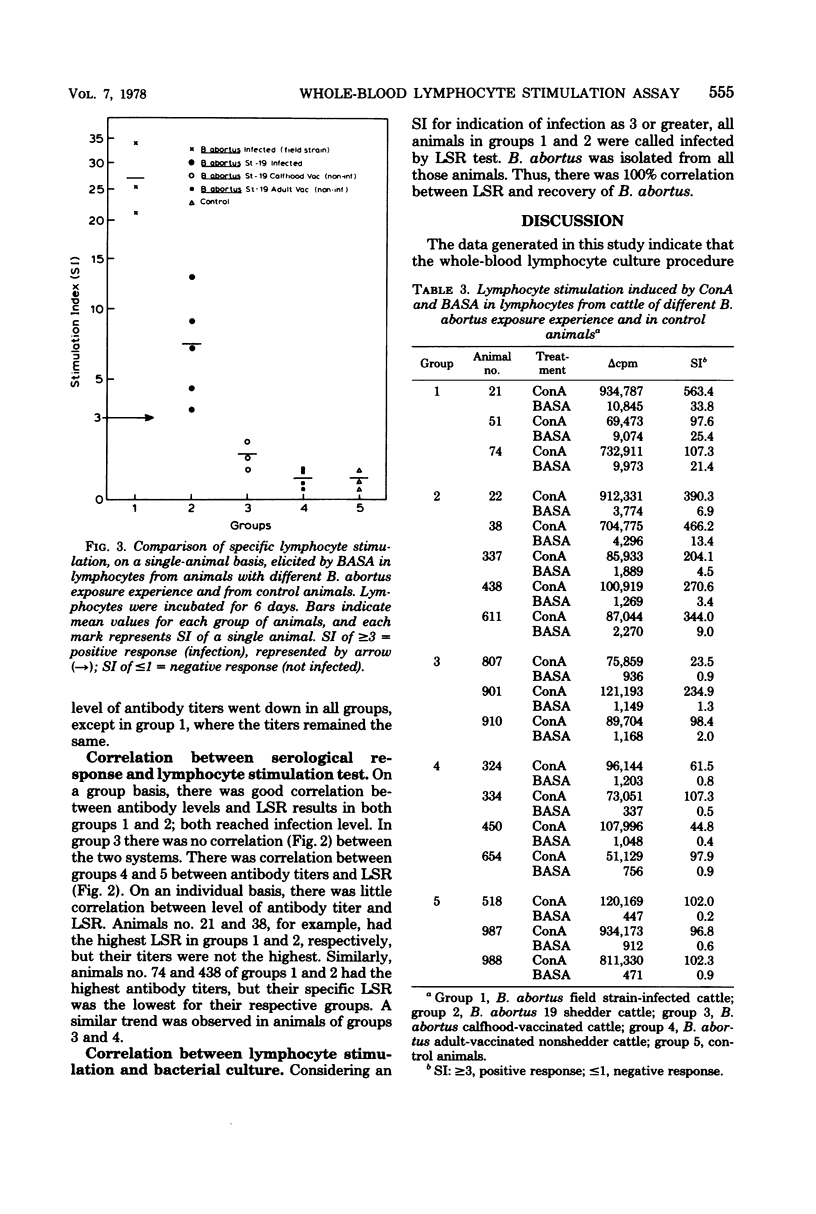
Abstract
A study was conducted to develop an in vitro whole-blood lymphocyte stimulation assay for measurement of cell-mediated immune response in bovine brucellosis. A soluble antigen (BASA) prepared from killed cells of Brucella abortus 1119-3 was used. Cattle infected with B. abortus field strains, B. abortus 19 calfhood- and adult-vaccinated cattle, and nonexposed cattle were tested. Blood was diluted 10-fold in RPMI-1640 medium (without added serum) and cultured with BASA (at a concentration of 2.2 microgram per culture) at varying times of incubation. Results were assayed for [3H]thymidine incorporation into deoxyribonucleic acid. A 6-day period was found to be optimal for incubating blood cultures to achieve maximum specific lymphocyte stimulation. Serological tests and bacteriological isolation attempts were conducted simultaneously with lymphocyte stimulation tests, and there was a significant correlation between cell-mediated immune response and bacteriological findings. There was a significant correlation between cell-mediated immune response and the level of serum antibodies on a group basis, but there was little correlation between the two systems on individual infected animals. Among vaccinated animals there was little or no correlation between cell-mediated immune and humoral responses. The whole-blood assay was found to be simple, fast, sensitive, and reproducible.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bascoul S., Peraldi M., Merino A. L., Lacave C., Cannat A., Serre A. Stimulating activity of Brucella fractions in a human lymphocyte transformation test. Correlation with humoral and cellular immunity. Immunology. 1976 Nov;31(5):717–722. [PMC free article] [PubMed] [Google Scholar]
- Chaparas S. D., Hedrick S. R., Clark R. G., Garman R. Comparison of the lymphocyte transformation test with the tuberculin test in rhesus monkeys and chimpanzees. Am J Vet Res. 1970 Aug;31(8):1437–1441. [PubMed] [Google Scholar]
- Daguillard F. Immunologic significance of in vitro lymphocyte responses. Med Clin North Am. 1972 Mar;56(2):293–303. doi: 10.1016/s0025-7125(16)32397-5. [DOI] [PubMed] [Google Scholar]
- Daniel T. M., Hinz C. F., Jr Reactivity of purified proteins and polysaccharides from Mycobacterium tuberculosis in delayed skin test and cultured lymphocyte mitogenesis assays. Infect Immun. 1974 Jan;9(1):44–47. doi: 10.1128/iai.9.1.44-47.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartzman R. J., Bach M. L., Bach F. H., Thurman G. B., Sell K. W. Precipitation of radioactively labeled samples: a semi-automatic multiple-sample processor. Cell Immunol. 1972 Jun;4(2):182–186. doi: 10.1016/0008-8749(72)90018-4. [DOI] [PubMed] [Google Scholar]
- Kaneene J. M., Johnson D. W., Anderson R. K., Angus R. D., Pietz D. E., Muscoplat C. C. Kinetics of in vitro bovine lymphocyte immunostimulation with a Brucella abortus antigen. Am J Vet Res. 1978 Feb;39(2):235–239. [PubMed] [Google Scholar]
- Kaneene J. M., Johnson D. W., Anderson R. K., Angus R. D., Pietz D. E., Muscoplat C. C. Specific lymphocyte stimulation in cattle naturally infected with strains of Brucella abortus and cattle vaccinated with Brucella abortus strain 19. Am J Vet Res. 1978 Apr;39(4):585–589. [PubMed] [Google Scholar]
- Miller S. D., Jones H. E. Correlation of lymphocyte transformation with tuberculin skin-test sensitivity. Am Rev Respir Dis. 1973 Apr;107(4):530–538. doi: 10.1164/arrd.1973.107.4.530. [DOI] [PubMed] [Google Scholar]
- Mills J. A. The immunologic significance of antigen induced lymphocyte transformation in vitro. J Immunol. 1966 Aug;97(2):239–247. [PubMed] [Google Scholar]
- Muscoplat C. C., Thoen C. O., McLaughlin R. M., Thoenig J. R., Chen A. W., Johnson D. W. Comparison of lymphocyte stimulation and tuberculin skin reactivity in Mycobacterium bovis-infected Macaca mulatta. Am J Vet Res. 1975 May;36(5):699–701. [PubMed] [Google Scholar]
- Paty D. W., Hughes D. Lymphocyte transformation using whole blood cultures: an analysis of responses. J Immunol Methods. 1972 Nov;2(1):99–114. doi: 10.1016/0022-1759(72)90022-1. [DOI] [PubMed] [Google Scholar]
- Pauly J. L., Sokal J. E. A simplified technique for in vitro studies of lymphocyte reactivity. Proc Soc Exp Biol Med. 1972 May;140(1):40–44. doi: 10.3181/00379727-140-36391. [DOI] [PubMed] [Google Scholar]
- Pauly J. L., Sokal J. E., Han T. Whole-blood culture technique for functional studies of lymphocyte reactivity to mitogens, antigens, and homologous lymphocytes. J Lab Clin Med. 1973 Sep;82(3):500–512. [PubMed] [Google Scholar]
- Rocklin R. E., Rosen F. S., David J. R. In vitro lymphocyte response of patients with immunologic deficiency diseases. N Engl J Med. 1970 Jun 11;282(24):1340–1343. doi: 10.1056/NEJM197006112822404. [DOI] [PubMed] [Google Scholar]
- Rosenberg G. L., Farber P. A., Notkins A. L. In vitro stimulation of sensitized lymphocytes by herpes simplex virus and vaccinia virus. Proc Natl Acad Sci U S A. 1972 Mar;69(3):756–760. doi: 10.1073/pnas.69.3.756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wyler D. J., Oppenheim J. J. Lymphocyte transformation in human Plasmodium falciparum malaria. J Immunol. 1974 Aug;113(2):449–454. [PubMed] [Google Scholar]



