Abstract
Recent research on brain correlates of cognitive processes revealed the occurrence of global synchronization during conscious processing of sensory stimuli. In spite of technological progress in brain imaging, an explanation of the computational role of synchrony is still a highly controversial issue. In this study, we depart from an analysis of the usage of blood-oxygen-level-dependent functional magnetic resonance imaging for the study of cognitive processing, leading to the identification of evoked local field potentials as the vehicle for sensory patterns that compose conscious episodes. Assuming the “astrocentric hypothesis” formulated by James M. Robertson (astrocytes being the final stage of conscious processing), we propose that the role of global synchrony in perceptual conscious processing is to induce the transfer of information patterns embodied in local field potentials to astrocytic calcium waves, further suggesting that these waves are responsible for the “binding” of spatially distributed patterns into unitary conscious episodes.
Keywords: Astrocytes, Perceptual consciousness, Local field potentials, BOLD signal, Functional magnetic resonance, Global synchrony, Calcium waves
Introduction
Astrocytes are star-shaped cells that compose one half of brain tissue volume. Until recently, only passive functions were attributed to them, such as giving structural, metabolic, and functional support for differentiation, proliferation, and survival of neurons.
In the last two decades, a growing number of results support a new view that astrocytes are also excitable cells and play important (perhaps crucial) roles in information processing in the brain [1]. They participate in synaptic transmission and integrate the activity of neural assemblies. The term “tripartite synapse” was coined to refer to the functional complex composed of presynaptic neurons, astrocytes, and postsynaptic neurons [2]. The functional unit composed by one astrocyte connected to a half dozen neurons, enveloping thousands of synapses, was called “synaptic island” [3].
A widespread class of tripartite synapses uses glutamate as their main excitatory transmitter. In this functional unit, astrocytes receive glutamate inputs from the presynaptic neuron and release gliotransmitters that provide feedback to both presynaptic and postsynaptic neurons. The physiological (excitable) response of astrocytes to presynaptic excitatory transmission is the calcium wave, a rising in the intracellular level of calcium, processing information from input (presynaptic neuron) to output (postsynaptic neuron).
In this paper, we discuss the kind of information processing performed by astrocytes and cognitive functions carried by information patterns embodied in astrocyte calcium waves. Inspired by recent findings about astrocyte sensitivity to sensory stimulation, we hypothesize that information patterns embodied in astrocytic calcium waves could be involved with the formation of a multimodal “conscious scene” that may be used for the coordination of adaptive behavior. Based on this hypothesis, we clarify the putative role of neural synchrony for neuro-astroglial communication.
The generation of coherent astrocytic calcium waves by the surrounding neuronal population requires multiple and coordinated inputs reaching a threshold of excitation. Synchronization of neuronal activity (including both graded and action potentials, i.e., oscillatory and spiking synchrony) can increase the communication from neurons to astrocytes by means of phase coordination of excitatory inputs. In summary, we claim that global synchrony facilitates the transfer of a multitude of cognitive patterns embodied in neural local field potentials (LFP) to astroglial calcium waves where they are possibly integrated into conscious episodes.
Astrocyte morphology, physiology, and sensitivity to sensory stimulation
Astrocytes are the most numerous glial cells. Collectively, they form the astroglia. They are extensively coupled by gap junctions forming a functional syncytium that allow intense exchange of intracellular fluid between neighboring cells. They display an irregular star-like shape, projecting their processes to neuropil and encephalic microcirculation. A number of astrocytic projections, called perivascular endfeet, expand to the surfaces of arterioles and capillaries, indicating that astrocytes are prompt to extract nutrients from the blood and distribute them to neural cells. Indeed, a significant amount of studies have shown that astrocytes function as a link for energetic supply to neurons by drawing glucose out from the plasma and—after partially metabolizing it—delivering the product for neuronal utilization [4].
The vast majority of astrocytic processes extend to radial directions, establishing a close proximity with neuron axon terminals, cell bodies, and dendrites. In many cases, astrocyte terminations wrap the synaptic cleft. In different brain regions, each astrocyte can contact 100,000 [5] or even up to 140,000 synapses [6]. These synapses belong to, on average, six neurons, composing “synaptic islands” [3].
At the synaptic level, astrocytes respond to presynaptic input through neurotransmitter-induced calcium waves, leading to the release of chemical transmitters (gliotransmitters) that alter pre- and postsynaptic neuronal activity (for a review, see [6]). The term “tripartite synapse” [7] has been used to identify such a novel synaptic unity. These findings suggest that astrocytes may act as a hub connecting thousands of synapses and also—as we propose—integrating distributed information patterns.
Astrocytes are not excitable cells in the same way as neurons or muscular fibers. When chemically or physically stimulated, they do not generate the typical nonlinear membrane electrical changes observed in those cells, but they show oscillations in cytosolic calcium levels as a noteworthy response. Such calcium waves can be driven by distinct mediators as acetylcholine [8], GABA [9], and ATP [10].
Astrocytes are also highly sensitive to glutamate [11–14]. Although astrocytes express ionotropic receptors [15] at the glutamatergic tripartite synapse, the astrocytic response depends mostly on presynaptic-released glutamate binding to metabotropic receptors. The active receptors raise intracellular levels of phospholipase-dependent inositol triphospate (IP3), which induces calcium waves mainly by means of the release of calcium from internal stores [13, 14, 16]. Depending on the status of intracellular biophysical parameters (such as the rate of calcium leakage from internal stores), the dynamics of these waves may encode information about external stimuli in amplitude and/or frequency modulation [17].
The results of astrocytic information processing define the feedback signal to tripartite synapses and to target cells located in encephalic microcirculation, leading to vasodilation or constriction. Several gliotransmitters released by astrocytes interact with receptors located at both pre- and postsynaptic neurons [4], including glutamate, d-serine [18], and ATP [19]. In this paper, we focus only on glutamate due its clear relation with cognitive functions. There is a causal relation between the dynamics of calcium waves in astrocytes and the corresponding rise of calcium entry in adjacent neurons. The first publications suggesting this relationship were published around 15 years ago [14, 20–23], and a number of those experiments indicated that the mechanism underlying such a response might include glutamate release from astrocytes and the consequent activation of ionotropic glutamatergic receptors at neuronal locations [14, 21–23]. Since then, this hypothesis has been corroborated.
In a remarkable finding, Fellin et al. [24] showed that astrocytic glutamate acts preferentially on the NR1/NR2B subtype of N-methyl-d-aspartate receptors (NMDAR). NMDAR are heteromeric complexes believed to comprise one or two NR1 plus one or two NR2 subunits [25]. There are at least four possible types of NR2 subunits (NR2A, NR2B, NR2C, and NR2D) which confer distinct kinetic properties to NMDAR [26].
The density of NMDAR subtypes along the postsynaptic membrane is not regular. At the end of synaptogenesis, fast kinetic NR1/NR2A NMDAR are placed mainly in synapses, while the majority of slow kinetic NR1/NR2B NMDAR is distributed in extrasynaptic locations. These latter receptors are the preferential target for the binding of the glutamate released by astrocytes [4, 24].
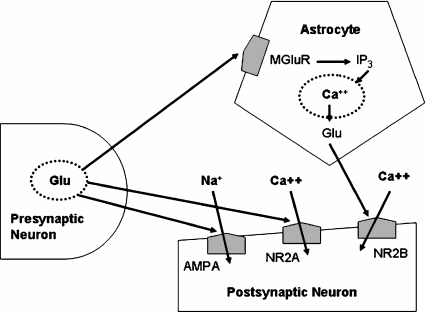
Activation of extrasynaptic NR1/NR2B NMDAR by astrocytic glutamate results in slow inward currents in the postsynaptic neuron [24, 27, 28]. These currents have higher amplitude and slower kinetics than the influx currents mediated by NR1/NR2A NMDAR, showing larger rise and decay times [4, 24]. One single astrocyte induces slow inward currents simultaneously in several neighboring cells (Fig. 1), leading to local neuronal coordination that may support cognitive processing [29].
Fig. 1.
The glutamatergic tripartite synapse. Glutamate (Glu) released by the presynaptic neuron binds with both astroglial (MGluR) and postsynaptic neuronal (AMPA and NR2A) receptors. MGluR activate the inositol triphosphate (IP3) pathway, inducing the release of calcium ions from internal stores (mitochondria and endoplasmatic reticulum) to prompt Glu release. Astroglial Glu binds mostly with neuronal NMDA receptors containing the NR2B subunit (NR2B), causing calcium ion entry (slow inward currents) and then sustaining excitatory activity of the postsynaptic neuron
Astrocytes and perceptual conscious processing: a testable hypothesis
There are several possibilities of interpretation of putative biological and cognitive functions of astrocytes. As a functional partner in the tripartite synapse, astrocytes modulate both excitatory and inhibitory transmission between neurons, thus mediating Hebbian learning [30]. They also function as a “gatekeeper” by decreasing the transfer of information carried by spikes after a period of high-frequency presynaptic stimulation [31].
Astrocytic release of glutamate, when coupled to a postsynaptic depolarization, leads to long-term potentiation [32]. Gibbs et al. [33] showed that memory consolidation depends on astrocyte metabolism, and Caudle [34], in a theoretical approach, argues that ion channels located in astrocytes and their syncytium can store information for long periods of time more efficiently than neuronal synapses.
Astrocytes can coordinate the activity of a local population of neighboring neurons by synchronously releasing glutamate to the synapses located in their territorial domain [24, 26]. Such a synchronization may contribute to perceptual processing (as shown by Schummers et al. [35] and discussed below in this article) as well as to the genesis of pathological responses, as seen in epilepsy [36, 37]. Additionally, in a recent paper, Halassa et al. [38] proved that astrocytes can modulate behavior by playing a role in sleep regulation.
Nevertheless, in the growing number of studies addressed to this novel field, little or no attention has been dedicated to the possible role of astrocytes in conscious processing. Based on an analysis of the above findings, we make a testable hypothesis in this direction: the informational content of perceptual conscious processes is embodied in astrocytic calcium waves. This idea was originally proposed as “the astrocentric hypothesis” by Robertson [1] and developed into a biophysical model by Pereira Jr. [39].
Possible tests of the astrocentric hypothesis can be made by means of new technologies, such as two-photon laser microscopy combined with labeling of calcium receptors with fluorescent dyes and genetic engineering of astroglial proteins. In few years, we predict that experimental results will allow the testing of the following hypotheses:
whether cognitive/conscious processing elicits measurable astrocytic responses and if they are more fine-tuned than neuronal responses (as advanced by Schummers et al. [35]), or
whether knocking out or enhancement of the expression of proteins supporting specific astrocytic functions in transgenic animals causes the impairment or improvement of cognitive/conscious functioning (this kind of result can be obtained by applying to astroglial processes the methodology used by Wulff et al. [40] for the study of GABAergic transmission).
At this moment, our argumentation in favor of the hypothesis is theoretical, but based on two important empirical findings about brain correlates of conscious processing: blood-oxygen-level-dependent (BOLD) functional magnetic resonance imaging (fMRI) and oscillatory synchrony of graded and action potentials.
The BOLD fMRI signal, astrocytes, and cognitive processing
Functional neurovascular coupling is a local increase in cerebral blood flow resulting from the dilation of microcirculation vessels in an area of elevated neuronal activity. This response provides an adequate supply of oxygen and energetic substrates to active neurons proportionally to their degree of activation.
BOLD fMRI is an imaging technique relying on such activity-dependent hemodynamic changes. In brief, the BOLD signal measures changes in the relative concentration of oxygenated and deoxygenated blood. Upon neural activation during cognitive tasks, in spite of the expected elevation in deoxygenated blood level, there is an increase in local cerebral blood flow. This increase occurs in such a degree that the delivery of oxygenated blood surpasses oxygen consumption in those more active areas. As a consequence, the BOLD signal is enhanced [41]. In the last two decades, this tool has been widely used in experiments for the mapping of brain activity and has greatly improved our comprehension of cognitive processes.
What kind of neural event underlies BOLD fMRI? Answers to this question have been provided by experimental approaches that simultaneously register spontaneous or sensory-driven BOLD signals and extracellular field potentials at the same neural site. When a microelectrode is placed at a neural site, the measured extracellular field potential may represent distinct kinds or neuronal activity (for a review, see [41, 42]). Single-unit activity is registered if the tip of the microelectrode is inserted close enough to the soma or axon of a neuron so that the spiking activity of that neuron and only a few of its neighbors is recorded, drowning out any other currents driven by subthreshold processes in the cell or even spikes from distant cells.
If a microelectrode is placed out of the field of the spike-generating source, the totality of potentials in a spherical domain—with the tip of the electrode in the center—will be recorded. This signal is composed of the weighted sum of synaptic (dendritic, subthreshold) events and spikes of hundreds of neurons. The resulting signal can be then separated using band-pass filters. Multiple-unit spiking activity is obtained by applying a high-pass filter with a cutoff of 400 Hz. Recordings obtained with low-pass filtering (cutoff <200 Hz) reflect synaptic events, and the waveform produced is the Local Field Potential (LFP). While spiking signals correspond to the output of a neural population, LFPs are composed of local dendrosomatic integrative events as well as processes generated by input information [42].
In 2001, Logothetis et al. [43] reported a strong correlation between the BOLD signal and LFP, but not with other neural events. When compared to single- and multi-unit spiking activity, LFPs are better predictors of the BOLD response. Moreover, in visual-stimulus-driven trials, spiking activity showed strong adaptation, returning to baseline level soon after stimulus onset even in trials when stimulus presentation lasted for many seconds. Conversely, both the BOLD signal and the LFP remain both elevated for the whole duration of stimulus presentation. So, even though the neural basis of BOLD fMRI is not yet fully understood, there is evidence that the LFP response underlies it, suggesting that this correlate of cognition reflects input and local processing at a neural site.
How are LFPs linked to hemodynamic changes observed in neurovascular coupling? Currently, there is an agreement about the critical role of astrocytes in such processes. Zonta et al. [44] showed in cortical slices that arteriolar dilation following neuronal activation is dependent on astrocytic calcium waves: inhibition of such a signal decreases vasodilation, while direct stimulation of astrocytes results in vascular expansion. Others authors reported similar responses in vitro in cortical [45] and retinal [46] tissue and in vivo in the somatosensory cortex [47] and primary visual cortex [35]. The fact that astrocytes release vasoactive substances such as nitric oxide and metabolites of arachidonic acid [4] reinforces their likely role in neurovascular coupling.
Today, there is conclusive evidence that the BOLD signal is a direct consequence of astrocytic activity, since astrocytes—not neurons—drive the hemodynamic changes that supply energetic demands of more active brain areas. In perceptual processes, sensory (‘bottom-up’) information reaches the central nervous system and elicits (with internal, top-down signaling) the formation of LFPs correlated with BOLD fMRI signals.
Additionally, in the interpretation of neuroimaging results, some authors—using the “subtraction” methodology to contrast brain activity in the presence and absence of a stimulus—have suggested a close correlation of active brain circuits with conscious processing, as proposed in the “mind-reading” paradigm (see [48]). For instance, Kamitani and Tong [49] investigated the perception of edge orientation, an important visual feature. Using statistical algorithms to classify brain states, they found that “ensemble fMRI signals in early visual areas could reliably predict in individual trials which of eight stimulus orientations the subject was seeing” (p. 679).
Prototypes are information patterns emerging from the activity of a cognitive system interacting with the environment, as proposed—among others—by Rosch [50] and Gärdenfors [51, 52]. The “mind-reading” research paradigm has contributed to show that prototypical cognitive patterns, such as hunger, thirst, sensations of hot and cold, pain and pleasure, visual features such as color, shape and movement, auditory features such as tone, intensity and timbre, etc., can be detected with an adequate use of fMRI. However, it is well known that fMRI cannot detect conscious episodes, which are composed by such prototypes, but including supplementary pattern selection and integration into a unitary meaningful episode (this process is called “the binding problem” in the consciousness studies literature; see below).
As BOLD fMRI signals correlate with the processing of conscious prototypes, we reason that the LFP embodies information patterns that, in a given moment, are ready and able to compose conscious episodes. According to our hypothesis, these patterns become conscious only when they are integrated by astrocytic calcium waves. Considering the experimental correlation of hemodynamic responses with LFP, we propose that the transfer of LFP patterns to astrocytes and further conscious information processing occurs only when an adequate state of global neuronal synchronization is reached.
Global synchrony and the “binding problem”
The stimulus-driven hemodynamic response, obtained in contexts when perceptual and cognitive tasks are performed, also correlates with the phenomenon of synchrony (see [53]). Synchronization of neural activity involves both graded and action potentials, or—in other words—oscillatory and spike synchronies (see [54]). Graded potentials are generated by the flow of ions across ligand and voltage-gated channels in dendrites and soma of the neuron, while action potentials are generated by the flow of ions across sequentially located voltage-gated channels in the axon. The well-known relation between them is that action potentials are triggered in the axon hillock when summed graded potentials reach a threshold.
The term “oscillatory synchrony” refers to recordings of graded potentials in a population of neurons, indicating that peaks of activity are time-locked. Of course, the presence of oscillatory synchrony in a population of neurons induces the production of synchronized neuron firing by the same population (see [55]). However, the spikes produced by these neurons, although synchronized at their origin, may arrive not synchronized at their targets, since those cells may be located in different parts of the brain and body.
It is likely that the co-occurrence of both phenomena—synchrony of graded and action potentials in a neuronal population—form positive feedback loops in recurrent neural circuits including the same population. For instance, a sequence of action potentials from neuron A to B cause graded potentials in B, which sends a sequence of action potentials to A. The local integration of graded potentials is expressed in the resulting LFP. Synchronization of excited neurons belonging to the LFP increases synchronized firing, impacting other neurons that share recurrent connections with the first ones.
However, there is a problem for the maintenance of synchrony during this kind of process. Since different neurons have different axonal lengths and respective conductance rates, interactions in a large population composed of locally synchronized circuits may lead to two scenarios (see [56]):
a mechanism of temporal tuning guarantees the time locking of circuits, leading to a resonance that induces robust global synchronization;
in the absence of such a tuning process, electromagnetic waves of the local synchronized circuits cancel each other, precluding the formation of global synchrony.
Therefore, the existence of global synchrony in the brain indicates the operation of a tuning mechanism that accounts for the coordination of local circuits. Izhikevich [56] introduced the concept of polychronization to refer to the time locking in ensembles of recurrent local circuits, allowing the emergence of global synchrony.
Local circuits have being previously conceptualized by Abeles [57] as “synfire chains” (chains of neurons that fire together). Polychrony is the time locking of many synfire chains. In this framework, the occurrence of synchrony is considered to be not trivial, but rather so “rare and difficult to occur by chance that when it happens, even transiently in a small subset of the network, it would signify something important” (Izhikevich [56], p. 269).
An important question that raised the interest of cognitive brain scientists in the last two decades regards the role of synchrony in conscious binding. The so-called binding problem [58] in consciousness studies refers to a lack of understanding of how the brain binds information patterns processed in spatially distributed brain circuits into a unitary pattern that is experienced as a coherent episode by the conscious subject. As the occurrence of global brain synchronization, mostly in the range of high-frequency and low-amplitude waveforms of electroencephalogram (EEG), correlates well with conscious perceptual processing (see [59]), a widespread belief that synchronization of neural activity contributes to conscious binding was formed. However, as far as we know, the role of global synchrony in conscious binding has not been completely explained (for advances in this field of research, see, e.g., [60] and [61]).
A partial explanation was given by Bienenstock [62] that “dynamic binding of various features of a stimulus corresponds to the synchronization of synfire waves propagating along distinct chains. The synchronization is induced by weak reentrant synaptic coupling between these chains” ([56], p. 271). It is not clear, in this explanation, how synchrony integrates information contents embodied in a variety of local circuits. Possibly, synchrony itself is not the computational medium that integrates the content that appears to the conscious subject as a coherent episode, but only an intermediary step necessary to cause an effect in another medium.
Reaching the threshold to elicit coherent calcium waves in one astrocyte
Astrocytes do not have direct access to sensory information. Informationally, they depend on neurons. However, neurons do not seem to perform all the integration of information that conscious processing requires, as shown by the patent difficulty of solving the “binding problem” in the context of neuroscience. Neurons are specialized to receive and process specific kinds of patterns and operations, but not able to put them all together. The neuron is, computationally, a filter that converts analog to digital-like information, while astrocytes can be described as being like a hub able to integrate patterns from around 100,000 to 140,000 synapses. They integrate excitatory inputs received from neurons connected to their tips.
The explanation we propose for the “binding problem” is limited to perceptual processing. In this explanation, high-frequency low-amplitude synchrony has the role of boosting communication between sensory neural LFP and astrocytes, inducing the formation of coherent calcium waves that embody the information content of a multimodal “conscious scene” [63]. This communication process involves two steps:
locally eliciting coherent calcium waves in one astrocyte and
eliciting global coherent calcium dynamics in an astrocytic network that involves several spatially separated and functionally distinct brain circuits.
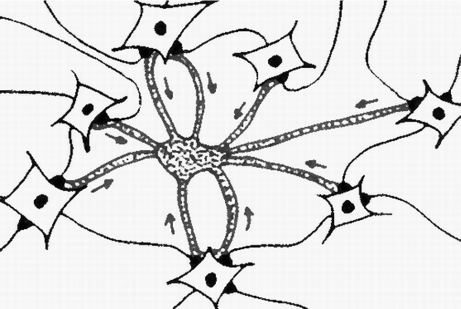
The induction of coherent calcium waves in one astrocyte participating in an active ensemble of glutamatergic tripartite synapses requires an adequate level of presynaptic activity to excite the astrocyte to the point of eliciting coherent calcium waves that integrate information from all input neurons. This assumption was modeled by Nadkarni and Jung [64], concluding that the generation of astrocytic calcium waves depends on the intensity of the input signal, which must be sufficient to raise calcium concentration above a self-amplification threshold. We propose that local neural synchrony has a central role in this process (Fig. 2).
Fig. 2.
Induction of an astrocyte calcium wave by means of coordinated excitatory input from a surrounding neuronal population. Arrows indicate concentric excitation promoted by synchronized neurons impacting the target astrocyte
For instance, in perceptual processes, sensory information would be first transduced to cortical LFPs and then, by means of excitatory connections (glutamate release by the presynaptic neuron and its binding with astroglial metabotropic receptors), transferred to astrocytes. This explanation requires astrocytes in sensory cortex to be responsive to sensory information mediated by neurons. Recent findings by Schummers et al. [35] showed that astrocytes in the visual cortex of ferrets are more sharply tuned to visual stimuli than neurons. Astrocytes’ responses arise from neuronal presynaptic activity, but the authors suggest that such an exquisite tuning is due to an existing threshold level that must be crossed to elicit calcium waves. Such a level would be reached only with neuronal activity coordination. In fact, when synaptic transmission was only slightly decreased, a significant fall in the astrocytes’ responses was observed [35].
The above finding gives empirical support to mathematical and computational models of the generation of calcium waves in one astrocyte participating in an ensemble of tripartite synapses [31]. Further hypotheses about the threshold for induction of informationally coherent calcium waves in astrocytes can be formulated in the framework provided by the Li–Rinzel model developed by De Pittà et al. [17]. This model covers the process by which external (e.g., sensory) signals modulate IP3 activity, imprinting a form on calcium release and resulting waves. The authors identify three possibilities for information encoding in astrocytic calcium waves: amplitude modulation (AM), frequency modulation (FM), and mixed (AFM). AM begins after a bifurcation when an unstable state of IP3 activity inducing calcium release reaches a fixed point. FM occurs when calcium is kept at low levels, and AFM depends on a complex bifurcation dynamics.
Combining our approach to the role of neuronal synchrony for the formation of astrocytic calcium waves with the above findings, we suggest that the summation process that results from synchronized multiple inputs is more likely to induce AM than FM. Therefore, our proposed threshold would correspond to the fixed point that is crossed in the process of AM generation. In this interpretation, FM would serve to other functions parallel to conscious processing, as the triggering of glutamate release back to neurons. AFM may also have a role in conscious processing by establishing a connection between the conscious information content and the triggering of glutamate release to neurons, which can control learning and memory formation processes.
Local synchrony in several frequencies—depending on the properties of specialized circuits, e.g., theta frequency in the hippocampus—can boost neuro-astroglial communication in each brain region, allowing the participation of target astrocytes in a larger kind of process that we discuss in the next sections: large-scale integration of information supporting conscious processing.
Phase-locked alpha, beta and gamma synchrony, and conscious processing
All possible brain frequencies can occur together in the EEG spectrum [65]. It is well known that a “clean” recording, highlighting a given frequency range, requires the usage of filters to be obtained. When analyzing the biophysical conditions for conscious processing, the crucial parameter is the EEG power spectrum: dominant high-frequency and low-amplitude ranges correlate positively with conscious processing, while dominant low-frequency and high-amplitude waves are negatively correlated (e.g., the latter are registered during dreamless slow-wave sleep and loss of consciousness in seizures absence epilepsy; see [66]).
Most of the evidence about the correlation of high-frequency oscillatory synchrony and conscious binding was obtained with recordings of gamma activity [67–69]. Recently, a new framework has been proposed that focuses on phase locking of gamma with alpha and beta rhythms [70]. In this new framework, a more dynamical picture can be formed, where, for example, gamma is considered as being related to the stabilization of attentional focus on perceptual patterns, while alpha rhythms mediate transitions between different patterns [71]. In mnemonic processes, it is also possible that theta rhythms in the medial temporal lobe are phase-locked with the higher frequency rhythms found in other regions of neocortex and eventually coordinate them (see [72]).
Within our framework, we can explain the putative functions of different rhythms in conscious processing. The frequency of coordinated spiking determines the amount of glutamate release from neurons to astrocytes. Accordingly, global delta synchrony is well correlated to unconsciousness. On the other side of the spectrum, dominant global gamma synchrony relates to the stabilization of patterns in conscious perceptual processing. We interpret these facts as meaning that in gamma, the amount of glutamatergic release reaches the threshold necessary to produce coherent calcium waves in astrocytes, while in delta, this level of activity remains subthreshold.
Interpretation of brain-wide EEG, in our proposed framework, assumes that phase-locked high-frequency rhythms operate together to boost neuro-astroglial communication, transferring LFP patterns to astrocytic activity where these patterns are integrated, forming conscious episodes. The dominance of one of the high-frequency rhythms, or even the dominance of theta, indicates the mode of operation of conscious processing: perceptual mode, with attention to external stimulation; creative mode, when the subject attends to his/her own imagination; mnemonic mode, when the subject retrieves previously learned and stored patterns to conscious attention; executive mode, when attending to a strategy to reach a planned goal, etc. These operations, as well as the rhythms that support them, cannot be considered as being separated from each other; they form an integrated system supporting conscious processes.
Developing the interpretation, we find that theta may be sufficient for memory consolidation and retrieval, but not for perceptual processing; alpha may be sufficient for the generation of a state of consciousness poor of sensory content (and sometimes rich of images), while beta and gamma may be adequate for prompting the transfer of sensory content from neurons to astrocytes (gamma possibly required for faster transfer of information, as in visual processing).
Integration of conscious information in the astrocytic syncytium
The information processing made by astrocytes is a complex and—at this moment—speculative issue (see [6] for a review of the state of art in this area). According to the astrocentric hypothesis, such a processing supports the formation of conscious states. Pereira Jr. [39] proposed that astrocytes perform the function of integration or “binding” of distributed LFP patterns into a quantum-like macro coherent state that putatively supports conscious episodes.
In our proposed explanation, the occurrence of local synchrony does not imply conscious processing, since the output of activity of these neurons may diverge to different brain areas. Global synchrony (at least within limited brain regions) is required for conscious binding in this model because only in this case there is an effective convergence of excitatory activity from neurons to astrocytes by means of the simultaneous release of glutamate from the axon terminals to astroglial membrane receptors in distributed brain regions.
A detailed mathematical model would be useful to understand how this kind of complex system works. The process of information integration necessary to support conscious binding has already been studied [73, 74] using methods of statistical physics and mathematical information theory as the measure of informational content and its integration in quasi-closed systems. The application of these methods to systems composed of neurons interconnected by astrocytes would provide a better understanding of how spatially distributed patterns merge, forming a coherent calcium ion waveform. This approach would provide evidence about the capacity of this system to support the formation of a conscious scene, making it available for further processing in neuronal networks and coordination of adaptive behavior in the perceived environment.
A sketch of one possible explanation of conscious binding by a population of interconnected astrocytes would be the following. Each astrocyte has an internal reservoir of ions in the endoplasmic reticulum where the ions can interact and become entangled. Here, we consider “entanglement” as referring to the mutual dependence of the superposed electronic states in an ionic population, i.e., the internal state of each ion is dependent on the state obtained by the other ions. The idea of entanglement in this context has been developed theoretically in [39] and [75] and is partially supported by the trapped-ion quantum computing experiments of Kielpinski et al. [76].
When an astrocyte is excited, some of the ions are pumped to the region of interaction with the excitatory neuron. At this moment, according to our hypothetical picture, the pattern of the calcium wave elicited by neuronal excitation is affected electromagnetically by the LFP and by ions released by neurons into the intracellular space. In essence, we picture a scenario where the LFP signals play the roles of magnets and lasers in the model of Kielpinski et al. [76], in that the near electromagnetic field of the LFP affects nearby charged particles (the calcium ions). It is possible that in our picture, the vibrational properties of calcium, discussed in the quantum information and computing literature [77], could lead to resonance with nearby LFP signals, and the resonating calcium ions could transfer this resonance to their entangled partners still trapped in the reticulum. This proposed model is quite speculative, of course, but tantalizing nonetheless. Future experimental and theoretical studies will be needed in order to more deeply explore these ideas, and we hope that the present discussion will serve as a spur to such studies.
Biophysical models of calcium dynamics in intracellular domains predict a complexity of amplitude- and frequency-modulated wave patterns [17, 78], leaving open the possibility of quantum effects in the ion population (for a brief review of proposals of “hot” ion-trap quantum computing, see [39]). Registering the patterns of the ions trapped in the reticulum would allow the scientific observer to distinguish integrated patterns—each one corresponding to a subjectively experienced conscious scene—assuming there were a technology to accomplish this task. Recent advances in two-photon microscopy combined with fluorescent tagging of calcium receptors has allowed important discoveries about the concentration of astrocytic calcium in vivo [35, 79], but this technology does not allow the detection of calcium waveforms. Measuring these waveforms would require a new technology, as the adaptation of ultraviolet lasers for in vivo brain calcium imaging.
Any astrocyte, in any anatomic location, when impacted by high-frequency neural oscillatory synchrony to the point of crossing a calcium wave activation threshold, would participate in the conscious function. Naturally, high-frequency neural synchrony does not happen equally in the whole brain, but mostly in the thalamo-cortical system. The selection of astrocyte populations supporting conscious processing at each moment is presumably provided by the selectivity of neuronal networks. Therefore, the cell population that is more likely to be participating in the formation of a conscious scene is constituted by astrocytes connected to neurons that process the sensory patterns that compose the scene.
It is important to note that calcium waves can propagate non-linearly in the astrocytic syncytium. This dynamics was called “saltatory” [80]. We predict that when neural global synchrony occurs, there is a coupling of waves in all astrocytes impacted by the synchrony. Possibly, this kind of global effect occurs only for conscious events. Saltatory dynamics is like a “domino effect” between astrocytes, possibly mediated by lines of ions that fill gap junctions between the cells. Making the analogy, if domino pieces are very close to each other, the fall of the last domino occurs almost simultaneously to the movement of the first one. In the astrocytic syncytium, these dynamics imply that the movement of ionic populations from astrocyte to astrocyte is not necessary to generate the global dynamics. If it were necessary, the putative function we ascribe to brain-wide neuro-astroglial dynamics—the support of conscious binding—would be not viable, since a linear calcium wave between millions of astrocytes would take too much time to occur, compared to the timing of conscious processing.
Concluding remarks
In one century of neuroscience, scientists have failed to find fine-grained isomorphism between neuron activity and the contents of consciousness. All they have found is the coarse-grained correlation between the activation of some brain areas and some kinds of content. Recent availability of a technology for in vivo measurement of activity of another kind of cell, the astrocyte, offers an alternative for the search of isomorphism (or, at least, closer homeomorphism) between brain and conscious activity.
Assuming that astrocytes are involved in conscious processing, how would conscious patterns be “encoded” or embodied in their physiological activity? Our suggestion is that the patterns are embodied in calcium waves generated by glutamatergic excitation from neurons to astrocyte membrane receptors. However, not all astrocytic activities are assumed to support conscious processing. According to the proposal made here, there is a threshold for the activation of coherent calcium dynamics that enter the conscious spotlight.
In normal physiological conditions, we suggested that the presence of global high-frequency synchronization is necessary to induce supra-threshold coherent local calcium waves and global “saltatory” calcium dynamics. This result can be obtained in brain tissues that contain tripartite synapses with an adequate architecture for the summation of neuron-to-astrocyte inputs. Supporting conscious processes is surely not a function carried by all astrocytes of a living system. Only cell populations in the right set of biophysical conditions—as those tentatively described here—would carry that function.
Once a conscious coherent process is formed in the brain’s astrocytic network, the result of information integration performed by this subsystem can provide feedback to brain activity, inducing effects on perceptual, cognitive, endocrine, and motor systems. Such a feedback is carried by slow inward calcium currents from astrocytes to pre- and postsynaptic neurons, causing depolarizations that impact on brain activity and behavior. In cases of abnormal activity, astrocytes are involved in epileptic seizures [37, 78], schizophrenia [81], and depression [82], among other neurological and psychiatric phenomena [83], most of them accompanied by changes in conscious activity.
In a new study, we are going to review the variety of NMDAR involved in the mediation of slow inward currents and intra-neuronal targets of related signaling pathways. These mechanisms are important for the understanding of how the participation of astrocytes in learning and memory consolidation processes [33, 84] provides the possibility of “approval” (by means of further neuronal potentiation) or “disapproval” (by means of neuronal depression) of evoked LFP patterns.
Acknowledgements
The authors thank the Brazilian National Research Council (CNPQ) for a grant conceded to APJ; Dr. Bernard Baars, for discussion of an early draft of this paper in his Advanced Seminar (an activity of Consciousness: the Webcourse, supported by the Univ. of Arizona), and two anonymous reviewers for their constructive criticisms and suggestions.
Contributor Information
Alfredo Pereira, Jr., Email: apj@ibb.unesp.br
Fábio Augusto Furlan, Email: fabioaugustofurlan@yahoo.com.br.
References
- 1.Robertson JM. The astrocentric hypothesis: proposed role of astrocytes in consciousness and memory formation. J. Physiol. (Paris) 2002;96:251–255. doi: 10.1016/S0928-4257(02)00013-X. [DOI] [PubMed] [Google Scholar]
- 2.Perea G, Araque A. Properties of synaptically evoked astrocyte calcium signal reveal synaptic information processing by astrocytes. J. Neurosci. 2005;25:2192–2203. doi: 10.1523/JNEUROSCI.3965-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Halassa MM, Fellin T, Takano H, Dong JH, Haydon PG. Synaptic islands defined by the territory of a single astrocyte. J. Neurosci. 2007;27:6473–6477. doi: 10.1523/JNEUROSCI.1419-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Haydon PG, Carmignoto G. Astrocyte control of synaptic transmission and neurovascular coupling. Physiol. Rev. 2006;86:1009–1031. doi: 10.1152/physrev.00049.2005. [DOI] [PubMed] [Google Scholar]
- 5.Bushong EA, Martone ME, Jones YZ, Ellisman MH. Protoplasmic astrocytes in CA1 stratum radiatum occupy separate anatomical domains. J. Neurosci. 2002;22:183–192. doi: 10.1523/JNEUROSCI.22-01-00183.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Agulhon C, Petravicz J, McMullen AB, Sweger EJ, Minton SK, Taves SR, Casper KB, Fiacco TA, McCarthy KD. What is the role of astrocyte calcium in neurophysiology? Neuron. 2008;59:932–946. doi: 10.1016/j.neuron.2008.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Araque A, Parpura V, Sanzgiri RP, Haydon PG. Tripartite synapses: glia, the unacknowledged partner. Trends Neurosci. 1999;22:208–215. doi: 10.1016/S0166-2236(98)01349-6. [DOI] [PubMed] [Google Scholar]
- 8.Araque A, Martín ED, Perea G, Arellano JI, Buño W. Synaptically released acetylcholine evokes Ca2+ elevations in astrocytes in hippocampal slices. J. Neurosci. 2002;22:2443–2450. doi: 10.1523/JNEUROSCI.22-07-02443.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kang J, Jiang L, Goldman SA, Nedergaard M. Astrocyte-mediated potentiation of inhibitory synaptic transmission. Nat. Neurosci. 1998;1:683–692. doi: 10.1038/3684. [DOI] [PubMed] [Google Scholar]
- 10.Perea G, Araque A. Properties of synaptically evoked astrocyte calcium signal reveal synaptic information processing by astrocytes. J. Neurosci. 2005;25:2192–2203. doi: 10.1523/JNEUROSCI.3965-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cornell-Bell AH, Finkbeiner SM, Cooper MS, Smith SJ.Glutamate induces calcium waves in cultured astrocytes: long-range glial signaling Science 1990247470–473.1990Sci...247..470C 10.1126/science.1967852 [DOI] [PubMed] [Google Scholar]
- 12.Charles AC, Merrill JE, Dirksen ER, Sanderson MJ. Intercellular signaling in glial cells: calcium waves and oscillations in response to mechanical stimulation and glutamate. Neuron. 1991;6:983–992. doi: 10.1016/0896-6273(91)90238-U. [DOI] [PubMed] [Google Scholar]
- 13.Porter JT, McCarthy KD. Hippocampal astrocytes in situ respond to glutamate released from synaptic terminals. J. Neurosci. 1996;16:5073–5081. doi: 10.1523/JNEUROSCI.16-16-05073.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pasti L, Volterra A, Pozzan T, Carmignoto G. Intracellular calcium oscillations in astrocytes: a highly plastic, bidirectional form of communication between neurons and astrocytes in situ. J. Neurosci. 1997;17:7817–7830. doi: 10.1523/JNEUROSCI.17-20-07817.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Verkhratsky A, Steinhauser C. Ion channels in glial cells. Brain Res. Rev. 2000;32:380–412. doi: 10.1016/S0165-0173(99)00093-4. [DOI] [PubMed] [Google Scholar]
- 16.Reyes RC, Parpura V. The trinity of Ca2 + sources for the exocytotic glutamate release from astrocytes. Neurochem. Int. 2009 doi: 10.1016/j.neuint.2008.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pittà M, Volman V, Levine H, Pioggia G, Rossi D, Ben-Jacob E. Coexistence of amplitude and frequency modulations in intracellular calcium dynamics. Phys. Rev. E. 2008;77:030903-R. doi: 10.1103/PhysRevE.77.030903. [DOI] [PubMed] [Google Scholar]
- 18.Mothet JP, Pollegioni L, Ouanounou G, Martineau M, Fossier P, Baux G.Glutamate receptor activation triggers a calcium-dependent and SNARE protein-dependent release of the gliotransmitter d-serine Proc. Natl. Acad. Sci. U. S. A. 20051025606–5611.2005PNAS..102.5606M 10.1073/pnas.0408483102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Newman EA. Glial cell inhibition of neurons by release of ATP. J. Neurosci. 2003;23:1659–1666. doi: 10.1523/JNEUROSCI.23-05-01659.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Charles AC. Glia-neuron intercellular calcium signaling. Dev. Neurosci. 1994;16:196–206. doi: 10.1159/000112107. [DOI] [PubMed] [Google Scholar]
- 21.Parpura V, Basarsky TA, Liu F, Jeftinija K, Jeftinija S, Haydon PG.Glutamate-mediated astrocyte-neuron signalling Nature 1994369744–747.1994Natur.369..744P 10.1038/369744a0 [DOI] [PubMed] [Google Scholar]
- 22.Hassinger TD, Atkinson PB, Strecker GJ, Whalen LR, Dudek FE, Kossel AH, Kater SB. Evidence for glutamate-mediated activation of hippocampal neurons by glial calcium waves. J. Neurobiol. 1995;28:159–170. doi: 10.1002/neu.480280204. [DOI] [PubMed] [Google Scholar]
- 23.Bezzi P, Carmignoto G, Pasti L, Vesce S, Rossi D, Rizzini BL, Pozzan T, Volterra A.Prostaglandins stimulate calcium-dependent glutamate release in astrocytes Nature 1998391281–285.1998Natur.391..281B 10.1038/34651 [DOI] [PubMed] [Google Scholar]
- 24.Fellin T, Pascual O, Gobbo S, Pozzan T, Haydon PG, Carmignoto G. Neuronal synchrony mediated by astrocytic glutamate through activation of extrasynaptic NMDA receptors. Neuron. 2004;43:729–743. doi: 10.1016/j.neuron.2004.08.011. [DOI] [PubMed] [Google Scholar]
- 25.Bartlett TE, Bannister NJ, Collet VJ, Dargan SL, Massey PV, Bortolotto ZA, Fitzjohn SM, Bashir ZI, Collingridge GL, Lodge D. Differential roles of NR2A and NR2B-containing NMDA receptors in LTP and LTD in the CA1 region of two-week old rat hippocampus. Neuropharmacology. 2007;52:60–70. doi: 10.1016/j.neuropharm.2006.07.013. [DOI] [PubMed] [Google Scholar]
- 26.Dingledine R, Borges K, Bowie D, Traynelis SF. The glutamate receptor ion channels. Pharmacol. Rev. 1999;51:7–61. [PubMed] [Google Scholar]
- 27.Araque A, Parpura V, Sanzgiri RP, Haydon PG. Glutamate-dependent astrocyte modulation of synaptic transmission between cultured hippocampal neurons. Eur. J. Neurosci. 1998;10:2129–2142. doi: 10.1046/j.1460-9568.1998.00221.x. [DOI] [PubMed] [Google Scholar]
- 28.Parri HR, Gould TM, Crunelli V. Spontaneous astrocytic Ca2+ oscillations in situ drive NMDAR-mediated neuronal excitation. Nat. Neurosci. 2001;4:803–812. doi: 10.1038/90507. [DOI] [PubMed] [Google Scholar]
- 29.Angulo MC, Kozlov AS, Charpak S, Audinat E. Glutamate released from glial cells synchronizes neuronal activity in the hippocampus. J. Neurosci. 2004;24:6920–6927. doi: 10.1523/JNEUROSCI.0473-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fellin T. Communication between neurons and astrocytes: relevance to the modulation of synaptic and network activity. J. Neurochem. 2009;108:533–544. doi: 10.1111/j.1471-4159.2008.05830.x. [DOI] [PubMed] [Google Scholar]
- 31.Volman V, Ben-Jacob E, Levine H.The astrocyte as a gatekeeper of synaptic information transfer Neural Comput. 200719303–326.1121.920182278391 10.1162/neco.2007.19.2.303 [DOI] [PubMed] [Google Scholar]
- 32.Perea G, Araque A.Astrocytes potentiate transmitter release at single hippocampal synapses Science 20073171083–1086.2007Sci...317.1083P 10.1126/science.1144640 [DOI] [PubMed] [Google Scholar]
- 33.Gibbs ME, Hutchinson D, Hertz L. Astrocytic involvement in learning and memory consolidation. Neurosci. Biobehav. Rev. 2008;32:927–944. doi: 10.1016/j.neubiorev.2008.02.001. [DOI] [PubMed] [Google Scholar]
- 34.Caudle RM. Memory in astrocytes: a hypothesis. Theor. Biol. Med. Model. 2006;3:2. doi: 10.1186/1742-4682-3-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Schummers J, Yu H, Sur M.Tuned responses of astrocytes and their influence on hemodynamic signals in the visual cortex Science 20083201638–1643.2008Sci...320.1638S 10.1126/science.1156120 [DOI] [PubMed] [Google Scholar]
- 36.Tian G, Azmi H, Takano T, Xu Q, Peng W, Lin J, Oberheim N, Lou N, Wang X, Zielke H, Kang J, Nedergaard M. An astrocytic basis of epilepsy. Nat. Med. 2005;11:973–981. doi: 10.1038/nm1277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Silchenko AN, Tass PA.Computational modeling of paroxysmal depolarization shifts in neurons induced by the glutamate release from astrocytes Biol. Cybern. 20089861–74.1149.92008 10.1007/s00422-007-0196-7 [DOI] [PubMed] [Google Scholar]
- 38.Halassa M, Florian C, Fellin T, Munoz J, Lee S, Abel T, Haydon P, Frank M. Astrocytic modulation of sleep homeostasis and cognitive consequences of sleep loss. Neuron. 2009;61:213–219. doi: 10.1016/j.neuron.2008.11.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pereira A., Jr Astrocyte-trapped calcium ions: the hypothesis of a quantum-like conscious protectorate. Quantum Biosystems. 2007;2:80–92. [Google Scholar]
- 40.Wulff P, Goetz T, Leppä E, Linden AM, Renzi M, Swinny JD, Vekovischeva OY, Sieghart W, Somogyi P, Korpi ER, Farrant M, Wisden W. From synapse to behavior: rapid modulation of defined neuronal types with engineered GABAA receptors. Nat. Neurosci. 2007;10:923–929. doi: 10.1038/nn1927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Logothetis NK, Wandell BA. Interpreting the BOLD signal. Annu. Rev. Physiol. 2004;66:735–769. doi: 10.1146/annurev.physiol.66.082602.092845. [DOI] [PubMed] [Google Scholar]
- 42.Logothetis NK, Pfeuffer J. On the nature of BOLD fMRI contrast mechanism. Magn. Reson. Imaging. 2004;22:1517–1531. doi: 10.1016/j.mri.2004.10.018. [DOI] [PubMed] [Google Scholar]
- 43.Logothetis NK, Pauls J, Augath M, Trinath T, Oeltermann A.Neurophysiological investigation of the basis of the fMRI signal Nature 2001412150–157.2001Natur.412..150L 10.1038/35084005 [DOI] [PubMed] [Google Scholar]
- 44.Zonta M, Angulo MC, Gobbo S, Rosengarten B, Hossmann KA, Pozzan T, Carmignoto G. Neuron-to-astrocyte signaling is central to the dynamic control of brain microcirculation. Nat. Neurosci. 2003;6:43–50. doi: 10.1038/nn980. [DOI] [PubMed] [Google Scholar]
- 45.Filosa JA, Bonev AD, Nelson MT. Calcium dynamics in cortical astrocytes and arterioles during neurovascular coupling. Circ. Res. 2004;95:e73–e81. doi: 10.1161/01.RES.0000148636.60732.2e. [DOI] [PubMed] [Google Scholar]
- 46.Metea MR, Newman EA. Glial cells dilate and constrict blood vessels: a mechanism of neurovascular coupling. J. Neurosci. 2006;26:2862–2870. doi: 10.1523/JNEUROSCI.4048-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Takano T, Tian GF, Peng W, Lou N, Libionka W, Han X, Nedergaard M. Astrocyte-mediated control of cerebral blood flow. Nat. Neurosci. 2006;9:159–161. doi: 10.1038/nn1623. [DOI] [PubMed] [Google Scholar]
- 48.Haynes JD, Rees G. Decoding mental states from brain activity in humans. Nat. Rev. Neurosci. 2006;7:523–534. doi: 10.1038/nrn1931. [DOI] [PubMed] [Google Scholar]
- 49.Kamitani Y, Tong F. Decoding the visual and subjective contents of the human brain. Nat. Neurosci. 2005;8:679–685. doi: 10.1038/nn1444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Rosch E. Cognitive representations of semantic categories. J. Exp. Psychol. (Gen.) 1975;104:192–233. doi: 10.1037/0096-3445.104.3.192. [DOI] [Google Scholar]
- 51.Gärdenfors P. Conceptual Spaces: The Geometry of Thought. Cambridge: MIT Press; 2000. [Google Scholar]
- 52.Gärdenfors P. Conceptual spaces as a framework for knowledge representations. Mind Matter. 2004;2:9–27. [Google Scholar]
- 53.Niessing J, Ebisch B, Schmidt KE, Niessing M, Singer W, Galuske RA.Hemodynamic signals correlate tightly with synchronized gamma oscillations Science 2005309948–951.2005Sci...309..948N 10.1126/science.1110948 [DOI] [PubMed] [Google Scholar]
- 54.Jermakowicz WJ, Casagrande VA. Neural networks a century after Cajal. Brain Res. Rev. 2007;55:264–284. doi: 10.1016/j.brainresrev.2007.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Samonds JM, Bonds AB. Gamma oscillation maintains stimulus structure-dependent synchronization in cat visual cortex. J. Neurophysiol. 2005;93:223–236. doi: 10.1152/jn.00548.2004. [DOI] [PubMed] [Google Scholar]
- 56.Izhikevich E.Polychronization: computation with spikes Neural Comput. 200618245–282.1090.920062188057 10.1162/089976606775093882 [DOI] [PubMed] [Google Scholar]
- 57.Abeles M. Corticonics: Neural Circuits of the Cerebral Cortex. New York: Cambridge University Press; 1991. [Google Scholar]
- 58.Treisman A. Solutions to the binding problem: progress through controversy and convergence. Neuron. 1999;24:105–110. doi: 10.1016/S0896-6273(00)80826-0. [DOI] [PubMed] [Google Scholar]
- 59.Buzsáki G.The structure of consciousness Nature 20074462672007Natur.446..267B 10.1038/446267a [DOI] [PubMed] [Google Scholar]
- 60.Roskies AL. The binding problem. Neuron. 1999;24:7–9. doi: 10.1016/S0896-6273(00)80817-X. [DOI] [PubMed] [Google Scholar]
- 61.Seth AK, McKinstry JL, Edelman GM, Krichmar JL. Visual binding through reentrant connectivity and dynamic synchronization in a brain-based device. Cereb. Cortex. 2004;14:1185–1199. doi: 10.1093/cercor/bhh079. [DOI] [PubMed] [Google Scholar]
- 62.Bienenstock E.A model of neocortex Network: Comput. Neural Syst. 19956179–224.0826.92004 10.1088/0954-898X/6/2/004 [DOI] [Google Scholar]
- 63.Seth AK, Izhikevich E, Reeke GN, Edelman GM.Theories and measures of consciousness: an extended framework Proc. Natl. Acad. Sci. U. S. A. 200610310799–10804.2006PNAS..10310799S 10.1073/pnas.0604347103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Nadkarni S, Jung P.Spontaneous oscillations of dressed neurons: a new mechanism for epilepsy? Phys. Rev. Lett. 2003912681012003PhRvL..91z8101N 10.1103/PhysRevLett.91.268101 [DOI] [PubMed] [Google Scholar]
- 65.Basar E, Basar-Eroglu S, Karaka S, Schurmann M. Gamma, alpha, delta, and theta oscillations govern cognitive processes. Int. J. Psychophysiol. 1991;39:241–248. doi: 10.1016/S0167-8760(00)00145-8. [DOI] [PubMed] [Google Scholar]
- 66.Blumenfeld H, Taylor J. Why do seizures cause loss of consciousness? Neurosci. 2003;9:301–310. doi: 10.1177/1073858403255624. [DOI] [PubMed] [Google Scholar]
- 67.König P, Engel AK, Singer W.Relation between oscillatory activity and long-range synchronization in cat visual cortex Proc. Natl. Acad. Sci. U. S. A. 199592290–294.1995PNAS...92..290K 10.1073/pnas.92.1.290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Engel AK, Singer W. Temporal binding and the neural correlates of sensory awareness. Trends Cogn. Sci. 2001;5:16–25. doi: 10.1016/S1364-6613(00)01568-0. [DOI] [PubMed] [Google Scholar]
- 69.Melloni L, Molina C, Pena M, Torres D, Singer W, Rodriguez E. Synchronization of neural activity across cortical areas correlates with conscious perception. J. Neurosci. 2007;27:2858–2865. doi: 10.1523/JNEUROSCI.4623-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Palva S, Palva JM. New vistas for alpha-frequency band oscillations. Trends Neurosci. 2007;30:150–158. doi: 10.1016/j.tins.2007.02.001. [DOI] [PubMed] [Google Scholar]
- 71.Sandkühler S, Bhattacharya J.Deconstructing insight: EEG correlates of insightful problem solving PLoS One 20083e14592008PLoSO...3.1459S 10.1371/journal.pone.0001459 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Jensen O. Reading the hippocampal code by theta phase-locking. Trends Cogn. Sci. 2005;9:551–554. doi: 10.1016/j.tics.2005.10.003. [DOI] [PubMed] [Google Scholar]
- 73.Tononi G. An information integration theory of consciousness. BMC Neurosci. 2004;5:42. doi: 10.1186/1471-2202-5-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Tononi G. Consciousness, information integration, and the brain. Prog. Brain Res. 2005;150:109–126. doi: 10.1016/S0079-6123(05)50009-8. [DOI] [PubMed] [Google Scholar]
- 75.Rocha A, Massad E, Pereira A., Jr . The Brain: From Fuzzy Grammar to Quantum Computing. Berlin: Springer; 2005. [Google Scholar]
- 76.Kielpinski D, Monroe C, Wineland DJ.Architecture for a large-scale ion-trap quantum computer Nature 2002417709–711.2002Natur.417..709K 10.1038/nature00784 [DOI] [PubMed] [Google Scholar]
- 77.Hughes RJ, James DFV, Gomez JJ, Gulley MS, Holzscheiter MH, Kwiat PG, Lamoreaux SK, Peterson CG, Sandberg VD, Schauer MM, Simmons CM, Thorburn CE, Tupa D, Wang PZ, White AG. The Los Alamos trapped ion quantum computer experiment. Prog. Phys. 1998;46:329–361. [Google Scholar]
- 78.Reyes RC, Parpura V. Models of astrocytic Ca2+ dynamics and epilepsy. Drug Discov. Today. 2008;5:13–18. doi: 10.1016/j.ddmod.2008.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Hirase H, Qian L, Barthó P, Buzsáki G. Calcium dynamics of cortical astrocytic networks in vivo. PLoS Biol. 2004;4:e96. doi: 10.1371/journal.pbio.0020096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Roth BJ, Yagodin SV, Holtzclaw L, Russell JT. A mathematical model of agonist-induced propagation of calcium waves in astrocytes. Cell Calcium. 1995;17:53–64. doi: 10.1016/0143-4160(95)90102-7. [DOI] [PubMed] [Google Scholar]
- 81.Halassa MM, Fellin T, Haydon PG. The tripartite synapse: roles for gliotransmission in health and disease. Trends Mol. Med. 2007;13:54–63. doi: 10.1016/j.molmed.2006.12.005. [DOI] [PubMed] [Google Scholar]
- 82.McNally L, Bhagwagar Z, Hannestad J. Inflammation, glutamate, and glia in depression: a literature review. CNS Spectr. 2008;13:501–510. doi: 10.1017/s1092852900016734. [DOI] [PubMed] [Google Scholar]
- 83.Keyser J, Mostert JP, Koch MW. Dysfunctional astrocytes as key players in the pathogenesis of central nervous system disorders. J. Neurol. Sci. 2008;267:3–16. doi: 10.1016/j.jns.2007.08.044. [DOI] [PubMed] [Google Scholar]
- 84.Laming PR.Potassium signaling in the brain: its role in behaviour Neurochem. Int. 200036271–290.2000yCat.2231....0L 10.1016/S0197-0186(99)00136-9 [DOI] [PubMed] [Google Scholar]