Abstract
A superficial lesion of the articular cartilage does not spontaneously self-repair and has been suggested to be partly due to lack of progenitor cells within the joint that can reach the site of injury. To study whether progenitor cells are present within the joint, 3-month-old New Zealand white rabbits were exposed to bromodeoxyuridine (BrdU) for 12 consecutive days and were then sacrificed 4, 6, 10, 14, 28 and 56 days after the first BrdU administration. Presence of BrdU and localization of progenitor markers were detected using immunohistochemistry. After 10 days of BrdU exposure, BrdU-positive cells, i.e. proliferating cells, were abundantly detected in the epiphyseal plate, the perichondrial groove of Ranvier, and in all zones of the articular cartilage. After a wash-out period, BrdU-positive cells were still present, i.e. those considered to be progenitor cells, in these regions of the knee except for the proliferative zone of the epiphyseal plate. Cells in the perichondrial groove of Ranvier were further positive for several markers associated with progenitor cells and stem cell niches, including Stro-1, Jagged1, and BMPr1a. Our results demonstrate that a small population of progenitor cells is present in the perichondrial groove of Ranvier as well as within the articular cartilage in the knee. The perichondrial groove of Ranvier also demonstrates the properties of a stem cell niche.
Keywords: progenitor cells, knee joint, BrdU, articular cartilage, perichondrial groove of Ranvier
Introduction
Joints are complex entities and the existence of potential chondrogenic stem cells and their locations within the joints are debatable. However, the development and maintenance of epiphyseal cartilage as well as articular cartilage is rather well documented. The epiphyseal cartilage has been well examined and it is accepted that cells are recruited from the stem cell layer/germinal zone for further proliferation preceded by hypertrophy and bone formation (Ianotti 1990; reviewed in Rauch 2005). The growth plate is surrounded by an encircling fibrochondrosseous structure. This anatomical structure consists of the zone of Ranvier and the ring of LaCroix. The area has been postulated to harbor prechondrocytes responsible for the circumferential growth of cartilage. In contrast, the poor capacity for self-repair of articular cartilage has been explained by cell senescence and the lack of chondroprogenitor cells (Elliott 1936; Hunziker 1999). However, it has recently been demonstrated that human articular chondrocytes cultured in vitro display phenotypic plasticity with chondrogenic, adipogenic and osteogenic potential (Barbero et al. 2003; Dell’Accio et al. 2003; Tallheden et al. 2003; Thornemo et al. 2005). Whether articular cartilage contains progenitor cells or the phenotypic plasticity detected in vitro is due to de-differentiation of the cells during expansion is debatable.
Several studies have been performed in vitro using different culture systems and selection methods to establish cell populations with stem cell characteristics from different types of tissues (Eriksson et al. 1998; Kajstura et al. 1998; Beltrami et al. 2007). Several studies have been performed in which potential stem cells have been localized in different types of tissues. The existence and anatomical location of potential stem cells is not well explored in the joint. In some tissues, it is well described that stem cells are located in a special microenvironment termed stem cell niches. The location and nature of these niches can vary depending on the tissue type. Extensively studied niches in mammals are the bulge area of hair follicles where epithelial stem cells are resident and the intestinal stem cell niche is located near the crypt base (Potten & Loeffler, 1990; Watt, 2001; Marshman et al. 2002; Cotsarelis et al. 2006).
An area of potential interest with regard to progenitor cells in joints is the circumferential anatomical structure first described by Ranvier in 1873, formerly named the perichondrial groove of Ranvier. This area is located at the periphery of the epiphyseal growth plate and has been demonstrated to contain proliferating cells (Shapiro et al. 1977). It has also been suggested that perichondrial cells from the ring of LaCroix, which is a fibrous band that surrounds the groove of Ranvier and is continuous with the periosteum of the metaphysis, serve as a reservoir for precartilaginous cells in the germinal layer of the epiphyseal growth plate (Fenichel et al. 2006). The important role of an intact epiphyseal growth plate, and especially intact perichondrial zone, for longitudinal bone growth is well documented. Salter-Harris type IV fractures within the groove of Ranvier have demonstrated severe growth disturbances (Riseborough et al. 1983; Ilharreborde et al. 2006).
An established method to identify stem cells within different tissues is labelling of slow cycling cells, viz. the stem cell population, with bromodeoxyuridine (BrdU) (Allen et al. 1978; Naylor et al. 2005; Barreto Henriksson et al. 2009). Progenitor cells/stem cells can further be identified and characterized by their expression of specific proteins, although no unique marker for this type of cell exists today. Markers associated with stem cells and stem cell niches in the literature include Stro-1, bone morphogenetic protein receptor 1a (BMPr1a), Patched, Notch1, integrin β1, and N-cadherin. Stro-1 is a widely accepted marker for mesenchymal stem cells and is also present on stem cells in the native bone niche (Simmons & Torok-Storb 1991; Song et al. 2007). BMPr1a has been characterized in epithelial cells in which inactivation of this protein results in overproduction of stem cells (Zhang et al. 2006). As with BMPr1a, it has been demonstrated that mice lacking one allele of the Sonic hedgehog receptor Patched had a decreased number of neural progenitors (Moshiri & Reh 2004). Notch1 is a regulator of great importance for cell fate commitment during both early growth and adult tissues. Notch1 has also been shown to play a decisive role in the cell–cell interactions and as cell fate determinant in different stem cell niche structures, e.g. dermis, drosophila ovary, tooth and during neurogenesis (reviewed in Watt & Hogan 2000; Fuchs et al. 2004; Song et al. 2007; Mitsiadis et al. 2007). Integrin β1 has been identified on committed progenitor cells of the hematopoietic hierarchy as well as on more primitive stem cells that efficiently bind to extracellular matrix components through beta 1 integrins. The integrin β1 protein is of great importance for cell-to-cell and cell-to-matrix adhesion as well as cell migration (Gottschling et al. 2007). N-cadherin mediates cell-to-cell adhesion via homophilic binding interactions, playing an important role in the long-term maintenance of tissue-specific stem cells within the bone marrow niche (Zhang et al. 2003; Hayashi et al. 2007).
The purpose of this study was to address the hypothesis that potential progenitor cells are present within the joint and to characterize this population of cells and adjacent cells using a panel of stem cell niche markers.
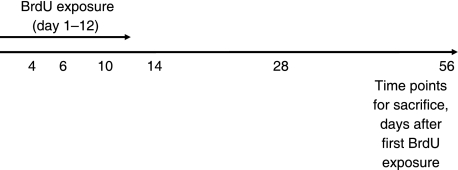
Materials and methods
Animals
Labelling with BrdU was used to identify label-retaining cells, i.e. progenitor cells, in rabbit knees. A total of 21 female New Zealand white rabbits were used for this study. The Zealand white rabbit has previously been extensively studied from the age of embryonic limb bud until skeletal maturity (Masoud et al. 1986; Rivas & Shapiro 2002). The rabbits were exposed to BrdU (Sigma-Aldrich) at 3 months of age. At this age the knee joint articular cartilage of rabbits reaches structural maturity, displaying the anisotropic structure characteristic of articular cartilage (Hunziker et al. 2007). With animals of this age it is possible to use the epiphyseal growth plate as an internal control for BrdU labelling. As BrdU has a very short half-life it must be administered on a daily basis for a period that covers the estimated cycling time of the stem cells, i.e. 12 days (Hunziker et al. 2007). To achieve this, the animals were exposed to BrdU (20 mg kg−1 bodyweight) which added to the rabbits’ daily supply of drinking water. During the 12 days of BrdU administration, the animals were kept in separate cages to monitor their BrdU intake. The animals (three per time point) were then sacrificed 4, 6, 10, 14, 28 and 56 days after the first BrdU administration (Fig. 1) by an overdose of Mebural followed by KCl injected into the heart. Three control animals, which were not exposed to BrdU, were kept simultaneously during the experiments. Ethical permission was permitted from the ethical committee at the Medical Faculty at Gothenburg University, diary no. 338-2006.
Fig. 1.
Schematic picture showing the experimental set-up of BrdU exposure and sacrifice of animals.
Immunohistochemical detection of BrdU
BrdU was detected immunohistochemically as follows: the rabbit knees were fixed in 4% formaldehyde (Histolab, Gothenburg, Sweden) before embedding in paraffin. The sections (5 µm) were then deparaffinized with xylene and rehydrated in decreasing concentrations of ethanol. An antigen retrieval step was performed using 10 mm citrate buffer pH 6.0 and incubation in 2 m HCl for 1 h at 37 °C. Unspecific binding of the antibody was prevented by incubation with a blocking solution containing 2% bovine serum albumin (BSA; Sigma-Aldrich) and 0.3% Triton-X100 dissolved in phosphate-buffered saline (PBS) for 30 min at room temperature. The sections were then incubated with an anti-BrdU antibody (DAKO, Glostrup, Denmark) (1 : 250 dilution) overnight at 4 °C. The primary antibody was visualized using a secondary antibody conjugated to Alexa Fluor 546 (1 : 200 dilution, Invitrogen, Carlsbad, CA) incubated for 3 h at room temperature. The nuclei were then stained with 4′,6-diamidino-2-phenyldole solution (DAPI; Sigma-Aldrich) for 3 min and the samples were mounted using Prolong Gold antifade mounting medium (Invitrogen). The sections were analyzed with a Nikon fluorescence microscope Eclipse 90i and digital pictures were taken using the NIS-elements software. Positive controls were sections from skin obtained from rabbits administered with BrdU, and negative controls were sections incubated with secondary antibody only, as well as rabbits not exposed to BrdU.
Immunohistochemical staining of stem cell-related markers
The tissue was fixed, sectioned and deparaffinized as described above. For the membrane-bound proteins, an antigen retrieval step using 10 mm citrate buffer pH 6.0 followed by incubation for 20 min at 90 °C was performed. To detect Notch1, two additional enzymatic steps were performed using 8000 U mL−1 hyaluronidase (Sigma-Aldrich) in PBS for 1 h at 37 °C and thereafter chondroitinase AC (Sigma-Aldrich. St Louis, MO) dissolved in PBS for 1 h at 37 °C. For the cytoplasmic and nuclear markers, a permeabilization step was performed by incubating the sections with 0.1% Triton X-100 diluted in PBS for 10 min. The sections were then blocked with 3% BSA. The epitope of interest was then labelled with one of the following primary antibodies diluted in 3% BSA; Stro-1 (mab, 1038, R&D Systems, diluted 1 : 100), BMPr1a (SC-5676, Santa Cruz, diluted 1 : 250), Patched (sc-6147, Santa Cruz, diluted 1 : 250), Notch1 (sc-23301, Santa Cruz, diluted 1 : 100), Delta4 (sc-18639, Santa Cruz, diluted 1 : 100), Jagged1 (sc-11376, Santa Cruz, diluted 1 : 100), integrin β1 (sc-9970, Santa Cruz, in dilution 1 : 100), and N-cadherin (sc-31031,Santa Cruz, in dilution 1 : 100). All incubations were performed overnight at 4 °C. Prior to the application of the secondary antibody, endogenous peroxidase activity was blocked with 3% H2O2 for 10 min. The primary antibodies were visualized using a horseradish peroxidase (HRP)-conjugated secondary antibody (donkey anti-goat or donkey anti-mouse, Santa Cruz Biotechnology) diluted 1 : 150, with the exception of Stro-1, which was visualized using an Alexa Fluor 568-conjugated goat anti-mouse antibody (A21043, Invitrogen). All incubations were performed at room temperature in a humidified chamber for 1 h. The HRP-antibody complex was then visualized using the TSA-Direct Cy3 kit (Perkin Elmer, Boston, MA) according to the manufacturer's instructions. The nuclei were then stained with DAPI (Sigma-Aldrich) solution for 3 min and the samples mounted using Prolong Gold antifade mounting medium. The sections were analyzed with a Nikon fluorescence microscope Microphot–FX and digital pictures were taken using the ACT-1 software. As positive controls, all markers were examined in dermal tissue and intestinal epithelium from the rabbits as these tissues contain a well characterized stem cell niche. Negative controls were sections incubated with secondary antibody only.
Results
BrdU localization
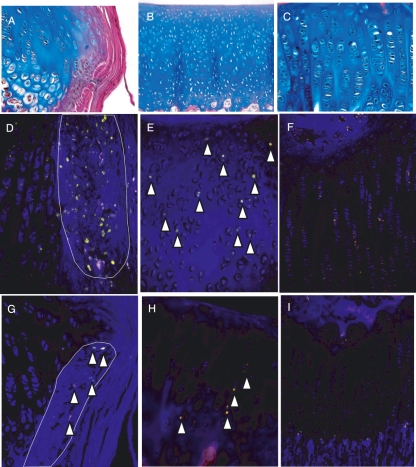
After 10–12 days of BrdU exposure, cells incorporating BrdU into their DNA were detected, especially in the epiphyseal plate, perichondrial groove of Ranvier, and all zones of the articular cartilage (Fig. 2D–F). These cells are thus either rapidly proliferating cells (also called transient amplifying cells) or slowly proliferating stem cells. To discern between these two populations of cells, BrdU-positive cells were also studied at later time points after BrdU administration to detect only the slowly proliferating cells. In all locations the number of BrdU-positive cells detected 56 days after the first administration of BrdU was significantly decreased compared to days 10 and 14 (Fig. 2G–I). In the germinal zone of the growth plate, a few cells were still detected after 28 and 56 days, whereas no positive cells could be detected in the proliferative or hypertrophic zone (Fig. 2I). A similar decrease in the number of cells detected between day 14 and the later time points (days 28 and 56) was also detected in the perichondrial groove of Ranvier, where the abundant expression after 10 days was changed to only a few BrdU-positive cells at the apical part of the groove of Ranvier at the later time points (Fig. 2D,G). Interestingly, a more abundant expression of BrdU-positive cells was detected in the growth plate near the perichondrial groove of Ranvier compared to centrally in the growth plate at later time points. Cells incorporating BrdU in the articular cartilage were detected in all cartilage zones at days 10 and 14, although at a low level (Fig. 2E). After 28 and 56 days, the numbers of BrdU-positive cells in these zones were decreased, but label-retaining cells were still detectable throughout the articular cartilage (Fig. 2H). It was also noted that these positive cells often had a columnar appearance.
Fig. 2.
Histological figures showing localization of the three areas of interest in the joint: (A) perichondrial groove of Ranvier, (B) articular cartilage, (C) growth plate. Detection of cells labelled with BrdU using immunohistochemistry. Samples after 10 days of consecutive exposure to BrdU (D–F) and after a wash-out period of 44 days (G–I). n = 3. Magnifications: (A,B) ×20, (C,F,H,I) ×100, (D,E,G) ×200.
Localization of progenitor markers in the knee
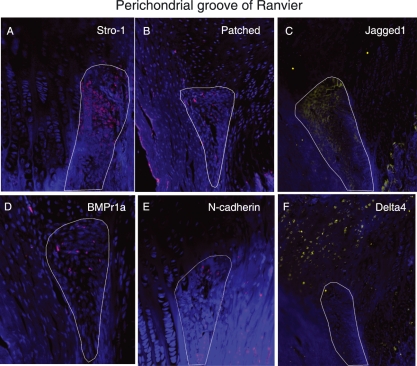
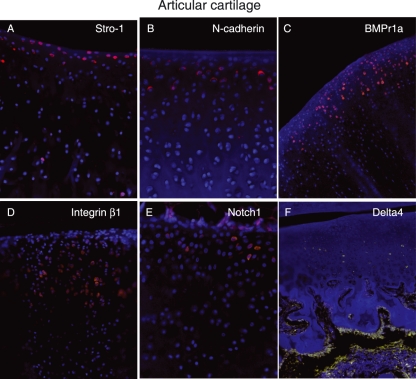
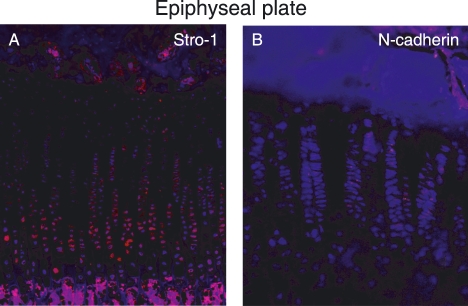
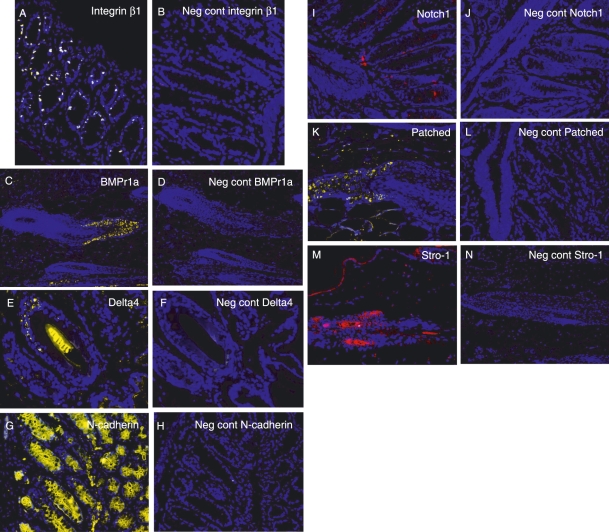
Cells in the perichondrial groove of Ranvier were positive for several of the antibodies used in this study, including Stro-1, Patched, Jagged1, BMPr1a, and N-cadherin (Fig. 3A,B,C,D,E). The most abundantly expressed markers were Jagged1 and Stro-1, which were detected in almost all cells in the perichondrial groove of Ranvier, whereas cells in the growth plate directly adjacent to the perichondrial groove of Ranvier did not express these markers (Fig. 3A,C). Cells expressing Jagged1 formed a distinct boundary between cells in the perichondrial groove of Ranvier and the surrounding tissue (Fig. 3C). The other markers detected in this area (N-cadherin, Patched and BMPr1a) were detected on fewer cells compared to Stro-1 and Jagged1, but their expression was more abundant compared to the BrdU-labelled cells detected at the later time points. Delta4 was expressed on cells just adjacent to the perichondrial groove of Ranvier (Fig. 3F). In the articular cartilage, Notch1, Stro-1 and N-cadherin were exclusively expressed on cells in the superficial zone (Fig. 4A,B,E). Interestingly, whereas Stro-1-positive cells were detected in the uppermost part of the articular cartilage, Notch1 and N-cadherin were detected in deeper parts of the superficial zone. These markers thus were not exclusively expressed on the label-retaining cells. BMRr1a and integrinβ1 were located in the superficial cells but positive cells were also detected deeper into the cartilage compared to Notch1, Stro-1, and N-cadherin (Fig. 4). Delta4 was expressed in the transitional and deeper zones of the articular cartilage (Fig. 4F). An abundant expression of Stro-1 was detected in the epiphyseal plate, whereas only a few of the cells were positive for N-cadherin (Fig. 5). No cells expressing Patched or BMPr1a could be detected in the epiphyseal plate. The specificity of the antibodies used was demonstrated on rabbit skin and intestinal epithelium (Fig. 6).
Fig. 3.
Immunohistochemical localization of Stro-1 (A), Patched (B), Jagged1 (C), BMPr1 a (D), N-cadherin (E) and Delta4 (F) in the perichondrial groove of Ranvier in New Zealand white rabbits. n = 3. Magnifications: (A,D,E) ×200, (B,C,F) ×100.
Fig. 4.
Immunohistochemical localization of Stro-1 (A), N-cadherin (B), BMPr1a (C) Integrin β1 (D), Notch1 (E) and Delta4 (F) in articular cartilage in New Zealand white rabbits. n = 3. Magnifications: (A,B,E) ×200, (C,D) ×100, (F) ×20.
Fig. 5.
Immunohistochemical localization of Stro-1 (A) and N-cadherin (B) in the epiphyseal plate in New Zealand white rabbits. n = 3. Magnifications: (A) ×100, (B) ×200.
Fig. 6.
Positive (A,C,E,G,I,K,M) and negative (B,D,F,H,J,L,N) controls for the antibodies used to detect progenitor cells in the rabbit joint. As controls, either rabbit intestine (A,B,G–L) or rabbit dermal tissue (C–F, M and N) was used.
Discussion
Tissues with a high cell turnover have an improved capacity to repair damaged areas. Bone, liver and intestine are examples of tissues with high turnover rates, and are thus capable of rapid, successful repair. Articular cartilage, on the other hand, is thought to be a post-mitotic tissue, with virtually no cellular turnover (Elliott 1936). Regeneration of a tissue is also dependent on the accessibility and number of stem cells available. It is still debatable whether stem cells are localized within the articular cartilage. There is evidence that articular cartilage growth during development is achieved by apposition from the articular surface until puberty (Hayes et al. 2001). For such a mechanism to occur a population of progenitor cells must reside within the surface zone to provide transient amplifying progenitors. Using BrdU injections it has been demonstrated in 3-month-old Monodelphis domestica that there are slow-cycling cells in the surface zone of the articular cartilage. Our data demonstrate that label-retaining cells exist within the articular cartilage, but there is no specific zonal localization of these cells. One could instead see that these cells often were located perpendicular to each other. The M. domesticareach puberty at the age of 6–8 months, whereas the rabbit reaches puberty at the age of 3 months. The animals used in our study were thus more mature compared to the M. domestica.The different developmental stages of the animals used in the two studies likely explain the different location of the progenitor cells, and it is of importance to gain knowledge about the presence and localization of progenitor cells also in sexually mature animals. Our rabbits further had a thicker cartilage compared to the M. domestica.Hunziker et al. (2007) demonstrated that before 3 months of age, the rabbit articular chondrocytes are very isotropically arranged. At the age of 3 months, a dramatic change in the structural organization of the articular cartilage layer was detected and individual chondrocytes were highly oriented in space; the tissue attained structural maturity at the time of sexual maturity (3 months of age). The fact that we administered BrdU to animals whose articular cartilage is structurally maturing likely explains the differences between our results and the data from Hayes et al. (2001). The progenitor cells, which according to the theory of appositional growth are present in the surface zone of articular cartilage, might thus not be present only in the superficial zone at a later stage. As the cartilage grows, the progenitor cells initially located in the superficial zone of the articular cartilage, where they are responsible for the appositional growth, either lose their progenitor properties or become more dispersed throughout the cartilage as the tissue becomes less isotropic. We could also detect a notable change in the arrangement of the chondrocytes, demonstrating the maturation of the tissue from an isotropic structure to a well-organized tissue. These differences in the location of the progenitor cells between immature and mature animals likely, at least to some extent, explain the differences detected in self-reparative capacity of superficial lesions in young and old animals (Namba et al. 1998; Vasara et al. 2006).
The BrdU-positive cells in the M. domesticafurther expressed the proposed progenitor marker Notch1. Our results as well as previously published data demonstrate that Notch1 is exclusively localized on cells in the superficial zone of the articular cartilage in adult bovines and humans (Dowthwaite et al. 2004; Karlsson et al. 2007, 2008). In agreement with our results, Dowthwaite et al. (2004) demonstrated that in 7-day-old calves, 86% of the chondrocytes in the surface zone express Notch1. However, in contrast to our data, they further demonstrated that Notch1 also was detected in the transitional (10%) and radial (34%) zones of the articular cartilage. These discrepancies might be explained by the different developmental stage of the animals used in the different studies. We have demonstrated earlier that Notch1 is not a marker for a population of chondrogenic progenitor cells in adult bovines (Karlsson et al. 2008). These results are thus in accordance with the results obtained in this study. The Notch1-positive cells detected in the superficial zone in sexually mature animals might instead be a population of less mature cells, not true progenitor cells. N-cadherin and Stro-1 were also localized to the superficial zone and thus were not solely expressed on the BrdU-positive cells; thus there seem to be no specific markers for progenitor cells in articular cartilage.
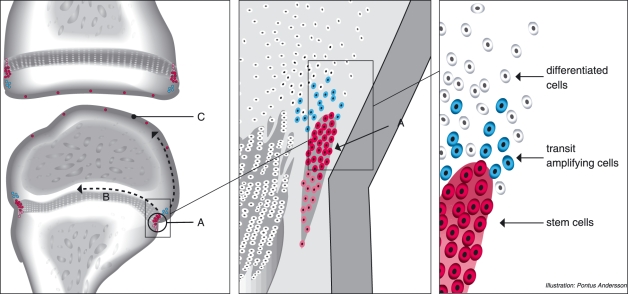
Our data demonstrated that proliferating cells were abundant in the epiphyseal growth plate but no slow-cycling cells were detected, except for in the germinal zone, which is an established stem cell area. Furthermore, label-retaining cells were detected in the apical part of the perichondrial groove of Ranvier, adjacent to the epiphyseal plate. Our results thus reinforce the results from Shapiro et al. (1977) demonstrating that this area contains densely packed cells with morphology similar to mesenchymal stem cells. Our results further show that cells located in this area express several known markers associated with progenitor cells and stem cell niches, suggesting a microenvironment for stem cells, i.e. a stem cell niche in the perichondrial groove of Ranvier. The distinct expression of markers for Notch signalling, known to create boundaries, in the perichondrial groove of Ranvier demonstrate that this population of cells has a separate phenotype compared to the surrounding cells. The coexistence of markers for progenitor cells as well as markers that are known to decrease the number of progenitor cells in the perichondrial groove of Ranvier suggests a delicate balance of stem cell renewal. The fact that these markers were more abundantly expressed than the slow-cycling cells demonstrates that these markers per se are not specific progenitor markers. These cells further express the fibroblast growth factor receptor-3 (FGFR-3), have a high proliferative potential, and appear to be responsible for bone growth (Robinson et al. 1999). If the perichondrial groove of Ranvier is removed, the longitudinal growth of the bone is significantly diminished (Rodríguez et al. 1985). Of interest is also that the population of cells located above the perichondrial groove of Ranvier did not contain any label-retaining cells but were strongly positive for Delta4. This population of cells thus also have a phenotype that differs from the surrounding cells, likely transient amplifying cells that later on will differentiate into terminally differentiated cells. We could detect a larger number of BrdU-positive cells in the epiphyseal plate near the perichondrial groove of Ranvier than in the central area of the epiphyseal plate. This might be explained migration of cells from the perichondrial groove of Ranvier into the epiphysis. Robinson et al. (1999) also suggested this, demonstrating that injection of LacZ-transfected cells from the perichondrial groove of Ranvier migrate initially to the perichondrial groove of Ranvier and later deeper into the epiphysis. The cells in the perichondrial groove of Ranvier might thus function as a reservoir of mesenchymal progenitor stem cells and the cells migrating from the perichondrial groove of Ranvier might be the label-retaining progenitor cells. It is also possible that these cells can migrate to the surface of the articular cartilage (Fig. 7).
Fig. 7.
Schematic overview of the localization of the BrdU-positive cells in the knee. (A) perichondrial groove of Ranvier, (B) growth plate, (C) articular cartilage. Label-retaining cells were localized in the perichondrial groove of Ranvier as well as the articular cartilage. Of special interest are the cells in the perichondrial groove of Ranvier, in which label-retaining cells were detected as well as cells positive for progenitor markers. Cells above the perichondrial groove of Ranvier further proved to be a distinct population of cells compared to the surrounding tissue, and are thus likely transient amplifying cells that later will mature into terminally differentiated cells. We also propose that it is these progenitor cells that have the ability to migrate (arrows).
Our results also demonstrate that there are different subpopulations of progenitor cells. Whereas the progenitor cells in the surface layer of the articular cartilage seem to lose their progenitor properties and/or become dispersed throughout the articular cartilage as they grow older, the progenitor cells in the perichondrial groove of Ranvier maintain their progenitor properties and localization. The cellular structure, cellular markers and label-retaining cell traffic have the properties of a stem cell niche and we therefore suggest that the groove of Ranvier should be regarded as such a niche.
In conclusion we have demonstrated that progenitor cells exist in the knee of sexually mature rabbits and are mainly localized to the perichondrial groove of Ranvier as well as detected in small numbers dispersed in the articular cartilage. The observations in this study raise a number of questions for further investigations: do the progenitor cells found behave like true mesenchymal stem cells and does the stem cell niche within the groove of Ranvier support the articular cartilage with progenitor cells and/or the epiphyseal plate? These issues would be of interest to investigate further.
References
- Allen JW, Shuler CF, Latt SA. Bromodeoxyuridine tablet methodology for in vivo studies of DNA synthesis. Somatic Cell Genet. 1978;4:393–405. doi: 10.1007/BF01538862. [DOI] [PubMed] [Google Scholar]
- Barbero A, Ploegert S, Heberer M, et al. Plasticity of clonal populations of dedifferentiated adult human articular chondrocytes. Arthritis Rheum. 2003;48:1315–1325. doi: 10.1002/art.10950. [DOI] [PubMed] [Google Scholar]
- Barreto Henriksson H, Thornemo M, Karlsson C, et al. Identification of cell proliferation zones, progenitor cells and a potential stem cells niche in the intervertebral disc region. A study in four species. Spine. 2009 doi: 10.1097/BRS.0b013e3181a95ad2. in press. [DOI] [PubMed] [Google Scholar]
- Beltrami AP, Cesselli D, Bergamin N, et al. Multipotent cells can be generated in vitrofrom several adult human organs (heart, liver and bone marrow) Blood. 2007;110:3438–3446. doi: 10.1182/blood-2006-11-055566. [DOI] [PubMed] [Google Scholar]
- Cotsarelis G. Gene expression profiling gets to the root of human hair follicle stem cells. J Clin Invest. 2006;116:19–22. doi: 10.1172/JCI27490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dell’Accio F, De Bari C, Luyten FP. Microenvironment and phenotypic stability specify tissue formation by human articular cartilage-derived cells in vivo. Exp Cell Res. 2003;287:16–27. doi: 10.1016/s0014-4827(03)00036-3. [DOI] [PubMed] [Google Scholar]
- Dowthwaite GP, Bishop JC, Redman SN. The surface of articular cartilage contains a progenitor cell population. J Cell Sci. 2004;117:889–897. doi: 10.1242/jcs.00912. [DOI] [PubMed] [Google Scholar]
- Elliott HC. Studies on articular cartilage I growth Mechanisms. Am J Anat. 1936;58:127. [Google Scholar]
- Eriksson PS, Perfilieva E, Bjork-Eriksson T. Neurogenesis in the adult human hippocampus. Nat Med. 1998;4:1313–1317. doi: 10.1038/3305. [DOI] [PubMed] [Google Scholar]
- Fenichel F, Evron Z, Nevo Z. The perichondrial ring as a reservoir for precartilaginous cells. In vivo model in young chicks’ epiphysis. Int Orthop. 2006;30:353–356. doi: 10.1007/s00264-006-0082-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuchs E, Tumbar T, Guasch G. Socializing with the neighbors: stem cells and their niche. Cell Mar. 2004;19:769–778. doi: 10.1016/s0092-8674(04)00255-7. [DOI] [PubMed] [Google Scholar]
- Gottschling S, Saffrich R, Seckinger A, et al. Human mesenchymal stromal cells regulate initial self-renewing divisions of hematopoietic progenitor cells by a β1-integrin-dependent mechanism. Stem Cells. 2007;3:798–806. doi: 10.1634/stemcells.2006-0513. [DOI] [PubMed] [Google Scholar]
- Hayashi R, Yamato M, Sugiyama H, et al. N-Cadherin is expressed by putative stem/progenitor cells and melanocytes in the human limbal epithelial stem cell niche. Stem Cells. 2007;2:289–296. doi: 10.1634/stemcells.2006-0167. [DOI] [PubMed] [Google Scholar]
- Hayes AJ, MacPherson S, Morrison H, et al. The development of articular cartilage: evidence for an appositional growth mechanism. Anat Embryol. 2001;203:469–479. doi: 10.1007/s004290100178. [DOI] [PubMed] [Google Scholar]
- Hunziker EB. Articular cartilage repair: are the intrinsic biological constraints undermining this process insuperable? Osteoarthr Cartil. 1999;7:15–28. doi: 10.1053/joca.1998.0159. [DOI] [PubMed] [Google Scholar]
- Hunziker EB, Kapfinger E, Geiss J. The structural architecture of adult mammalian articular cartilage evolves by a synchronized process of tissue resorption and neoformation during postnatal development. Osteoarthr Cartil. 2007;15:403–413. doi: 10.1016/j.joca.2006.09.010. [DOI] [PubMed] [Google Scholar]
- Ianotti JP. Growth plate physiology and pathology. Orthop Clin N Am. 1990;21:1–17. [PubMed] [Google Scholar]
- Ilharreborde B, Raquillet C, Morel E, et al. Long-term prognosis of Salter–Harris Type 2 injuries of the distal femoral physis. J Pediatr Orthop. 2006;15:433–438. doi: 10.1097/01.bpb.0000228384.01690.aa. [DOI] [PubMed] [Google Scholar]
- Kajstura J, Leri A, Finato N. Myocyte proliferation in end-stage cardiac failure in humans. Proc Natl Acad Sci U S A. 1998;95:8801–8805. doi: 10.1073/pnas.95.15.8801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karlsson C, Jonsson M, Asp J, et al. Notch and HES5 are regulated during human cartilage differentiation. Cell Tissue Res. 2007;327:539–551. doi: 10.1007/s00441-006-0307-0. [DOI] [PubMed] [Google Scholar]
- Karlsson C, Stenhamre H, Sandstedt J, et al. Neither Notch1 expression nor cellular size correlate with mesenchymal stem cell properties of adult articular chondrocytes. Cells Tissues Organs. 2008;187:275–285. doi: 10.1159/000113409. [DOI] [PubMed] [Google Scholar]
- Marshman E, Booth C, Potten CS. The intestinal epithelial stem cell. Bioessays. 2002;24:91–98. doi: 10.1002/bies.10028. [DOI] [PubMed] [Google Scholar]
- Masoud I, Shapiro F, Kent R, Moses A. A longitudinal study of the growth of the New Zealand white rabbit: cumulative and biweekly incremental growth rates for body length, body weight, femoral length and tibial length. J Orthop Res. 1986;4:221–231. doi: 10.1002/jor.1100040211. [DOI] [PubMed] [Google Scholar]
- Mitsiadis TA, Barrandonb O, Rochatc A, et al. Stem cell niches in mammals. Exp Cell Res. 2007;313:3377–3385. doi: 10.1016/j.yexcr.2007.07.027. [DOI] [PubMed] [Google Scholar]
- Moshiri A, Reh TA. Persistent progenitors at the retinal margin of ptcmice. J Neurosci. 2004;24:229–237. doi: 10.1523/JNEUROSCI.2980-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Namba RS, Meuli M, Sullivan KM, et al. Spontaneous repair of superficial defects in articular cartilage in a fetal lamb model. J Bone Joint Surg Am. 1998;80:4–10. doi: 10.2106/00004623-199801000-00003. [DOI] [PubMed] [Google Scholar]
- Naylor AS, Persson AI, Eriksson PS, et al. Extended voluntary running inhibits exercise-induced adult hippocampal progenitor proliferation in the spontaneously hypertensive rat. J Neurophysiol. 2005;93:2406–2414. doi: 10.1152/jn.01085.2004. [DOI] [PubMed] [Google Scholar]
- Potten CS, Loeffler M. Stem cells: attributes, cycles, spirals, pitfalls and uncertainties. Lessons for and from the crypt. Development. 1990;110:1001–1020. doi: 10.1242/dev.110.4.1001. [DOI] [PubMed] [Google Scholar]
- Rauch F. Bone growth in length and width: The Yin and Yang of bone stability. J Musculoskelet Neuronal Interact. 2005;5:194–201. [PubMed] [Google Scholar]
- Riseborough EJ, Barrett IR, Shapiro F. Growth disturbances following distal femoral physeal fracture separations. J Bone Joint Surg Am. 1983;65:885–893. [PubMed] [Google Scholar]
- Rivas R, Shapiro F. Structural stages in the development of the long bones and epiphyses: a study in the New Zealand white rabbit. J Bone Joint Surg Am. 2002;Jan 84-A(1):85–100. doi: 10.2106/00004623-200201000-00013. [DOI] [PubMed] [Google Scholar]
- Robinson D, Hasharoni A, Cohen N, et al. Fibroblast growth factor receptor-3 as a marker for precartilaginous stem cells. Clin Orthop Relat Res. 1999;367:163–175. doi: 10.1097/00003086-199910001-00018. [DOI] [PubMed] [Google Scholar]
- Rodríguez JI, Delgado E, Paniagua R. Changes in young rat radius following excision of the perichondrial ring. Calcif Tissue Int. 1985;37:677–683. doi: 10.1007/BF02554930. [DOI] [PubMed] [Google Scholar]
- Shapiro F, Holtrop ME, Glimcher MJ. Organization and cellular biology of the perichondrial ossification groove of Ranvier: a morphological study in rabbits. Organization. J Bone Joint Surg Am. 1977;59:703–723. [PubMed] [Google Scholar]
- Simmons PJ, Torok-Storb B. Identification of stromal cell precursors in human bone marrow by a novel monoclonal antibody, STRO-1. Blood. 1991;78:55–62. [PubMed] [Google Scholar]
- Song X, Call GB, Kirilly D, et al. Notch signaling controls germline stem cell niche formation in the Drosophilaovary. Development. 2007;134:1071–1080. doi: 10.1242/dev.003392. [DOI] [PubMed] [Google Scholar]
- Tallheden T, Dennis JE, Lennon DP, et al. Phenotypic plasticity of human articular chondrocytes. J Bone Joint Surg Am. 2003;85:93–100. doi: 10.2106/00004623-200300002-00012. [DOI] [PubMed] [Google Scholar]
- Thornemo M, Tallheden T, Sjögren-Jansson E, et al. Clonal populations of chondrocytes with progenitor properties identified within human articular cartilage. Cells Tissues Organs. 2005;180:141–150. doi: 10.1159/000088242. [DOI] [PubMed] [Google Scholar]
- Vasara AI, Hyttinen MM, Pulliainen O, et al. Immature porcine knee cartilage lesions show good healing with or without autologous chondrocyte transplantation. Osteoarthr Cartil. 2006;14:1066–1074. doi: 10.1016/j.joca.2006.04.003. [DOI] [PubMed] [Google Scholar]
- Watt FM. Stem cell fate and patterning in mammalian epidermis. Curr Opin Genet Dev. 2001;11:410–417. doi: 10.1016/s0959-437x(00)00211-2. [DOI] [PubMed] [Google Scholar]
- Watt FM, Hogan BL. Out of Eden: stem cells and their niches. Science. 2000;287:1427–1430. doi: 10.1126/science.287.5457.1427. [DOI] [PubMed] [Google Scholar]
- Zhang J, Niu C, Ye L, et al. Identification of the haematopoietic stem cell niche and control of the niche size. Nature. 2003;425:836–841. doi: 10.1038/nature02041. [DOI] [PubMed] [Google Scholar]
- Zhang J, He XC, Tong WG, et al. Bone morphogenetic protein signaling inhibits hair follicle anagen induction by restricting epithelial stem/progenitor cell activation and expansion. Stem Cells. 2006;24:2826–2839. doi: 10.1634/stemcells.2005-0544. [DOI] [PubMed] [Google Scholar]