Abstract
Differentiation, maturation, and repair of skeletal muscle requires ongoing cooperation between signaling cascades activated by hormones and growth factors, and intrinsic regulatory programs controlled by myogenic transcription factors. The insulin-like growth factor - phosphatidylinositol-3 kinase - Akt pathway has been implicated in muscle growth and regeneration after injury, in counteracting sarcopenia during aging, and in maintaining muscle cell viability. Here we present evidence for distinct roles for Akt1 and Akt2 in different phases of muscle differentiation. Targeted knockdown of either Akt had no effect on C2 myoblast proliferation, even though Akt1 concentrations are markedly higher than Akt2 levels under growth-promoting conditions. Akt2 concentrations rose by nearly an order of magnitude during muscle differentiation, while Akt1 levels remained constant, yet loss of either protein did not increase myoblast death. Rather, knockdown or genetic knockout of Akt1 blocked differentiation at its earliest stages, preventing induction of muscle-specific proteins and inhibiting formation of multinucleated myofibers, while myoblasts lacking Akt2 differentiated normally, although resultant myofibers were thinner and incorporated fewer nuclei than controls. Forced expression of knockdown-resistant Akt1 partially reversed the deficit in differentiation seen in myoblasts lacking Akt1. Our results define isoform-specific Akt actions in muscle cells, and demonstrate that both Akts are necessary for full myoblast differentiation and maturation.
Keywords: muscle differentiation, Akt, Akt1, Akt2, protein kinase B, PKB
Introduction
The Akt or PKB family of serine - threonine protein kinases (Akt1, Akt2, Akt3) has been subjected to intensive study since its initial discovery in the early 1990’s (Brazil and Hemmings, 2001; Brazil et al., 2004; Woodgett, 2005). The three Akts are highly related to each other in both structure and amino acid sequence (~85% identity), and are activated by common biochemical pathways that act downstream of growth factor and hormone receptors via the signaling enzyme, phosphatidylinositol-3 kinase (Brazil and Hemmings, 2001; Manning and Cantley, 2007). Initial analysis of Akt actions demonstrated common effects on cell proliferation, survival, and intermediary metabolism (Datta et al., 1999), while more recent studies have revealed distinct functions for each protein. In mice, global deficiency of Akt1 caused diminished somatic growth (Cho et al., 2001b), while knockout of Akt2 led to insulin resistance and diabetes mellitus (Cho et al., 2001a), and loss of Akt3 caused a decline in brain size (Easton et al., 2005). In addition, combined deficiency of Akt1 and Akt2 led to severe growth deficits, and neonatal death (Peng et al., 2003), while loss of Akt1 and Akt3 caused fetal death (Yang et al., 2005). In confirmation of observations in mice, a family with an inherited mis-sense mutation in Akt2 was shown to develop diabetes mellitus with severe insulin resistance (George et al., 2004).
Studies in cultured cells have identified potentially specific actions of each Akt. In human keratinocytes, engineered knockdown of Akt1 enhanced cell death and thereby prevented differentiation, while loss of Akt2 had no effect (Thrash et al., 2006). In mouse osteoclast precursor cells, reduction of either Akt1 or Akt2 inhibited differentiation, but had no impact on cell viability or proliferation (Sugatani and Hruska, 2005). In human breast cancer cell lines, over-expression of a constitutively-active Akt stimulated cell proliferation and tumor growth in nude mice (Liu et al., 2006), but paradoxically caused reduced cell motility and led to diminished invasiveness (Yoeli-Lerner et al., 2005; Liu et al., 2006). By contrast, loss of Akt1 in breast cancer cells promoted increased motility, and enhanced invasiveness by mechanisms dependent on the continued expression of Akt2 (Irie et al., 2005). Thus, based on a range of studies, each Akt appears to have both distinct and overlapping functions that may vary depending on the cell or tissue type analyzed.
Skeletal muscle is also highly responsive to Akt signaling (Nader, 2005), with several effector pathways acting downstream of Akts being important in muscle development, regeneration, and hypertrophy through a combination of effects including Akt-initiated stimulation of protein synthesis, inhibition of muscle cell atrophy, and prevention of apoptotic cell death (Glass, 2003; Hoffman and Nader, 2004; Sartorelli and Fulco, 2004). In this context, over-expression of a constitutively active Akt has been shown to enhance muscle hypertrophy in transgenic mice (Lai et al., 2004), while dual systemic knockout of Akt1 and Akt2 in mice has been found to result in severe muscle hypoplasia that was incompatible with post-natal life (Peng et al., 2003).
The specific roles of individual Akts have not been elucidated in muscle, despite several recent but potentially contradictory observations. Our laboratory has found through selective gene knockdown in a model system of MyoD-mediated myoblast commitment and differentiation that Akt1 was necessary for both initiation and maintenance of differentiation but that Akt2 appeared dispensable (Wilson and Rotwein, 2007). In contrast, others have reported that Akt2 is important for myoblast differentiation in established muscle cell lines (Gonzalez et al., 2004; Heron-Milhavet et al., 2008), while still others have suggested specialized metabolic roles for each Akt in muscle, with Akt2 being important for glucose and Akt1 for lipid metabolism (Bouzakri et al., 2006; Cleasby et al., 2007).
Here we have attempted to address the question of specific Akt actions in muscle by studying differentiation after selective Akt loss in an established myogenic cell line and in embryonic fibroblasts from mice with individual Akt deficiencies. In each case, we find that lack of Akt1 completely prevented muscle differentiation at its earliest phases; forced expression of Akt1 could partially reverse the deficit in differentiation seen its absence. In contrast, myoblasts with Akt2 deficiency differentiated relatively normally, although the myotubes that developed were immature, as they were thinner and incorporated fewer nuclei than controls. Thus, each Akt may exert distinct biological effects in muscle development and function, with Akt1 being essential early in differentiation, and Akt2 being important later during myotube maturation.
Materials and Methods
Materials
Fetal bovine serum was purchased from Hyclone (Logan, UT), and newborn calf serum, horse serum, and trypsin from Invitrogen (Carlsbad, CA). Dulbecco’s modified Eagle’s medium (DMEM) and phosphate-buffered saline were from Mediatech-Cellgrow (Herndon, VA). Protease inhibitor tablets were from Roche Applied Sciences (Indianapolis, IN), okadaic acid from Alexis Biochemicals (San Diego, CA), and sodium orthovanadate from Sigma-Aldrich (St. Louis, MO). The BCA protein assay kit was from Pierce Biotechnologies (Rockford, IL) and PVDF (Immobilon-FL) was from Millipore Corp. (Billerica, MA). Restriction enzymes, buffers, ligases, and polymerases were purchased from Roche Applied Sciences, BD Biosciences-Clontech (Palo Alto, CA), and Fermentas (Hanover, MD). Hoechst 33258 nuclear dye was from Polysciences (Warrington, PA). The following monoclonal antibodies were from the Developmental Studies Hybridoma Bank (Iowa City, IA): F5D (anti-myogenin, W. E. Wright) and CT3 (anti-troponin-T, J. J-C. Lin). A monoclonal antibody to MyoD was from BD Biosciences-PharMingen (San Diego, CA). Polyclonal antibodies to Akt, Akt2, phospho-AktSer473, phospho-AktThr308, and Akt substrates were from Cell Signaling Technology (Beverly, MA), as were monoclonal antibodies to Akt1 and phospho-Erk. A polyclonal antibody to α-tubulin was from Sigma-Aldrich. Polyclonal antibodies against phospho-tyrosine and the IGF-I receptor β-subunit were from Santa Cruz Biotechnology (Santa Cruz, CA). Antibody conjugates were from Molecular Probes (Eugene, OR), including goat anti-mouse IgG1-Alexa 488, goat anti-mouse IgG2b-Alexa 594, and goat-anti-mouse IgG-Alexa 680. Goat-anti-rabbit IgG-IR800 was from Rockland Immunochemicals Inc. (Gilbertsville, PA). R3IGF-I was obtained from GroPep (Adelaide, Australia). All other chemicals were purchased from commercial suppliers.
Cell culture
Mouse embryo fibroblasts (MEFs) from wild-type mice and mice lacking either Akt1 or Akt2 were obtained from Dr. Morris Birnbaum of the University of Pennsylvania, and have been described (Zhou et al., 2006). MEFs, C3H10T1/2 mouse mesenchymal stem cells (ATCC catalog #CCL226), and C2 myoblasts (Yaffe and Saxel, 1977) were incubated on gelatin-coated tissue culture dishes in growth medium (DMEM with 10% fetal bovine serum and 10% newborn calf serum) at 37°C in humidified air with 5% CO2, until they reached ~95% of confluent density. Differentiation was initiated after washing cells with phosphate-buffered saline by addition of differentiation medium (DM - DMEM plus 2% horse serum (Wilson et al., 2003)). Cell counting experiments were performed as described previously (Wilson and Rotwein, 2007), as were studies of incorporation of bromodeoxyuridine (BrdU) into DNA (Stewart and Rotwein, 1996).
Construction and use of recombinant adenoviruses
The following recombinant adenoviruses have been described: mouse MyoD (Ad-MyoD), β-galactosidase (Ad-β-Gal) (Wilson et al., 2003; Wilson et al., 2004), and Ad-shAkt1 and Ad-shAkt2 (encoding short hairpin inhibitor RNAs targeting the 3’ un-translated regions of mouse Akt1 or Akt2 mRNAs (Wilson and Rotwein, 2007)). A recombinant adenovirus was prepared expressing the coding region of mouse Akt1, and was engineered to contain an influenza hemagglutinin (HA) tag at its COOH-terminus. Adenoviruses were purified on discontinuous cesium chloride gradients, and titered by optical density. Prior to use all adenoviruses were diluted in DMEM plus 2% fetal calf serum, and filtered through a Gelman syringe filter (0.45 mM).
MEFs were infected at ~50% of confluent density with Ad-MyoD at a multiplicity of infectivity (MOI) of 250. Viruses were added to cells at 37°C for 120 min followed by addition of an equal volume of DMEM with 20% fetal bovine serum, and incubation for a further 24 hr. Cells then were washed with phosphate-buffered saline, and DM was added. C3H10T1/2 cells were infected with Ad-MyoD (MOI of 125) at ~50% of confluent density. After an additional 24 hr in growth medium, cells were washed and DM added. Under these conditions, 80 – 90% of cells were infected. For infections of C2 myoblasts with Ad-β-gal, Ad-shAkt1, or Ad-shAkt2, viruses were added at MOIs of 5000 to cells plated at various concentrations as indicated in individual figure legends. After incubation for an additional 48 – 72 hr in growth medium, cells were washed and DM was added as described above.
Immunoblotting
Whole cell protein lysates were prepared and aliquots stored at −80°C until use (Tureckova et al., 2001; Wilson et al., 2003). Immunoblotting was performed with 20 – 30 µg of cellular protein per sample, using methods described previously (Wilson and Rotwein, 2006). Results were scanned and analyzed on a LiCoR Odyssey Infrared Imaging System, using software version 1.2 (LiCoR, Lincoln, NE). Antibodies were used at the following dilutions: anti-myogenin supernatant (1:100), anti-troponin-T (1:1000), anti-Akt (1:2000), anti-phospho-AktSer473 (1:1000), anti-phospho-AktThr308 (1:1000), anti-Akt1 (1:2000), anti-Akt2 (1:1000), anti-Akt substrate (1:1000), anti-MyoD (1:3000), anti-α-tubulin (1:5000), and secondary antibodies conjugated to Alexa 680 or IR-800 (1:5000). For immunoprecipitation studies, cellular protein lysates (150 µg) were incubated overnight at 4°C with antibody to phospho-tyrosine (1 µg), followed by SDS-PAGE and immunoblotting with an antibody to the IGF-I receptor β subunit (1:1000 dilution), and other steps as described above.
Measurement of Akt concentrations
Purified Akt1 and Akt2 were obtained from Dr. Morris Birnbaum of the University of Pennsylvania, and stored at −80°C until use. Whole cell protein lysates (5 – 10 µg for Akt1, 30 µg for Akt2) and graded amounts of purified Akts (0.1 – 10 ng) were separated by SDS-PAGE, transferred to PVDF, and used for immunoblotting as above. The same lots of monoclonal Akt1 antibody (Cell Signaling Technology, 2H10, #2967) and polyclonal Akt2 antibody (Cell Signaling Technology, 5B5, #2964) were used for all experiments. After analysis with LiCoR Odyssey software, a standard curve was established, and the amount of Akt1 and Akt2 in cell lysates was calculated, and corrected for sample loading by immunoblotting for α-tubulin. Results are expressed as ng Akt/mg protein.
Immunocytochemistry
Cells were fixed, permeabilized, blocked, and incubated with antibodies as described (Wilson et al., 2003). Primary antibodies were added in blocking buffer for 16 hr at 4°C (anti-troponin-T, 1:1000; anti-myogenin, 1:50; secondary antibodies at 1:1000). Images were captured using a Roper Scientific Cool Snap FX CCD camera attached to a Nikon Eclipse T300 fluorescent microscope using IP Labs 3.5 software. The calculation of muscle cell fusion index and myotube area has been described (Wilson et al., 2004). Hoechst staining was performed as described (Wilson and Rotwein, 2006).
Statistical analysis
Data are presented as mean ± SD. Statistical significance was determined using paired Student’s t test. Results were considered significant when p < 0.01.
Results
Induction of Akt2 protein expression during muscle differentiation
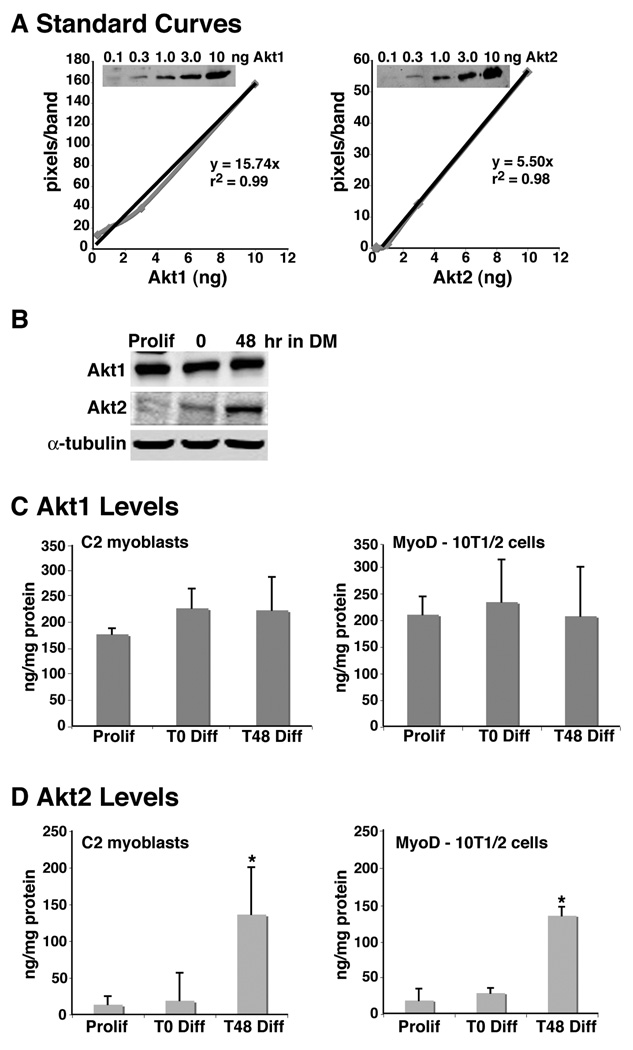
Several investigators have demonstrated that the abundance of Akt2 increases during muscle differentiation (Vandromme et al., 2001; Kaneko et al., 2002; Gonzalez et al., 2004), but the levels of each Akt in muscle cells have not been established. To rectify this deficiency, we have developed a semi-quantitative immunoblotting assay for Akt1 and Akt2 using purified standards and isoform-specific antibodies. As shown in Fig. 1A, each assay was linear over two orders of magnitude, and we were able to detect as little as 0.1 ng of each purified protein under the conditions used. Measurement of Akt1 was achieved readily in C2 myoblasts with 5 – 10 µg of whole cell protein extracts, although 30 µg was needed for Akt2 because of its lower abundance (Fig. 1B). Under the conditions of our assays we found that in C2 myoblasts and in Ad-MyoD infected 10T1/2 mesenchymal stem cells Akt1 levels were fairly constant in proliferating, confluent, and differentiated myoblasts (~200 ng/mg protein; Fig. 1B and C). In contrast, prior to differentiation, the amount of Akt2 in muscle cells was much less than Akt1 in the two cell lines (< 20 ng/mg protein), but levels increased substantially after 48 hr under differentiation-promoting conditions (to ~140 ng/mg protein, Fig. 1B and D). Thus, Akt1 is the predominant Akt prior to and during early phases of muscle differentiation, comprising most of the total. During the course of differentiation the amount of Akt2 increased by nearly an order of magnitude, and as a result, total Akt concentrations rose by ~75%.
Figure 1. Induction of Akt2 expression during myoblast differentiation.
A Standard curves for measuring Akt1 and Akt2 concentrations. The black line represents the best fit in each graph. The equation and r2 values are shown. A representative dilution series of purified Akt1 and Akt2 is depicted in each inset. B. Representative immunoblots for Akt1 and Akt2 from C2 myoblasts during proliferation in growth medium (Prolif), and after 0 and 48 hr in DM. C. Akt1 levels in C2 myoblasts and Ad-MyoD infected 10T1/2 cells (mean ± SD, n = 3). D. Akt2 levels in C2 myoblasts and Ad-MyoD infected 10T1/2 cells (mean ± SD, n = 3; * - p < 0.02 vs. other groups).
Knockdown of Akt1 prevents C2 myoblast differentiation
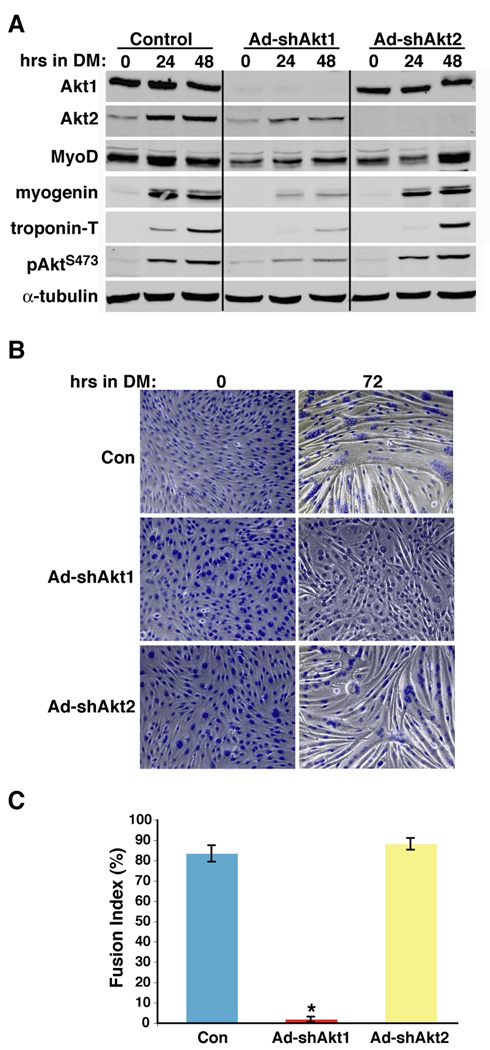
To address the roles of individual Akt isoforms in muscle differentiation, we used recombinant adenoviruses encoding short hairpin (sh) interfering RNAs targeting the 3’ noncoding regions of mouse Akt1 and Akt2 mRNAs (Wilson and Rotwein, 2007). As depicted in Fig. 2A, Ad-shAkt1 specifically decreased the amount of Akt1 by > 95% in C2 myoblasts, while Ad-shAkt2 caused the nearly complete elimination of Akt2. Loss of Akt1 had no immediate effect on Akt2 levels (Fig. 2A, 0 hr in DM), but reduced the induction of Akt2 expression seen during myoblast differentiation (Vandromme et al., 2001; Kaneko et al., 2002; Gonzalez et al., 2004), and thus led to a ~80% decrease in total Akt phosphorylation after incubation in DM for 24 or 48 hr (Fig. 2A). Akt1 knockdown caused a ~90% decline in accumulation of myogenin and troponin-T, markers of early and later differentiation, respectively, a ~50% decrease in MyoD (Fig. 2A), and a 90% reduction in myotube formation, as measured by myocyte fusion index, in comparison with control cells infected with Ad-β-gal (Fig. 2B and C). By contrast, targeted loss of Akt2 had little effect on Akt1 levels or Akt phosphorylation, or on the rate or extent of induction of muscle proteins myogenin or troponin-T, and did not inhibit myofiber formation or the degree of fusion (Fig. 2B and C), although as seen in Fig. 2B (and in Fig. 8) overall myotube size and the number of nuclei incorporated into each myofiber was diminished in Akt2-deficient myocytes compared with controls.
Figure 2. Akt1 deficiency prevents differentiation of C2 myoblasts.
C2 muscle cells were infected either with Ad-β-gal (control - Con), Ad-shAkt1, or Ad-shAkt2 at ~ 25% of confluent density, followed by incubation in DM starting 2 days later for up to 72 hr. A. Results are shown of time course experiments measuring Akt1, Akt2, MyoD, myogenin, troponin-T, pAktS473, and α-tubulin by immunoblotting. B. Phase-contrast micrographs of cells stained with Hoechst 33258 dye after incubation for 0 or 72 hr in DM. Magnification is 200X. C. Assessment of fusion index after 72 hr in DM (mean ± SD, n = 4; * - p < 0.00002 vs. other groups).
Figure 8. Loss of Akt2 inhibits myofiber maturation.
C2 myoblasts were infected with Ad-β-gal (control - Con) or Ad-shAkt2 at ~ 25% of confluent density. Two days later, cells were washed and incubated in DM for up to 3 days. A. Representative images showing immunocytochemistry for myogenin in nuclei (green) and for troponin-T (red) after 3 days in DM. Magnification is 200X. B. Assessment of myotube diameter (mean ± SD, n = 25; * - p < 0.000002 vs. Ad-shAkt2). C. Measurement of the number of nuclei incorporated per myotube (mean ± SD, n = 20; ** - p < 0.0000001 vs. Ad-shAkt2).
Akt1 deficiency does not impair myoblast proliferation or reduce myoblast survival
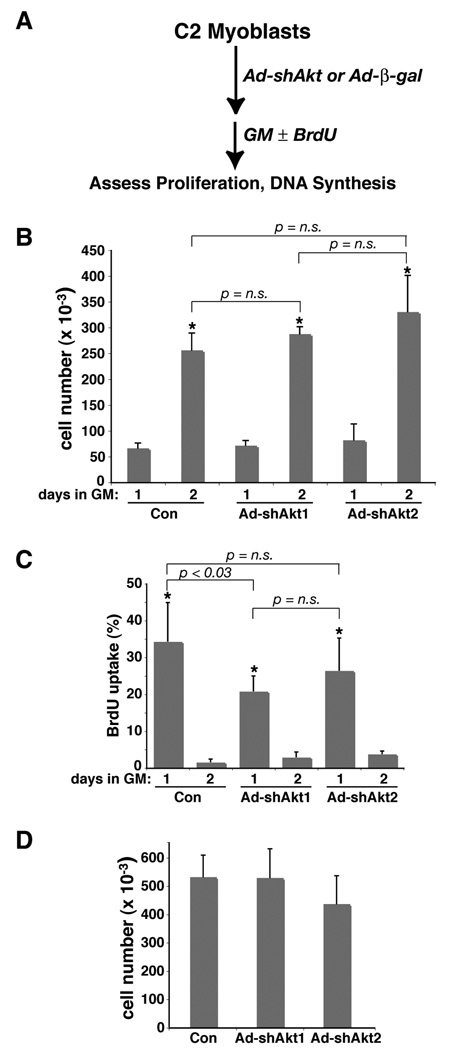
We considered the possibility that shRNA-mediated knockdown of Akt1 inhibited muscle differentiation as a secondary consequence of diminished muscle cell proliferation, as loss of Akt1 by siRNA micro-injection was shown by Héron-Milhavet et al to cause reduced DNA synthesis in C2 myoblasts (Heron-Milhavet et al., 2006). To address this question, we infected C2 myoblasts with Ad-β-gal, Ad-shAkt1, or Ad-shAkt2 shortly after cells were plated at low concentrations (25,000 per well; approximately 5% of confluent density), and assessed growth rates starting one day later (see experimental scheme, Fig. 3A), when these cells would be ordinarily at their maximal proliferation rate (doubling time of ~ 12 hr, twice the rate originally reported (Yaffe and Saxel, 1977)). In each case, cell numbers increased by 4-fold during the second day, which is equivalent to two cell doublings (Fig. 3B, compare day 2 to day 1). In other experiments, starting with higher cell numbers (50,000 per well), we measured DNA synthesis rates beginning the day after adenoviral infection by BrdU incorporation into DNA. In cells infected with Ad-β-gal, 34% of myoblasts entered S-phase of the cell cycle during day 1, but only 2% during day 2 (Fig. 3C). Myoblasts infected with Ad-shAkt1 or Ad-shAkt2 had lower rates of DNA synthesis on the day after infection, 21% and 26% respectively, which dropped on the following day to 3% and 4%, respectively (Fig. 3C). Despite the somewhat reduced rates of S-phase entry in cells lacking Akt1 compared with controls, both groups of myoblasts had similar cell counts at the end of day 2 (Fig. 3D), and did not proliferate further (data not shown). Of note, Akt2-deficient myoblast had consistently lower cell counts at confluent density (Fig. 3D, Fig. 4, and data not shown). We thus conclude that diminished proliferation is not the cause of reduced differentiation in C2 myoblasts lacking Akt1.
Figure 3. Knockdown of Akt1 or Akt2 does not inhibit C2 myoblast proliferation.
A. Experimental scheme. C2 myoblasts were infected at 25,000 cells per well (~5% of confluent density) with Ad-β-gal (control - Con), Ad-shAkt1, or Ad-shAkt2 (B), or at 50,000 cells per well (C, D), followed by incubation in growth medium (GM) for 1 – 3 days. B. Cells were counted at 1 and 2 days after viral infection (mean ± SD, n = 6; * - p < 0.0003 vs. day 1; other statistics are as indicated). C. BrdU incorporation into DNA was measured at 1 and 2 days after viral infection (mean ± SD, n = 6; * - p < 0.0003 vs. day 2; other statistics are as indicated). D. Cells were counted at 2 days after viral infection (mean ± SD, n = 6; p = n.s. for all pair-wise comparisons).
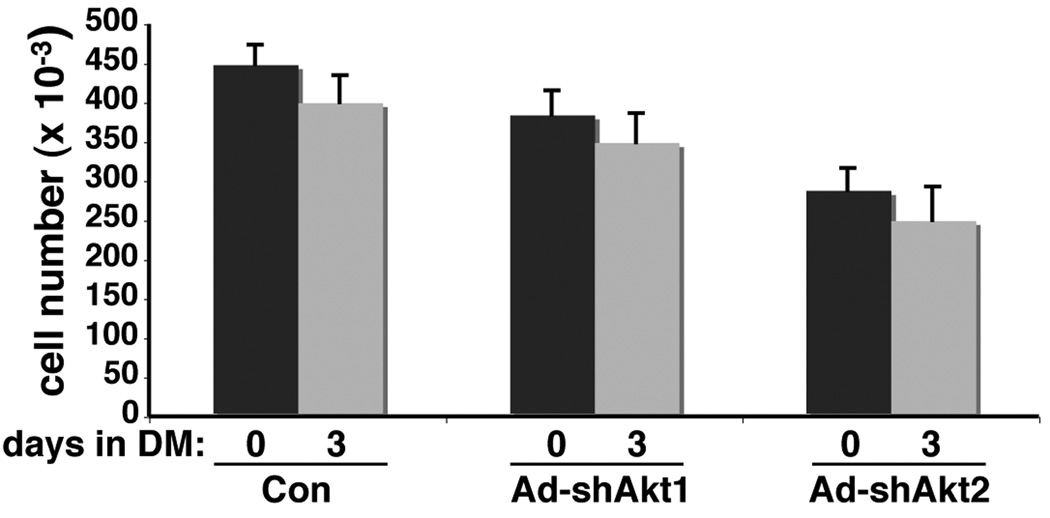
Figure 4. Loss of Akt1 or Akt2 does not promote C2 myoblast death.
C2 myoblasts were infected with Ad-β-gal (control - Con), Ad-shAkt1, or Ad-shAkt2 at ~ 25% of confluent density. Two days later, cells were washed and incubated in DM for up to 3 days. Cells were counted at the time of addition of DM (day 0) or 3 days later (mean ± SD, n = 4).
Autocrine production of IGF-II and the subsequent activation of the IGF-I receptor, PI3-kinase, and Akt are necessary to prevent apoptotic death of C2 myoblasts during incubation in low serum differentiation medium (Lawlor and Rotwein, 2000). To address the possibility that inhibition of muscle differentiation by Akt1 deficiency was secondary to diminished myoblast viability, we counted living cells before and after a 3-day incubation in DM after infection several days earlier with Ad-β-gal, Ad-shAkt1, or Ad-shAkt2. In all cases, cell numbers were reduced by only ~ 10% from values at the time of addition of DM (Fig. 4). Thus, loss of either Akt1 or Akt2 did not increase myoblast death.
Genetic deficiency of Akt1 impairs MyoD-mediated myogenic differentiation
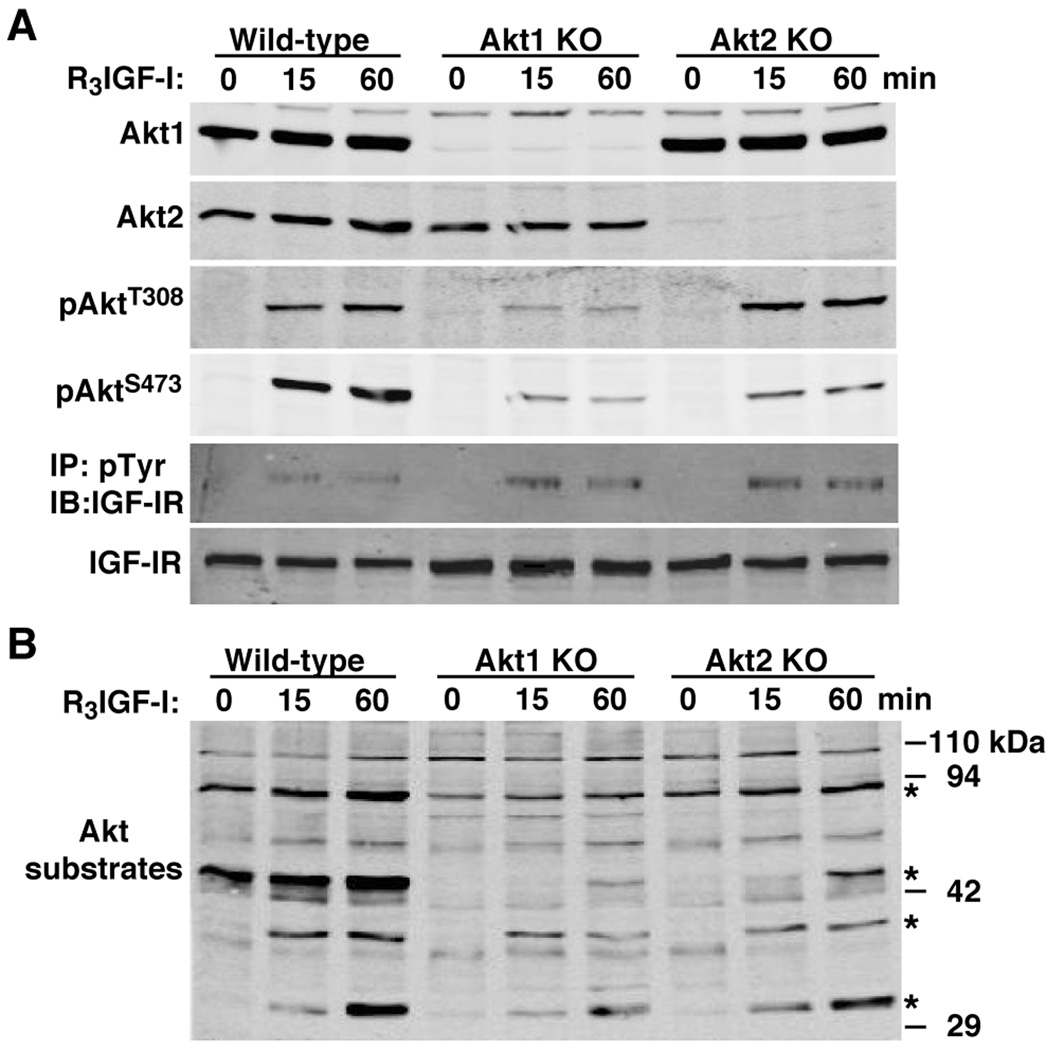
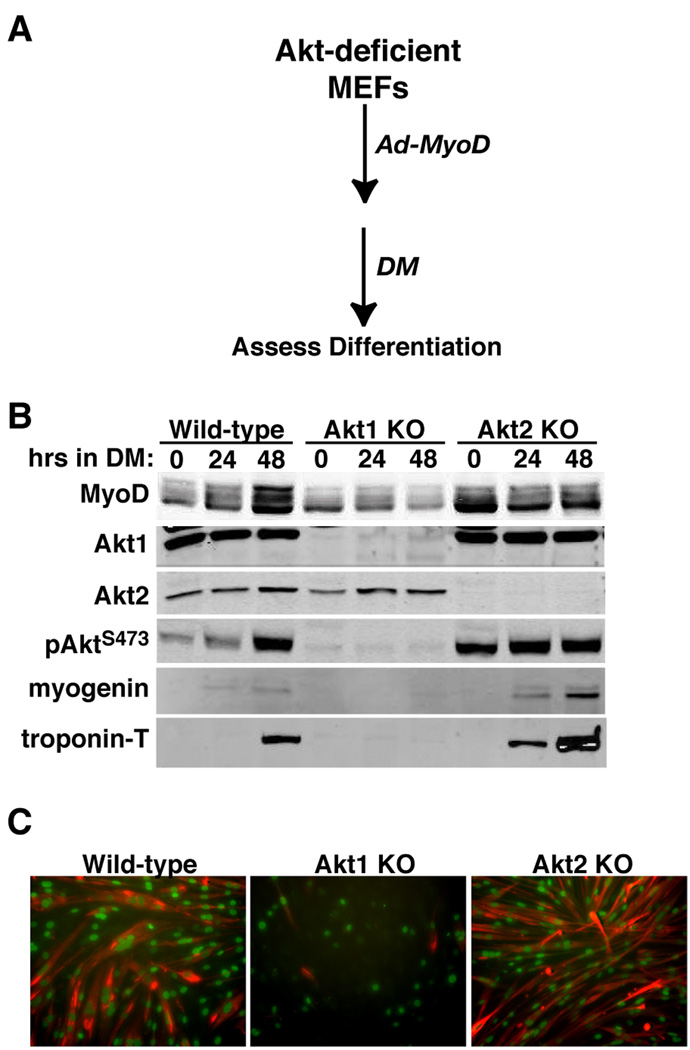
To extend results seen with C2 myoblasts, we analyzed MyoD-initiated differentiation in MEFs from mice lacking either Akt1 or Akt2. As depicted in Fig. 5A, in these cells loss of either Akt did not lead to a compensatory change in the amount of the other protein, but elimination of Akt1 caused a greater decline in acute IGF-I-stimulated Akt phosphorylation than did loss of Akt2, even though lack of each Akt led to a similar reduction in IGF-induced Akt substrate phosphorylation (Fig. 5B). Akt1 deficiency dramatically impaired MyoD-mediated muscle differentiation of these MEFs, as is evident by minimal expression of myogenin and troponin-T, and by the nearly complete lack of myocyte fusion compared with wild-type control MEFs (Fig. 6). In contrast, Akt2 deficiency had little effect on MyoD-stimulated biochemical differentiation, as shown by normal increases in muscle proteins (Fig. 6), and did not prevent morphological differentiation, as demonstrated by relatively normal myofiber formation, although, as in C2 myoblasts, myotubes were thinner and incorporated fewer nuclei than controls (Fig. 6C, compare Akt2 KO with wild-type).
Figure 5. Analysis of MEFs from mice lacking Akt1 or Akt2.
MEFs from wild-type mice, and from mice lacking either Akt1 or Akt2 were incubated with R3IGF-I [1 nM] for 0, 15, or 60 min. A. Results of time course experiments measuring Akt1, Akt2, pAktT308, pAktS473, and the IGF-I receptor β subunit (IGF-IR) by immunoblotting, and assessing tyrosine phosphorylation of the IGF-IR in anti-phosphotyrosine (pTyr) immunoprecipitates. B. Results of time course experiments for potential Akt substrates by immunoblotting. Asterisks indicate phospho-proteins showing reduced intensity after incubation with R3IGF-I in Akt1- or Akt2-deficient MEFs compared with wild-type controls.
Figure 6. Loss of Akt1 prevents MyoD-mediated muscle differentiation in MEFs.
A. Experimental scheme. B. Results of time course experiments measuring MyoD, Akt1, Akt2, pAktS473, myogenin, and troponin-T in Ad-MyoD infected MEFs by immunoblotting. C. Immunocytochemistry for myogenin in nuclei (green) and troponin-T (red) after 48 hr in DM. Magnification is 200X.
Forced expression of Akt1 promotes differentiation in Akt1-deficient C2 myoblasts
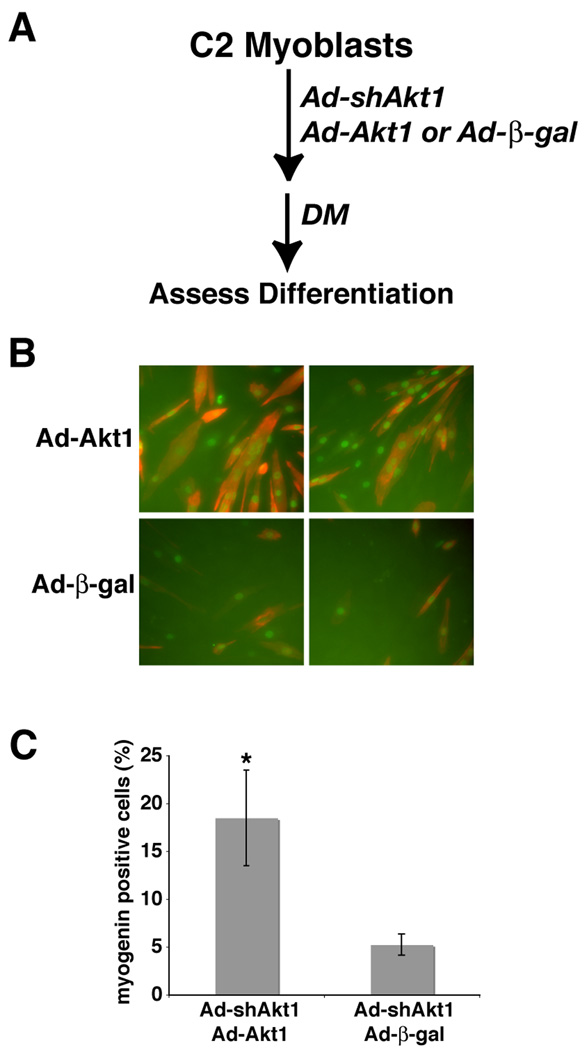
We next asked if addition of Akt1 could restore muscle differentiation in myoblasts in which expression of endogenous Akt1 was reduced. To test this idea, we first generated a recombinant adenovirus for mouse Akt1 that did not contain the 3’ un-translated sequence targeted by shAkt1. C2 myoblasts infected with Ad-shAkt1 were also infected with Ad-Akt1 or with Ad-β-gal (as a negative control), and incubated under differentiation-promoting conditions for up to 48 hr, followed by assessment of muscle protein expression and myotube formation (see experimental scheme in Fig. 7A). As pictured in Fig. 7B and C, Akt1-deficient C2 myoblasts infected with Ad-β-gal underwent minimal differentiation, with few myogenin positive cells and no myotubes (even after longer incubations in DM, data not shown). By contrast, adenovirus-mediated expression of Akt1 stimulated production of both myogenin and troponin-T, and restored the normal rate of myocyte fusion and myotube formation (Fig. 7B and C), even though the extent of adenovirus infection was fairly low (~ 25%, data not shown). Thus, under these conditions, an shRNA-insensitive Akt1 can rescue the defect in muscle differentiation caused by Akt1 knockdown.
Figure 7. Partial restoration of muscle differentiation by forced expression of shRNA-resistant Akt1 in C2 myoblasts lacking Akt1.
C2 myoblasts were infected at ~ 25% of confluent density with Ad-shAkt1 followed 24 hr later by infection either with Ad-β-gal or Ad-Akt1, and 1 day later by incubation in DM. A. Experimental scheme. B. Results showing immunocytochemistry for myogenin in nuclei (green) and for troponin-T (red) after 48 hr in DM. Magnification is 200X. B. Analysis of myogenin positive cells after 48 hr in DM (mean ± SD, n = 6; * - p < 0.001 vs. Ad-β-gal).
Impaired myotube maturation after loss of Akt2
Targeted reduction or genetic loss of Akt2 did not impair the early events of muscle differentiation nor inhibit myotube formation, but the myofibers appeared to be less robust than in control cells (Fig. 2 and Fig 6). To address this observation more quantitatively, we measured myotube width and counted the number of nuclei per myofiber in C2 myoblasts infected with Ad-shAkt2 or Ad-β-gal, after incubation for 72 hr in DM (Fig. 8). Myotubes from controls expressing β-gal were more twice the diameter of myofibers lacking Akt2 (Fig. 8B), and contained 2.5-times more nuclei (Fig. 8C). Thus, based on defects seen after it targeted loss, Akt2 may be important in the later phases of muscle differentiation, particularly for myofiber maturation.
Discussion
Multiple lines of evidence support the hypothesis that Akt actions are critical for skeletal muscle development, growth, repair, and metabolism (Glass, 2003; Hoffman and Nader, 2004; Sartorelli and Fulco, 2004; Nader, 2007). Here we show that of the two Akts produced by muscle cells, Akt1 is substantially more abundant than Akt2 at the onset of differentiation, and its elimination both cripples Akt activity and impairs differentiation at its earliest steps. Although myoblasts lacking Akt1, either through shRNA-mediated knockdown or genetic knockout, exhibit a profound defect in production of muscle-specific proteins, and have a markedly reduced capability to form multinucleated myofibers, their rate of proliferation under growth-promoting conditions appeared to be relatively normal. By contrast, in myoblasts in which Akt2 has been reduced or eliminated, the early and intermediate stages of muscle differentiation appeared to proceed normally, although deficits in the size and nuclear content of myotubes lead us to postulate that Akt2 may be important for myofiber maturation.
Our results extend observations from several groups who have shown that the abundance of Akt2 increases in differentiating muscle cells (Vandromme et al., 2001; Kaneko et al., 2002; Gonzalez et al., 2004), by providing more quantitative support for their data. While it is perhaps surprising to discover that Akt1 comprises most of the total Akt content in proliferating myoblasts, these observations are consistent with our demonstration that elimination of Akt1, and hence the vast majority of Akt activity, blocks differentiation. We also found that the amount of Akt2 increased by almost an order of magnitude during the normal course of differentiation, and that as a consequence total Akt concentrations nearly doubled. As shown previously, this rise in Akt2 levels appears to be secondary to induction of Akt2 gene expression (Kaneko et al., 2002; Wilson and Rotwein, 2007), potentially caused by an increase in Akt2 gene promoter activity in differentiating myoblasts (Kaneko et al., 2002).
Despite the evidence presented here and in our previous study (Wilson and Rotwein, 2007) that Akt1 is the key Akt for muscle differentiation, our results are at odds with some published observations. In an approach analogous to ours, Héron-Milhavet et al performed siRNA-mediated knockdown of Akts in C2 myoblasts and 10T1/2 mesenchymal stem cells, and obtained data that led them to implicate Akt2 in differentiation (Heron-Milhavet et al., 2008), and Akt1 in proliferation (Heron-Milhavet et al., 2006). While it is difficult to explain these two disparate conclusions, one possibility may be the different methodology. Héron-Milhavet et al micro-injected siRNAs or performed sequential transfections of expression plasmids encoding Akt siRNAs, while we used adenoviral gene delivery and also studied cells genetically lacking each Akt. However, this same group showed previously that micro-injected antibodies to Akt2 also could inhibit muscle differentiation more effectively than could antibodies to Akt1 (Vandromme et al., 2001). Also of note are observations of Bouzakri et al, who transfected primary myoblasts derived from human muscle biopsies with siRNAs to Akt1 and Akt2, and found that Akt2 deficiency caused cell death, but Akt1 knockdown had little effect on either viability or differentiation (Bouzakri et al., 2006). In the absence of information on the amount of each Akt in human muscle cells, these data are difficult to interpret, or to reconcile with our studies.
Based on overall reduction in myotube size and diminished incorporation of nuclei into myofibers in Akt2-deficient muscle cells compared with controls, we postulate that Akt2 may play a role in myotube maturation. Myotube formation in mammals occurs in two phases (Horsley and Pavlath, 2004). Individual myoblasts first fuse with one another to form small myofibers, which subsequently increase in size as additional myoblasts join with these nascent myotubes (Horsley and Pavlath, 2004). Recent studies have identified several different signaling pathways that are able to influence this second maturation phase of myotube development. The cytokine IL-4, which is produced and secreted by differentiating myoblasts (Horsley et al., 2003), has been found to stimulate expression of the cell-surface mannose receptor (Jansen and Pavlath, 2006). Myoblasts lacking the mannose receptor can undergo primary fusion but were unable to form large myotubes (Jansen and Pavlath, 2006). As yet, however, there is no evidence connecting Akt2 with the IL-4 - mannose receptor pathway. Inhibition of the mTor - p70 S6 kinase cascade with rapamycin blocked myotube hypertrophy induced by exogenous IGF-I, potentially by interfering with production of an as yet unidentified secreted signaling molecule (Park and Chen, 2005). Although Akts are well-known upstream activators of the mTor - p70 S6 kinase pathway in many cell types including muscle (Bodine et al., 2001; Erbay and Chen, 2001; Rommel et al., 2001), there is little information on specificity. In fact, over-expressed constitutively-active Akt1 or Akt2 each led to stimulation of p70 S6 kinase and similar degrees of myofiber hypertrophy in rat muscles (Cleasby et al., 2007). Knockdown of Rho kinase 2 (Rock2), which acts downstream of the small GTP binding protein, RhoA, or loss of its muscle specific variant, Rock2m, also inhibited myofiber hypertrophy caused by IGF-I (Pelosi et al., 2007), but to date there is no evidence implicating Rock2 as an Akt-regulated protein or demonstrating specific actions in secondary myotube formation. Thus, it remains to be established how Akt2-stimulated signaling pathways are involved in myofiber maturation.
We were able to partially restore muscle differentiation in myoblasts in which Akt1 was knocked down by addition of a wild-type (and shRNA-resistant) Akt1. Many previous studies have shown the importance of Akts in muscle cell viability, growth, or hypertrophy in cell culture models and in vivo but only by using constitutively-active versions of the protein (Rommel et al., 1999; Lawlor et al., 2000; Bodine et al., 2001; Fujio et al., 2001; Rommel et al., 2001; Pallafacchina et al., 2002; Lai et al., 2004; Wilson et al., 2004), and it is likely that the over-expressed Akt in these situations does not faithfully recapitulate physiologically-relevant signaling pathways or feedback control mechanisms. Further understanding of key signaling molecules regulated by each Akt along with elucidation of their specific actions has the potential to enhance therapeutic options for muscle disorders and for treatment of sarcopenia in aging humans and in systemic disease (Glass, 2003; Hoffman and Nader, 2004).
Acknowledgements
We thank Dr. Morris Birnbaum of the University of Pennsylvania for supplying MEFs and purified Akt standards. These studies were supported by National Institutes of Health research grant, R01 DK42748 (to P. R.).
Literature Cited
- Bodine SC, Stitt TN, Gonzalez M, Kline WO, Stover GL, Bauerlein R, Zlotchenko E, Scrimgeour A, Lawrence JC, Glass DJ, Yancopoulos GD. Akt/mTOR pathway is a crucial regulator of skeletal muscle hypertrophy and can prevent muscle atrophy in vivo. Nat Cell Biol. 2001;3:1014–1019. doi: 10.1038/ncb1101-1014. [DOI] [PubMed] [Google Scholar]
- Bouzakri K, Zachrisson A, Al-Khalili L, Zhang BB, Koistinen HA, Krook A, Zierath JR. siRNA-based gene silencing reveals specialized roles of IRS-1/Akt2 and IRS-2/Akt1 in glucose and lipid metabolism in human skeletal muscle. Cell Metab. 2006;4:89–96. doi: 10.1016/j.cmet.2006.04.008. [DOI] [PubMed] [Google Scholar]
- Brazil DP, Hemmings BA. Ten years of protein kinase B signalling: a hard Akt to follow. Trends Biochem Sci. 2001;26:657–664. doi: 10.1016/s0968-0004(01)01958-2. [DOI] [PubMed] [Google Scholar]
- Brazil DP, Yang ZZ, Hemmings BA. Advances in protein kinase B signalling: AKTion on multiple fronts. Trends Biochem Sci. 2004;29:233–242. doi: 10.1016/j.tibs.2004.03.006. [DOI] [PubMed] [Google Scholar]
- Cho H, Mu J, Kim JK, Thorvaldsen JL, Chu Q, Crenshaw EBr, Kaestner KH, Bartolomei MS, Shulman GI, Birnbaum MJ. Insulin resistance and a diabetes mellitus-like syndrome in mice lacking the protein kinase Akt2 (PKB beta) Science. 2001a;292:1728–1731. doi: 10.1126/science.292.5522.1728. [DOI] [PubMed] [Google Scholar]
- Cho H, Thorvaldsen JL, Chu Q, Feng F, Birnbaum MJ. Akt1/PKBalpha is required for normal growth but dispensable for maintenance of glucose homeostasis in mice. J Biol Chem. 2001b;276:38349–38352. doi: 10.1074/jbc.C100462200. [DOI] [PubMed] [Google Scholar]
- Cleasby ME, Reinten TA, Cooney GJ, James DE, Kraegen EW. Functional studies of Akt isoform specificity in skeletal muscle in vivo; maintained insulin sensitivity despite reduced insulin receptor substrate-1 expression. Mol Endocrinol. 2007;21:215–228. doi: 10.1210/me.2006-0154. [DOI] [PubMed] [Google Scholar]
- Datta SR, Brunet A, Greenberg ME. Cellular survival: a play in three Akts. Genes Dev. 1999;13:2905–2927. doi: 10.1101/gad.13.22.2905. [DOI] [PubMed] [Google Scholar]
- Easton RM, Cho H, Roovers K, Shineman DW, Mizrahi M, Forman MS, Lee VM, Szabolcs M, de Jong R, Oltersdorf T, Ludwig T, Efstratiadis A, Birnbaum MJ. Role for Akt3/protein kinase Bgamma in attainment of normal brain size. Mol Cell Biol. 2005;25:1869–1878. doi: 10.1128/MCB.25.5.1869-1878.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erbay E, Chen J. The mammalian target of rapamycin regulates C2C12 myogenesis via a kinase-independent mechanism. J Biol Chem. 2001;276:36079–36082. doi: 10.1074/jbc.C100406200. [DOI] [PubMed] [Google Scholar]
- Fujio Y, Mitsuuchi Y, Testa JR, Walsh K. Activation of Akt2 Inhibits anoikis and apoptosis induced by myogenic differentiation. Cell Death Differ. 2001;8:1207–1212. doi: 10.1038/sj.cdd.4400919. [DOI] [PubMed] [Google Scholar]
- George S, Rochford JJ, Wolfrum C, Gray SL, Schinner S, Wilson JC, Soos MA, Murgatroyd PR, Williams RM, Acerini CL, Dunger DB, Barford D, Umpleby AM, Wareham NJ, Davies HA, Schafer AJ, Stoffel M, O'Rahilly S, Barroso I. A family with severe insulin resistance and diabetes due to a mutation in AKT2. Science. 2004;304:1325–1328. doi: 10.1126/science.1096706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glass DJ. Signalling pathways that mediate skeletal muscle hypertrophy and atrophy. Nat Cell Biol. 2003;5:87–90. doi: 10.1038/ncb0203-87. [DOI] [PubMed] [Google Scholar]
- Gonzalez I, Tripathi G, Carter EJ, Cobb LJ, Salih DA, Lovett FA, Holding C, Pell JM. Akt2, a novel functional link between p38 mitogen-activated protein kinase and phosphatidylinositol 3-kinase pathways in myogenesis. Mol Cell Biol. 2004;24:3607–3622. doi: 10.1128/MCB.24.9.3607-3622.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heron-Milhavet L, Franckhauser C, Rana V, Berthenet C, Fisher D, Hemmings BA, Fernandez A, Lamb NJ. Only Akt1 is required for proliferation, while Akt2 promotes cell cycle exit through p21 binding. Mol Cell Biol. 2006;26:8267–8280. doi: 10.1128/MCB.00201-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heron-Milhavet L, Mamaeva D, Rochat A, Lamb NJ, Fernandez A. Akt2 is implicated in skeletal muscle differentiation and specifically binds Prohibitin2/REA. J Cell Physiol. 2008;214:158–165. doi: 10.1002/jcp.21177. [DOI] [PubMed] [Google Scholar]
- Hoffman EP, Nader GA. Balancing muscle hypertrophy and atrophy. Nat Med. 2004;10:584–585. doi: 10.1038/nm0604-584. [DOI] [PubMed] [Google Scholar]
- Horsley V, Jansen KM, Mills ST, Pavlath GK. IL-4 acts as a myoblast recruitment factor during mammalian muscle growth. Cell. 2003;113:483–494. doi: 10.1016/s0092-8674(03)00319-2. [DOI] [PubMed] [Google Scholar]
- Horsley V, Pavlath GK. Forming a multinucleated cell: molecules that regulate myoblast fusion. Cells Tissues Organs. 2004;176:67–78. doi: 10.1159/000075028. [DOI] [PubMed] [Google Scholar]
- Irie HY, Pearline RV, Grueneberg D, Hsia M, Ravichandran P, Kothari N, Natesan S, Brugge JS. Distinct roles of Akt1 and Akt2 in regulating cell migration and epithelial-mesenchymal transition. J Cell Biol. 2005;171:1023–1034. doi: 10.1083/jcb.200505087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jansen KM, Pavlath GK. Mannose receptor regulates myoblast motility and muscle growth. J Cell Biol. 2006;174:403–413. doi: 10.1083/jcb.200601102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaneko S, Feldman RI, Yu L, Wu Z, Gritsko T, Shelley SA, Nicosia SV, Nobori T, Cheng JQ. Positive feedback regulation between Akt2 and MyoD during muscle differentiation. Cloning of Akt2 promoter. J Biol Chem. 2002;277:23230–23235. doi: 10.1074/jbc.M201733200. [DOI] [PubMed] [Google Scholar]
- Lai KM, Gonzalez M, Poueymirou WT, Kline WO, Na E, Zlotchenko E, Stitt TN, Economides AN, Yancopoulos GD, Glass DJ. Conditional activation of akt in adult skeletal muscle induces rapid hypertrophy. Mol Cell Biol. 2004;24:9295–9304. doi: 10.1128/MCB.24.21.9295-9304.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawlor MA, Feng X, Everding DR, Sieger K, Stewart CE, Rotwein P. Dual control of muscle cell survival by distinct growth factor-regulated signaling pathways. Mol Cell Biol. 2000;20:3256–3265. doi: 10.1128/mcb.20.9.3256-3265.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawlor MA, Rotwein P. Insulin-like growth factor-mediated muscle cell survival: central roles for Akt and cyclin-dependent kinase inhibitor p21. Mol Cell Biol. 2000;20:8983–8995. doi: 10.1128/mcb.20.23.8983-8995.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu H, Radisky DC, Nelson CM, Zhang H, Fata JE, Roth RA, Bissell MJ. Mechanism of Akt1 inhibition of breast cancer cell invasion reveals a protumorigenic role for TSC2. Proc Natl Acad Sci U S A. 2006;103:4134–4139. doi: 10.1073/pnas.0511342103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manning BD, Cantley LC. AKT/PKB signaling: navigating downstream. Cell. 2007;129:1261–1274. doi: 10.1016/j.cell.2007.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nader GA. Molecular determinants of skeletal muscle mass: getting the "AKT" together. Int J Biochem Cell Biol. 2005;37:1985–1996. doi: 10.1016/j.biocel.2005.02.026. [DOI] [PubMed] [Google Scholar]
- Nader GA. Muscle growth learns new tricks from an old dog. Nat Med. 2007;13:1016–1018. doi: 10.1038/nm0907-1016. [DOI] [PubMed] [Google Scholar]
- Pallafacchina G, Calabria E, Serrano AL, Kalhovde JM, Schiaffino S. A protein kinase B-dependent and rapamycin-sensitive pathway controls skeletal muscle growth but not fiber type specification. Proc Natl Acad Sci U S A. 2002;99:9213–9218. doi: 10.1073/pnas.142166599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park IH, Chen J. Mammalian target of rapamycin (mTOR) signaling is required for a late-stage fusion process during skeletal myotube maturation. J Biol Chem. 2005;280:32009–32017. doi: 10.1074/jbc.M506120200. [DOI] [PubMed] [Google Scholar]
- Pelosi M, Marampon F, Zani BM, Prudente S, Perlas E, Caputo V, Cianetti L, Berno V, Narumiya S, Kang SW, Musaro A, Rosenthal N. ROCK2 and its alternatively spliced isoform ROCK2m positively control the maturation of the myogenic program. Mol Cell Biol. 2007;27:6163–6176. doi: 10.1128/MCB.01735-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng XD, Xu PZ, Chen ML, Hahn-Windgassen A, Skeen J, Jacobs J, Sundararajan D, Chen WS, Crawford SE, Coleman KG, Hay N. Dwarfism, impaired skin development, skeletal muscle atrophy, delayed bone development, and impeded adipogenesis in mice lacking Akt1 and Akt2. Genes Dev. 2003;17:1352–1365. doi: 10.1101/gad.1089403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rommel C, Bodine SC, Clarke BA, Rossman R, Nunez L, Stitt TN, Yancopoulos GD, Glass DJ. Mediation of IGF-1-induced skeletal myotube hypertrophy by PI(3)K/Akt/mTOR and PI(3)K/Akt/GSK3 pathways. Nat Cell Biol. 2001;3:1009–1013. doi: 10.1038/ncb1101-1009. [DOI] [PubMed] [Google Scholar]
- Rommel C, Clarke BA, Zimmermann S, Nunez L, Rossman R, Reid K, Moelling K, Yancopoulos GD, Glass DJ. Differentiation stage-specific inhibition of the Raf-MEK-ERK pathway by Akt. Science. 1999;286:1738–1741. doi: 10.1126/science.286.5445.1738. [DOI] [PubMed] [Google Scholar]
- Sartorelli V, Fulco M. Molecular and cellular determinants of skeletal muscle atrophy and hypertrophy. Sci STKE. 2004;2004:re11. doi: 10.1126/stke.2442004re11. [DOI] [PubMed] [Google Scholar]
- Stewart CE, Rotwein P. Insulin-like growth factor-II is an autocrine survival factor for differentiating myoblasts. J Biol Chem. 1996;271:11330–11338. doi: 10.1074/jbc.271.19.11330. [DOI] [PubMed] [Google Scholar]
- Sugatani T, Hruska KA. Akt1/Akt2 and mammalian target of rapamycin/Bim play critical roles in osteoclast differentiation and survival, respectively, whereas Akt is dispensable for cell survival in isolated osteoclast precursors. J Biol Chem. 2005;280:3583–3589. doi: 10.1074/jbc.M410480200. [DOI] [PubMed] [Google Scholar]
- Thrash BR, Menges CW, Pierce RH, McCance DJ. AKT1 provides an essential survival signal required for differentiation and stratification of primary human keratinocytes. J Biol Chem. 2006;281:12155–12162. doi: 10.1074/jbc.M512116200. [DOI] [PubMed] [Google Scholar]
- Tureckova J, Wilson EM, Cappalonga JL, Rotwein P. Insulin-like growth factor-mediated muscle differentiation: collaboration between phosphatidylinositol 3-kinase-Akt-signaling pathways and myogenin. J Biol Chem. 2001;276:39264–39270. doi: 10.1074/jbc.M104991200. [DOI] [PubMed] [Google Scholar]
- Vandromme M, Rochat A, Meier R, Carnac G, Besser D, Hemmings BA, Fernandez A, Lamb NJ. Protein kinase B beta/Akt2 plays a specific role in muscle differentiation. J Biol Chem. 2001;276:8173–8179. doi: 10.1074/jbc.M005587200. [DOI] [PubMed] [Google Scholar]
- Wilson EM, Hsieh MM, Rotwein P. Autocrine growth factor signaling by insulin-like growth factor-II mediates MyoD-stimulated myocyte maturation. J Biol Chem. 2003;278:41109–41113. doi: 10.1074/jbc.C300299200. [DOI] [PubMed] [Google Scholar]
- Wilson EM, Rotwein P. Control of MyoD function during initiation of muscle differentiation by an autocrine signaling pathway activated by insulin-like growth factor-II. J Biol Chem. 2006;281:29962–29971. doi: 10.1074/jbc.M605445200. [DOI] [PubMed] [Google Scholar]
- Wilson EM, Rotwein P. Selective control of skeletal muscle differentiation by Akt1. J Biol Chem. 2007;282:5106–5110. doi: 10.1074/jbc.C600315200. [DOI] [PubMed] [Google Scholar]
- Wilson EM, Tureckova J, Rotwein P. Permissive roles of phosphatidyl inositol 3-kinase and Akt in skeletal myocyte maturation. Mol Biol Cell. 2004;15:497–505. doi: 10.1091/mbc.E03-05-0351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodgett JR. Recent advances in the protein kinase B signaling pathway. Curr Opin Cell Biol. 2005;17:150–157. doi: 10.1016/j.ceb.2005.02.010. [DOI] [PubMed] [Google Scholar]
- Yaffe D, Saxel O. Serial passaging and differentiation of myogenic cells isolated from dystrophic mouse muscle. Nature. 1977;270:725–727. doi: 10.1038/270725a0. [DOI] [PubMed] [Google Scholar]
- Yang ZZ, Tschopp O, Di-Poi N, Bruder E, Baudry A, Dummler B, Wahli W, Hemmings BA. Dosage-dependent effects of Akt1/protein kinase Balpha (PKBalpha) and Akt3/PKBgamma on thymus, skin, and cardiovascular and nervous system development in mice. Mol Cell Biol. 2005;25:10407–10418. doi: 10.1128/MCB.25.23.10407-10418.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoeli-Lerner M, Yiu GK, Rabinovitz I, Erhardt P, Jauliac S, Toker A. Akt blocks breast cancer cell motility and invasion through the transcription factor NFAT. Mol Cell. 2005;20:539–550. doi: 10.1016/j.molcel.2005.10.033. [DOI] [PubMed] [Google Scholar]
- Zhou GL, Tucker DF, Bae SS, Bhatheja K, Birnbaum MJ, Field J. Opposing roles for Akt1 and Akt2 in Rac/Pak signaling and cell migration. J Biol Chem. 2006;281:36443–36453. doi: 10.1074/jbc.M600788200. [DOI] [PubMed] [Google Scholar]