SUMMARY
Differentiating cells can dedifferentiate to replace stem cells in aged or damaged tissues, but the underlying mechanisms are unknown. In the Drosophila testis a cluster of stromal cells called the hub creates a niche by locally activating Janus-Kinase, Signal Transducer and Activator of Transcription (Jak-STAT) signaling in adjacent germline and somatic stem cells. Here we establish a novel system to study spermatogonial dedifferentiation. Ectopically expressing the differentiation factor Bag-of-marbles (Bam) removes germline stem cells from the niche. However, withdrawing ectopic Bam causes interconnected spermatogonia to fragment, move into the niche, exchange positions with resident somatic stem cells and establish contact with the hub. Concomitantly, Actin-based protrusions appear on subsets of spermatogonia, suggesting acquired motility. Furthermore, global downregulation of Jak-STAT signaling inhibits dedifferentiation, indicating that normal levels of pathway activation are required to promote movement of spermatogonia into the niche during dedifferentiation, where they outcompete somatic stem cells for niche occupancy.
Keywords: Stem cell niche, spermatogonia, dedifferentiation, Jak-STAT signaling, niche occupancy, cell motility, stem cell competition, Drosophila testis
INTRODUCTION
Adult stem cells divide asymmetrically, producing both stem cells and differentiating cells that usually undergo clonal amplification (or transit-amplification) before fully differentiating to regenerate tissues. Differentiation is generally considered irreversible. However, increasing evidence suggests that transit-amplifying cells show flexibility in their commitment towards differentiation (Raff, 2003). The reversion of a more differentiated cell back to a less differentiated state, or dedifferentiation, has classically been studied during amphibian regeneration (Straube and Tanaka, 2006). Recent examples include the conversion of cultured adult human fibroblasts into induced pluripotent stem cells (Nishikawa et al., 2008). Within stem cell niches, most of what is known of dedifferentiation comes from studies of the Drosophila ovary and testis (Fuller and Spradling, 2007; Morrison and Spradling, 2008). In these tissues, lost germline stem cells (GSCs) are replenished by the reversion of differentiating germ cells (Brawley and Matunis, 2004; Kai and Spradling, 2004). Dedifferentiation also sustains GSC replacement in wild-type testes during aging (Cheng et al., 2008), and has recently been shown to occur in the mouse testis (Nakagawa et al., 2007; Barroca et al., 2008), indicating that this process is a highly conserved feature of stem cell niches.
The cellular and molecular composition of the Drosophila testis niche is well characterized (Fuller, 1998; Li and Xie, 2005). The testis apex contains approximately 9 GSCs that adhere to a cluster of quiescent somatic cells called the hub. The hub creates a stem cell niche by secreting the ligand Unpaired (Upd), which activates the Jak-STAT signaling pathway in adjacent cells to promote stem cell maintenance (Kiger et al., 2001; Tulina and Matunis, 2001; Leatherman and Dinardo, 2008). GSC divisions are oriented such that one daughter maintains contact with the hub while the other is displaced, becoming a gonialblast (Yamashita et al., 2003). Gonialblasts undergo 4 mitotic divisions with incomplete cytokinesis, producing clusters of 16 interconnected spermatogonia which give rise to spermatocytes and ultimately to sperm (Fuller, 1998). Approximately 20 cyst progenitor cells (CPCs) also attach to the hub, generating a continuous supply of non-mitotic somatic support cells called cyst cells (Hardy et al., 1979; Gönczy and DiNardo, 1996). A pair of cyst cells envelops each gonialblast and its progeny, providing signals mediating differentiation (Matunis et al., 1997).
We serendipitously found that spermatogonia can revert to GSCs by manipulating the levels of the stem cell maintenance factor STAT92E. Conditionally removing STAT92E causes GSCs to differentiate into spermatogonia. However, if STAT92E function is restored while spermatogonia remain in the tissue, testes completely lacking GSCs regain new GSCs. Spermatogonial cysts, which remain adjacent to the hub under these conditions, fragment into single cells that become functional GSCs (Brawley and Matunis, 2004). To elucidate the mechanisms underlying dedifferentiation, we sought to identify additional cases in which it occurs. Here we find that spermatogonial dedifferentiation can be induced at high levels in the Drosophila testis by conditionally manipulating the differentiation factor Bag-of-marbles (Bam).
Bam is a novel protein required for germ cell differentiation. Ovaries or testes lacking Bam accumulate undifferentiated stem-like cells or spermatogonia, respectively (McKearin and Spradling, 1990; Gönczy et al., 1997). In both systems, Bone Morphogenic Protein signaling from surrounding somatic cells inhibits bam transcription in GSCs (Chen and McKearin, 2003; Kawase et al., 2004). Consistent with this finding, ectopic Bam is necessary and sufficient to induce differentiation of GSCs in the ovary and loss of the germline lineage in the testis (Ohlstein and McKearin, 1997; Kawase et al., 2004; Schulz et al., 2004 Flatt et al., 2008). Furthermore, conditional manipulation of Bam expression causes differentiating germ cells to revert to GSCs in the larval ovary (Kai and Spradling, 2004). However, it was not known whether manipulating Bam expression in the testis could induce spermatogonial dedifferentiation. Here we show that conditional manipulation of Bam expression triggers dedifferentiation, and that this process involves both signaling from the stem cell niche and dynamic cellular rearrangements, including displacement of somatic stem cells by spermatogonia.
RESULTS
Ectopic expression of Bam induces GSC differentiation in the testis
Spermatogonia can revert to GSCs in wild-type testes, replenishing GSCs lost due to turnover (Wallenfang et al., 2006; Cheng et al., 2008). This process occurs infrequently and is not understood mechanistically; therefore we sought to establish a novel system in which multiple dedifferentiation events could be studied. Ectopic expression of the differentiation factor Bam causes GSC loss. We hypothesized that if testes subjected to ectopic Bam still contained spermatogonia, withdrawal of ectopic Bam could stimulate their reversion to GSCs. Since this would require dose-dependent, reversible manipulation of Bam levels, we assessed the level and distribution of this protein before, during, and after administration of heat-shocks in testes from flies expressing bam under the control of the Hsp70 promoter (referred to as Hs-bam) (Ohlstein and McKearin, 1997). Before heat-shock, Bam was enriched in the cytoplasm of late 2-cell through late 8-cell spermatogonia, while GSCs and older spermatocytes lacked Bam (Fig. S1A) - a distribution indistinguishable from that seen in wild-type (Gönczy et al., 1997; Kiger et al., 2001; Brawley and Matunis, 2004; Kawase et al., 2004; Schulz et al., 2004). After 2 heat-shocks, Bam was detected in most cells within the testis, notably in GSCs (Fig. S1B). Bam levels increased with additional heat-shocks (Fig. S1C). However, the level and distribution of Bam returned to wild-type after heat-shocked flies were returned to 18°C for 6 days (Fig. S1D). We conclude that this approach produces high levels of transient ectopic Bam throughout the testis.
We next asked if incrementally increasing the level of ectopic Bam yields a corresponding change in the number of GSCs in the testis. Progressively higher numbers of heat-shocks were administered to Hs-bam flies, and GSCs and spermatogonia were quantified by immunostaining and serial confocal microscopy. A cell was classified as a GSC if it was located adjacent to the hub, expressed the germ cell-specific marker Vasa, and contained a spherical fusome. The fusome is a germ cell-specific organelle rich in cytoskeletal components that is spherical in GSCs and gonialblasts, but elongates and eventually branches in 2 to 16-cell spermatogonia (Lin et al., 1994; Hime et al., 1996; de Cuevas et al., 1997). Testes from Hs-bam flies contained a full complement of GSCs before heat-shocks (Fig. 1A, arrowhead), but progressively lost GSCs with increasing numbers of heat-shocks (Fig. 1B-G). GSC numbers were unaffected in control flies processed in parallel (Fig. 1G), indicating that GSC loss was specifically due to ectopic Bam expression.
Figure 1. Ectopic Bam expression causes progressive GSC loss due to differentiation.
(A-F) Hs-bam testes stained with Vasa (red, germ cells); Armadillo (green, hub, *); 1B1 (green, fusomes); and DAPI (blue, nuclei). (A) Without heat-shocks, testes are indistinguishable from wild-type and contain GSCs (arrowhead) and CPCs (arrow); spermatogonial cysts and spermatocytes are positioned progressively further from the hub. (B-F) Increasing heat-shocks depletes GSCs, but spermatogonial cysts and Vasa-negative cells (arrows) remain. (G) The average number of GSCs/testis (± standard deviation) falls with increasing ectopic Bam expression. (H) The average number of GSCs, gonialblasts and 2-cell cysts/testis (± standard deviation) inversely correlates with the number of heat-shocks. (I-J) Testes stained with TUNEL labeling (green, apoptotic cells); DAPI (blue, nuclei); Fasciclin III (red, hub, *). TUNEL-positive GSCs were not detected in wild-type or Hs-bam testes after 5 heat-shocks; apoptotic spermatogonial cysts were observed as expected. Scale bars = 10μm.
Lost GSCs could differentiate into spermatogonia or die. To distinguish between these possibilities, we assayed for apoptotic cells within testes using TUNEL (Terminal Deoxynucleotidyl Transferase Fluorescein-dUTP Nick End Labeling). TUNEL-positive GSCs were not observed in heat-shocked testes from either control or Hs-bam flies (n>46) (Fig. 1I-J), suggesting that GSCs differentiated, rather than died. However, the assay was working appropriately, since TUNEL-positive spermatogonial cysts were found in both control and Hs-bam testes as expected (Fig. 1I-J) (Brawley and Matunis, 2004). Furthermore, as the number of heat-shocks increased, there was a gradual loss of early germ cells, rather than an accumulation of any particular stage (Fig. 1H). Thus, GSCs likely differentiate under these conditions.
Previous experiments have shown that spermatogonia, but not spermatocytes, can revert to functional GSCs (Brawley and Matunis, 2004). Thus, we sought conditions in which Hs-bam testes lacked GSCs yet contained spermatogonia. After 5 heat-shocks, Hs-bam flies contained an average of 0.7±1.0 GSCs/testis (n=74), and 53% of testes had no GSCs. However, the average number of spermatogonial cysts/testis was 9.9±2.2 (n=25). Since additional heat-shocks impaired adult viability and yielded testes containing few spermatogonia (see below), 5 heat-shocks were used for all subsequent experiments.
Somatic stem cells fill the area adjacent to the hub as GSCs are lost
Upon ectopic Bam expression, differentiating spermatogonia were not always found next to the hub, as occurs when STAT92E function is withdrawn (Brawley and Matunis, 2004). In many Hs-bam testes, spermatogonia were displaced from the niche, and as the number of GSCs decreased, a population of Vasa-negative somatic cells became apparent in the region surrounding the hub (Fig. 1B-F, arrows). To characterize these somatic cells, we immunostained testes for the transcription factor Zinc Finger Homeodomain 1 (Zfh-1) which is highly enriched in CPCs (Leatherman and DiNardo, 2008). Before heat-shock, Hs-bam testes contained 28.5±1.9 (n=24) Zfh-1-positive cells/testis apex (Fig. 2A). After heat-shock, this number did not change significantly (26.9±2.1, n=29, p=0.59) (Fig. 2B). Since CPCs are mitotically active, unlike their differentiating daughters (cyst cells), an additional hallmark of CPC identity is the ability to incorporate BrdU (Gönczy and DiNardo, 1996). BrdU-positive somatic cells were detected adjacent to the hub in 77% (n=23) of Hs-bam testes following GSC depletion (Fig. 2B, inset). Finally, the transcription factor Eyes Absent (Eya), which marks cyst cells but not CPCs (Fabrizio et al., 2003; Leatherman and DiNardo, 2008), was undetectable in the somatic cells surrounding the hub both before and after heat-shock (Fig. 2C-D). Together these data indicate that ectopic Bam does not alter the number of CPCs or trigger their premature differentiation.
Figure 2. Ectopic Bam expression alters the position, but not the number or identity, of somatic cells within the niche.
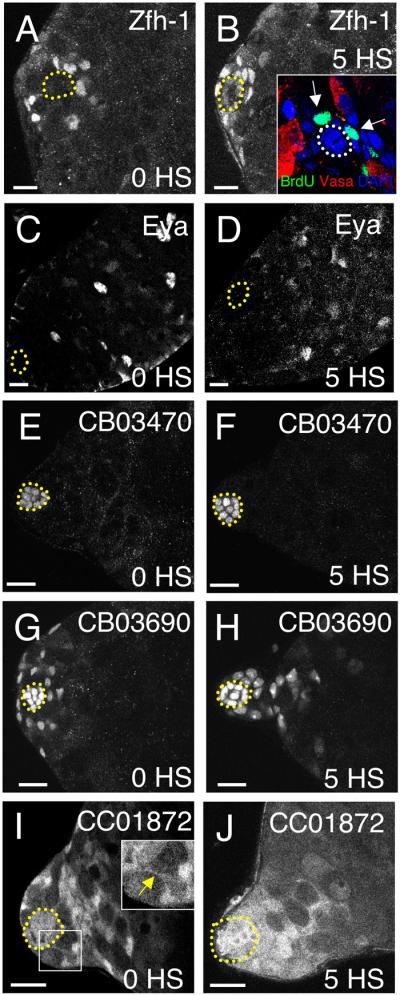
(A-J) Expression of markers in testes from Hs-bam flies before (A,C,E,G,I) or after (B,D,F,H,J) induction of ectopic Bam. Hubs are outlined in all panels. (A-B) Zfh-1 marks CPC nuclei before heat-shock; these appear closer to the hub following heat-shock. (B, inset) Somatic (Vasa-negative) nuclei adjacent to the hub incorporate BrdU (green) in vitro after 5 heat-shocks. (C-D) Mature cyst cells normally express Eya; this marker remains off in somatic cells adjacent to the hub after induction of ectopic Bam. (E-F) The hub marker CB03470 is unchanged after heat-shocks, indicating that somatic cells adjacent to the hub do not acquire hub cell identity under these conditions. (G-H) CB03690 marks CPC and hub cell nuclei. CPC nuclei are closer to the hub after expression of ectopic Bam. (I-J) CC01872 is predominantly expressed throughout somatic cells, revealing thin cytoplasmic extensions on CPCs (arrow, inset) contacting the hub. After heat-shock, the distribution of CC01872 is altered, with GFP-positive somatic cells surrounding and broadly contacting the hub. Scale bars = 10μm.
These results were confirmed and extended by introducing three different GFP-protein trap lines into Hs-bam flies (Fig. 2E-L). Expression of the hub-specific GFP-protein trap line CB03470 revealed that the number and appearance of hub cells were unaffected in our assay (Fig. 2E-F) (22.8±1.6, n=10, versus 24.6±1.6, n=9, hub cells/testis before and after heat-shock, p=0.96). Line CC03690, which marks the nuclei of hub and early somatic cells, appeared similar before and after heat-shock, except that the early somatic cell nuclei were closer to the hub after heat-shock (Fig. 2G-H). Line CC01872, which highlights the cytoplasm of hub and early somatic cells, revealed that the fine extensions CPCs normally extend toward the hub (Fig. 2I, inset, arrowhead) (Hardy et al., 1979) were replaced by broad areas of contact (Fig. 2J). In conclusion, although ectopic Bam causes GSCs to differentiate, hub cells and CPCs remain at constant levels in the niche but CPCs move in to fill the area surrounding the hub upon GSC loss.
Spermatogonial dedifferentiation gives rise to new GSCs upon withdrawal of ectopic Bam
We next asked if testes from Hs-bam flies that had been depleted of GSCs could regain them after recovery at 18°C. After 1 day of recovery, the average number of GSCs fell to 0.1±0.3 and only 10% of testes contained any GSCs (Fig. 3D-E). This observation suggests that GSCs continue to differentiate after the last heat-shock, likely due to perdurance of ectopic Bam. Consistent with this finding, the area adjacent to the hub remained full of somatic cells in most testes (Fig. 3A). However, after 4 days of recovery, most testes contained both GSCs and spermatogonial cysts adjacent to the hub (Fig. 3B), and by 6 days of recovery, most were phenotypically indistinguishable from wild-type (Fig. 3C-D).
Figure 3. Spermatogonial dedifferentiation occurs upon withdrawal of ectopic Bam.
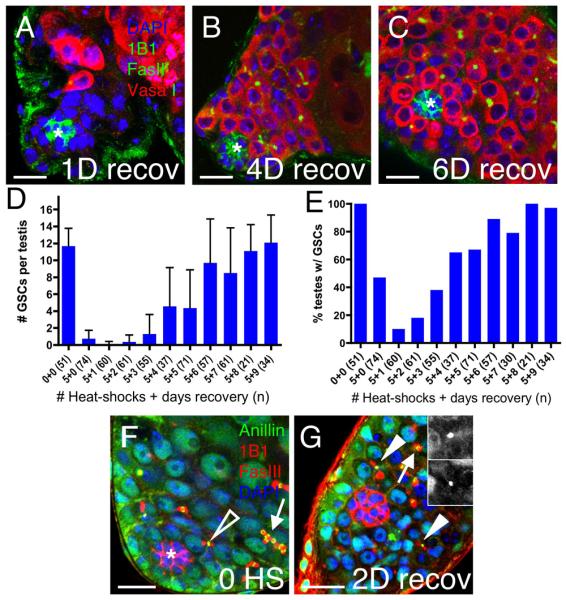
(A-C) Vasa (red) marks germ cells, Fasciclin III (green) marks the hub (asterisk), 1B1 (green) marks fusomes, and DAPI (blue) marks nuclei. (A) One day after withdrawal of ectopic Bam, niches are often filled with somatic cells but become indistinguishable from wild-type by (B) 4 and (C) 6 days of recovery. (D) The average number of GSCs/testis (± standard deviation) and (E) the percentage of testes containing GSCs rise during recovery. (F-G) Anillin (green) marks ring canals, Fasciclin III and 1B1 (red) mark the hub and fusomes, respectively, and DAPI (blue) marks nuclei in testes from Hs-bam flies. (F) Before heat-shock, stable ring canals are observed in spermatogonial cysts (arrow); smaller transient ring canal remnants are only apparent between GSCgonialblast pairs (open arrowhead). (G) After 5 heat-shocks and 2 days of recovery, ring canal remnants appear in cells near the hub (2 visible in this focal plane, arrowheads, insets); open ring canals remain in spermatogonial cysts displaced from the hub (arrow). Scale bars = 10μm.
Missing GSCs could be regained either by dedifferentiation of spermatogonia or by division of remaining GSCs. To distinguish between these possibilities, we determined the percentage of testes containing any GSCs during the entire experiment (Fig. 3E). If new GSCs arise via spermatogonial dedifferentiation, testes devoid of GSCs (but not spermatogonia) before recovery could acquire new GSCs. In contrast, if GSCs are the sole source of new GSCs, then the percentage of testes containing GSCs should not rise above that seen after GSC depletion. Comparing the percentage of testes containing any GSCs before and after recovery eliminated the second possibility. After 1 day of recovery, only 10% of testes (n=60) contained any GSCs; however, 86% contained GSCs after 5 or more days of recovery (n=203). Therefore, testes completely lacking GSCs are able to generate new stem cells even in niches fully occupied by CPCs.
Our previous studies showed that spermatogonia but not spermatocytes can regenerate missing GSCs (Brawley and Matunis, 2004). Here, the presence of spermatogonia but not spermatocytes also correlates with the ability of the testis to regain missing GSCs. Administering 10 heat-shocks reduced the number of testes containing spermatogonia to 48% (n=21), and only 36% (n=88) regained GSCs upon withdrawal of ectopic Bam. Administering 15 heat-shocks depleted spermatogonia from all testes (n=28), and none regained GSCs (n=33).
Previous experiments have demonstrated that cysts in the testis or ovary fragment during dedifferentiation, generating multiple ring canal remnants near the stem cell niche (Brawley and Matunis, 2004; Kai and Spradling, 2004; Cheng et al., 2008). Ring canals, which are normally transient between GSCs and their daughters but stable between differentiating germ cells, can be detected by immunostaining for the Anillin protein (Field and Alberts, 1995; Hime et al., 1996; de Cuevas and Spradling, 1998). Before ectopic Bam expression, Hs-bam testes contain ring canals indistinguishable from wild-type, with transient remnants between GSCs and gonialblasts (Fig. 3F, open arrowhead) and stable, open ring canals between interconnected spermatogonia (Fig. 3F, arrow). Importantly, remnants are only detected between GSC-gonialblast pairs adjacent to the hub. In contrast, when testes depleted of GSCs via ectopic Bam expression are allowed to recover, multiple ring canal remnants are detected in germ cells near, but not necessarily adjacent to the hub beginning at 2 days of recovery (Fig. 3G, arrowheads; Table 1). Such remnants could be products of either GSC division or spermatogonial breakdown. However, at 2 days of recovery, we observed 26 ring canal remnants in 20 recovering testes containing an estimated total number of 7 GSCs. These remnants cannot all be products of GSC divisions, since there are more than three times as many remnants as GSCs. Instead, most must be products of spermatogonial breakdown. Consistent with our observation that most testes regain a full complement of GSCs by 6 days of recovery, remnants were not readily apparent at this time-point (Table 1). Thus, withdrawal of ectopic Bam causes spermatogonial cysts near the hub to fragment and regenerate missing GSCs in a gradual process of dedifferentiation spanning 5 or 6 days.
Table 1.
Ring Canal Remnants Indicate Spermatogonial Fragmentation During Recovery
Ring canal remnants were identified by immunostaining with Anillin; germ cell ring canals were distinguished from their somatic counterparts by co-localization of the germ-cell specific marker 1B1.
| # Heat-shocks, days recovery | # Testes | # GSCs1 | # Ring canal remnants |
|---|---|---|---|
| 0, 0 | 22 | 257 | 3 |
| 5, 0 | 23 | 17 | 0 |
| 5, 1 | 21 | 2 | 0 |
| 5, 2 | 20 | 7 | 26 |
| 5, 3 | 18 | 23 | 8 |
| 5, 4 | 23 | 104 | 7 |
| 5, 6 | 18 | 175 | 0 |
calculated by multiplying the average number of GSCs (Fig. 5D) by the number of testes at each time-point.
Spermatogonia do not require contact with the hub to initiate dedifferentiation
When GSCs are depleted via conditional loss of STAT92E, each testis contains spermatogonia in close association with the hub, and these cysts are thought to undergo dedifferentiation (Brawley and Matunis, 2004). In contrast, many Hs-bam testes depleted of GSCs appeared to lack germ cells contacting the hub (e.g. Fig. 1D), suggesting that spermatogonia do not require intimate contact with the hub in order to dedifferentiate. However, accurate determination of germ cell position was difficult using the cytoplasmic marker Vasa. Thus, we assessed the distribution of the Actin cytoskeleton in germ cells by expressing the Actin-binding domain of Moesin fused to GFP (GMA) using the germ cell-specific nanos-Gal4-VP16 driver (Van Doren et al., 1998; Dutta et al., 2002). GMA localized to the cortex of germ cells, facilitating accurate quantification of germ cell position with respect to the hub in Hs-Bam flies (Fig. 4A).
Figure 4. Actin-based protrusions appear in 4- and 8-cell spermatogonial cysts in testes undergoing dedifferentiation.
(A,B,D,E) In Hs-bam testes, GMA driven marks cortical F-Actin in germ cells (green) 1B1 (red) marks fusomes; Armadillo (red) marks the hub; and DAPI (blue) marks nuclei (genotype: Hs-bam; nanos-Gal4-VP16/+; UAS-GMA/+) (A′,B′,D′,E′). GMA (green) channel. (A) Before expression of ectopic Bam, GSCs extend short Actin-rich projections (arrowhead) into the hub, while 4- and 8-cell spermatogonia lack protrusions (arrow), and gonialblasts display randomly oriented protrusions (open arrowhead). (B) After expression of ectopic Bam, spermatogonial cysts (arrow) often do not contact the hub. (C) The percentage of testes containing germ cells less than 3μm from the hub throughout the time course of ectopic Bam expression. (D) Testes appear phenotypically normal after 5 days of recovery; a rosette of polarized GSCs (arrowhead) flanked by CPCs surrounds the hub and spermatogonia (arrow) fill the testis. (E) Fine Actin-based protrusions on the surface of a 4-cell spermatogonial cyst (inset) at 1 day of recovery. (F) The percentage of testes containing 4-cell (blue) and 8-cell (purple) spermatogonial cysts with protrusions before, during and after recovery. Scale bars = 10μm.
All testes from both Hs-bam; nanos-Gal4-VP16; UAS-GMA and control flies contained germ cells located within 3 μm of the hub (n=46) before expression of ectopic Bam. After ectopic Bam expression only 60% of Hs-bam; nanos-Gal4-VP16; UAS-GMA testes still contained germ cells within 3 μm of the hub (n=46). Thus, the remaining 40% of testes contained hubs entirely surrounded by somatic cells (Fig. 4B-C). Nonetheless, upon withdrawal of ectopic Bam, germ cells returned to the hub, and all testes contained germ cells at the hub by 7 days of recovery (n=51, Fig. 4C-D). A Fisher exact test showed that the percentage of testes containing germ cells contacting the hub was significantly different before and after 7 days of recovery (p=6×10−8). Thus, even testes with no germ cells contacting the hub regain a full complement of new stem cells upon withdrawal of ectopic Bam.
A subset of spermatogonia acquire dynamic actin-based protrusions in testes undergoing dedifferentiation
As our results indicated that cellular rearrangement accompanies dedifferentiation, we further characterized the localization of GMA within germ cells during this process. In control testes, GMA localized to the cell cortex of all germ cells and was enriched at cell contacts between GSCs and the hub, and between interconnected spermatogonia (Fig. 4A). GMA was unpolarized with respect to the hub in gonialblasts and their descendants. Interestingly, short protrusions were apparent on all gonialblasts (Fig. 4A-A′, open arrowhead) and some early 2-cell cysts. These protrusions may be indicative of interactions of these germ cells with CPCs or cyst cells and merit future studies.
In control testes, we did not see 4, 8, or 16-cell spermatogonia with protrusions (n=21, Fig. 4A,F). In contrast, testes regaining GSCs contained 4 and 8-cell spermatogonial cysts with fine Actin-rich protrusions (Fig. 4E, inset) whose appearance correlated with the timecourse of dedifferentiation (Fig. 4F). Protrusions were found on one or two cysts in testes where they occurred, appeared on one or multiple spermatogonia within a cyst, and were approximately 0.2 microns in diameter and 1 micron in length. Time-lapse imaging of live testes undergoing dedifferentiation for 1.5 h revealed that the protrusions were dynamic (Fig. S2). With this technique, 51% of testes undergoing dedifferentiation contained 4-cell spermatogtonial cysts with protrusions (n=35, Fig. S2B-C), compared to 8.3% of control testes (n=24, Fig. S2A). Together, our data suggest that the ability of differentiating germ cells to outcompete CPCs for niche occupancy may be an active process involving the acquisition of motility within a small subset of spermatogonia.
Partial inhibition of Jak-STAT signaling disrupts spermatogonial dedifferentiation
Since spermatogonia extending protrusions are found near, but not necessarily contacting, the hub in testes undergoing dedifferentiation, we considered that local signals from the niche could facilitate dedifferentiation. Although Jak-STAT signaling maintains GSCs and CPCs in this tissue (Kiger et al., 2001; Tulina and Matunis, 2001; Leatherman and DiNardo, 2008), the role of this or any other signaling pathway during spermatogonial dedifferentiation was unknown. Having established a way to induce dedifferentiation without manipulating Jak-STAT signaling, we could now ask if this pathway acts during the process.
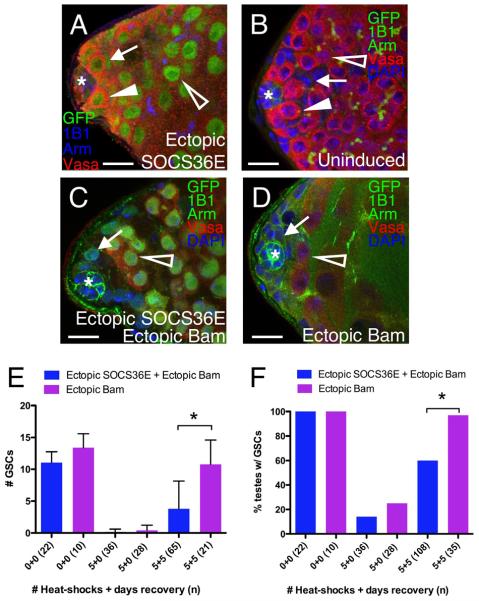
Since complete loss of Jak-STAT signaling forces both somatic and germline stem cells to differentiate, we sought to partially inhibit this pathway by misexpressing the Jak-STAT inhibitor Suppressor of cytokine signaling 36E (SOCS36E) using a UAS-SOCS36E transgene (Callus and Mathey-Prevot, 2002). When combined with a strong germline driver, this transgene recapitulated previous findings and caused GSC loss (0.4±0.3 GSCs/testis, n=12), a result consistent with loss of Jak-STAT signaling (Kiger et al., 2001; Tulina and Matunis, 2001; Brawley and Matunis, 2004; Terry et al., 2006). However, when combined with an inducible Gal4 driver, this transgene gives moderate constitutive expression of SOCS36E misexpression as revealed by co-expression of a UAS-GFP marker (Fig. 5A) (see methods for details). This moderate misexpression did not cause a change in cell morphology or a significant loss of GSCs compared to similarly heat-shocked sibling controls (p=0.71), suggesting that Jak-STAT signaling was not fully abolished. Although both SOCS36E misexpressing testes and sibling controls had the same number of GSCs/testis (6.4±0.3, n=52, versus 6.3±0.2 n=35), they both had significantly fewer GSCs compared to un-heat-shocked flies of the same genotypes (11.8±2.1 GSCs/testis, n=32, p<0.05), indicating that the heat-shock regime reduces GSC numbers. Importantly, this GSC loss is not due to SOCS36E misexpression, so these flies can be used to study spermatogonial dedifferentiation.
Figure 5. Attenuation of Jak-STAT signaling inhibits spermatogonial dedifferentiation.
(A-D) Examples of GSCs (arrowheads), spermatogonia (open arrowheads) and CPC nuclei (arrow) are indicated. (A) Testes from flies misexpressing moderate levels of UAS-SOCS36E maintain GSCs, as revealed by immunostaining for GFP (green, marks nuclei of cells misexpressing SOCS36E), Armadillo and 1B1 (blue, marks the hub and fusomes, respectively), and Vasa (red, marks germ cells). (B-D) Testes immunostained for GFP (SOCS36E misexpressing cells), 1B1, marks fusomes, and Armadillo, marks the hub (green); Vasa (red), marks germ cells; and DAPI (blue). (B) Before heat-shock, testes from flies containing Hs-bam and UAS-SOCS36E have wild-type morphology; GFP is undetectable as expected. Heat-shock-induced misexpression of (C) both SOCS36E (green nuclei) and Bam or (D) Bam alone yields morphologically indistinguishable testes: GSCs are lost but CPCs (arrows) and spermatogonia (open arrowheads) remain. (E) The average number of GSCs (± standard deviation) and (F) the percentage of testes containing GSCs from testes represented in panels B-D. GSCs in testes misexpressing SOCS36E and Bam (blue) are significantly reduced (asterisks) at 5 days of recovery compared to testes misexpressing Bam alone (purple). Scale bars = 10μm.
To determine the effect of ectopic SOCS36E on dedifferentiation, we introduced the same inducible system to moderately misexpress UAS-SOCS36E (described above) into the Hs-Bam background. Testes from these flies were indistinguishable from wild-type before heat-shock (Fig. 5B). Furthermore, upon heat-shock (which induced ectopic Bam, SOCS36E and a GFP marker) testes lost GSCs but retained spermatogonia in a manner indistinguishable from siblings expressing Bam alone (Fig. 5C-D, open arrowhead). In contrast, when assayed for dedifferentiation via withdrawal of ectopic Bam, testes expressing ectopic SOCS36E displayed a marked decrease in their ability to regain new GSCs. The average number of GSCs was significantly decreased after recovery compared to controls (Fig. 5E), and only 60% (n=108) of SOCS36E-expressing testes regained GSCs compared to 97% (n=35) of control testes (Fig. 5F). Together these data strongly suggest that global inhibition of Jak-STAT signaling inhibits spermatogonial dedifferentiation.
To further confirm the requirement for Jak-STAT signaling during dedifferentiation, we examined the distribution of the STAT92E protein in testes, which reflects Jak-STAT pathway activity and is enriched in GSCs and GSC-gonialblast pairs, but not spermatogonia (Chen et al., 2002; Boyle et al., 2007). The distribution of STAT92E was indistinguishable from wild-type in Hs-bam testes before heat-shock. However, after heat-shock STAT92E became visible in a few 4-16 cell spermatogonial cysts, and we found a significant percentage of testes containing 4-cell spermatogonia with enriched STAT92E expression during recovery (Fig. S3, Table S1). In all cases, spermatogonia enriched in STAT92E were located adjacent to the hub, suggesting that Jak-STAT signaling is activated locally in spermatogonia near the testis apex during spermatogonial dedifferentiation. The pathway is likely upregulated in somatic cells as well, since STAT92E-positive somatic cells became apparent after GSC depletion (Fig. S3B).
These results above suggested that cells entering the GSC-depleted niche are programmed to activate the Jak-STAT pathway, implying that the ligand Unpaired might be present during this process. We found that the level and distribution of Upd mRNA was indistinguishable from wild-type in Hs-bam testes throughout the dedifferentiation assay (Fig. S4). These results confirm our finding that Jak-STAT activation is involved in dedifferentiation and suggest that syncytial spermatogonia entering the niche respond to local Jak-STAT signaling by expressing GSC factors before and/or during cyst fragmentation. This is the first signaling pathway implicated in dedifferentiation, and while future studies are needed to determine the cellular cause(s) for this requirement, our results suggest that spermatogonial dedifferentiation is sensitive to partially reduced levels of Jak-STAT signaling.
DISCUSSION
How cells committed to differentiate can revert to earlier, less differentiated cell types is a central question in stem cell biology. Although dedifferentiation has been observed in both invertebrates and vertebrates, much remains unknown about the underlying cellular and molecular mechanisms. Here we establish a novel strategy for studying the reversion of spermatogonia to GSCs in the Drosophila testis. This work reveals that a subset of spermatogonia gain access to niches completely filled with somatic stem cells and regenerate missing GSCs in an active process involving both cell movement and Jak-STAT signaling.
Dedifferentiation may involve concomitant homing into the niche and reversion to stem cell identity
Selectively removing germline but not somatic stem cells from the testis has yielded insight into unexpected properties of spermatogonia that likely facilitate their reversion to GSCs. It is surprising that these cells can come from a distance to re-enter niches completely full of somatic stem cells, since spermatogonia in the Drosophila testis were previously considered immotile. However, because germ cells ultimately exchange positions with somatic stem cells during spermatogonial dedifferentiation, cellular movement must be occurring. The presence of dynamic protrusions on select spermatogonial cysts during this process further supports this hypothesis, and suggests that some germ cells in testes lacking GSCs re-acquire intrinsic properties of cell movement similar to those found in their embryonic precursors, the primordial germ cells (Kunwar et al., 2006). However, it is also plausible that protrusions do not represent motility associated with movement into the niche, but instead reflect changes in encystment that must occur during dedifferentiation. In this case, spermatogonia could remain immotile but become ‘pushed’ back into the niche by neighboring somatic cells, which likely play an integral role in dedifferentiation, as discussed below. Interestingly, the spermatogonial protrusions appear similar to those found on migrating somatic cells but distinct from those seen on migrating primordial germ cells in Drosophila (Dutta et al., 2002; Kunwar et al., 2006). The ability to acquire motility may be a conserved feature of spermatogonia, as undifferentiated spermatogonia in mouse testes actively migrate along the basement membrane (Yoshida et al., 2007). Since spermatogonial dedifferentiation also occurs in mammalian testes (Barroca et al., 2008), but has not yet been visualized in vivo in any system, combining our genetic system for inducing spermatogonial dedifferentiation with techniques for sustained imaging of this tissue in vivo should provide important mechanistic insights.
CPCs may promote spermatogonial dedifferentiation
In general, stem cell transplantation is more efficient when endogenous stem cells are first depleted from the tissue, suggesting it is necessary to create ‘space’ within niches to accommodate incoming cells (Bhattacharya et al., 2008; Oatley and Brinster, 2008). Thus, it is surprising that niches filled with somatic stem cells readily accept incoming germ cells in our assay. Rather than obstructing the niche and preventing GSCs from returning, CPCs may be conducive or even required for niche repopulation. In support of this hypothesis, the presence of somatic cells within the niche correlates positively with repopulation efficiency. For example, manipulating the stem cell maintenance factor STAT92E triggers spermatogonial dedifferentiation, but depletes CPCs from the niche. In this case, only 77% of testes can recover GSCs (Brawley and Matunis, 2004). In contrast, manipulation of Bam triggers spermatogonial dedifferentiation but leaves the pool of CPCs intact, and nearly all testes (98%) recover GSCs. Somatic cells play a role in spermatogonial homing in the mammalian testis: β1 integrin is required in both germline and somatic (Sertoli) cells during this process (Kanatsu-Shinohara et al., 2008). In addition to providing regulatory cues, somatic cells could also physically participate in spermatogonial dedifferentiation by actively breaking apart interconnected spermatogonia. Finally, since the correct 2:1 ratio of CPCs to GSCs reappears following dedifferentiation, spermatogonial cysts must lose their association with accompanying cyst cells and gain close associations with the hub and CPCs during this process, necessitating rearrangements; perhaps the spermatogonial protrusions discussed above reflect these events. Although somatic cells have not yet been characterized in live adult gonads, somatic stem cells in the Drosophila ovary are thought to exchange positions within the Drosophila germarium, suggesting they can acquire a previously unexpected degree of cell motility (Nystul and Spradling, 2007). It will be interesting to determine whether similar phenomena occur in additional niches.
Altered signaling, rather than physical space within the niche, may guide spermatogonia to acquire niche occupancy
Although much remains to be learned about mechanisms underlying spermatogonial dedifferentiation in this or any other system, our finding that partially reducing Jak-STAT signaling interferes with dedifferentiation indicates signals from the niche are involved. Ectopic SOCS36E may affect the ability of spermatogonia to upregulate STAT92E and transition into GSCs, or it may inhibit the ability of germ cells to establish contact with the hub. Likewise, excess SOCS36E may affect the CPCs' ability to upregulate STAT92E, re-encyst the germline or to be displaced by incoming spermatogonia. There is precedent for the involvement of Jak-STAT signaling in cell movement in Drosophila: it sustains cell motility during primordial germ cell migration (Li, 2003; Li, 2004; Brown et al., 2006) and border cell migration in the ovary (Silver and Montell, 2001). While further work is needed to establish whether spermatogonia undergo directed movements during dedifferentiation, a candidate attractant is Unpaired (Upd). While the distribution of Upd protein in the testis is not known, it is thought to be limited, perhaps via binding to its receptor or to the extracellular matrix (Arbouzova and Zeidler, 2006). Our analysis of Jak-STAT signaling activity within the niche during dedifferentiation suggests that ligand production remains constant while pathway activation occurs in a limited domain of select spermatogonia near the hub. Perhaps without GSCs acting as a ‘sink’ for Upd, these spermatogonia are now able to receive Upd and activate Jak-STAT signaling. Niche signals may also promote spermatogonial dedifferentiation in the mouse testis: Glial-cell-derived neurotrophic factor, which is produced by Sertoli cells and required for spermatogonial stem cell maintenance (Meng et al., 2000), may promote spermatogonial dedifferentiation in vitro (Barroca et al., 2008). Together, our findings suggest that spermatogonial dedifferentiation is a regulated process involving local niche signals, rather than a stochastic one whereby random cells encounter space within the niche and then subsequently remain there as stem cells. Since dedifferentiation may be a highly conserved feature of many stem cell niches, and could be a more prevalent means of stem cell maintenance than is currently appreciated, building on these findings to uncover the underlying regulatory mechanisms should greatly add to our understanding of stem cell biology.
EXPERIMENTAL PROCEDURES
Fly stocks
Hs-bam flies contain the P[w+; Hsp70-bam+]18d transgene inserted on the X chromosome (Ohlstein and McKearin, 1997). nanos-Gal4-VP16 flies (Van Doren et al., 1998) (from E. Selva) were crossed to UAS-GMA flies (Bloor and Kiehart, 2001) (from D. Kiehart) to drive expression of GMA in germ cells. To generate Hs-bam flies containing GFP-marked cells, Hs-bam virgins were crossed to the following GFP-protein trap lines (Buszczak et al., 2007) (from M. Buszczak and A. Spradling): CB03470 (hub), CC01872 (enriched in somatic cell cytoplasm) and CB03960 (hub and CPCs). UAS-pBS-SOCS36Ewt was from B. Callus (Callus and Mathey-Prevot, 2002). y1w*;; p{wmC=Gal4-Act5c(FRT.CD2).P}S, P{w+mC UAS-GFP::nls 8} flies (Neufeld et al., 1998) (abbreviated: Actin5c>CD2>Gal4, UAS-GFP) were crossed to pr1pwn1{ry[+t7.2]=hsFLP}38/CyO; ki1ry506 flies (Pignoni and Zipursky, 1997) (abbreviated: Hs-FLP) to create Hs-FLP/CyO; Actin5c>CD2>Gal4, UASGFP/TM6B, Tb flies. Flies were from the Bloomington Stock Center unless otherwise noted.
Heat-shock protocol
Approximately twenty 0-3 day-old adult males raised in a humidified 18°C incubator were placed into vials containing Drosophila food that had previously air-dried for 24 h. Vials were partially submerged in a 37°C water bath for 30 min. at approximately 9 AM and 5 PM daily, placed in a 29°C incubator between heat-shocks and then returned to 18°C after the final heat-shock. Flies receiving 5 or 10 heat-shocks were heat-shocked over 48 or 96 h respectively; flies receiving 15 heat-shocks were heat-shocked three times daily over 96 h.
SOCS36E misexpression during dedifferentiation
Males containing both Hs-FLP and the inducible Actin5c>CD2>Gal4, UAS-GFP transgenes were crossed to Hs-bam; UAS-SOCS36E/CyO virgins at 25°C to create experimental flies which transiently overexpress Bam and permanently overexpress SOCS36E upon heat-shock (genotype: Hs-bam/Y; UAS-SOCS36E/Hs-FLP; Actin5c>CD2>Gal4,UAS-GFP/+). Sibling controls were Hs-bam/Y; UASSOCS36E/CyO; Actin5c>CD2>Gal4,UAS-GFP/+ or Hs-bam/Y; Hs-FLP/CyO; Actin5c<CD2<Gal4,UAS-GFP/+. Flies were heat-shocked and allowed to recover at 18°C or 25°C as described above.
Immunostaining and apoptosis detection
Immunostaining was performed as described (Matunis et al., 1997), except anti-STAT92E was incubated 48 h at 4°C. Primary antibodies were: rat anti-Bam at 1:1000 (McKearin and Ohlstein, 1995) (from D. McKearin); rat anti-BrdU at 1:40 (Serotec MC2060); guinea pig anti-zfh-1 at 1:1000 (Lai et al., 1991) (from J. Skeath); rabbit anti-STAT92E at 1:400 (from E. Bach); rabbit anti-STAT92E at 1:800 (from S. Hou); rabbit anti-Vasa at 1:5000 (Lasko and Ashburner, 1990) (from P. Lasko); rabbit anti-GFP at 1:10000 (Torrey Pines Biolabs); rabbit anti-Anillin at 1:500 (Field and Alberts, 1995) (from C. Field); rabbit anti-Phospho-Histone H3 at 1:200 (Upstate Cell Signaling Solutions); chick anti-Vasa at 1:10000 (from K. Howard); mouse anti-1B1 at 1:25, mouse anti-Fasciclin III at 1:50, mouse anti-Armadillo at 1:50, mouse anti-EYA 10H6 at 1:50 (all from Developmental Studies Hybridoma Bank, University of Iowa). Alexa fluor-conjugated secondary IgG (H+L) antibodies were used at 1:200 for 568 and 633 conjugates, and 1:400 for 488 conjugates. Secondary antisera were: goat anti-rat 488 and 555; goat anti-rabbit 488 and 568, goat anti-mouse 488 and 568, goat anti-chick 568 and 633, and goat anti-guinea-pig 488 (Molecular Probes/Invitrogen). Nuclei were counterstained using 1 μg/ml 4′-6-diamidino-2-phenylindole (DAPI)(Roche Molecular Biochemical). Apoptosis was detected via TUNEL with the Apoptag Fluorescein Direct In Situ kit (Chemicon International) according to the manufacturer's instructions with the following modifications: 15 min. fixation in 4% paraformaldehyde, 15 min. wash in equilibration buffer, and 1h incubation with terminal deoxytransferase (TDT) at 37°C.
In vitro BrdU incorporation
Testes were incubated in Schneider's medium containing 20 μM BrdU for 30 min. at RT, and BrdU incorporation detected as described (Brawley and Matunis, 2004).
Analysis of confocal images
Fixed testes were mounted in Vectashield (Vector), imaged with a LSM 5 Pascal or LSM 510 Meta (Zeiss), and analyzed using the Zeiss software; panels are single confocal sections unless stated otherwise. To estimate the number of spermatogonia, the testis apex was optically sectioned, and spermatogonia in the z-stack were counted based on Vasa expression and fusome morphology (not all spermatogonia were included in the z-stack, but stack size and area were consistent for all testes imaged). To determine CPC number, we counted bright (but not dim) Zfh-1 positive cells in the testis apex in Hs-bam; nanos-Gal4-VP16; UAS-GMA flies. Graphing and statistical analysis was performed using Prism software (Graphpad). Averages were compared using two-tailed Student's t-test assuming unequal variances, and percentages were analyzed for statistical significance using a Fisher exact test or chi-squared test.
Supplementary Material
ACKNOWLEDGEMENTS
We thank our colleagues who have supplied us with stocks, technical assistance, suggestions and insightful comments during the course of this work. We thank Dr. M. de Cuevas and anonymous reviewers for comments and the Andrew lab for use of their Zeiss Axiophot. This work was supported by NIH grants HD052937 and HD040307-07 (EM).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Arbouzova NI, Zeidler MP. JAK/STAT signalling in Drosophila: insights into conserved regulatory and cellular functions. Development. 2006;133:2605–2616. doi: 10.1242/dev.02411. [DOI] [PubMed] [Google Scholar]
- Barroca V, Lassalle B, Coureuil M, Louis JP, Le Page F, Testart J, Allemand I, Riou L, Fouchet P. Mouse differentiating spermatogonia can generate germinal stem cells in vivo. Nat Cell Biol. 2008 doi: 10.1038/ncb1826. [DOI] [PubMed] [Google Scholar]
- Bhattacharya D, Ehrlich LI, Weissman IL. Space-time considerations for hematopoietic stem cell transplantation. Eur J Immunol. 2008;38:2060–2067. doi: 10.1002/eji.200838383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bloor JW, Kiehart DP. zipper Nonmuscle myosin-II functions downstream of PS2 integrin in Drosophila myogenesis and is necessary for myofibril formation. Dev Biol. 2001;239:215–228. doi: 10.1006/dbio.2001.0452. [DOI] [PubMed] [Google Scholar]
- Boyle M, Wong C, Rocha M, Jones DL. Decline in self-renewal factors contributes to aging of the stem cell niche in the Drosophila testis. Cell Stem Cell. 2007;1:470–478. doi: 10.1016/j.stem.2007.08.002. [DOI] [PubMed] [Google Scholar]
- Brawley C, Matunis E. Regeneration of male germline stem cells by spermatogonial dedifferentiation in vivo. Science. 2004;304:1331–1334. doi: 10.1126/science.1097676. [DOI] [PubMed] [Google Scholar]
- Brown S, Zeidler MP, Hombria JE. JAK/STAT signalling in Drosophila controls cell motility during germ cell migration. Dev Dyn. 2006;235:958–966. doi: 10.1002/dvdy.20709. [DOI] [PubMed] [Google Scholar]
- Buszczak M, Paterno S, Lighthouse D, Bachman J, Planck J, Owen S, Skora AD, Nystul TG, Ohlstein B, Allen A, et al. The carnegie protein trap library: a versatile tool for Drosophila developmental studies. Genetics. 2007;175:1505–1531. doi: 10.1534/genetics.106.065961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Callus BA, Mathey-Prevot B. SOCS36E, a novel Drosophila SOCS protein, suppresses JAK/STAT and EGF-R signalling in the imaginal wing disc. Oncogene. 2002;21:4812–4821. doi: 10.1038/sj.onc.1205618. [DOI] [PubMed] [Google Scholar]
- Chen D, McKearin D. Dpp signaling silences bam transcription directly to establish asymmetric divisions of germline stem cells. Curr Biol. 2003;13:1786–1791. doi: 10.1016/j.cub.2003.09.033. [DOI] [PubMed] [Google Scholar]
- Chen HW, Chen X, Oh SW, Marinissen MJ, Gutkind JS, Hou SX. mom identifies a receptor for the Drosophila JAK/STAT signal transduction pathway and encodes a protein distantly related to the mammalian cytokine receptor family. Genes Dev. 2002;16:388–398. doi: 10.1101/gad.955202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng J, Turkel N, Hemati N, Fuller MT, Hunt AJ, Yamashita YM. Centrosome misorientation reduces stem cell division during ageing. Nature. 2008;456:599–604. doi: 10.1038/nature07386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Cuevas M, Lilly MA, Spradling AC. Germline cyst formation in Drosophila. Annu Rev Genet. 1997;31:405–428. doi: 10.1146/annurev.genet.31.1.405. [DOI] [PubMed] [Google Scholar]
- de Cuevas M, Spradling AC. Morphogenesis of the Drosophila fusome and its implications for oocyte specification. Development. 1998;125:2781–2789. doi: 10.1242/dev.125.15.2781. [DOI] [PubMed] [Google Scholar]
- Dutta D, Bloor JW, Ruiz-Gomez M, VijayRaghavan K, Kiehart DP. Real-time imaging of morphogenetic movements in Drosophila using Gal4-UAS-driven expression of GFP fused to the actin-binding domain of moesin. Genesis. 2002;34:146–151. doi: 10.1002/gene.10113. [DOI] [PubMed] [Google Scholar]
- Fabrizio JJ, Boyle M, DiNardo S. A somatic role for eyes absent (eya) and sine oculis (so) in Drosophila spermatocyte development. Dev Biol. 2003;258:117–128. doi: 10.1016/s0012-1606(03)00127-1. [DOI] [PubMed] [Google Scholar]
- Field CM, Alberts BM. Anillin, a contractile ring protein that cycles from the nucleus to the cell cortex. J Cell Biol. 1995;131:165–178. doi: 10.1083/jcb.131.1.165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flatt T, Min KJ, D'Alterio C, Villa-Cuesta E, Cumbers J, Lehmann R, Jones DL, Tatar M. Drosophila germ-line modulation of insulin signaling and lifespan. Proc Natl Acad Sci U S A. 2008;105:6368–6373. doi: 10.1073/pnas.0709128105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuller MT. Genetic control of cell proliferation and differentiation in Drosophila spermatogenesis. Semin Cell Dev Biol. 1998;9:433–444. doi: 10.1006/scdb.1998.0227. [DOI] [PubMed] [Google Scholar]
- Fuller MT, Spradling AC. Male and female Drosophila germline stem cells: two versions of immortality. Science. 2007;316:402–404. doi: 10.1126/science.1140861. [DOI] [PubMed] [Google Scholar]
- Gönczy P, DiNardo S. The germ line regulates somatic cyst cell proliferation and fate during Drosophila spermatogenesis. Development. 1996;122:2437–2447. doi: 10.1242/dev.122.8.2437. [DOI] [PubMed] [Google Scholar]
- Gönczy P, Matunis E, DiNardo S. bag-of-marbles and benign gonial cell neoplasm act in the germline to restrict proliferation during Drosophila spermatogenesis. Development. 1997;124:4361–4371. doi: 10.1242/dev.124.21.4361. [DOI] [PubMed] [Google Scholar]
- Hardy RW, Tokuyasu KT, Lindsley DL, Garavito M. The germinal proliferation center in the testis of Drosophila melanogaster. J Ultrastruct Res. 1979;69:180–190. doi: 10.1016/s0022-5320(79)90108-4. [DOI] [PubMed] [Google Scholar]
- Hime GR, Brill JA, Fuller MT. Assembly of ring canals in the male germ line from structural components of the contractile ring. J Cell Sci. 1996;109(Pt 12):2779–2788. doi: 10.1242/jcs.109.12.2779. [DOI] [PubMed] [Google Scholar]
- Kai T, Spradling A. An empty Drosophila stem cell niche reactivates the proliferation of ectopic cells. Proc Natl Acad Sci U S A. 2003;100:4633–4638. doi: 10.1073/pnas.0830856100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kai T, Spradling A. Differentiating germ cells can revert into functional stem cells in Drosophila melanogaster ovaries. Nature. 2004;428:564–569. doi: 10.1038/nature02436. [DOI] [PubMed] [Google Scholar]
- Kanatsu-Shinohara M, Takehashi M, Takashima S, Lee J, Morimoto H, Chuma S, Raducanu A, Nakatsuji N, Fassler R, Shinohara T. Homing of mouse spermatogonial stem cells to germline niche depends on beta1-integrin. Cell Stem Cell. 2008;3:533–542. doi: 10.1016/j.stem.2008.08.002. [DOI] [PubMed] [Google Scholar]
- Kawase E, Wong MD, Ding BC, Xie T. Gbb/Bmp signaling is essential for maintaining germline stem cells and for repressing bam transcription in the Drosophila testis. Development. 2004;131:1365–1375. doi: 10.1242/dev.01025. [DOI] [PubMed] [Google Scholar]
- Kiger AA, Jones DL, Schulz C, Rogers MB, Fuller MT. Stem cell self-renewal specified by JAK-STAT activation in response to a support cell cue. Science. 2001;294:2542–2545. doi: 10.1126/science.1066707. [DOI] [PubMed] [Google Scholar]
- Kunwar PS, Siekhaus DE, Lehmann R. In vivo migration: a germ cell perspective. Annu Rev Cell Dev Biol. 2006;22:237–265. doi: 10.1146/annurev.cellbio.22.010305.103337. [DOI] [PubMed] [Google Scholar]
- Lai ZC, Fortini ME, Rubin GM. The embryonic expression patterns of zfh-1 and zfh-2, two Drosophila genes encoding novel zinc-finger homeodomain proteins. Mech Dev. 1991;34:123–134. doi: 10.1016/0925-4773(91)90049-c. [DOI] [PubMed] [Google Scholar]
- Lasko PF, Ashburner M. Posterior localization of vasa protein correlates with, but is not sufficient for, pole cell development. Genes Dev. 1990;4:905–921. doi: 10.1101/gad.4.6.905. [DOI] [PubMed] [Google Scholar]
- Leatherman JL, DiNardo S. Zfh-1 controls somatic stem cell self-renewal in the Drosophila testis and nonautonomously influences germline stem cell self-renewal. Cell Stem Cell. 2008;3:44–54. doi: 10.1016/j.stem.2008.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li j., Xia Fan, Li Willis X. Coactivation of STAT and Ras is Required for Germ Cell Proliferation and invasive migration in drosophila. Developmental Cell. 2003;5:787–798. doi: 10.1016/s1534-5807(03)00328-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li L, Xie T. Stem cell niche: structure and function. Annu Rev Cell Dev Biol. 2005;21:605–631. doi: 10.1146/annurev.cellbio.21.012704.131525. [DOI] [PubMed] [Google Scholar]
- Li WX. Receptor tyrosine kinase signaling and primordial germ cell development. Cell Cycle. 2004;3:249–251. [PMC free article] [PubMed] [Google Scholar]
- Lin H, Yue L, Spradling AC. The Drosophila fusome, a germline-specific organelle, contains membrane skeletal proteins and functions in cyst formation. Development. 1994;120:947–956. doi: 10.1242/dev.120.4.947. [DOI] [PubMed] [Google Scholar]
- Matunis E, Tran J, Gönczy P, Caldwell K, DiNardo S. punt and schnurri regulate a somatically derived signal that restricts proliferation of committed progenitors in the germline. Development. 1997;124:4383–4391. doi: 10.1242/dev.124.21.4383. [DOI] [PubMed] [Google Scholar]
- McKearin D, Ohlstein B. A role for the Drosophila bag-of-marbles protein in the differentiation of cystoblasts from germline stem cells. Development. 1995;121:2937–2947. doi: 10.1242/dev.121.9.2937. [DOI] [PubMed] [Google Scholar]
- McKearin DM, Spradling AC. bag-of-marbles: a Drosophila gene required to initiate both male and female gametogenesis. Genes Dev. 1990;4:2242–2251. doi: 10.1101/gad.4.12b.2242. [DOI] [PubMed] [Google Scholar]
- Meng X, Lindahl M, Hyvonen ME, Parvinen M, de Rooij DG, Hess MW, Raatikainen-Ahokas A, Sainio K, Rauvala H, Lakso M, et al. Regulation of cell fate decision of undifferentiated spermatogonia by GDNF. Science. 2000;287:1489–1493. doi: 10.1126/science.287.5457.1489. [DOI] [PubMed] [Google Scholar]
- Morrison SJ, Spradling AC. Stem cells and niches: mechanisms that promote stem cell maintenance throughout life. Cell. 2008;132:598–611. doi: 10.1016/j.cell.2008.01.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakagawa T, Nabeshima Y, Yoshida S. Functional identification of the actual and potential stem cell compartments in mouse spermatogenesis. Dev Cell. 2007;12:195–206. doi: 10.1016/j.devcel.2007.01.002. [DOI] [PubMed] [Google Scholar]
- Neufeld TP, de la Cruz AF, Johnston LA, Edgar BA. Coordination of growth and cell division in the Drosophila wing. Cell. 1998;93:1183–1193. doi: 10.1016/s0092-8674(00)81462-2. [DOI] [PubMed] [Google Scholar]
- Nishikawa S, Goldstein RA, Nierras CR. The promise of human induced pluripotent stem cells for research and therapy. Nat Rev Mol Cell Biol. 2008;9:725–729. doi: 10.1038/nrm2466. [DOI] [PubMed] [Google Scholar]
- Nystul T, Spradling A. An epithelial niche in the Drosophila ovary undergoes long-range stem cell replacement. Cell Stem Cell. 2007;1:277–285. doi: 10.1016/j.stem.2007.07.009. [DOI] [PubMed] [Google Scholar]
- Oatley JM, Brinster RL. Regulation of spermatogonial stem cell self-renewal in mammals. Annu Rev Cell Dev Biol. 2008;24:263–286. doi: 10.1146/annurev.cellbio.24.110707.175355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohlstein B, McKearin D. Ectopic expression of the Drosophila Bam protein eliminates oogenic germline stem cells. Development. 1997;124:3651–3662. doi: 10.1242/dev.124.18.3651. [DOI] [PubMed] [Google Scholar]
- Pignoni F, Zipursky SL. Induction of Drosophila eye development by decapentaplegic. Development. 1997;124:271–278. doi: 10.1242/dev.124.2.271. [DOI] [PubMed] [Google Scholar]
- Raff M. Adult stem cell plasticity: fact or artifact? Annu Rev Cell Dev Biol. 2003;19:1–22. doi: 10.1146/annurev.cellbio.19.111301.143037. [DOI] [PubMed] [Google Scholar]
- Schulz C, Kiger AA, Tazuke SI, Yamashita YM, Pantalena-Filho LC, Jones DL, Wood CG, Fuller MT. A misexpression screen reveals effects of bag-of-marbles and TGF beta class signaling on the Drosophila male germ-line stem cell lineage. Genetics. 2004;167:707–723. doi: 10.1534/genetics.103.023184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silver DL, Montell DJ. Paracrine signaling through the JAK/STAT pathway activates invasive behavior of ovarian epithelial cells in Drosophila. Cell. 2001;107:831–841. doi: 10.1016/s0092-8674(01)00607-9. [DOI] [PubMed] [Google Scholar]
- Straube WL, Tanaka EM. Reversibility of the differentiated state: regeneration in amphibians. Artif Organs. 2006;30:743–755. doi: 10.1111/j.1525-1594.2006.00296.x. [DOI] [PubMed] [Google Scholar]
- Terry NA, Tulina N, Matunis E, DiNardo S. Novel regulators revealed by profiling Drosophila testis stem cells within their niche. Dev Biol. 2006;294:246–257. doi: 10.1016/j.ydbio.2006.02.048. [DOI] [PubMed] [Google Scholar]
- Tulina N, Matunis E. Control of stem cell self-renewal in Drosophila spermatogenesis by JAK-STAT signaling. Science. 2001;294:2546–2549. doi: 10.1126/science.1066700. [DOI] [PubMed] [Google Scholar]
- Van Doren M, Williamson AL, Lehmann R. Regulation of zygotic gene expression in Drosophila primordial germ cells. Curr Biol. 1998;8:243–246. doi: 10.1016/s0960-9822(98)70091-0. [DOI] [PubMed] [Google Scholar]
- Wallenfang MR, Nayak R, DiNardo S. Dynamics of the male germline stem cell population during aging of Drosophila melanogaster. Aging Cell. 2006;5:297–304. doi: 10.1111/j.1474-9726.2006.00221.x. [DOI] [PubMed] [Google Scholar]
- Yamashita YM, Jones DL, Fuller MT. Orientation of asymmetric stem cell division by the APC tumor suppressor and centrosome. Science. 2003;301:1547–1550. doi: 10.1126/science.1087795. [DOI] [PubMed] [Google Scholar]
- Yoshida S, Sukeno M, Nabeshima Y. A vasculature-associated niche for undifferentiated spermatogonia in the mouse testis. Science. 2007;317:1722–1726. doi: 10.1126/science.1144885. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.