Abstract
The sexual dimorphism of life span and caloric restriction effects in numerous species suggest that estradiol (E2) is protective against oxidative damage. The only direct test of E2's protective effect in mice against in vivo oxidative stress to date may have been confounded by E2's direct chemical action as an antioxidant because it was administered at very high dosages. Therefore, we have identified a low yet physiologically effective dose of E2. We then administered this dose using subcutaneous time-release pellets to ovariectomized mice. Two weeks after E2 pellet implantation, sham-operated, ovariectomized, and ovariectomized E2-supplemented female mice were injected with a lethal dose of paraquat and their survival was followed. It was observed that ovariectomy exacerbates paraquat-induced mortality and is rescued by E2 supplementation. An equivalent experiment was performed on sham-operated, orchidectomized, and E2-supplemented orchidectomized male mice. The survival of male mice was improved by orchidectomy, and E2 gave no further benefit. We interpret the results to mean that E2 is protective against oxidative stress through its regulatory role and that testosterone diminishes protection against oxidative stress.
Keywords: Estradiol, Paraquat, Oxidative Stress, Mice, Gonadectomy
INTRODUCTION
Reactive oxygen species (ROS) production and the effectiveness of physiological defenses against them may play a major role in susceptibility to many illnesses (1–3) and the aging process itself (4). In many species, there are sex differences in longevity (5–7). In humans, for instance, women live longer than men (8) and premenopausal women are less prone to cardiac disease (9–11), Alzheimer's disease (12), and progression of chronic renal disease to renal failure (13,14). During and after menopause, women's disease susceptibility to these illnesses rises (15–17) until, at later ages, it is indistinguishable from that observed in men (18). Menopause is characterized by depletion of ovarian follicles and the decline of the estrogens that they produce. As estrogen levels drop, they are no longer sufficient to repress follicle-stimulating hormone and luteinizing hormone production in the anterior pituitary, which results in elevated levels of these gonadotropins and a cessation of menstrual cycle (19).
Estrogens are a family of steroid hormones that include estrone, estriol, and 17-β2-estradiol (E2). Of these, E2 is the most abundant and biologically active hormone (19). In addition to its role in estrous cycling and the development of secondary sexual characteristics, E2 is involved in neuroprotection (20,21), reduction of blood pressure (22–24), lowering of low-density lipoprotein and total cholesterol (25–27), enhancement of learning and memory (28), lowering of plasma insulin and glucose levels (29,30), and possibly upregulation of antioxidative defense mechanisms (18,31–33).
In humans, plasma antioxidant capacity correlates with E2 levels throughout the menstrual cycle (34) as does the activity of the glutathione peroxidase (Gpx) family of antioxidant enzymes (35–37). Furthermore, the levels of lipid hydroperoxides, which are the products of oxidative lipid damage, correlate negatively with E2 levels (35). After menopause, GPX activity declines and lipid hydroperoxide levels increase (35). However, supplementation of E2 in women after menopause restores total plasma antioxidant capacity and decreases serum lipid peroxides (36–39) while increasing GPX activity and the levels of glutathione (GSH), an antioxidant and a cofactor of the Gpx enzymes (36–38,40). However, the Women's Health Initiative (41) reported that E2 supplementation may increase the risk of stroke (42) as well as cognitive decline and dementia (43,44). For this reason, there is debate in the medical community on the benefits of estrogen supplementation and whether hormone replacement therapy is a viable treatment option.
Rodent studies with treatments known to cause oxidative stress have mostly agreed with the human data. Female rats are more resistant to oxidative damage that accompanies copper deficiency (due to decrease in the activity of CuZn superoxide dismutase, Sod1, an antioxidant enzyme) than male rats, and ovariectomy abrogates this resistance (45). Ovariectomy also lowers total antioxidant capacity with a concomitant increase of N,N-diethyl-p-phenylene diamine radical in normal rats (46) and of H2O2 levels in female spontaneously hypertensive rats (47). This is in agreement with studies suggesting that E2 is protective against diquat, a compound similar to paraquat (48) in rats (49) and in cultured bovine endothelial cells (50). However, not all E2 data support the conclusion of a protective role. For example, several studies have found that E2 may actually exacerbate oxidative stress (51,52).
To date, few studies have directly probed E2's role in in vivo oxidative stress resistance. Munoz-Castenada et al. have reported that ovariectomized rats are more vulnerable to adriamycin (53) and that this effect can be reversed with E2 supplementation (54). To our knowledge, the only mouse study of E2's role in protection against in vivo oxidative stress was done by Baba et al. (55) in wild-type and insulin receptor hemizygous null (IR+/−) mice. In the course of studying the gender dimorphic effect of the mutant IR allele's protection against oxidative stress, they found that ovariectomized female mice of both genotypes were more susceptible to hyperoxia and that their resistance to it could be rescued by E2 supplementation. This was accompanied by changes in the expression and activity of manganese superoxide dismutase. The Baba group also reported survival data from a paraquat experiment but not on ovariectomized and E2-supplemented mice.
The problem with these studies is that the large doses of E2 that they administered could be providing antioxidant protection in a nonphysiological manner. For example, Baba et al. (55) administered 20 mg/kg of E2 per week subcutaneously. If the E2 from these injections was assimilated at a constant rate, this would be 2,300−3,600 ng/h, or more than 10-fold the highest dose we used in our experiment. Moreover, it is known that E2 administered in this manner is not released in a linear fashion and instead enters the circulation as a large and exponentially decreasing bolus dose (56). Mice that are injected with E2 subcutaneously are likely exposed to many orders of magnitude higher than physiological amounts of E2 with the levels only declining to the physiological range after several days. It has been shown that at sufficiently high doses E2 can directly scavenge free radicals (57). Therefore, it is not clear whether Baba et al.'s results are because of E2 acting through its normal physiological role, regulating specific genes through its receptors, or simply acting as an antioxidant, scavenging free radicals like any other antioxidant. For this reason, we measured the effect of E2 on the survival of C57BL/6 mice that were exposed to paraquat but at E2 dosages that approximate average physiological levels, which we established in a preliminary dosage study using well-established biological markers for estrogenicity and observing a response of those markers comparable with that of intact mice.
We used paraquat as an oxidative stressor in this study because it is one of the most widely used means of generating ROS in vivo with few confounding effects. Although several specific mechanisms have been proposed for paraquat toxicity (58), there is a long-standing consensus in the field of free radical biology that they all ultimately depend on ROS (59–62). Early pulse radiolysis (63,64) and electrochemical (65) studies have shown that paraquat spontaneously catalyzes the production of super-oxide from molecular oxygen in aqueous solution. In vivo, paraquat administration is followed by an increase in oxidative damage to macromolecules: proteins (66), DNA (67), and especially lipids (68–70). Within the cell, paraquat depletes the NADPH pool (71–73). As NADPH is needed by glutathione reductase to replenish GSH, paraquat thus delivers the dual blow of weakening antioxidant defenses while catalyzing superoxide production (74). When injected, paraquat is excreted in the urine unmodified (75), so any possible metabolites are unlikely to be significant contributors to its toxicity under our experimental conditions.
The strongest evidence for oxidative stress being the mechanism of paraquat toxicity comes from numerous tissue culture studies and in vivo studies in multiple species showing that antioxidants are protective against paraquat and their suppression makes the animal or cell more vulnerable to paraquat. Pretreatment with antioxidants was found to improve survival at the cellular or organismal level after paraquat administration (76–82). Conversely, deficiency of α-tocopherol in rats (83) and mice (69) sensitized them to paraquat. Data from genetically modified mice follow a similar pattern. Embryonic fibroblasts from Sod1- and catalase-overexpressing mice (84), embryonic fibroblasts from Sod2overexpressing mice (85), and mice that overexpressed Gpx1 (86,87) all had increased tolerance for paraquat. Mice that were homozygous or, in some cases, hemizygous for null alleles of genes coding for antioxidant enzymes (including Gpx1, Gpx4, Sod1, and Sod2) were found to have increased levels and accelerated rates of paraquat-induced mortality. Moreover, sublethal hypoxia protected mice against paraquat (88), whereas hyperoxia sensitized mice (89) and rats (73,90–93) to paraquat. Together, this evidence recommends paraquat as a robust and time-proven experimental model for in vivo and in vitro oxidative stress.
In this study, we showed for the first time that survival time of paraquat-exposed female mice was shortened by ovariectomy and E2 supplementation restored paraquat survival times to the same level as those of intact animals. Furthermore, we found that orchidectomy improves survival of paraquat-exposed male mice, but E2 confers no survival benefit on male mice.
MATERIALS AND METHODS
Animals
The mice in these experiments were derived from inbred C57BL/6 male and female breeders purchased from The Jackson Laboratory. All mice were fed a standard NIH-31 chow and maintained in micro-isolator cages on a 12-h dark/light cycle until paraquat injection. Age-matched groups of mice were chosen for all experiments. All procedures involving the mice were approved by the subcommittee for Animal Studies at the Audie L. Murphy Veterans Administration Hospital and the University of Texas Health Science Center at San Antonio IACUC.
Paraquat
Paraquat (methyl viologen; Sigma-Aldrich, St. Louis, MO) was stored in a dessicator at 4°C in dark containers. A consistent dosage was verified by measuring absorbance in saline solution at 308 nm. Two weeks after ovariectomy or orchidectomy and E2 capsule implantation, mice were injected interperitoneally with paraquat dissolved in 0.9% saline (25 mg/mL) at a dose of 75 mg/kg of animal body weight. A Hamilton syringe demarcated in 2.5 μL increments was used for the injection, making it possible to adjust dosage for body weight differences as small as 0.6 g (the volumes injected ranged from 75 to 135 μL).
To follow the survival of mice after paraquat treatment, the cages containing the treated mice were placed under an array of digital surveillance cameras (Strategic Vista, Ontario, Canada) attached to generic Pentium-series computers running Windows XP. These cameras monitored the animals continuously, and the footage was used to determine the time of death with a precision of within 1 min. The time of injection was subtracted from the recorded time of death to obtain the survival time for each animal.
Ovariectomy and E2 Supplementation
Ovariectomy was performed under isofluorane anesthesia. A single dorsal incision was made in the skin and through it two lateral incisions in the muscle layer were made, approximately half a centimeter below each kidney. The ovaries were extruded through the incision, ligated off, and removed (or, in the case of the sham-operated group, extruded and reinserted). During the procedure, the E2 group of mice was implanted with 21-day time-release capsules each containing 0.001 mg of β17-estradiol (Innovative Research of America, Sarasota, FL, USA). The capsules were placed below the skin of the upper back. The sham and ovariectomized mice were likewise implanted with placebo capsules. The muscle layer was sutured with 5−0 absorbable sutures. The skin was closed with surgical staples and treated with triple antibiotic cream. The mice were monitored daily for self-removed staples or complications from surgery. All mice were housed singly for 2 days or until the wounds healed, then the staples were removed and the mice were rehoused four per cage.
Orchidectomy and E2 Administration
Orchidectomy was performed under isofluorane anesthesia. An incision was made in the scrotum, the underlying muscle layer was cut, and the testes were extruded by massaging the lower abdomen (and reinserted in the sham-operated group). In the nonsham groups, the testes were ligated off and removed. During the procedure, an incision was made in the skin of the upper back, and the E2 group of mice was implanted with 21-day time-release capsules each containing 0.001 mg of β17-estradiol. The sham and orchidectomized mice were likewise implanted with placebo capsules. Both incisions were closed with surgical staples and treated with triple antibiotic cream. The mice were monitored daily for self-removed staples or complications from surgery. The staples were removed after the incisions healed. The mice were housed singly until the beginning of the paraquat experiment.
Statistical Analysis
The Student's t-test was used to compare the uterine weights in the dosage experiment. Multiple comparison correction was done using Holm's method (94). For survival, the predicted differences were observed only at late time points (after 100 h). We therefore used Fleming and Harrington's G-rho family test (95,96) with a negative rho to put the emphasis on later periods and detect the difference among the animals that had survived past 80 h. Each pair of treatments within each sex were compared, that is, sham versus gonadectomized, gonadectomized versus gonadectomized + E2, sham versus gonadectomized + E2. The R statistical language and survival analysis package were used for all statistics (97).
RESULTS
E2 Dosage and Bioeffectiveness
It is nontrivial to convert published information on physiological E2 levels (98) into concrete dosages because endogenous E2 is rapidly turned over by the liver (19), and for much of the 3- to 4-day rodent estrous cycle, circulating E2 is at or below the threshold of reliable detection. Therefore, instead of direct measurement of plasma E2 levels, two bioassays are commonly used as a standard for effectiveness of E2 dose because they are reliable, sensitive, and directly measure a major physiological effect of E2. These are vaginal cornification and increase in uterine weight (99). Obtaining vaginal smears is a minimally invasive procedure that can be performed daily without requiring anesthesia, but it has the disadvantage of being a qualitative assay: vaginal smears are graded according to whether they are noncornified, partly cornified, or highly cornified. The other assay is uterine weight, which is highly sensitive to E2 concentrations. We used both these assays in our range-finding experiment.
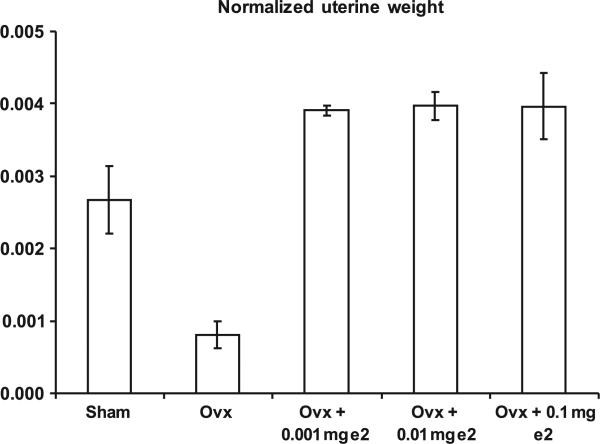
We first empirically determined the minimal effective dose for mice using E2 pellets in three different concentrations: 0.1, 0.01, and 0.001 mg (the latter was the smallest available dose for this type of pellet). According to the manufacturer, these pellets deliver the indicated dosage at a constant rate over a 21-day period, which corresponds to 198, 19.8, and 1.98 ng/h. The lowest available dose (1.98 ng/h) was sufficient to restore uterine weight to the same or higher levels as sham-treated mice (Figure 1). As all the E2 doses elicited a response, there remains the possibility that 1.98 ng/h is a supraphysiological dose. However, an earlier study reported the minimum effective dose in mice to be 100 μg/day (100), which was far in excess of the 48 ng/day that we used. Furthermore, another E2 dose–response study in mice showed dosages corresponding to 1.1 and 5.7 ng/h were within the range where uterine weight was increased and vaginal cornification was induced, yet the response was still close to physiological norms (101). Our dose falls near the bottom of that range. We observed more complete cornification in the E2-supplemented animals than in sham animals (data not shown), but this was expected because of estrous cycling in the sham animals. We also observed uterine weights between 85 and 101 mg in E2-supplemented animals, which fell between the values obtained in Cohen and Milligan's 1.1 and 5.7 ng/h dose levels (101).
FIGURE 1.
Response of ovariectomized female mice to various dosages of E2. Female mice (6 months) were overiectomized and implanted with E2 capsules (dosages indicated on graph) as described in the Experimental Procedures. Two weeks after ovariectomy and implantation with E2 capsules, uterine weight was measured. Each bar shows the mean and SEM uterine weight from 5 mice. The leftmost bar represents non-ovariectomized, sham operated mice. The data were analyzed using Student's t-test corrected for multiple comparisons as described in the Experimental Procedures.
The Effect of E2 on Paraquat Resistance
Our main experiment measured survival of male and female mice in their respective treatment groups after exposure to oxidative stress in the form of paraquat (75 mg/kg body weight). Paraquat participates in a cyclic reaction that produces superoxide radicals (102), and it has become a standard model for measuring oxidative stress resistance in vivo and in vitro as explained in the introductory section and reviewed in Suntres (58), Bus and Gibson (59), and Bus et al. (103). In vivo, paraquat is actively absorbed from the blood by lung pneumocytes (104–107), causing death within a week because of pulmonary edema or, after a longer period, pulmonary fibrosis.
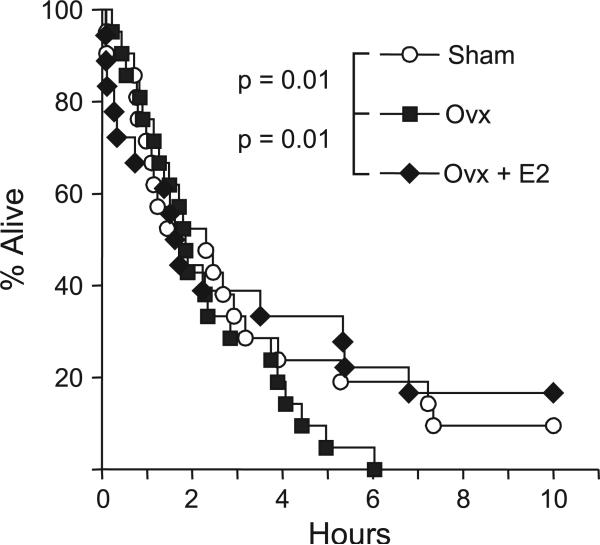
The survival of sham, ovariectomized (ovx), and ovariectomized E2-supplemented (ovx + E2) female mice was tracked for 10 days. During the first 3 days of the experiment, the three treatment groups had indistinguishable survival curves until approximately 72 h and then they started to diverge (Figure 2), with the sham and ovx + E2 groups surviving longer than the ovx group. Altogether, 20 of 21 sham mice, 15 of 18 E2 mice, and all 21 ovx mice died. There were significant differences (p = 0.01) in survival times between the sham and the ovx groups as well as between the ovx and the ovx + E2 groups. There was no significant difference between the ovx + E2 and the sham groups. These differences along with the statistical summary are reported in Table 1.
FIGURE 2.
Survival of sham, ovx, and ovx + E2 female mice after paraquat injection. Female mice (6 months old) were overiectomized and implanted with E2 time-release pellets (0.001 mg/21 days) as described in the Experimental Procedures. Two weeks after ovariectomy and implantation with E2 pel-lets, the mice were treated with paraquat (75 mg/kg). The sham mice are shown as white circles, the ovx mice as gray squares, and the ovx + E2 mice as black diamonds. The survival of the mice was followed for 10 days and statistically analyzed using the Fleming-Harrington G-rho test as described in the Experimental Procedures. The number of animals in each group is given in Table 1.
TABLE 1.
| Treatment | N | Expected | Observed | (O-E)^2/E | (O-E)^2/V | χ2 | df | p | Adjusted | p |
|---|---|---|---|---|---|---|---|---|---|---|
| Males | ||||||||||
| orx | 16 | 15.7 | 18.5 | 0.439 | 0.28 | 6.3 | 1 | 0.597 | 0.597 | |
| orx + E2 | 17 | 26.9 | 24.1 | 0.338 | 0.28 | |||||
| sham | 15 | 61.7 | 33.3 | 24.3 | 6.8 | 6.8 | 1 | 0.00909 | 0.01818 | * |
| orx + E2 | 17 | 27.1 | 55.5 | 14.6 | 6.8 | |||||
| sham | 15 | 64 | 34.1 | 26.2 | 8.44 | 8.4 | 1 | 0.00367 | 0.01101 | * |
| orx | 16 | 18.4 | 48.3 | 18.5 | 8.44 | |||||
| Females | ||||||||||
| ovx | 21 | 192 | 98.4 | 89.2 | 6.46 | 6.5 | 1 | 0.0111 | 0.0333 | |
| ovx + E2 | 18 | 201 | 294.8 | 29.8 | 6.46 | |||||
| sham | 21 | 145.7 | 121 | 5 | 0.538 | 0.5 | 1 | 0.463 | 0.463 | |
| ovx + E2 | 18 | 91.6 | 116 | 5.21 | 0.538 | |||||
| sham | 21 | 427 | 535 | 22 | 5.94 | 5.9 | 1 | 0.148 | 0.0333 | * |
| ovx | 21 | 228 | 120 | 98.2 | 5.94 | |||||
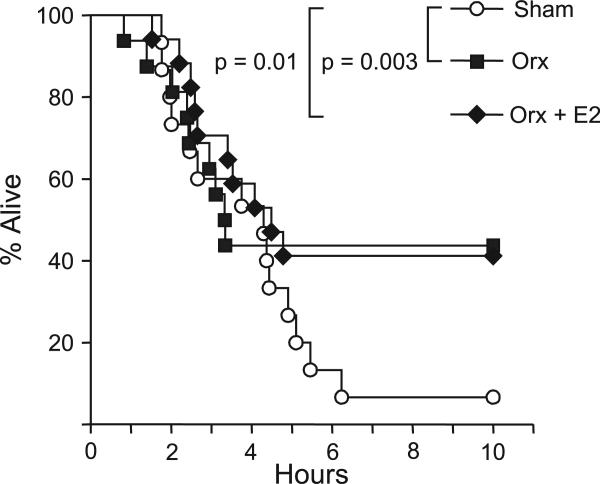
Similarly to the female mice, the survival curves of the three groups of male mice did not diverge from each other for the first 5 days of the experiment, although deaths were occurring (Figure 3). The last of the nine deaths in the orchidectomized (orx) group occurred 80.2 h into the experiment, and the remaining seven mice in that group survived the experiment. Then, at 114.8 h, the last of the 10 deaths in the orx + E2 group occurred, and the remaining seven mice in that group also survived the experiment. The mice in the sham group continued dying until the last mouse died at 149.7 h into the experiment, leaving only one sham mouse that survived the paraquat injection. We found a significant difference between the survival curves of the sham and the orx + E2 groups as well as between the survival curves of the sham and the orx groups. However, there was no significant difference between the survival curves of the orx + E2 and the orx groups. In short, the sham male mice died faster than both the orx and the E2 mice, and the latter two groups were not significantly different from each other. These differences along with the statistical summary are reported in Table 1.
FIGURE 3.
Survival of sham, orx, and orx + E2 male mice after paraquat injection. Male mice (10 months old) were orchidectomized and implanted with E2 time-release pellets (0.001 mg/21 days) as described in the Experimental Procedures. Two weeks after orchidectomy and implantation with E2 pel-lets, the mice were treated with paraquat (75 mg/kg). The sham mice are shown as white circles, the orx mice as gray squares, and the orx + E2 mice as black diamonds. The survival of the mice was followed for 10 days and statistically analyzed using the Fleming-Harrington G-rho test as described in the Experimental Procedures. The number of animals in each group is given in Table 1.
DISCUSSION
The survival curves in our experiment did not diverge until 3 or 4 days after the injection. This may seem surprising, but there is in fact an excellent biological reason why mortality rates change over the course of a paraquat experiment. Paraquat is cleared from the body within 72 h (104). Yet paraquat-induced deaths can continue for more than a week. Clearly, the animals are dying from tissue damage sustained during the period that paraquat was in their bodies. Conversely, animals that cross a critical threshold of pulmonary damage do not all die immediately but rather over the course of several days.
In detecting the differences of survival, we use a variation of the popular log-rank test that seems to be underutilized in the oxidative stress field, the Fleming–Harrington test (95,96). It incorporates the assumption of a changing mortality rate over the course of an experiment, improving the power and detecting the differences in survival rate in the later period.
We chose a relatively low yet biologically effective dose of E2 for this study in order to avoid confounding effects from E2 acting as an antioxidant at high concentrations. The fact that E2 did not cause any additional improvement in the survival of orx males beyond that already conferred by their orx status suggests that direct antioxidant activity of E2 had no significant impact on the outcome. Given that most of the orx males nonetheless were killed by paraquat, just at a lower rate than sham-operated males, and given that previous studies have shown antioxidant compounds are protective against paraquat (108,109), if E2 was acting as an antioxidant, it would have had an effect in the males as well.
Published data on the relationship between testosterone and oxidative stress are more narrowly focused than is the case for E2. Most of the data come from studies of the prostate and testes (110). Nevertheless, in contrast with E2, the data that are available suggest that testosterone potentiates oxidative damage and can suppress the activities of SOD, catalase, and GPX in rats (110). Furthermore, urine and kidney H2O2 are higher in men than in women (111), and such is also the case in male hypertensive rats compared with females of the same strain. This difference can be abolished by orchidectomizing the males (47). Orchidectomy also resulted in decreased DNA damage in the brain and lymphocytes of dogs (112) and, according to a 1969 retrospective study of castrated and noncastrated mental asylum inmates, in humans as well (113).
Our study strongly implicates testosterone in sensitizing male mice to oxidative stress, in agreement with Waters et al. (112) and with Hamilton and Mestler (113), suggesting a trade-off between fertility and oxidative stress resistance like that predicted by the antagonistic pleiotropy theory of aging (114). We have not at this time attempted the converse of this study, namely testosterone replacement in ovariectomized males to test whether it would restore their paraquat sensitivity, but this would be an interesting future direction to pursue.
Our results raise the question of why the hormonal action of E2 failed to protect the males, given that the antagonism of testosterone (19) was eliminated by ovariectomy. It is likely that even with unimpeded E2 signaling, male mice have less ability to respond because of lower receptor expression or sex-specific epigenetic silencing. In support of this, E2 increases SOD, catalase, GPX, and GR in cultured female bovine aortic endothelial cells with a concomitant protection against paraquat, but not in male cells (50). It has also been reported that E2 is only protective against ischemic brain injury when administered immediately after ovariectomy, but not 10 weeks after (115). A similar effect may be occurring here. We did in fact implant E2 pel-lets in the mice immediately after gonadectomy, so for the E2 group of female mice there was no interruption in exposure. The male mice, however, had never been exposed to these levels of E2 since birth.
These results directly demonstrate that gonadal hormones, most likely E2 and testosterone, play an important signaling role in resistance to oxidative stress and are certain to be involved in the sexual dimorphism of oxidative stress resistance in animals ranging from Caenorhabditis elegans (116), to Drosophila (117), to humans (8).
DECLARATION OF INTEREST
The authors gratefully acknowledge support from the National Institutes of Health: AGO-21890 (A.F.B); AG-19316, AG-13319, AG-23843, and AG-26557 (A.R.); the San Antonio Nathan Shock Aging Center (1P30-AG-13319) (A.R.); Department of Veterans Affairs Merit Grant (A.R.); and a Research Enhancement Award Program from the Department of Veterans Affairs (A.R.)
Footnotes
Publisher's Disclaimer: Full terms and conditions of use: http://www.informaworld.com/terms-and-conditions-of-access.pdf This article may be used for research, teaching and private study purposes. Any substantial or systematic reproduction, re-distribution, re-selling, loan or sub-licensing, systematic supply or distribution in any form to anyone is expressly forbidden. The publisher does not give any warranty express or implied or make any representation that the contents will be complete or accurate or up to date. The accuracy of any instructions, formulae and drug doses should be independently verified with primary sources. The publisher shall not be liable for any loss, actions, claims, proceedings, demand or costs or damages whatsoever or howsoever caused arising directly or indirectly in connection with or arising out of the use of this material.
Contributor Information
Alex F. Bokov, Department of Epidemiology and Biostatistics, University of Texas Health Science Center at San Antonio, San Antonio, Texas, USA
Daijin Ko, Department of Management Science and Statistics, College of Business, University of Texas at San Antonio, San Antonio, Texas, USA.
Arlan Richardson, Departments of Physiology and Cellular and Structural Biology, University of Texas Health Science Center at San Antonio, San Antonio, Texas, USA and Geriatric Research Education and Clinical Center, South Texas Veterans Health Care System, San Antonio, Texas, USA.
REFERENCES
- 1.Harman D. The aging process: major risk factor for disease and death. Proc Natl Acad Sci USA. 1991;88:5360–5363. doi: 10.1073/pnas.88.12.5360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Harman D. Role of free radicals in aging and disease. Ann NY Acad Sci. 1992;673:126–141. doi: 10.1111/j.1749-6632.1992.tb27444.x. [DOI] [PubMed] [Google Scholar]
- 3.Stadtman ER, Berlett BS. Reactive oxygen-mediated protein oxidation in aging and disease. Drug Metab Rev. 1998;30(2):225–243. doi: 10.3109/03602539808996310. [DOI] [PubMed] [Google Scholar]
- 4.Harman D. Aging: a theory based on free radical and radiation chemistry. J Gerontol. 1956;11:298–300. doi: 10.1093/geronj/11.3.298. [DOI] [PubMed] [Google Scholar]
- 5.DaCunha GL, Dacruz IBM, Fiorino P, DeOliveira AK. Paraquat resistance and starvation conditions in the selection for longevity extremes in Drosophila melanogaster populations previously selected for long and short developmental period. Dev Genet. 1995;17(4):352–361. doi: 10.1002/dvg.1020170408. [DOI] [PubMed] [Google Scholar]
- 6.Tower J. Sex-specific regulation of aging and apoptosis. Mech Ageing Dev. 2006;127(9):705–718. doi: 10.1016/j.mad.2006.05.001. [DOI] [PubMed] [Google Scholar]
- 7.Austad SN. Why women live longer than men: sex differences in longevity. Gend Med. 2006;3(2):79–92. doi: 10.1016/s1550-8579(06)80198-1. [DOI] [PubMed] [Google Scholar]
- 8.United States Bureau of the Census . Statistical Abstract of the United States. 126th ed. Bernan Press; Blue Ridge Summit, PA: 2007. ISBN 978 1598880 7933. [Google Scholar]
- 9.Adams KF, Sueta CA, Gheorghiade M, et al. Gender differences in survival in advanced heart failure – insights from the FIRST study. Circulation. 1999;99(14):1816–1821. doi: 10.1161/01.cir.99.14.1816. [DOI] [PubMed] [Google Scholar]
- 10.Carroll JD, Carroll EP, Feldman T, et al. Sex-associated differences in left-ventricular function in aortic-stenosis of the elderly. Circulation. 1992;86(4):1099–1107. doi: 10.1161/01.cir.86.4.1099. [DOI] [PubMed] [Google Scholar]
- 11.Olivetti G, Giordano G, Corradi D, et al. Gender differences and aging – effects on the human heart. J Am Coll Cardiol. 1995;26(4):1068–1079. doi: 10.1016/0735-1097(95)00282-8. [DOI] [PubMed] [Google Scholar]
- 12.Lapane KL, Gambassi G, Landi F, Sgadari A, Mor V, Bernabei R. Gender differences in predictors of mortality in nursing home residents with AD. Neurology. 2001;56(5):650–654. doi: 10.1212/wnl.56.5.650. [DOI] [PubMed] [Google Scholar]
- 13.Silbiger SR, Neugarten J. The impact of gender on the progression of chronic renal-disease. Am J Kidney Dis. 1995;25(4):515–533. doi: 10.1016/0272-6386(95)90119-1. [DOI] [PubMed] [Google Scholar]
- 14.Silbiger SR, Neugarten J. The role of gender in the progression of renal disease. Adv Ren Replac Ther. 2003;10(1):3–14. doi: 10.1053/jarr.2003.50001. [DOI] [PubMed] [Google Scholar]
- 15.Colditz GA, Willett WC, Stampfer MJ, Rosner B, Speizer FE, Hennekens CH. Menopause and the risk of coronary heart-disease in women. N Engl J Med. 1987;316(18):1105–1110. doi: 10.1056/NEJM198704303161801. [DOI] [PubMed] [Google Scholar]
- 16.Gordon T, Kannel WB, Hjortland MC, Mcnamara PM. Menopause and coronary heart-disease – Framingham study. Ann Intern Med. 1978;89(2):157–161. doi: 10.7326/0003-4819-89-2-157. [DOI] [PubMed] [Google Scholar]
- 17.vanderSchouw YT, vanderGraaf Y, Steyerberg EW, Eijkemans MJC, Banga JD. Age at menopause as a risk factor for cardiovascular mortality. Lancet. 1996;347(9003):714–718. doi: 10.1016/s0140-6736(96)90075-6. [DOI] [PubMed] [Google Scholar]
- 18.Dimitrova KR, DeGroot K, Myers AK, Kim YD. Estrogen and homocysteine. Cardiovasc Res. 2002;53(3):577–588. doi: 10.1016/s0008-6363(01)00462-x. [DOI] [PubMed] [Google Scholar]
- 19.Guyton AC. Textbook of Medical Physiology. 4th ed W. B. Saunders Company; Philadelphia: 1973. [Google Scholar]
- 20.Hurn PD, Macrae IM. Estrogen as a neuroprotectant in stroke. J Cereb Blood Flow Metab. 2000;20(4):631–652. doi: 10.1097/00004647-200004000-00001. [DOI] [PubMed] [Google Scholar]
- 21.Wise PM, Dubal DB, Wilson ME, Rau SW, Liu Y. Estrogens: trophic and protective factors in the adult brain. Front Neuroendocrinol. 2001;22(1):33–66. doi: 10.1006/frne.2000.0207. [DOI] [PubMed] [Google Scholar]
- 22.Hazzard WR. Estrogen replacement and cardiovascular-disease – serum-lipids and blood-pressure effects. Am J Obstet Gynecol. 1989;161(6):1847–1853. doi: 10.1016/s0002-9378(89)80005-5. [DOI] [PubMed] [Google Scholar]
- 23.Hassager C, Riis BJ, Strom V, Guyene TT, Christiansen C. The long-term effect of oral and percutaneous estradiol on plasma-renin substrate and blood-pressure. Circulation. 1987;76(4):753–758. doi: 10.1161/01.cir.76.4.753. [DOI] [PubMed] [Google Scholar]
- 24.Wren BG, Brown LB, Routledge DA. Differential clinical-response to estrogens after menopause. Med J Aust. 1982;2(7):329–332. doi: 10.5694/j.1326-5377.1982.tb132453.x. [DOI] [PubMed] [Google Scholar]
- 25.Walsh BW, Schiff I, Rosner B, Greenberg L, Ravnikar V, Sacks FM. Effects of postmenopausal estrogen replacement on the concentrations and metabolism of plasma-lipoproteins. N Engl J Med. 1991;325(17):1196–1204. doi: 10.1056/NEJM199110243251702. [DOI] [PubMed] [Google Scholar]
- 26.Wahl P, Walden C, Knopp R, et al. Effect of estrogen progestin potency on lipid lipoprotein cholesterol. N Engl J Med. 1983;308(15):862–867. doi: 10.1056/NEJM198304143081502. [DOI] [PubMed] [Google Scholar]
- 27.Rijpkema AHM, Vandersanden AA, Ruijs AHC. Effects of postmenopausal estrogen-progestogen replacement therapy on serum-lipids and lipoproteins – a review. Maturitas. 1990;12(3):259–285. doi: 10.1016/0378-5122(90)90007-s. [DOI] [PubMed] [Google Scholar]
- 28.Iivonen S, Heikkinen T, Puolivali J, et al. Effects of estradiol on spatial learning, hippocampal cytochrome P450 19, and estrogen alpha and beta mRNA levels in ovariectomized female mice. Neuroscience. 2006;137(4):1143–1152. doi: 10.1016/j.neuroscience.2005.10.023. [DOI] [PubMed] [Google Scholar]
- 29.Cagnacci A, Soldani R, Carriero PL, Paoletti AM, Fioretti P, Melis GB. Effects of low-doses of transdermal 17-beta-estradiol on carbohydrate-metabolism in postmenopausal women. J Clin Endocrinol Metab. 1992;74(6):1396–1400. doi: 10.1210/jcem.74.6.1317387. [DOI] [PubMed] [Google Scholar]
- 30.Barrett-Connor E, Laakso M. Ischemic-heart-disease risk in postmenopausal women – effects of estrogen use on glucose and insulin levels. Arteriosclerosis. 1990;10(4):531–534. doi: 10.1161/01.atv.10.4.531. [DOI] [PubMed] [Google Scholar]
- 31.Amantea D, Russo R, Bagetta G, Corasaniti MT. From clinical evidence to molecular mechanisms underlying neuroprotection afforded by estrogens. Pharmacol Res. 2005;52(2):119–132. doi: 10.1016/j.phrs.2005.03.002. [DOI] [PubMed] [Google Scholar]
- 32.Brunet A, Bonni A, Zigmond MJ, et al. Akt promotes cell survival by phosphorylating and inhibiting a forkhead transcription factor. Cell. 1999;96(6):857–868. doi: 10.1016/s0092-8674(00)80595-4. [DOI] [PubMed] [Google Scholar]
- 33.Suzuki S, Brown CM, Wise PM. Mechanisms of neuroprotection by estrogen. Endocrine. 2006;29(2):209–215. doi: 10.1385/ENDO:29:2:209. [DOI] [PubMed] [Google Scholar]
- 34.Michos C, Kiortsis DN, Evangelou A, Karkabounas S. Antioxidant protection during the menstrual cycle: the effects of estradiol on ascorbic-dehydroascorbic acid plasma levels and total antioxidant plasma status in eumenorrhoic women during the menstrual cycle. Acta Obstet Gynecol Scand. 2006;85(8):960–965. doi: 10.1080/00016340500432812. [DOI] [PubMed] [Google Scholar]
- 35.Bednarek-Tupikowska G, Bohdanowicz-Pawlak A, Bidzinska B, Milewicz A, Antonowicz-Juchniewicz J, Andrzejak R. Serum lipid peroxide levels and erythrocyte glutathione peroxidase and superoxide dismutase activity in premenopausal and postmenopausal women. Gynecol Endocrinol. 2001;15(4):298–303. [PubMed] [Google Scholar]
- 36.Ha EJ, Smith AM. Plasma selenium and plasma and erythrocyte glutathione peroxidase activity increase with estrogen during the menstrual cycle. J Am Coll Nutr. 2003;22(1):43–51. doi: 10.1080/07315724.2003.10719274. [DOI] [PubMed] [Google Scholar]
- 37.Massafra C, Gioia D, De Felice C, et al. Effects of estrogens and androgens on erythrocyte antioxidant superoxide dismutase, catalase and glutathione peroxidase activities during the menstrual cycle. J Endocrinol. 2000;167(3):447–452. doi: 10.1677/joe.0.1670447. [DOI] [PubMed] [Google Scholar]
- 38.Chang SP, Yang WS, Lee SK, Min WK, Park JS, Kim SB. Effects of hormonal replacement therapy on oxidative stress and total antioxidant capacity in postmenopausal hemodialysis patients. Ren Fail. 2002;24(1):49–57. doi: 10.1081/jdi-120002660. [DOI] [PubMed] [Google Scholar]
- 39.Bednarek-Tupikowska G, Tupikowski K, Bidzinska B, et al. Serum lipid peroxides and total antioxidant status in postmenopausal women on hormone replacement therapy. Gynecol Endocrinol. 2004;19(2):57–63. doi: 10.1080/09513590412331272328. [DOI] [PubMed] [Google Scholar]
- 40.Bednarek-Tupikowska G, Tworowska U, Jedrychowska I, et al. Effects of oestradiol and oestroprogestin on erythrocyte antioxidative enzyme system activity in postmenopausal women. Clin Endocrinol. 2006;64(4):463–468. doi: 10.1111/j.1365-2265.2006.02494.x. [DOI] [PubMed] [Google Scholar]
- 41.Rossouw JE, Anderson GL, Prentice RL, et al. Risks and benefits of estrogen plus progestin in healthy postmenopausal women - principal results from the Women's Health Initiative randomized controlled trial. JAMA. 2002;288(3):321–333. doi: 10.1001/jama.288.3.321. [DOI] [PubMed] [Google Scholar]
- 42.Anderson GL, Limacher M, Assaf AR, et al. Effects of conjugated, equine estrogen in postmenopausal women with hysterectomy - the Women's Health Initiative randomized controlled trial. JAMA. 2004;291(14):1701–1712. doi: 10.1001/jama.291.14.1701. [DOI] [PubMed] [Google Scholar]
- 43.Rapp SR, Espeland MA, Shumaker SA, et al. Effect of estrogen plus progestin on global cognitive function in postmenopausal women - the Women's Health Initiative memory study: a randomized controlled trial. JAMA. 2003;289(20):2663–2672. doi: 10.1001/jama.289.20.2663. [DOI] [PubMed] [Google Scholar]
- 44.Shumaker SA, Legault C, Rapp SR, et al. Estrogen plus progestin and the incidence of dementia and mild cognitive impairment in postmenopausal women - the Women's Health Initiative memory study: a randomized controlled trial. JAMA. 2003;289(20):2651–2662. doi: 10.1001/jama.289.20.2651. [DOI] [PubMed] [Google Scholar]
- 45.Bureau I, Gueux E, Mazur A, Rock E, Roussel AM, Rayssiguier Y. Female rats are protected against oxidative stress during copper deficiency. J Am Coll Nutr. 2003;22(3):239–246. doi: 10.1080/07315724.2003.10719299. [DOI] [PubMed] [Google Scholar]
- 46.Kankofer M, Radzki RP, Bienko M, Albera E. Anti-oxidative/oxidative status of rat liver after ovariectomy. J Vet Med A Physiol Pathol Clin Med. 2007;54(5):225–229. doi: 10.1111/j.1439-0442.2007.00916.x. [DOI] [PubMed] [Google Scholar]
- 47.Sullivan JC, Sasser JM, Pollock DM, Pollock JS. Sexual dimorphism in renal production of prostanoids in spontaneously hypertensive rats. Hypertension. 2005;45(3):406–411. doi: 10.1161/01.HYP.0000156879.83448.93. [DOI] [PubMed] [Google Scholar]
- 48.Stancliffe TC, Pirie A. The production of superoxide radicals in reactions of the herbicide diquat. FEBS Lett. 1971;17(2):297–299. doi: 10.1016/0014-5793(71)80168-0. [DOI] [PubMed] [Google Scholar]
- 49.Gupta S, Husser RC, Geske RS, Welty SE, Smith CV. Sex differences in diquat-induced hepatic necrosis and DNA fragmentation in fischer 344 rats. Toxicol Sci. 2000;54(1):203–211. doi: 10.1093/toxsci/54.1.203. [DOI] [PubMed] [Google Scholar]
- 50.Ejima K, Nanri H, Araki M, Uchida K, Kashimura M, Ikeda M. 17 beta-estradiol induces protein thiol disulfide oxidoreductases and protects cultured bovine aortic endothelial cells from oxidative stress. Eur J Endocrinol. 1999;140(6):608–613. doi: 10.1530/eje.0.1400608. [DOI] [PubMed] [Google Scholar]
- 51.Gomez-Zubeldia MA, Arbues JJ, Hinchado G, Nogales AG, Millan JC. Influence of estrogen replacement therapy on plasma lipid peroxidation. Menopause J North Am Menopause Soc. 2001;8(4):274–280. doi: 10.1097/00042192-200107000-00009. [DOI] [PubMed] [Google Scholar]
- 52.Sverko V, Sobocanec S, Balog T, Marotti T. Age and gender differences in antioxidant enzyme activity: potential relationship to liver carcinogenesis in male mice. Biogerontology. 2004;5(4):235–242. doi: 10.1023/B:BGEN.0000038024.58911.6e. [DOI] [PubMed] [Google Scholar]
- 53.Munoz-Castaneda JR, Muntane J, Herencia C, et al. Ovariectomy exacerbates oxidative stress and cardiopathy induced by adriamycin. Gynecol Endocrinol. 2006;22(2):74–79. doi: 10.1080/09513590500490249. [DOI] [PubMed] [Google Scholar]
- 54.Munoz-Castaneda JR, Muntane J, Munoz MC, Bujalance I, Montilla P, Tunez I. Estradiol and catecholestrogens protect against adriamycin-induced oxidative stress in erythrocytes of ovariectomized rats. Toxicol Lett. 2006;160(3):196–203. doi: 10.1016/j.toxlet.2005.07.003. [DOI] [PubMed] [Google Scholar]
- 55.Baba T, Shimizu T, Suzuki Y, et al. Estrogen, insulin, and dietary signals cooperatively regulate longevity signals to enhance resistance to oxidative stress in mice. J Biol Chem. 2005;280(16):16417–16426. doi: 10.1074/jbc.M500924200. [DOI] [PubMed] [Google Scholar]
- 56.Butcher RL, Inskeep EK, Pope RS. Plasma concentrations of estradiol produced with 2 delivery systems in ovariectomized rats. Proc Soc Exp Biol Med. 1978;158(3):475–477. doi: 10.3181/00379727-158-40229. [DOI] [PubMed] [Google Scholar]
- 57.Behl C, Skutella T, Lezoualch F, et al. Neuroprotection against oxidative stress by estrogens: structure-activity relationship. Mol Pharmacol. 1997;51(4):535–541. [PubMed] [Google Scholar]
- 58.Suntres ZE. Role of antioxidants in paraquat toxicity. Toxicology. 2002;180(1):65–77. doi: 10.1016/s0300-483x(02)00382-7. [DOI] [PubMed] [Google Scholar]
- 59.Bus JS, Gibson JE. Paraquat - model for oxidant-initiated toxicity. Environ Health Perspect. 1984;55:37–46. doi: 10.1289/ehp.845537. APR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Fridovich I, Hassan HM. Paraquat and the exacerbation of oxygen-toxicity. Trends Biochem Sci. 1979;4(5):113–115. [Google Scholar]
- 61.Mohammadi-Bardbori A, Ghazi-Khansari M. Alternative electron acceptors: proposed mechanism of paraquat mitochondrial toxicity. Environ Toxicol Pharmacol. 2008;26(1):1–5. doi: 10.1016/j.etap.2008.02.009. [DOI] [PubMed] [Google Scholar]
- 62.Smith LL. Paraquat toxicity. Philos Trans R Soc Lond B Biol Sci. 1985;311(1152):647–657. doi: 10.1098/rstb.1985.0170. [DOI] [PubMed] [Google Scholar]
- 63.Patterson LK, Small RD, Scaiano JC. Reaction of paraquat radical cations with oxygen - pulse-radiolysis and laser photolysis study. Radiat Res. 1977;72(2):218–225. [Google Scholar]
- 64.Farrington JA, Ebert M, Land EJ, Fletcher K. Bipyridylium quaternary-salts and related compounds. 5. Pulse-radiolysis studies of reaction of paraquat radical with oxygen - implications for mode of action of bipyridyl herbicides. Biochim Biophys Acta. 1973;314(3):372–381. doi: 10.1016/0005-2728(73)90121-7. [DOI] [PubMed] [Google Scholar]
- 65.Thornele RN. Convenient electrochemical preparation of reduced methyl viologen and a kinetic study of reaction with oxygen using an anaerobic stopped-flow apparatus. Biochim Biophys Acta. 1974;333(3):487–496. doi: 10.1016/0005-2728(74)90133-9. [DOI] [PubMed] [Google Scholar]
- 66.Li Q, Yang XP, Sreejayan N, Ren J. Insulin-like growth factor I survival and antagonizes deficiency prolongs paraquat-induced cardiomyocyte dysfunction: role of oxidative stress. Rejuvenation Res. 2007;10(4):501–511. doi: 10.1089/rej.2007.0552. [DOI] [PubMed] [Google Scholar]
- 67.Ross WE, Block ER, Chang RY. Paraquat-induced DNA damage in mammalian-cells. Biochem Biophys Res Commun. 1979;91(4):1302–1308. doi: 10.1016/0006-291x(79)91208-7. [DOI] [PubMed] [Google Scholar]
- 68.Bus JS, Gibson JE, Aust SD. Superoxide-catalyzed and singlet oxygen-catalyzed lipid peroxidation as a possible mechanism for paraquat (methyl viologen) toxicity. Biochem Biophys Res Commun. 1974;58(3):749–755. doi: 10.1016/s0006-291x(74)80481-x. [DOI] [PubMed] [Google Scholar]
- 69.Bus JS, Aust SD, Gibson JE. Lipid peroxidation – possible mechanism for paraquat toxicity. Res Commun Chem Pathol Pharmacol. 1975;11(1):31–38. [PubMed] [Google Scholar]
- 70.Shu H, Talcott RE, Rice SA, Wei ET. Lipid peroxidation and paraquat toxicity. Biochem Pharmacol. 1979;28(2):327–331. doi: 10.1016/0006-2952(79)90523-9. [DOI] [PubMed] [Google Scholar]
- 71.Comporti M. Glutathione depleting agents and lipid-peroxidation. Chem Phys Lipids. 1987;45(2−4):143–169. doi: 10.1016/0009-3084(87)90064-8. [DOI] [PubMed] [Google Scholar]
- 72.Fisher HK, Clements JA, Tierney DF, Wright RR. Pulmonary effects of paraquat in first day after injection. Am J Physiol. 1975;228(4):1217–1223. doi: 10.1152/ajplegacy.1975.228.4.1217. [DOI] [PubMed] [Google Scholar]
- 73.Witschi H, Kacew S, Hirai KI, Cote MG. In vivo oxidation of reduced nicotinamide-adenine dinucleotide phosphate by paraquat and diquat in rat lung. Chem Biol Interact. 1977;19(2):143–160. doi: 10.1016/0009-2797(77)90027-8. [DOI] [PubMed] [Google Scholar]
- 74.Takizawa M, Komori K, Tampo Y, Yonaha M. Paraquat-induced oxidative stress and dysfunction of cellular redox systems including antioxidative defense enzymes glutathione peroxidase and thioredoxin reductase. Toxicol In Vitro. 2007;21(3):355–363. doi: 10.1016/j.tiv.2006.09.003. [DOI] [PubMed] [Google Scholar]
- 75.Daniel JW, Gage JC. Absorption and excretion of diquat and paraquat in rats. Br J Ind Med. 1966;23(2):133–136. doi: 10.1136/oem.23.2.133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Suntres ZE, Shek PN. Alleviation of paraquat-induced lung injury by pretreatment with bifunctional liposomes containing alpha-tocopherol and glutathione. Biochem Pharmacol. 1996;52(10):1515–1520. doi: 10.1016/s0006-2952(96)89626-2. [DOI] [PubMed] [Google Scholar]
- 77.Wegener T, Sandhagen B, Chan KW, Saldeen T. N-Acetylcysteine in paraquat toxicity – toxicological and histological-evaluation in rats. Ups J Med Sci. 1988;93(1):81–89. doi: 10.1517/03009734000000041. [DOI] [PubMed] [Google Scholar]
- 78.Hoffer E, Baum Y, Tabak A, Taitelman U. N-Acetylcysteine increases the glutathione content and protects rat alveolar type II cells against paraquat-induced cytotoxicity. Toxicol Lett. 1996;84(1):7–12. doi: 10.1016/0378-4274(95)03446-3. [DOI] [PubMed] [Google Scholar]
- 79.Cappelletti G, Maggioni MG, Maci R. Apoptosis in human lung epithelial cells: triggering by paraquat and modulation by antioxidants. Cell Biol Int. 1998;22(9−10):671–678. doi: 10.1006/cbir.1998.0305. [DOI] [PubMed] [Google Scholar]
- 80.Day BJ, Crapo JD. A metalloporphyrin superoxide dismutase mimetic protects against paraquat-induced lung injury in vivo. Toxicol Appl Pharmacol. 1996;140(1):94–100. doi: 10.1006/taap.1996.0201. [DOI] [PubMed] [Google Scholar]
- 81.Day BJ, Shawen S, Liochev SI, Crapo JD. A metalloporphyrin superoxide dismutase mimetic protects against paraquat-induced endothelial cell injury, in vitro. J Pharmacol Exp Ther. 1995;275(3):1227–1232. [PubMed] [Google Scholar]
- 82.Kang SA, Jang YJ, Park H. In vivo dual effects of vitamin C on paraquat-induced lung damage: dependence on released metals from the damaged tissue. Free Radic Res. 1998;28(1):93–107. doi: 10.3109/10715769809097880. [DOI] [PubMed] [Google Scholar]
- 83.Block ER. Potentiation of acute paraquat toxicity by vitamin-E-deficiency. Lung. 1979;156(3):195–203. doi: 10.1007/BF02714010. [DOI] [PubMed] [Google Scholar]
- 84.Mele J, Van Remmen H, Vijg J, Richardson A. Characterization of transgenic mice that overexpress both copper zinc superoxide dismutase and catalase. Antioxid Redox Signal. 2006;8(3−4):628–638. doi: 10.1089/ars.2006.8.628. [DOI] [PubMed] [Google Scholar]
- 85.Jang YC, Pérez VI, Song W, et al. Overexpression of Mn superoxide dismutase protects against oxidative stress but does not increase lifespan in mice. J Gerontol. 2009 doi: 10.1093/gerona/glp100. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Lei XG. Glutathione peroxidase-1 gene knockout on body antioxidant defense in mice. Biofactors. 2001;14(1−4):93–99. doi: 10.1002/biof.5520140113. [DOI] [PubMed] [Google Scholar]
- 87.Cheng WH, Ho YS, Valentine BA, Ross DA, Combs GF, Lei XG. Cellular glutathione peroxidase is the mediator of body selenium to protect against paraquat lethality in transgenic mice. J Nutr. 1998;128(7):1070–1076. doi: 10.1093/jn/128.7.1070. [DOI] [PubMed] [Google Scholar]
- 88.Rhodes ML, Zavala DC, Brown D. Hypoxic protection in paraquat poisoning. Lab Invest. 1976;35(5):496–500. [PubMed] [Google Scholar]
- 89.Bus JS, Gibson JE. Postnatal toxicity of chronically administered paraquat in mice and interactions with oxygen and bromobenzene. Toxicol Appl Pharmacol. 1975;33(3):461–470. doi: 10.1016/0041-008x(75)90072-1. [DOI] [PubMed] [Google Scholar]
- 90.Fisher HK, Clements JA, Wright RR. Enhancement of oxygen toxicity by herbicide paraquat. Am Rev Respir Dis. 1973;107(2):246–252. doi: 10.1164/arrd.1973.107.2.246. [DOI] [PubMed] [Google Scholar]
- 91.Autor AP. Reduction of paraquat toxicity by superoxide-dismutase. Life Sci. 1974;14(7):1309–1319. doi: 10.1016/0024-3205(74)90439-1. [DOI] [PubMed] [Google Scholar]
- 92.Douze JMC, Vanheijst ANP. Paraquat intoxication – oxygen a real poison. Acta Pharmacol Toxicol. 1977;41:241–245. [PubMed] [Google Scholar]
- 93.Kehrer JP, Haschek WM, Witschi H. Influence of hyperoxia on the acute toxicity of paraquat and diquat. Drug Chem Toxicol. 1979;2(4):397–408. doi: 10.3109/01480547909016033. [DOI] [PubMed] [Google Scholar]
- 94.Holm S. A simple sequentially rejective multiple test procedure. Scand J Stat. 1979;6:65–70. [Google Scholar]
- 95.Fleming TH, Harrington DP. Nonparametric estimation of the survival distribution in censored data. Commun Stat. 1984;13:2469–2486. [Google Scholar]
- 96.Harrington DP, Fleming TR. A class of rank test procedures for censored survival-data. Biometrika. 1982;69(3):553–566. [Google Scholar]
- 97.R Development Core Team . R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing; Vienna, Austria: 2007. [Google Scholar]
- 98.Nelson JF, Felicio LS, Osterburg HH, Finch CE. Altered profiles of estradiol and progesterone associated with prolonged estrous cycles and persistent vaginal cornification in aging C57Bl-6J mice. Biol Reprod. 1981;24(4):784–794. doi: 10.1095/biolreprod24.4.784. [DOI] [PubMed] [Google Scholar]
- 99.Schwartz NB. Acute effects of ovariectomy on pituitary LH uterine weight + vaginal cornification. Am J Physiol. 1964;207(6):1251–1259. doi: 10.1152/ajplegacy.1964.207.6.1251. [DOI] [PubMed] [Google Scholar]
- 100.Sullivan TR, Karas RH, Aronovitz M, et al. Estrogen inhibits the response-to-injury in a mouse carotid-artery model. J Clin Invest. 1995;96(5):2482–2488. doi: 10.1172/JCI118307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Cohen PE, Milligan SR. Silastic implants for delivery of estradiol to mice. J Reprod Fertil. 1993;99(1):219–223. doi: 10.1530/jrf.0.0990219. [DOI] [PubMed] [Google Scholar]
- 102.Hirai KI, Ikeda K, Wang GY. Paraquat damage of rat liver mitochondria by superoxide production depends on extramitochondrial NADH. Toxicology. 1992;72:1–16. doi: 10.1016/0300-483x(92)90081-o. [DOI] [PubMed] [Google Scholar]
- 103.Bus JS, Cagen SZ, Olgaard M, Gibson JE. Mechanism of paraquat toxicity in mice and rats. Toxicol Appl Pharmacol. 1976;35(3):501–513. doi: 10.1016/0041-008x(76)90073-9. [DOI] [PubMed] [Google Scholar]
- 104.Litchfield MH, Daniel JW, Longshaw S. The tissue distribution of the bipyridylium herbicides diquat and paraquat in rats and mice. Toxicology. 1973;1:155–165. doi: 10.1016/0300-483x(73)90029-2. [DOI] [PubMed] [Google Scholar]
- 105.Hoet PHM, Lewis CPL, Dinsdale D, Demedts M, Nemery B. Putrescine uptake in hamster lung slices and primary cultures of type-II pneumocytes. Am J Physiol Lung Cell Mol Physiol. 1995;13(5):L681–L689. doi: 10.1152/ajplung.1995.269.5.L681. [DOI] [PubMed] [Google Scholar]
- 106.Smith LL, Wyatt I. The accumulation of putrescine into slices of rat lung and brain and its relationship to the accumulation of paraquat. Biochem Pharmacol. 1981;30(10):1053–1058. doi: 10.1016/0006-2952(81)90441-x. [DOI] [PubMed] [Google Scholar]
- 107.Rose MS, Smith LL, Wyatt I. Evidence for energy-dependent accumulation of paraquat into rat lung. Nature. 1974;252(5481):314–315. doi: 10.1038/252314b0. [DOI] [PubMed] [Google Scholar]
- 108.Saibara T, Toda K, Wakasuki A, Ogawa Y, Ono M, Onishi S. Protective effect of 3-methyl-1-phenyl-2-pyrazolin-5-one, a free radical scavenger, on acute toxicity of paraquat in mice. Toxicol Lett. 2003;143(1):51–54. doi: 10.1016/s0378-4274(03)00113-9. [DOI] [PubMed] [Google Scholar]
- 109.Nagano N, Yagi M, Nishikori K. Protective effects of antioxidants on paraquat-induced acute-renal-failure in mice. Jpn J Pharmacol. 1992;59(4):481–483. doi: 10.1254/jjp.59.481. [DOI] [PubMed] [Google Scholar]
- 110.Chainy GBN, Samantaray S, Samanta L. Testosterone-induced changes in testicular antioxidant system. Andrologia. 1997;29(6):343–349. doi: 10.1111/j.1439-0272.1997.tb00328.x. [DOI] [PubMed] [Google Scholar]
- 111.Lacy F, Kailasam MT, O'Connor DT, Schmid-Schonbein GW, Parmer RJ. Plasma hydrogen peroxide production in human essential hypertension - role of heredity, gender, and ethnicity. Hypertension. 2000;36(5):878–884. doi: 10.1161/01.hyp.36.5.878. [DOI] [PubMed] [Google Scholar]
- 112.Waters DJ, Shen SR, Glickman LT. Life expectancy, antagonistic pleiotropy, and the testis of dogs and men. Prostate. 2000;43(4):272–277. doi: 10.1002/1097-0045(20000601)43:4<272::aid-pros6>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- 113.Hamilton JB, Mestler GE. Mortality and survival. Comparison of eunuchs with intact men and women in a mentally retarded population. J Gerontol. 1969;24(4):395–411. doi: 10.1093/geronj/24.4.395. [DOI] [PubMed] [Google Scholar]
- 114.Williams GC. Pleiotropy, natural selection, and the evolution of senescence. Evolution. 1957;11:398–411. [Google Scholar]
- 115.Suzuki S, Brown CM, Dela Cruz CD, Yang EH, Bridwell DA, Wise PM. Timing of estrogen therapy after ovariectomy dictates the efficacy of its neuroprotective and anti inflammatory actions. Proc Natl Acad Sci U S A. 2007;104(14):6013–6018. doi: 10.1073/pnas.0610394104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Johnson TE. Increased life-span of age-1 mutants in Caenorhabditis elegans and lower Gompertz rate of aging. Science. 1990;249(4971):908–912. doi: 10.1126/science.2392681. [DOI] [PubMed] [Google Scholar]
- 117.Bharathi NS, Prasad NG, Shakarad M, Joshi A. Variation in adult life history and stress resistance across five species of Drosophila. J Genet. 2003;82(3):191–205. doi: 10.1007/BF02715818. [DOI] [PubMed] [Google Scholar]