Abstract
Purpose
To provide a clinical tool for calculating a patient's future risk for developing cognitive impairment based on age, family history, and AVLT retention.
Participants
1019 cognitively normal persons followed for an average of 5 years. 159 participants were eventually diagnosed with cognitive impairment.
Results
Risk of developing cognitive impairment increases with age and family history, but decreases with better memory performance. A nomogram is provided for calculation of relative risk of developing cognitive impairment in combinations of age, family history, and memory performance.
Conclusions
These results enhance clinicians' ability to provide information to a patient about risk of cognitive impairment.
Keywords: cognitive decline, dementia, risk, AVLT, family history
The investigation of factors that place an individual at risk for developing cognitive impairment is an important area of dementia research. Genetic polymorphisms such as the apolipoprotein E-4 gene (Bondi, Salmon, Galasko, Thomas & Thal, 1999; Petersen et al., 1995; Smith et al., 1998), anatomic variables such as hippocampal volume (Jack et al., 1999; Killiany et al., 2000), and functional neuroimaging variables (Bookheimer et al., 2000; Machulda et al., 2003) have all been identified as risk markers. Also of interest, especially to the public, is the risk of dementia associated with demographic factors, such as age or family history of dementia. Older age and a family history of dementia have been shown to increase risk (Mendez & Cummings, 2003; Silverman, Smith, Marin, Mohs, & Propper, 2003).
There have been numerous studies that suggest that initial performance on verbal memory testing is a strong predictor of whether an individual will be diagnosed with cognitive impairment (Albert, Moss, Tanzi, & Jones, 2001; Chen, Ratcliff, Belle, Cauley, DeKosky, & Ganguli, 2001; Collie & Maruff, 2000; Powell et al., 2006; Rubin et al. 1998). Formal memory testing is usually part of a detailed evaluation of cognitive status conducted by clinical neuropsychologists. The goal of this study is to demonstrate how formal memory testing might aide clinicians in providing more specific information about a patient's future risk for developing cognitive impairment in addition to quantifying a patient's current neurocognitive status. Specifically, we provide a clinical tool for calculating a patient's future risk for developing cognitive impairment based on age, family history, and a formal memory retention measure.
Method
Participants
Participants in the Mayo Alzheimer's Disease Patient Registry (ADPR) were utilized in this analysis. Recruitment and requirements for acceptance to the ADPR are described in detail elsewhere (Malec, Ivnik, & Smith, 1993; Petersen, Kokmen, Tangalos, Ivnik, & Kurland, 1990) and are briefly described here. The ADPR project was approved by the Mayo Institutional review board, and all participants provided written informed consent.
Participants were Olmsted County, Minnesota residents identified during routine general medical examination in Primary Care Internal Medicine at the Mayo Clinic. After a review of their medical record, if no cognitive concerns were raised, these persons became eligible to serve as cognitively normal controls and were referred to the ADPR. They qualified as normal controls if they were judged by their clinician to be functioning normally in the community and did not have cognitive impairment. In addition, to be qualified as normal, participants did not have any active neurologic, psychiatric, or other illnesses believed to affect cognition (as determined by the neurologist's interview), and were not taking any psychoactive medications. All referred persons underwent neurological and neuropsychological evaluation. Thus, it is important to note that all participants included in these analyses were cognitively, functionally, neurologically, and psychiatrically normal at the time of recruitment.
A total of 1019 participants were followed for an average of 5 years (median 3.6 years) with approximately annual comprehensive neurological and neuropsychological re-evaluation. The ADPR involves continual enrollment with participants enrolled over a period of more than 10 years. At the completion of each annual assessment, evaluations were reviewed in a consensus conference consisting of neurologists (D.S.K., B.F.B., and R.C.P.), neuropsychologists (G.E.S. and R.J.I.), nurses, and geriatricians. A final clinical diagnosis was made for that visit based on all available information.
For the purposes of this study, we evaluated each participant's cognitive status at their last or most recent assessment, including participants who later died or withdrew from longitudinal evaluation. We ascertained each person's cognitive status at that last examination (normal or abnormal), then we used each person's original testing to predict status at that last examination. Over the course of longitudinal assessment, 159 of the participants who were cognitively normal at their first assessment became cognitively impaired by their most recent evaluation.
To provide more externally valid information for clinicians of patients presenting with cognitive complaints, the primary condition of interest in these analyses was new onset cognitive impairment. Thus, we included dementia syndromes other than Alzheimer's disease (AD) and mild cognitive impairment (MCI), as well as AD, as outcomes in this study. Kaplan-Meier analysis yielded a 5-year cumulative incidence of 12.9% for cognitive impairment in this sample. Forty-nine percent were diagnosed with dementia (APA, 1994) and fifty-one percent with MCI (Petersen, Smith, Waring, Ivnik, Tangalos, & Kokmen, 1999). Of those with dementia, 40% were diagnosed with clinically possible or clinically probable AD (McKhann, Drachman, Folstein, Katzman, Price, & Stadlan, 1984). Nineteen percent were diagnosed with vascular related dementia (Román et al., 1993). The remaining 41% were diagnosed with other dementia syndromes such as Lewy Body dementia (McKeith, Perry, & Perry, 1999).
Measures
Family history questionnaire
At the initial visit, the participant or a family informant provided information on family history of dementia via a clinical interview (Lautenschlager et al., 1996; Li et al., 1997; Silverman et al., 2003). For this analysis, family history was reported as the percentage of the patient's parents and siblings with a history of cognitive impairment or dementia. This methodology takes into account the extent of family history rather than presence or absence of dementia. Using this methodology, 35% of the sample reported a family history of dementia (n = 359).
Rey Auditory Verbal Learning Test
(AVLT; Rey, 1958; Schmidt, 1996). This 15-item list learning test was included in a larger neuropsychological test battery. Percent retention at 30-minute delay (AVLT PR) was the memory variable of interest given prior research demonstrating its diagnostic utility. MOANS age-adjusted scaled scores (Ivnik et al., 1992) were used with a normative mean of 10 and a standard deviation of 3. To facilitate ease of use of the results of this paper for clinicians, Table 1 provides age adjusted scaled scores for AVLT PR scores.
Table 1.
Auditory Verbal Learning Test long term percent retention age adjusted scaled scores
| MOANS | AVLT Percent Retention Values Age Range |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Scaled Score | 56-66 | 59-69 | 62-72 | 65-75 | 68-78 | 71-81 | 74-84 | 77-87 | 80-90 | 83+ |
| 2 | 0-10 | 0-10 | - | - | - | - | - | - | - | - |
| 3 | 11-13 | 11-13 | 0-5 | 0-5 | - | - | - | - | - | - |
| 4 | 14-15 | 14-15 | 6-12 | 6-12 | 0-10 | 0-5 | - | - | - | - |
| 5 | 16-37 | 16-30 | 13-21 | 13-21 | 11-19 | 6-20 | 0-7 | - | - | - |
| 6 | 38-53 | 31-45 | 22-42 | 22-38 | 20-36 | 21-36 | 8-27 | 0-24 | 0-24 | 0-19 |
| 7 | 54-58 | 46-54 | 43-53 | 39-49 | 37-49 | 37-49 | 28-44 | 25-36 | 25-32 | 20-32 |
| 8 | 59-64 | 55-62 | 54-60 | 50-59 | 50-56 | 50-56 | 45-56 | 37-49 | 33-49 | 33-45 |
| 9 | 65-75 | 63-71 | 61-70 | 60-65 | 57-63 | 57-62 | 57-61 | 50-60 | 50-59 | 46-54 |
| 10 | 76-83 | 72-82 | 71-80 | 66-76 | 64-74 | 63-74 | 62-71 | 61-69 | 60-69 | 55-65 |
| 11 | 84-88 | 83-88 | 81-84 | 77-83 | 75-80 | 75-80 | 72-79 | 70-75 | 70-75 | 66-70 |
| 12 | 89-93 | 89-92 | 85-90 | 84-89 | 81-86 | 81-86 | 80-86 | 76-84 | 76-84 | 71-80 |
| 13 | 94+ | 93-95 | 91-94 | 90-92 | 87-90 | 87-90 | 87-90 | 85-90 | 85-90 | 81-86 |
| 14 | - | 96+ | 95+ | 93-94 | 91-94 | 91-94 | 91-94 | 91-94 | 91-94 | 87-92 |
| 15 | - | - | - | 95+ | 95+ | 95+ | 95+ | 95+ | 95+ | 93+ |
Note. Compiled from Ivnik et al. (1992)25 by permission of Mayo Foundation for Medical Education and Research. All rights reserved. Norms utilize overlapping age ranges as explained in the original text. AVLT = Auditory Verbal Learning Test; MOANS=Mayo's Older Americans Normative Studies.
Results
Sample characteristics are presented in Table 2. A total of 159 of the 1019 participants were eventually diagnosed with cognitive impairment. The remaining 860 participants remained cognitively normal across the longitudinal follow-up period. Univariate analyses showed age, extent of family history, and AVLT percent retention at first evaluation to be significant individual predictors of developing cognitive impairment at last evaluation. Gender and education were not significant predictors of developing cognitive impairment.
Table 2.
Sample characteristics
| Variable | Mean (SD) | Median | Min | Max |
|---|---|---|---|---|
| Entire Sample (n=1019) | ||||
| Age at recruitment | 78 (6.6) years | 78 | 46 | 100 |
| Length of follow up | 5.0 (3.3) years | 3.6 | 1 | 16 |
| Education | 13 (3.0) years | 13 | 4 | 20 |
| # of family members | 8.4 (3.5) | 8 | 2 | 26 |
| % of family with dementia | 8.8 (14.3) | 0 | 0 | 75 |
| 35% of sample with positive family history (n=359) | ||||
| # of family members | 8.9 (3.8) | 8 | 2 | 26 |
| % of family with dementia | 25 (13.5) | 20 | 6 | 75 |
Note. Family history was calculated as the percentage of the patient's parents and siblings with a history of cognitive impairment or dementia.
Multivariate Cox regression modeling was performed to determine how the significant individual predictors function collectively. In this model, risk of developing cognitive impairment increased with each year of age (Risk Ratio = 1.102; C.I. = 1.074-1.130) and proportion of family members with a history of dementia (Risk Ratio = 1.011; C.I. = 1.001-1.021). However, each one MOANS scaled score point improvement in memory performance attenuated that risk (Risk Ratio = .848; C.I. = .794-.906).
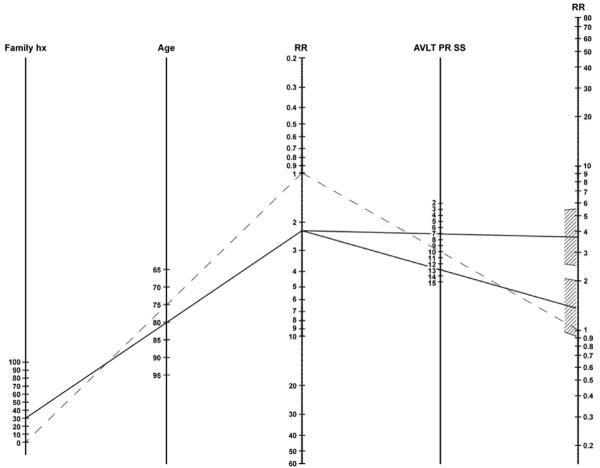
The coefficients from the multivariate analysis were used to create a risk nomogram that combined information about age, family history, and AVLT performance to offer information about relative risk of developing cognitive impairment (see Figure 1). The reference case is a 75-year-old, with no family history of dementia, and an AVLT percent retention MOANS scaled score of 10 (see dotted line on nomogram). The nomogram permits calculation of risk of developing cognitive impairment compared to this referent for any other combination of age, family history, and AVLT performance.
Figure 1. Relative Risk Nomogram.
Family hx = % of parents and siblings with a history of dementia; RR = Relative risk; AVLT PR SS = Auditory Verbal Learning Test percent retention scaled score. In order to use the nomogram, a straight line should be drawn connecting the desired age and family history proportion (percent of first degree relatives with a positive history) to the intermediate relative risk estimate. Then a second straight line should be drawn from the intermediate relative risk estimate through the AVLT percent retention scaled score to the final relative risk estimate. The final relative risk estimate represents relative risk of developing cognitive impairment over the future five year period compared to the reference patient described in text.
For example (see solid line of Figure 1), an 80-year-old patient with 30% of his/her parents and siblings affected by dementia has approximately a 2.2 times greater risk of developing dementia than the referent. If that patient also scores below a scaled score of 10 on the AVLT, the risk increases relative to risk based on age and family history alone (e.g., if AVLT = 7, RR = 3.68; C.I. = 2.48-5.46). However, if that person scores one standard deviation above the mean on the AVLT, the patient's risk based on age and family history is attenuated, and approaches the risk of the referent patient (RR=1.37; C.I. = 0.93-2.00).
Discussion
The primary purpose of the current study was investigation of the added value of assessing memory performance in combination with age and family history of dementia in describing future risk of developing cognitive impairment. To our knowledge, the current study is the first study to provide individual risk information based on the combination of all three of these variables. Using the nomogram provided here, the clinician can provide the patient information about relative risk of developing cognitive impairment utilizing all these pieces of information concurrently.
More elaborate predictive models are certainly possible and we encourage research in this direction. However, our goal for this project was to provide a relatively simple and easy to use clinically relevant model. Therefore, this model does not include all possible factors that may be related to risk for cognitive impairment, such as genetic vulnerability related to apolipoprotein E-4 gene. Certainly, an obvious direction for future research is development of a more elaborate predictive model with multiple medical and demographic variables, genetic factors, biomarker data, along with multiple cognitive test scores beyond memory.
Some may feel that the presentation of relative risk instead of absolute risk of cognitive impairment is a limitation of this study. However, we present relative risk as opposed to absolute risk because our primary aim was to show how memory test scores, as a more precise estimate of current memory functioning, modify risk estimate. Also base rate of cognitive impairment in other populations may be different than in our own. Relative risks can be applied in settings without regard to base rate, allowing this nomogram to be a tool directly applicable by clinicians in their settings for responding to a patient's concern about subsequent risk for cognitive decline. Utilization of absolute risk tables would not be applicable in populations other than our own.
It is also important to note that our data are based on a longitudinal analysis with average follow-up time of five years. Thus, the relative risk values presented here pertain to risk in a comparable time period. These results do not reflect risk for periods exceeding roughly five years.
Similarly, it is important to note that the outcome variable is cognitive impairment in general with a number of different etiologies possible. Cognitive impairment can refer to Alzheimer's disease or other neurodegenerative condition, Mild Cognitive Impairment, or vascular cognitive impairment or other non-neurodegenerative etiologies. An area of future research could certainly be development of similar models with clinically useful tools for specific diagnostic outcomes such as Alzheimer's disease or Lewy Body disease individually. This requires a larger longitudinal sample than was available for this analysis.
We acknowledge that there is not full independence between memory scores at initial evaluation and memory scores that contributed to clinical diagnosis a few years later. We utilized AVLT score at the time of enrollment as a normal control to develop a model predicting clinical diagnosis on average 3.6 year later. Clinical diagnosis was informed by the results of neuropsychological evaluation in addition to other variables, but not the initial AVLT score used in the risk analysis. Correlation between AVLT at entry and AVLT at time of clinical diagnosis is 0.38 (p <0.0001) for the sample as a whole, 0.37 (p<0.0001) for those who remain clinically normal at last follow-up, and 0.32 (p<0.0001) for those who convert to cognitive impairment at last follow-up. Thus, there is only modest shared variance between AVLT at entry and AVLT at last follow-up. The correlation is low enough that this model is not simply prediction of future AVLT from past AVLT. Rather, we show that obtaining a person's memory score can contribute to not only to knowing their current status but also to assessing risk for future change in clinical status.
In summary, increasing age and family history are associated with increased risk of developing cognitive impairment; however, that risk can be significantly increased or significantly attenuated dependent on the patient's performance on a memory retention measure. The nomogram provided here allows the clinician to provide an individual patient with information about his/her risk of developing cognitive impairment, over the next five years, based on these factors in combination.
Acknowledgments
This research was supported by National Institute of Aging grants P50 AG16574 and U01 AG06786, by the Robert H. and Clarice Smith and Abigail Van Buren Alzheimer's Disease Research program, by the Mayo Foundation, and the Rochester Epidemioogy Project Grant AR30582 (Dr. Walter Rocca, PI).
Footnotes
There are no conflicts of interest for any author relevant to the subject matter or materials discussed in the manuscript.
References
- Albert M, Moss M, Tanzi R, Jones K. Preclinical prediction of AD using neuropsychological tests. Journal of the International Neuropsychological Society. 2001;7:631–639. doi: 10.1017/s1355617701755105. [DOI] [PubMed] [Google Scholar]
- American Psychiatric Association . Diagnostic and statistical manual of mental disorders. 4th ed. Author; Washington, D.C.: 1994. [Google Scholar]
- Bondi M, Salmon D, Galasko D, Thomas R, Thal L. Neuropsychological function and apolipoprotein E genotype in the preclinical detection of Alzheimer's disease. Psychology & Aging. 1999;14:295–303. doi: 10.1037//0882-7974.14.2.295. [DOI] [PubMed] [Google Scholar]
- Bookheimer S, Strojwas M, Cohen M, Saunders A, Pericak-Vance M, Mazziotta J, et al. Patterns of brain activation in people at risk for Alzheimer's disease. New England Journal of Medicine. 2000;343:450–456. doi: 10.1056/NEJM200008173430701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen P, Ratcliff G, Belle S, Cauley J, DeKosky S, Ganguli M. Patterns of cognitive decline in pre-symptomatic Alzheimer disease: a prospective community study. Archives of Genera Psychiatry. 2001;58:853–858. doi: 10.1001/archpsyc.58.9.853. [DOI] [PubMed] [Google Scholar]
- Collie A, Maruff P. The neuropsychology of preclinical Alzheimer's disease and mild cognitive impairment. Neuroscience & Biobehavioral Reviews. 2000;24:365–374. doi: 10.1016/s0149-7634(00)00012-9. [DOI] [PubMed] [Google Scholar]
- Ivnik R, Malec J, Smith G, Tangalos E, Petersen R, Kokmen E, et al. Mayo's Older Americans Normative Studies: Updated AVLT norms for ages 56 to 97. The Clinical Neuropsychologist. 1992;6(Suppl):83–104. [Google Scholar]
- Jack C, Petersen R, Xu Y, O'Brien P, Smith G, Ivnik R, et al. Prediction of AD with MRI-based hippocampal volume in mild cognitive impairment. Neurology. 1999;52:1397–1403. doi: 10.1212/wnl.52.7.1397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Killiany R, Gomez-Isla T, Moss M, Kikinis R, Sandor T, Jolesz F, et al. Use of structural magnetic resonance imaging to predict who will get Alzheimer's disease. Annals of Neurology. 2000;47:430–439. [PubMed] [Google Scholar]
- Lautenschlager NT, Cupples LA, Rao SV, Auerbach SA, Becker R, Burke J, et al. Risk of dementia among relatives of Alzheimer's disease patients in the MIRAGE study: what is in store for the oldest old? Neurology. 1996;46:641–650. doi: 10.1212/wnl.46.3.641. [DOI] [PubMed] [Google Scholar]
- Li G, Aryan M, Silverman JM, Haroutunian V, Perl D, Birstein S, et al. The validity of the family history method for identifying Alzheimer's disease. Archives of Neurology. 1997;54:634–640. doi: 10.1001/archneur.1997.00550170104021. [DOI] [PubMed] [Google Scholar]
- Machulda M, Ward H, Borowski B, Gunter J, Cha R, O'Brien P, et al. Comparison of memory fMRI response among normal, MCI, and Alzheimer's patients. Neurology. 2003;61:500–506. doi: 10.1212/01.wnl.0000079052.01016.78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malec JF, Ivnik RJ, Smith GE. Neuropsychology and normal aging. In: Parks R, Zec R, Wilson RS, editors. Neuropsychology of Alzheimer's disease and other dementias. Oxford University Press; New York: 1993. pp. 81–111. [Google Scholar]
- McKeith IG, Perry EK, Perry RH. Report of the Second Dementia with Lewy Body International Workshop: diagnosis and treatment. Consortium on Dementia with Lewy Bodies. Neurology. 1999;53:902–905. doi: 10.1212/wnl.53.5.902. [DOI] [PubMed] [Google Scholar]
- McKhann G, Drachman D, Folstein M, Katzman R, Price D, Stadlan EM. Clinical diagnosis of Alzheimer's disease: report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer's Disease. Neurology. 1984;34:939–944. doi: 10.1212/wnl.34.7.939. [DOI] [PubMed] [Google Scholar]
- Mendez MF, Cummings JL. Dementia: a clinical approach. 3rd ed. Butterworth-Heinemann; New York: 2003. [Google Scholar]
- Petersen R, Kokmen E, Tangalos EG, Ivnik RJ, Kurland LT. Mayo Clinic's Alzheimer's Disease Patient Registry. Aging. 1990;2:408–415. doi: 10.1007/BF03323961. [DOI] [PubMed] [Google Scholar]
- Petersen RC, Smith GE, Ivnik RJ, Tangalos EG, Schaid DJ, Thibodeau SN, et al. Apolipoprotein E status as a predictor of the development of Alzheimer's disease in memory-impaired individuals. Journal of the American Medical Association. 1995;273:1274–1278. [PubMed] [Google Scholar]
- Petersen RC, Smith GE, Waring SC, Ivnik RJ, Tangalos EG, Kokmen E. Mild cognitive impairment: Clinical characterization and outcome. Archives of Neurology. 1999;56:303–308. doi: 10.1001/archneur.56.3.303. [DOI] [PubMed] [Google Scholar]
- Powell MR, Smith GE, Knopman DS, Parisi JE, Boeve BF, Petersen RC, et al. Cognitive measures predict pathologic Alzheimer disease. Archives of Neurology. 2006;63:865–868. doi: 10.1001/archneur.63.6.865. [DOI] [PubMed] [Google Scholar]
- Rey A. L'examen clinique en psychologie. Presse Universitaire de France; Paris: 1958. [Google Scholar]
- Román GC, Tatemichi T, Erkinjuntti T, Cummings JL, Masdue JC, Garcia JH. Vascular dementia: Diagnostic criteria for research studies. Report of the NINDS-AIREN International Workshop. Neurology. 1993;43:250–260. doi: 10.1212/wnl.43.2.250. [DOI] [PubMed] [Google Scholar]
- Rubin E, Storandt M, Miller P, Kinscherf D, Grant E, Morris J, et al. A prospective study of cognitive function and onset of dementia in cognitively healthy elders. Archives of Neurology. 1998;55:395–401. doi: 10.1001/archneur.55.3.395. [DOI] [PubMed] [Google Scholar]
- Schmidt M. Rey auditory-verbal learning test. Western Psychological Services; Los Angeles: 1996. [Google Scholar]
- Silverman JM, Smith CJ, Marin DB, Mohs RC, Propper CB. Familial patterns of risk in very late onset Alzheimer's disease. Archives of General Psychiatry. 2003;60:190–197. doi: 10.1001/archpsyc.60.2.190. [DOI] [PubMed] [Google Scholar]
- Smith GE, Bohac DL, Waring SC, Kokmen EG, Tangalos RJ, Ivnik RJ, et al. Apolipoprotein E genotype influences cognitive `phenotype' in Alzheimer's disease patients but not normal controls. Neurology. 1998;50:355–362. doi: 10.1212/wnl.50.2.355. [DOI] [PubMed] [Google Scholar]