Abstract
We studied the effect of chronic hypobaric hypoxia (CHx; 10-11% O2) on the response to hypercapnia (15% CO2) of individual solitary complex (SC) neurons from adult rats. We simultaneously measured the intracellular pH and firing rate responses to hypercapnia of SC neurons in superfused medullary slices from control and CHx-adapted adult rats using the blind whole cell patch clamp technique and fluorescence imaging microscopy. We found that CHx caused the percentage of SC neurons inhibited by hypercapnia to significantly increase from about 10% up to about 30%, but did not significantly alter the percentage of SC neurons activated by hypercapnia (50% in control versus 35% in CHx). Further, the magnitudes of the responses of SC neurons from control rats (chemosensitivity index for activated neurons of 166±11% and for inhibited neurons of 45±15%) were the same in SC neurons from CHx-adapted rats. This plasticity induced in chemosensitive SC neurons by CHx appears to involve intrinsic changes in neuronal properties since they were the same in synaptic blockade medium.
Keywords: Intracellular pH, pHi, membrane potential, Vm, ventilation, chemosensitivity
1. Introduction
The ventilatory response to CO2 depends on CO2-sensitive chemoreceptors in the central nervous system (CNS). Several anatomically distinct areas of central chemosensitivity have been identified in the brainstem but the significance of a distributed chemosensitive network is not clear (Feldman et al., 2003). Functional differences exist between these sites, such as focal acidosis of the medullary raphé affecting ventilation during non-REM sleep but not wakefulness, while focal acidosis of the retrotrapezoid nucleus affects ventilation only during wakefulness (Feldman et al., 2003). There may also be differences in the cellular mechanisms of chemoreception between different CO2-sensitive sites in the CNS (Putnam et al., 2004). At the very least, the phenotype of central chemoreceptors appears to differ between sites, such as the glutamatergic neurons described for the retrotrapezoid nucleus (Guyenet et al., 2008) and serotonergic neurons from the raphé nucleus (Richerson et al., 2001).
One way to study central chemosensitivity and the significance of different groups of central chemoreceptors is to adapt animals to chronic changes in environmental gas levels and investigate changes in chemoreceptors at the cellular level. In this study, we investigated the effects of chronic hypoxia (CHx) on central chemoreceptors in the solitary complex (SC: including the nucleus tractus solitarius, NTS, and dorsal motor nucleus of the vagus, DMV).
We studied the SC because it is a well-known area of central chemosensitivity. The functional significance of SC chemoreceptors has been demonstrated by the increased ventilation induced by focal acidosis in the caudal SC (Coates et al., 1993; Nattie and Li, 2002). Of SC neurons recorded with single-cell electrophysiological methods, 30 to 50% are excited by CO2, while 10 to 15% are inhibited and the balance show no response to CO2 (Huang et al., 1997; Conrad et al., 2009; Nichols et al., 2009).
We chose hypoxia as a chronic stimulus because it is expected to have multiple effects on the SC. First, CHx alters CO2 regulation with time-dependent decreases in arterial PCO2 during ventilatory acclimatization to hypoxia, and arterial PCO2 remains below control levels for days after return to normoxia (Weil, 1986). This may involve plasticity of central chemoreceptors. Second, CHx results in tonic increases in afferent input from O2-sensitive chemoreceptors in the carotid body, and the first synapse for these afferents in the CNS is in the caudal NTS (Zhang and Mifflin, 2007). Also, there is a time-dependent increase in the O2-sensitivity of the carotid bodies in CHx that further increases afferent input to NTS neurons (Bisgard and Neubauer, 1995). Finally, there is evidence for plasticity in the CNS with ventilatory acclimatization to hypoxia that involves the NTS (Dwinell and Powell, 1999; Chung et al., 2006).
Hence, we hypothesized that the NTS is a unique site for integrating information about chronic changes in both O2 and CO2 and will exhibit plasticity during CHx. We investigated the effects of CHx on the CO2 responsiveness (percentage of neurons responding to hypercapnia with an altered firing rate and the magnitude of that response as determined by the chemosensitivity index (CI)) of neurons within the SC. The main findings of this study are that CHx induces plasticity in chemosensitive SC neurons, significantly increasing the percentage of SC neurons that are inhibited by hypercapnia, but not altering significantly the percentage of activated SC neurons. We saw the same changes in percentages of SC neurons from CHx-adapted animals in the presence of chemical synaptic block medium, suggesting that this plasticity is due to changes in intrinsic neuronal properties. We found that CHx does not affect the CI of SC neurons.
A preliminary report of these findings has previously been published (Nichols et al., 2008).
2. Methods
2.1 Chronic adaptation to hypoxia
Adult male Sprague-Dawley rats (∼P50) were exposed to hypobaric hypoxia (0.5 atmosphere for PO2 of approximately 75 Torr), which we term chronic hypoxia (CHx), for ≥ 7 days. We chose this protocol for CHx since it allows us to compare our findings to those made previously (Powell et al., 2000; Wilkinson et al., 2009) and it has been shown that 7 days is a sufficient period for ventilatory acclimatization to hypoxia (Olson and Dempsey, 1978) and for a change in the central gain of the hypoxic ventilatory response (Powell et al., 2000). It is possible that had we used a longer period of acclimatization that our results would have been different.
Sixteen groups of 4-6 adult male rats each were adapted to CHx and the body weights were recorded for each animal prior to euthanasia. We used 16 groups, all treated identically, since our chamber would only accommodate a maximum of 6 male rats at a time and we needed 16 groups to perform all of our experiments. Briefly, 4-6 Sprague-Dawley male adult rats were placed in a plexiglas chamber (cylinder 91.4 cm long and 27.9 cm in diameter) in which they were exposed to approximately 75 Torr O2 starting at ∼P50. O2 was monitored with an electrode connected to an O2 gauge (Teledyne Analytical Instruments, Industry, CA) and pressure was monitored with a differential electronic manometer (HHP91, Omega, Stamford, CT). All animals were maintained on a 12:12 light:dark cycle and had free access to food and water. Chamber pressure was interrupted for 20-30 minutes 3 times a week for cleaning and replacement of food and water. Each group was left in the chamber for 7 days and then exposure continued until each animal was sacrificed and tested. For controls, 4-6 male adult rats were placed in the chamber with a continuous flow of room air (21% O2) at normal barometric pressure. Temperature and relative humidity were recorded continuously for all groups with HOBO HO8 RH recorders (Onset Computer Corporation, Bourne, MA) placed in the Plexiglas chamber. Temperature ranged from 17-23° C and relative humidity ranged from 23-60%.
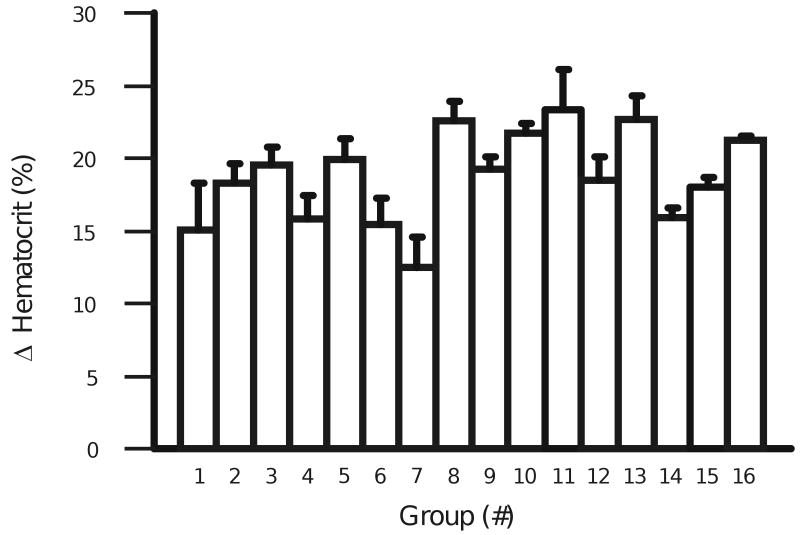
We measured hematocrit as an index for acclimatization to hypoxia. In order to measure hematocrit, blood samples were collected after decapitation by filling heparinized capillary tubes (6 samples per animal) with blood from the carotid artery. These samples were then spun for 3-5 minutes at maximal speed (13,460×g using a hematocrit centrifuge (IEC)). It was found that the change in hematocrit above control induced by CHx was significant for each group of rats (Fig. 1) (47.2 ± 0.2% for controls (n=44) which increased 18.7 ± 0.8% for CHx animals) (n=79) (P<0.0001), and body weight was significantly lower for rats adapted to CHx (269 ± 8g for control animals and 230 ± 3g for CHx animals) (P<0.0001). The change in hematocrit that was induced by CHx is similar to the findings of others (Olson and Dempsey, 1978; Villafuerte et al., 2007), indicating that our rats were showing typical signs of acclimatization to CHx.
Figure 1.
The average increase in hematocrit for each of the 16 groups of rats exposed to CHx. Hematocrit was recorded in both chamber control adult rats and adult rats adapted to CHx to verify that hypoxia had been achieved in the adult rats adapted to CHx. The hematocrit was 47.2 ± 0.2% for chamber control animals (n=44). The average change in hematocrit (18.7 ± 0.8% for all CHx animals (n=79) (P<0.0001)) was significantly increased in all sixteen groups of adult rats adapted to CHx. The height of each bar represents the mean change in hematocrit for each group adapted to CHx and the error bars represent 1 SEM (n=4-6 for each group).
All procedures involving animals were approved by the Institutional Animal Care and Use Committee at Wright State University and were in agreement with standards set forth in the National Institutes of Health Guide for Care and Use of Laboratory Animals. Wright State University is accredited by AAALAC and is covered by NIH Assurance (no. A3632-01).
2.2 Solutions
In this study we used standard solutions employed in our previous studies. Artificial cerebral spinal fluid (aCSF) contained the following (in mM): 124 NaCl, 5.0 KCl, 2.4 CaCl2, 1.3 MgSO4, 1.24 KH2PO4, 26 NaHCO3, and 10 glucose, and was equilibrated with 95 % O2/ 5% CO2 (pH ∼ 7.45 at 37°C). Synaptic blockade (SNB) solution was modified from aCSF with 0.2 mM CaCl2 and 11.4 mM MgSO4 ([NaCl] was adjusted to maintain osmotic balance) in order to block chemical synapses. The whole cell patch intracellular solution contained (in mM): 130 K+-gluconate, 10 K+-HEPES, 0.4 EGTA, 1 MgCl2, 0.3 GTP, and 2 ATP (pH = 7.45 at room temperature) (Filosa and Putnam, 2003). The high K+/nigericin solution that was used for calibration in the imaging studies contained (in mM): 104 KCl, 2.4 CaCl2, 1.3 MgSO4, 1.24 KH2PO4, 25 N-methyl-D-glucamine (NMDG)-HEPES, 25 K-HEPES, 10 glucose, and 0.004 nigericin titrated with KOH or HCl to a pH value of 7.4. The pH-sensitive fluorescent dye 8-hydroxypyrene-1,3,6-trisulfonic acid, trisodium salt (1 mM) (HPTS, pyranine) was added to the whole cell patch intracellular solution and was purchased from Invitrogen (Eugene, OR). All other chemicals were purchased from Sigma (St. Louis, MO) except where indicated.
2.3 Slice preparation
Slices were prepared and used as previously described (Nichols et al., 2009). Briefly, control and CHx adult male rats were anesthetized with a brief exposure to CO2 (100 %), which was followed by rapid decapitation. The brainstem was then removed and submerged in aCSF equilibrated with 5% CO2 / 95% O2 gas mixture. Transverse slices (300 μm) were prepared on a vibratome (Pelco 101, series 1000) beginning at the obex and extending rostrally for ∼ 1mm and were allowed to recover for at least 1 hour at room temperature in aCSF equilibrated with 5% CO2 / 95% O2. In this study we used 3 SC slices centered on the area postrema (slices 1-3 from figure 2 in Ritucci et al, 1997). Individual slices for study from control and CHx adult male rats were placed in a superfusion chamber on the stage of an upright Nikon Optiphot-2 microscope. Slices were immobilized with a nylon grid and superfused at ∼2.4 ml/min with aCSF equilibrated with 5% CO2 / 95% O2 (pH 7.45 at 37° C).
Individual neurons from the SC were then studied. To study the response of these neurons to hypercapnia, we used a protocol that consisted of a 5 minute exposure to aCSF equilibrated with 5% CO2 / 95% O2, a 10-15 minute exposure to aCSF equilibrated with 15% CO2 / 85% O2 (pHo ∼ 6.8-6.9 at 37°C), and a 5-10 minute exposure to aCSF equilibrated with 5% CO2 / 95% O2. We used very high hypercapnic challenges in order to differentiate chemosensitive neurons and possibly detect small changes in percentages induced by CHx. We also used 15% CO2 as the hypercapnic challenge so that we could compare our results to those previously reported for SC neurons from adult rats (Dean et al., 1989, 1990; Nichols et al., 2009). We believe that the percentages of CO2 activated neurons that we observe in this study would have been the same had we used lower levels of hypercapnic challenges since Dean et al. (1990) previously showed that SC neurons that were activated by 15% CO2 were also activated by 10% and 7% CO2 as well. For SNB studies, this protocol was repeated in the same neuron with SNB solution.
2.4 Imaging of fluorescent neurons
pHi was measured as previously described (Mulkey et al., 2004a; Ritucci et al., 2005). Individual SC neurons were loaded with 1mM of the pH-sensitive dye, pyranine, through a whole cell patch pipette. Loaded neurons were excited and alternately exposed for 1s to light of wavelength 450 ± 10 and 410 ± 10 nm using a Sutter Lambda 10-2 filter wheel. Emitted fluorescence at 515 ± 10 nm was collected and processed using MetaFluor 7.1.4.0 software (Molecular Devices), and the 450/410 fluorescence ratio (Rfl) was determined. We used a one point calibration (pH of 7.4) using the high K+/ nigericin technique (Thomas et al., 1979) to determine initial pHi of SC neurons from adult rats that had been adapted to CHx. Rfl values measured during the experiment were divided by the Rfl value for pH 7.4, which gave normalized Rfl (Nfl). We used the equation (pH = 7.4969 + log (Nfl -0.2003)/(2.0194 - Nfl); r2 = 0.99) (Nichols et al., 2009) to convert Nfl into pHi. A pHi versus time plot was made in Microsoft Excel, from which the pHi recovery rate from CO2-induced acidification of an individual neuron could be estimated from the slope of a linear fit to the pHi versus time trace during acute hypercapnia (at least 5 points starting at minimum pHi).
2.5 Electrophysiological studies
The blind whole-cell patch clamp technique was used to measure neuronal membrane potential (Vm) and integrated firing rate as described previously (Blanton et al. 1989; Nichols et al., 2009). The experimental setup that was used has been previously described (Dean et al., 1997; Huang et al., 1997; Filosa et al., 2002; Conrad et al., 2009; Nichols et al., 2009). Briefly, a whole cell patch pipette (5 MΩ) was fabricated from borosilicate glass using a Narishige PP-830 dual stage pipette puller. The pipette was filled with whole cell patch solution (see above). Positive pressure was maintained on the pipette while a -0.1nA pulse (30 msec, 5 Hz) was applied. Tip impedance increased as the pipette approached a neuron, which was indicated by a 1-2 mV downward deflection. Negative pressure was applied to the pipette to obtain a giga-ohm seal, brief suction was applied to the pipette to rupture the membrane, and then Vm and integrated firing rate were measured throughout the experiment. Integrated firing rate (Hz) was determined from the Vm trace in 10s bins using a window discriminator (FHC model 700B), which was then analyzed using pClamp 8.2 software. Viable neurons had a stable Vm of between -40 and -60 mV and fired action potentials that crossed through zero. The electrophysiological response to hypercapnia was quantified using two measures: percentage of neurons either activated or inhibited by acute hypercapnia, and the magnitude of the firing rate response to acute hypercapnia, calculated as the CI according to the equation of Wang and Richerson (1999):
Where FRhc and FRnc are the neuronal firing rates at 15% and 5% CO2, respectively, and pH5 and pH15 are the solution pH at 5% and 15% CO2, respectively. A neuron was designated as activated if its CI was greater than 120% or inhibited if its CI was less than 80%.
2.6 Statistical analysis
Values are reported as means ± S.E.M. Fisher's exact tests were used to compare differences between the percentages of neurons that respond to hypercapnia. Paired t-tests were used to compare differences between two means with a level of significance of P<0.05. Differences between three or more means were determined by one-way ANOVA. If significant differences existed, then multiple comparisons were done using Tukey's method with a level of significance of P<0.05.
3. Results
3.1 pHi and electrophysiological responses to acute hypercapnia of SC neurons from control and CHx-adapted adult rats in aCSF
The initial pHi of SC neurons from control (7.30 ± 0.03; n = 27 neurons from 9 rats) and CHx-adapted (7.35 ± 0.06 pH unit; n = 12 neurons from 3 rats) adult rats did not differ. Both values for adults were somewhat less alkaline than previously reported values for SC neurons from neonates (7.49 ± 0.02; Ritucci et al., 1997), as previously noted (Nichols et al., 2009).
The typical response to hypercapnia (15% CO2) of an SC neuron from a control (Fig. 2A) and a CHx-adapted rat (Fig. 2B) is a maintained acidification without pHi recovery and a reversible increase in firing rate. On average, the pHi response to 15% CO2 was the same in SC neurons from CHx-adapted vs. control rats, acidifying by 0.26 ± 0.013 pH unit (CHx, n = 89 neurons from 24 rats) and by 0.28 ± 0.018 pH unit (control, n = 47 neurons from 10 rats). SC neurons from both control and CHx-adapted adult rats exhibited a lack of pHi recovery (-0.005 ± 0.001 pH unit/min; n = 47 and -0.009 ± 0.002 pH unit/min; n = 89, respectively). Upon return to normocapnia, the pHi of SC neurons from control and CHx adapted adult rats returned to initial values with no apparent overshoot (Fig. 2A and B), consistent with a lack of pHi recovery during hypercapnia (Boron and De Weer, 1976; Ritucci et al., 1997).
Figure 2.
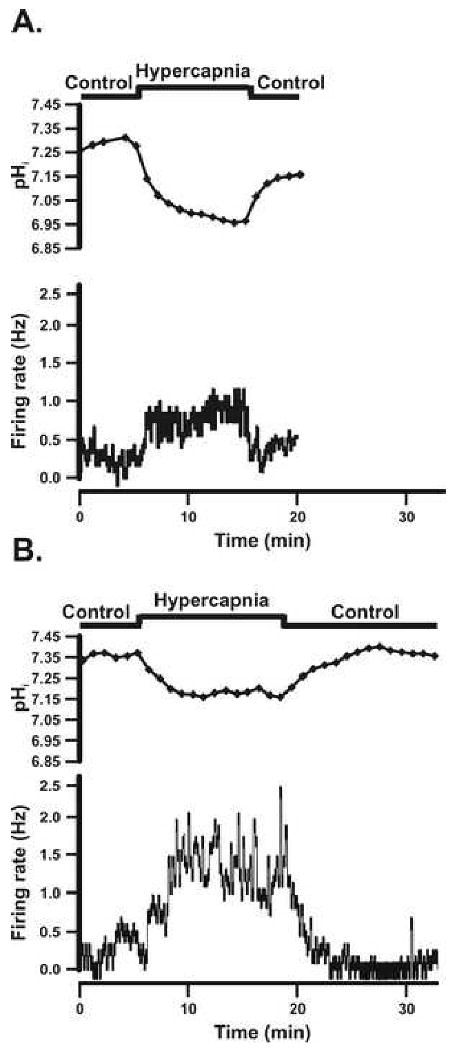
A: The pHi and firing rate responses of an individual SC neuron that was activated by hypercapnic acidosis from a chamber control adult rat. B: The pHi and firing rate responses of an individual SC neuron that was activated by hypercapnic acidosis from an adult rat adapted to CHx. The top panel shows the experimental protocol used. The second panel shows the pHi response of the SC neuron to hypercapnic acidosis over time, which was a maintained acidification with a lack of pHi recovery. Notice that once the hypercapnic solution was removed, pHi returned back towards initial pHi. The bottom panel shows the firing rate response of the SC neuron to hypercapnic acidosis over time, which was a reversible increase in firing rate in response to hypercapnic acidosis.
The typical responses of a control SC neuron (Fig. 3A) and one from a CHx-adapted rat (Fig. 3B) that are inhibited by hypercapnia are a maintained intracellular acidification but with a reversible reduction of firing rate.
Figure 3.
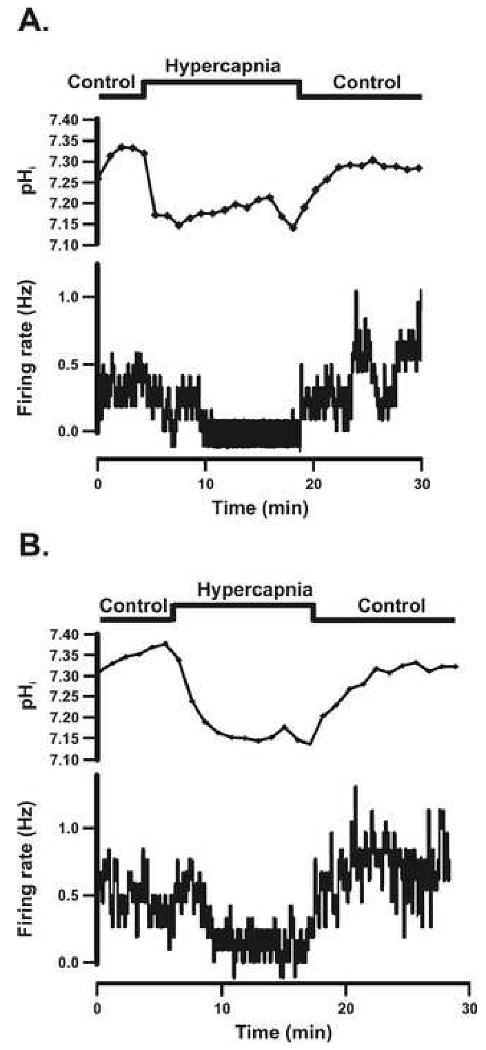
A: The pHi and firing rate responses of an individual SC neuron that was inhibited by hypercapnic acidosis from a chamber control adult rat. B: The pHi and firing rate responses of an individual SC neuron that was inhibited by hypercapnic acidosis in an adult rat adapted to CHx. The top panel shows the experimental protocol used. The second panel shows the pHi response of the SC neuron to hypercapnic acidosis over time, which was a maintained acidification with a lack of pHi recovery. Notice that once the hypercapnic solution was removed, pHi returned back towards initial pHi. The bottom panel shows the firing rate response of the SC neuron to hypercapnic acidosis over time, which was a reversible decrease in response to hypercapnic acidosis.
SC neurons were classified (based on their integrated firing rate response to hypercapnia) as activated (CI > 120%) (Fig. 2A and B), inhibited (CI < 80%) (Fig. 3A and B), or non-chemosensitive (80% < CI < 120%) (sample not shown). Activated SC neurons in 5% CO2 from control and CHx-adapted rats had a similar spontaneous integrated firing rate (0.76 ± 0.10 Hz and 0.56 ± 0.07 Hz respectively) that increased significantly in response to 15% CO2 (2.42 ± 0.56 Hz and 1.58 ± 0.19 Hz respectively), and returned towards initial values of 1.37 ± 0.39 Hz (control) and 0.88 ± 0.12 Hz (CHx) upon return to 5% CO2. There were no significant differences in firing rates between control and CHx values in activated SC neurons at any level of CO2. Inhibited SC neurons from control and CHx rats had a similar basal integrated firing rate in 5% CO2 (0.55 ± 0.13 Hz and 0.94 ± 0.22 Hz respectively) that fell significantly in response to 15% CO2 (0.03 ± 0.03 Hz and 0.32 ± 0.10 Hz respectively), and returned towards initial values of 0.79 ± 0.54 Hz (control) and 0.90 ± 0.19 Hz (CHx) upon return to 5% CO2. The firing rate in response to 15% CO2 was significantly lower for control rats compared to CHx rats (P=0.0055) and was the only significant difference in firing rates between control and CHx values. Non-chemosensitive neurons from control and CHx animals had a similar basal integrated firing rate in 5% CO2 (1.75 ± 0.28 Hz and 1.09 ± 0.24 Hz respectively), which did not change significantly in 15% CO2 (1.69 ± 0.29 Hz and 1.12 ± 0.23 Hz respectively), and remain unchanged upon return to 5% CO2 (1.60 ± 0.26 Hz and 1.26 ± 0.24 Hz respectively).
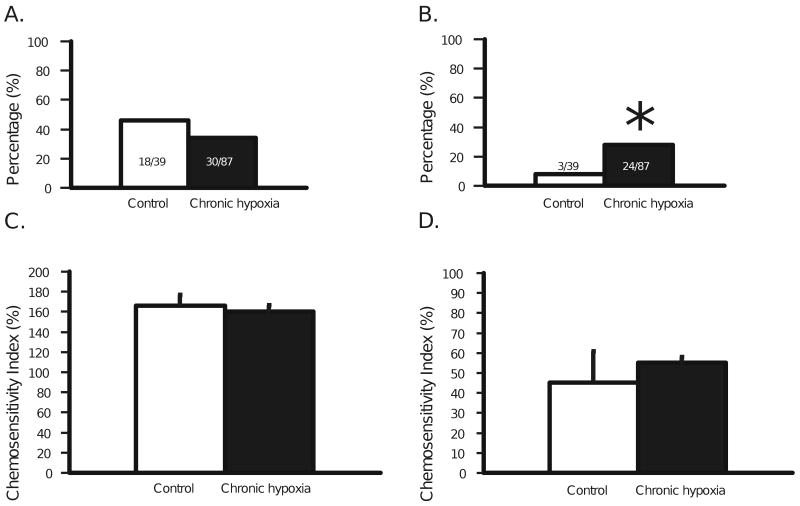
We assessed neuronal responses to acute hypercapnia in two ways, determining the percentage of neurons that respond to and the magnitude of their response to acute hypercapnia. Previously, we found that 57% of SC neurons from adult rats were activated (n = 32) and 9% were inhibited (n = 5) (Nichols et al., 2009) by hypercapnia. In this study we found that our controls showed similar percentages with 46% activated (n = 18; Fig. 4A) and 8% inhibited (n = 3; Fig. 4B). Interestingly, we did not find any significant change in the percentage of SC neurons that were activated by hypercapnia 34% (n = 30; Fig. 4A) but we did find a significant (P=0.017) increase in the percentage inhibited to 28% (n = 24; Fig. 4B). The magnitude of the response of SC neurons to hypercapnia was quantified by calculating the CI (Wang and Richerson, 1999). Previously, we showed that the average CI for activated and inhibited SC neurons from adult rats was 177 ± 8% and 63 ± 10% respectively (Nichols et al., 2009). In this study, we found similar control values for CI for activated (166 ± 11%; Fig. 4C) and for inhibited (45 ± 15%; Fig. 4D) SC neurons from adult rats. Values of CI were unchanged in CHx-adapted rats, with values of 160 ± 6% (Fig. 4C) and 55 ± 3% (Fig. 4D) for activated and inhibited SC neurons from CHx-adapted adult rats, respectively.
Figure 4.
A: The percentage of SC neurons from control adult rats (18 neurons from 9 rats) (white bar) and adult rats adapted to CHx (30 neurons from 20 rats) (black bar) that were activated by hypercapnic acidosis. The N values are denoted on each bar (39 neurons from 10 control rats and 87 neurons from 27 CHx rats). There is no significant change in the percentage of SC neurons activated by hypercapnia in CHx-adapted rats. B: The percentage of SC neurons from control adult rats (3 neurons from 3 rats) (white bar) and adult rats adapted to CHx (24 neurons from 18 rats) (black bar) that were inhibited by hypercapnic acidosis. The N values are denoted on each bar. The remaining, non-chemosensitive, neurons amounted to 18 neurons from 10 control rats and 33 neurons from 17 CHx rats. Notice that the percentage inhibited by hypercapnic acidosis is significantly increased by CHx (* indicates P = 0.017). C: The chemosensitivity index of SC neurons from control adult rats and adult rats adapted to CHx that were activated by hypercapnic acidosis. D: The chemosensitivity index of SC neurons from control adult rats and adult rats adapted to CHx that were inhibited by hypercapnic acidosis. Notice that CHx does not affect the chemosensitivity index of SC neurons activated (C) or inhibited (D) by hypercapnic acidosis. The height of each bar represents the mean chemosensitivity index for that group and the error bars represent 1 SEM.
3.2 pHi and firing rate responses to acute hypercapnia of SC neurons from control and CHx-adapted adult rats in synaptic blockade medium
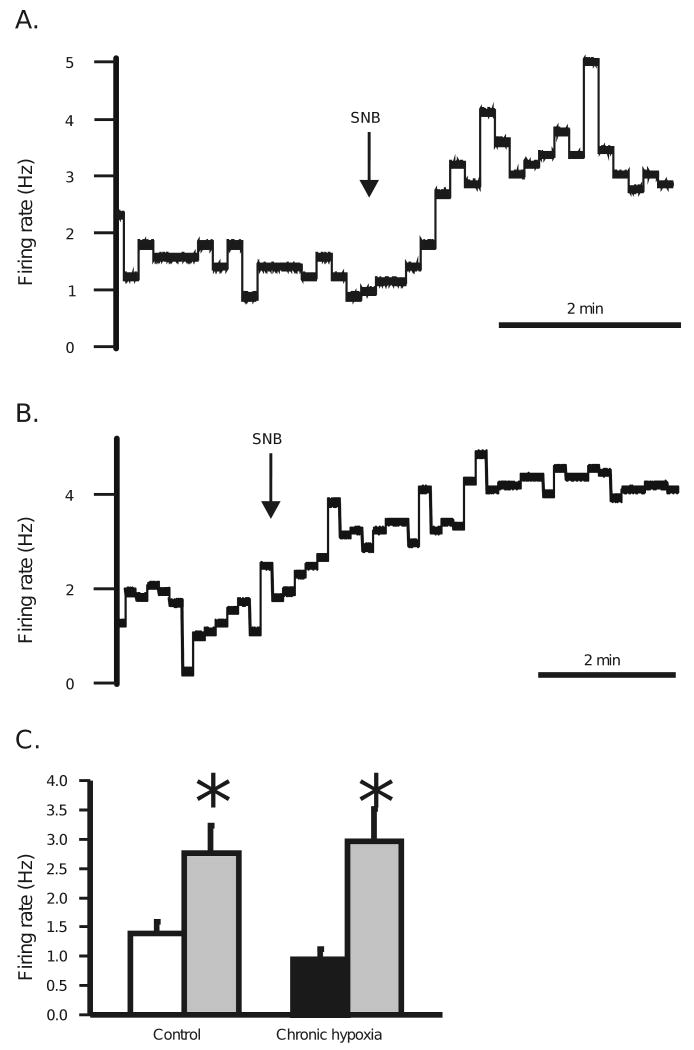
We measured the impact of blocking chemical synaptic transmission on the response to hypercapnia of SC neurons from CHx-adapted rats since we observed significant changes in the percentage of neurons that respond to hypercapnia in these rats (Fig. 4A and B). Basal firing rate increases in response to SNB in SC neurons from both control (Fig. 5A) and CHx-adapted (Fig. 5B) rats, as we have previously observed (Nichols et al., 2009). The average basal firing rate of SC neurons from control rats was 1.38 ± 0.21 Hz (n = 22) (Fig. 5C) and for CHx rats was 0.96 ± 0.17 Hz respectively (n = 22) (Fig. 5C). SC neuronal firing rate increased significantly in the presence of SNB to 2.76 ± 0.47 Hz for control (P=0.0035) and 2.96 ± 0.56 Hz for CHx-adapted rats (P=0.0004). These data suggest that SC neurons in slices from adult rats receive tonic inhibitory input.
Figure 5.
A: Effect of synaptic blockade medium (SNB—11.4 mM Mg2+ and 0.2 mM Ca2+) on basal firing rate of an SC neuron from a control adult rat. Exposure to SNB solution is denoted by the arrow. Notice that SNB causes an increase in basal firing rate. B: Effect of SNB on basal firing rate of an SC neuron from an adult rat adapted to CHx. Exposure to SNB solution is denoted by the arrow. Notice that SNB causes an increase in basal firing rate. C: The average basal firing rate for SC neurons from control animals (white bar) significantly increases in the presence of SNB (22 neurons from 7 rats) (gray bar) (* indicates P = 0.0035). The average basal firing rate for SC neurons from adult rats adapted to CHx (black bar) also significantly increases in the presence of SNB (22 neurons from 9 rats) (gray bar) (* indicates P = 0.0004). The height of each bar represents the mean firing rate for that group and the error bars represent 1 SEM.
SNB had no effect on basal pHi of SC neurons from control animals (7.13 ± 0.03 pH unit for control animals in aCSF versus 7.10 ± 0.03 pH unit for control animals in the presence of SNB; n = 19 neurons from 7 rats). SNB slightly acidified basal pHi of SC neurons from CHx animals (7.21 ± 0.02 pH unit for CHx animals in aCSF versus 7.15 ± 0.02 pH unit for CHx animals in the presence of SNB; n = 26 neurons from 9 rats; P=0.0004).
For SNB experiments, a neuron's chemosensitive response was first studied in the absence of SNB, which was followed by the same experiment in the presence of SNB. Before the chemosensitive response was measured in the presence of SNB, the firing rate was decreased back towards initial firing rate by injecting negative DC current. SNB did not affect the magnitude of acidification caused by hypercapnia in SC neurons from either control animals or CHx animals (0.27 ± 0.03 pH unit for control animals and 0.27 ± 0.02 pH unit for CHx animals in the presence of aCSF and 0.32 ± 0.02 pH unit for control animals and 0.31 ± 0.02 pH unit for CHx animals in the presence of SNB; n = 19 for control and n = 26 for CHx). Additionally, there was a lack of pHi recovery in SC neurons from both control (-0.005 ± 0.002 pH unit/minute in aCSF and -0.011 ± 0.003 pH unit/minute in the presence of SNB) and CHx animals(-0.011 ± 0.002 pH unit/minute in aCSF and -0.009 ± 0.002 pH unit/minute in the presence of SNB). Upon return to normocapnia in aCSF and SNB, the pHi of SC neurons from both control and CHx animals returned towards initial values with no apparent overshoot (data not shown).
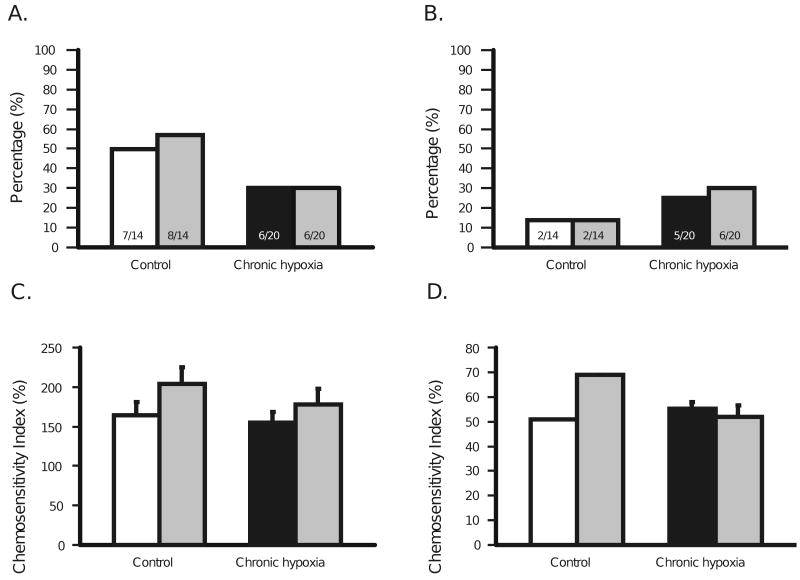
The integrated firing rate response to hypercapnic acidosis in SC neurons from control and CHx adult rats did not change in the presence of SNB. In the absence of SNB 50% of SC neurons from control animals were activated and 57% of SC neurons were activated in the presence of SNB, while 30% of SC neurons from CHx animals were activated both in the absence and presence of SNB (Fig. 6A). We found that 14% of SC neurons from control animals were inhibited both in the absence and the presence of SNB, whereas 25% of SC neurons from CHx animals were inhibited in the absence of SNB and 30% were inhibited in the presence of SNB (Fig. 6B). Notice that SNB does not affect the percentage of neurons that respond to hypercapnic acidosis in either control or CHx animals (Fig. 6A and B). The CI for activated SC neurons was 164 ± 17% in the absence of SNB and 204 ± 21% in the presence of SNB from control animals and 155 ± 13% in the absence of SNB and 178 ± 20% in the presence of SNB from CHx animals (Fig. 6C). Inhibited SC neurons from control animals had a CI of 51% in the absence of SNB and 69% in the presence of SNB (no error bar since n = 2), whereas those from CHx animals had a CI of 55 ± 3% in the absence of SNB and 52 ± 5% in the presence of SNB (Fig. 6D). Since SNB had no effect on the chemosensitive response we conclude that chemical synaptic transmission is not required for the SC neuronal chemosensitive response in control and CHx animals. Further, the CHx-induced plasticity in changing the percentage of SC neurons that are inhibited by hypercapnic acidosis is not due to changes in chemical synaptic transmission. Therefore, the responses to hypercapnic acidosis of SC neurons and the changes in these responses induced by CHx seem to involve changes of the intrinsic properties of SC neurons.
Figure 6.
A: The percentage of SC neurons from control adult rats that were activated by hypercapnic acidosis in the absence (7 neurons from 4 rats) (white bar) and presence of synaptic blockage medium (SNB—11.4 mM Mg2+ and 0.2 mM Ca2+) (8 neurons from 6 rats) (gray bar) and SC neurons from adult rats adapted to CHx that were activated by hypercapnic acidosis in the absence (6 neurons from 6 rats) (black bar) and the presence (gray bar) of SNB (6 neurons from 6 rats). The N values are denoted on each bar (14 neurons from 6 control rats and 20 neurons from 9 CHx rats). B: The percentage of SC neurons from control adult rats that were inhibited by hypercapnic acidosis in the absence (2 neurons from 2 rats) (white bar) and presence (2 neurons from 2 rats) of SNB (gray bar) and of SC neurons from adult rats adapted to CHx that were inhibited by hypercapnic acidosis in the absence (5 neurons from 4 rats) (black bar) and the presence (6 neurons from 3 rats) (gray bar) of SNB. The N values are denoted on each bar. The remaining, non-chemosensitive, neurons amounted to 5 neurons from 4 control rats and 7 neurons from 5 CHx rats. Notice that SNB does not affect the percentage of neurons that respond within control or CHx animals. C: The chemosensitivity index of SC neurons from control adult rats that were activated by hypercapnic acidosis in the absence (white bar) and presence (gray bar) of SNB and of SC neurons from adult rats adapted to CHx that were activated by hypercapnic acidosis in the absence (black bar) and presence (gray bar) of SNB. D: The chemosensitivity index of SC neurons from control adult rats that were inhibited by hypercapnic acidosis in the absence (white bar) and presence (gray bar) of SNB and of SC neurons from adult rats adapted to CHx that were inhibited by hypercapnic acidosis in the absence (black bar) and presence (gray bar) of SNB. The height of each bar represents the mean chemosensitivity index for that group and the error bars represent 1 SEM. There are no error bars for the CI of inhibited neurons from control animals in the absence and presence of SNB because the two inhibited neurons in the absence and presence of SNB had the same CI. Notice that CHx does not affect the chemosensitivity index of SC neurons activated or inhibited by hypercapnic acidosis.
4. Discussion
The main findings of this study are: 1) CHx results in a significant plasticity of adult SC neurons, with an increase in the percentage of CO2-inhibited SC neurons without a concomitant increase (if anything a small decrease was seen) in CO2-activated neurons, and 2) SNB does not alter the firing rate response to hypercapnia of adult SC neurons from either control or CHx-adapted rats. Thus, CHx induces plasticity in which the adult chemosensitive response of SC neurons to hypercapnia is suppressed and this suppression does not appear to depend on chemical synaptic transmission.
4.1 Ventilatory acclimatization to hypoxia
Upon exposure to hypoxia ventilation increases. If the hypoxic exposure is prolonged, there is a time-dependent additional increase in ventilation (ventilatory acclimatization to hypoxia; VAH) (Bisgard, 2000). Intact carotid bodies are necessary for VAH (Smith et al., 1986) and it is clear that VAH is at least in part due to increased sensitivity of carotid bodies to hypoxia since the carotid body afferent input to the CNS increases over time of exposure to hypoxia (Barnard et al., 1987; Vizek et al., 1987; Nielsen et al., 1988). Chronic hypoxia also induces an increase in the CNS gain, defined as the increase of ventilation for a given carotid body chemoreceptor afferent input to the CNS (Powell et al., 2000) but the basis for increased central gain is not known. It is known that caudal NTS neurons receive the afferent input from the carotid bodies (Donoghue et al., 1984; Mifflin, 1992) and therefore these NTS neurons will receive increased afferent input in response to CHx (Powell, 2007). These observations lead us to hypothesize that the increased gain could result from CHx-induced plasticity of caudal SC neurons.
Chronic hypoxia has also been shown to alter the hypercapnic ventilatory response (HCVR), which is defined by the ventilation vs. end-tidal CO2 curve. Depending on the hypoxic conditions and the animal being studied, chronic hypoxia results in either an increase in the slope, a decrease in the intercept (apneic threshold) or both of the ventilation vs. CO2 curve (Engwall and Bisgard, 1990; Fatemian and Robbins, 1998; Powell et al., 2000; Khodadadeh et al., 2006; Wilkinson et al., 2009). Since the caudal NTS is also a site of CO2 chemoreception (Dean et al., 1989, 1990; Nattie and Li, 2002), CHx-induced plasticity of SC neurons may also be the basis for altered HCVR in animals exposed to chronic hypoxia. The current study is the first to quantify the chemosensitive response of SC neurons in response to CHx exposure.
4.2 Hypoxic ventilatory response
CHx increases the O2 sensitivity of the carotid bodies (Nielsen et al., 1988), thus afferent input to the NTS should be tonically increased (Powell, 2007), which could cause central ventilatory plasticity resulting in increased CNS gain. However, it is difficult to relate our findings of increased percentage of CO2-inhibited neurons in CHx-adapted rats to plasticity of the hypoxic ventilatory response. Since CHx results in prolonged hyperventilation and therefore reduced PaCO2 (Weil, 1986), one interesting possibility is that CO2-inhibited SC neurons could be “activated” by this hypocapnia (Richerson et al, 2001) and could result in increased ventilatory drive from the SC under conditions of hypocapnia.
It is also possible that SC neurons from CHx-adapted rats have altered intrinsic properties unrelated to their CO2 responsiveness that contributes to increased central gain. In this regard, CO2-sensitive SC neurons have been shown to be sensitive also to hyperoxia (Mulkey et al., 2003) and to hypoxia (Dean et al., 1991; Pascual et al., 2002), and that O2 sensitivity involves a distinct cellular mechanism from CO2 sensitivity (Mulkey et al., 2003). Thus, it might be that CHx alters the O2 sensitivity of SC neurons, a possibility that we did not study.
4.3 Hypercapnic ventilatory response
We found that CHx resulted in plasticity of SC neurons, with a significant increase in CO2-inhibited SC neurons. This finding would suggest that HCVR should be decreased in CHx-adapted rats, which it is not (Engwall and Bisgard, 1990; Fatemian and Robbins, 1998; Powell et al., 2000; Wilkinson et al., 2009). Attempts to correlate cellular chemosensitive responses to hypercapnia of neurons from one brainstem region with whole animal ventilatory responses is complicated by the likelihood that there are many chemosensitive brainstem regions and the role of each in ventilatory control is not known (Nattie and Li, 2006; 2009). It could be argued that CO2-sensitive SC neurons do not contribute to the respiratory network (Guyenet et al., 2008). We believe this is untenable. We have recent evidence that focal acidosis of the caudal SC (centered on the area postrema, i.e. from the exact same are of the SC from which our slices were taken) from CHx-adapted rats reduces integrated phrenic nerve activity when compared with control rats (Wilkinson et al., 2009). This is consistent with CHx increasing CO2-inhibited neurons within the SC and reducing hypercapnic drive from the SC to the respiratory network.
At this time, it is not clear how our results correlate with the whole animal response to hypercapnia after CHx exposure. That HCVR is not decreased after CHx exposure while SC neuronal input is decreased suggests that CHx activates chemosensitive neurons located in another CO2-sensitive region. The carotid bodies have been shown to contribute substantially to CO2 chemoreception (Fatemian et al., 2003; Dempsey, 2004; Nattie, 2006; Smith et al., 2006). While it is known that CHx increases the O2 sensitivity of the carotid bodies (Nielsen et al., 1988), it is not known how their CO2-sensitivity is changed. Thus, it seems reasonable to postulate that the CO2- responsiveness of the carotid bodies may be elevated by CHx exposure.
Another CO2-sensitive region that may have an altered response after CHx exposure is the retrotrapezoid nucleus (RTN) (Mulkey et al., 2004b; Ritucci et al., 2005). It is known that RTN neurons receive input from peripheral chemoreceptors, although afferent output first gets relayed through the caudal NTS (Stornetta et al., 2006; Takakura et al., 2006). It would thus be of interest to know how CHx alters the CO2- responsiveness of RTN neurons.
4.4 Perspective
We observed an unexpected plasticity of SC neurons to CHx acclimatization. It is not clear what role this plasticity plays in the effects of acclimatization on the central gain of the HVR or on changes in the HCVR induced by CHx exposure. It is clear that we have identified a model system in which to study CO2-inhibited neurons. In most chemosensitive regions, inhibited neurons are rare, comprising about 10-15% of SC neurons (Conrad et al., 2009; Nichols et al., 2009), about 15% of medullary raphé neurons (Wang and Richerson, 1999) and 5-15% of LC neurons (Hartzler et al., 2007). Here we show that the percentage of SC neurons inhibited by hypercapnia increases to 30% after exposure to CHx, a sufficiently high proportion of CO2-inhibited neurons to allow the study of the previously unexplored mechanism of how hypercapnia can result in decreased neuronal firing rate. Additionally, we found that the shift in the percentage of inhibited neurons was not due to chemical synaptic input, so the response we observe in SC neurons from CHx-adapted rats must result from a shift in intrinsic neuronal properties. We suggest that the increase in the percentage of inhibited neurons is due to changes in the expression of CO2/H+-sensitive ion channels, converting CO2-insensitive SC neurons into CO2-inhibited neurons.
It appears that the relative increase in the percentage of SC neurons inhibited by CO2 results in reduced excitatory input to the respiratory network since focal acidification of the SC from CHx-adapted rats has a reduced phrenic nerve response (Wilkinson et al., 2009). Since the HCVR is not similarly reduced in CHx-adapted animals, CHx must increase the CO2 responsiveness of neurons in another part of the respiratory network. Studies of CHx-induced plasticity of CO2-responsive neurons from other brainstem regions should prove interesting.
Finally we propose the novel hypothesis that reduced SC neuronal response to acidosis implies increased responsiveness to alkalosis. We hypothesize that SC neurons that are inhibited by hypercapnic acidosis will be activated by hypocapnic alkalosis. Thus, the increased percentage of SC neurons that are inhibited by hypercapnia that occurs in CHx-adapted rats could result in increased respiratory drive from the SC during conditions of hypocapnia, as observed in rats chronically adapted to hypoxia. This novel hypothesis can be tested by studying the integrated phrenic nerve response to focal alkalosis of the SC in CHx-adapted rats, which we would hypothesize is greater than the integrated phrenic nerve response to focal alkalosis of the SC in control rats.
Acknowledgments
This work was supported by National Institutes of Health Grants RO1 HL56683 (RWP and JBD) and RO1 HL081823 (FLP, RWP, and JBD). NLN received support from the Biomedical Sciences Ph.D. program at Wright State University.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Barnard P, Andronikou S, Pokorski M, Smatresk N, Mokashi A, Lahiri S. Time-dependent effect of hypoxia on carotid body chemosensory function. J Appl Physiol. 1987;63:685–691. doi: 10.1152/jappl.1987.63.2.685. [DOI] [PubMed] [Google Scholar]
- Bisgard GE, Neubauer JA. Peripheral and central effects of hypoxia. In: Dempsey JA, Pack AI, editors. Regulation of Breathing. New York, Basel, Hong Kong: Marcel Dekker, Inc.; 1995. pp. 617–618. [Google Scholar]
- Bisgard GE. Carotid body mechanisms in acclimatization to hypoxia. Respir Physiol. 2000;121:237–46. doi: 10.1016/s0034-5687(00)00131-6. [DOI] [PubMed] [Google Scholar]
- Boron WF, De Weer P. Intracellular pH transients in squid giant axons caused by CO2, NH3, and metabolic inhibitors. J Gen Physiol. 1976;67:91–112. doi: 10.1085/jgp.67.1.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung S, Ivy GO, Reid SG. GABA-mediated neurotransmission in the nucleus of the solitary tract alters resting ventilation following exposure to chronic hypoxia in conscious rats. Am J Physiol Regul Integr Comp Physiol. 2006:R1449–R1456. doi: 10.1152/ajpregu.00645.2005. [DOI] [PubMed] [Google Scholar]
- Coates EL, Li A, Nattie EE. Widespread sites of brain stem ventilatory chemoreceptors. J Appl Physiol. 1993;75:5–14. doi: 10.1152/jappl.1993.75.1.5. [DOI] [PubMed] [Google Scholar]
- Conrad SC, Nichols NL, Ritucci NA, Dean JB, Putnam RW. Development of chemosensitivity in neurons from the nucleus tractus solitarius (NTS) of neonatal rats. Resp Physiol Neurobiol. 2009;166:4–12. doi: 10.1016/j.resp.2008.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dean JB, Bayliss DA, Erickson JT, Lawing WL, Millhorn DE. Depolarization and stimulation of neurons in nucleus tractus solitarii by carbon dioxide does not require chemical synaptic input. Neuroscience. 1990;36:207–216. doi: 10.1016/0306-4522(90)90363-9. [DOI] [PubMed] [Google Scholar]
- Dean JB, Gallman EA, Zhu WH, Millhorn DE. Multiple effects of hypoxia on neurons in dorsal motor nucleus (X) and nucleus tractus solitarii (NTS) Soc Neurosci Abstr. 1991;17:187.7. [Google Scholar]
- Dean JB, Huang RQ, Erlichman JS, Southard TL, Hellard DT. Cell-cell coupling occurs in dorsal medullary neurons after minimizing anatomical-coupling artifacts. Neuroscience. 1997;80:21–40. doi: 10.1016/s0306-4522(97)00016-x. [DOI] [PubMed] [Google Scholar]
- Dean JB, Lawing WL, Millhorn DE. CO2 decreases membrane conductance and depolarizes neurons in the nucleus tractus solitarii. Exp Brain Res. 1989;76:656–661. doi: 10.1007/BF00248922. [DOI] [PubMed] [Google Scholar]
- Dempsey JA. Crossing the apnoeic threshold: causes and consequences. (The Julius H Comroe Lecture) Exp Physiol. 2004;90:13–24. doi: 10.1113/expphysiol.2004.028985. [DOI] [PubMed] [Google Scholar]
- Donoghue S, Felder RB, Jordan D, Spyer KM. The central projections of carotid baroreceptors and chemoreceptors in the cat: a neurophysiological study. J Physiol. 1984;347:397–409. doi: 10.1113/jphysiol.1984.sp015072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dwinell MR, Powell FL. Chronic hypoxia enhances the phrenic nerve response to arterial chemoreceptor stimulation in anesthetized rats. J Appl Physiol. 1999;87:817–823. doi: 10.1152/jappl.1999.87.2.817. [DOI] [PubMed] [Google Scholar]
- Engwall MJA, Bisgard GE. Ventilatory responses to chemoreceptor stimulation after hypoxic acclimatization in awake goats. J Appl Physiol. 1990;69:1236–1243. doi: 10.1152/jappl.1990.69.4.1236. [DOI] [PubMed] [Google Scholar]
- Fatemian M, Nieuwenhuijs DJF, Teppema LJ, Meinesz S, van der Mey AGJ, Dahan A, Robbins PA. The respiratory response to carbon dioxide in humans with unilateral and bilateral resections of the carotid bodies. J Physiol. 2003;549:965–973. doi: 10.1113/jphysiol.2003.042259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fatemian M, Robbins PA. Human ventilatory response to CO2 after 8 h of isocapnic or poikilocapnic hypoxia. J Appl Physiol. 1998;85:1922–1928. doi: 10.1152/jappl.1998.85.5.1922. [DOI] [PubMed] [Google Scholar]
- Feldman JL, Mitchell GS, Nattie EE. Breathing: rhythmicity, plasticity, chemosensitivity. Ann Rev Neurosci. 2003;26:239–266. doi: 10.1146/annurev.neuro.26.041002.131103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filosa JA, Dean JB, Putnam RW. Role of intracellular and extracellular pH in the chemosensitive response of rat locus coeruleus neurons. J Physiol. 2002;541(2):493–509. doi: 10.1113/jphysiol.2001.014142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filosa JA, Putnam RW. Multiple targets of chemosensitive signaling in locus coeruleus neurons: role of K+ and Ca2+, channels. Am J Physiol Cell Physiol. 2003;284:C145–C155. doi: 10.1152/ajpcell.00346.2002. [DOI] [PubMed] [Google Scholar]
- Guyenet PG, Stornetta RL, Bayliss DA. Retrotrapezoid nucleus and central chemoreception. J Physiol. 2008;586:2043–2048. doi: 10.1113/jphysiol.2008.150870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartzler LK, Dean JB, Putnam RW. Developmental changes in the chemosensitive response in locus coeruleus neurons from neonatal rats. Soc Neurosci Abstr. 2007 Program No 297.8. [Google Scholar]
- Huang RQ, Erlichman JS, Dean JB. Cell-cell coupling between CO2 excited neurons in the dorsal medulla oblongata. Neuroscience. 1997;80:41–57. doi: 10.1016/s0306-4522(97)00017-1. [DOI] [PubMed] [Google Scholar]
- Khodadadeh B, Safwan Badr M, Mateika JH. The ventilatory response to carbon dioxide and sustained hypoxia is enhanced after episodic hypoxia in OSA patients. Resp Physiol Neurobiol. 2006;150:122–134. doi: 10.1016/j.resp.2005.04.019. [DOI] [PubMed] [Google Scholar]
- Mifflin SW. Arterial chemoreceptor input to nucleus tractus solitarius. Am J Physiol. 1992;263:R368–R375. doi: 10.1152/ajpregu.1992.263.2.R368. [DOI] [PubMed] [Google Scholar]
- Mulkey DK, Henderson RA, III, Putnam RW, Dean JB. Hyperbaric oxygen and chemical oxidants stimulate CO2/H+-sensitive neurons in rat brain stem slices. J Appl Physiol. 2003;95:910–921. doi: 10.1152/japplphysiol.00864.2002. [DOI] [PubMed] [Google Scholar]
- Mulkey DK, Henderson RA, III, Ritucci NA, Putnam RW, Dean JB. Oxidative stress decreases pHi and Na+/H+ exchange and increases excitability of solitary complex neurons from rat brain slices. Am J Physiol Cell Physiol. 2004a;286:C940–951. doi: 10.1152/ajpcell.00323.2003. [DOI] [PubMed] [Google Scholar]
- Mulkey DK, Stornetta RL, Weston MC, Simmons JR, Parker A, Bayliss DA, Guyenet PG. Respiratory control by ventral surface chemoreceptor neurons in rats. Nat Neurosci. 2004b;7:1360–1369. doi: 10.1038/nn1357. [DOI] [PubMed] [Google Scholar]
- Nattie E. Why do we have both peripheral and central chemoreceptors? J Appl Physiol. 2006;100:9–10. doi: 10.1152/japplphysiol.01097.2005. [DOI] [PubMed] [Google Scholar]
- Nattie EE, Li A. CO2 dialysis in nucleus tractus solitarius region of rat increases ventilation in sleep and wakefulness. J Appl Physiol. 2002;92:2119–2130. doi: 10.1152/japplphysiol.01128.2001. [DOI] [PubMed] [Google Scholar]
- Nattie E, Li A. Central chemoreception 2005: A brief review. Auton. Neurosci. 2006:126–127. 332–338. doi: 10.1016/j.autneu.2006.02.003. [DOI] [PubMed] [Google Scholar]
- Nattie E, Li A. Central chemoreception is a complex system function that involves multiple brainstem sites. J Appl Physiol. 2009;106:1464–1466. doi: 10.1152/japplphysiol.00112.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nichols NL, Mulkey DK, Wilkinson KA, Powell FL, Dean JB, Putnam RW. The characterization of the chemosensitive response of individual nucleus tractus solitarius (NTS) neurons from adult rats. Am J Physiol Regul Integr Comp Physiol. 2009;296:R763–R773. doi: 10.1152/ajpregu.90769.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nichols NL, Wilkinson KA, Powell FL, Putnam RW. Chronic hypoxia (CHx) suppresses the chemosensitive response of individual nucleus tractus solitarius (NTS) neurons from adult rats. FASEB J. 2008;22:1172.1. [Google Scholar]
- Nielsen AM, Bisgard GE, Vidruk EH. Carotid chemoreceptor activity during acute and sustained hypoxia in goats. J Appl Physiol. 1988;65:1796–1802. doi: 10.1152/jappl.1988.65.4.1796. [DOI] [PubMed] [Google Scholar]
- Olson EB, Jr, Dempsey JA. Rat as a model for humanlike ventilatory adaptation to chronic hypoxia. J Appl Physiol. 1978;44:763–769. doi: 10.1152/jappl.1978.44.5.763. [DOI] [PubMed] [Google Scholar]
- Pascual O, Morin-Surun MP, Barna B, Denavit-Saubié M, Peqquignot JM, Champagnat J. Progesterone reverses the neuronal responses to hypoxia in rat nucleus tractus solitarius in vitro. J Physiol. 2002;544:511–520. doi: 10.1113/jphysiol.2002.023994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powell FL. The influence of chronic hypoxia upon chemoreception. Respir Physiol Neurobiol. 2007;157:154–61. doi: 10.1016/j.resp.2007.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powell FL, Huey KA, Dwinell MR. Central nervous system mechanisms of ventilatory acclimatization to hypoxia. Resp Physiol. 2000;121:223–236. doi: 10.1016/s0034-5687(00)00130-4. [DOI] [PubMed] [Google Scholar]
- Putnam RW, Filosa JA, Ritucci NA. Cellular Mechanisms involved in CO2 and acid signaling in chemosensitive neurons. Am J Physiol Cell Physiol. 2004;287:C1493–C1526. doi: 10.1152/ajpcell.00282.2004. [DOI] [PubMed] [Google Scholar]
- Richerson GB, Wang W, Tiwari J, Bradley SR. Chemosensitivity of serotonergic neurons in the rostral ventral medulla. Resp Physiol. 2001;129:175–189. doi: 10.1016/s0034-5687(01)00289-4. [DOI] [PubMed] [Google Scholar]
- Ritucci NA, Dean JB, Putnam RW. Intracellular pH response to hypercapnia in neurons from chemosensitive areas of the medulla. Am J Physiol Regul Integr Comp Physiol. 1997;273:R433–441. doi: 10.1152/ajpregu.1997.273.1.R433. [DOI] [PubMed] [Google Scholar]
- Ritucci NA, Erlichman JS, Leiter JC, Putnam RW. Response of membrane potential and intracellular pH to hypercapnia in neurons and astrocytes from rat retrotrapezoid nucleus. Am J Physiol Regul Integr Comp Physiol. 2005;289:R851–R861. doi: 10.1152/ajpregu.00132.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith CA, Bisgard GE, Nielsen AM, Daristotle L, Kressin NA, Forster HV, Dempsey JA. Carotid bodies are required for ventilatory acclimatization to chronic hypoxia. J Appl Physiol. 1986;60:1003–10. doi: 10.1152/jappl.1986.60.3.1003. [DOI] [PubMed] [Google Scholar]
- Smith CA, Rodman JR, Chenuel BJA, Henderson KS, Dempsey JA. Response time and sensitivity of the ventilatory response to CO2 in unanesthetized intact dogs: central vs. peripheral chemoreceptors. J Appl Physiol. 2006;100:13–19. doi: 10.1152/japplphysiol.00926.2005. [DOI] [PubMed] [Google Scholar]
- Stornetta RL, Moreira TS, Takakura AC, Kang BJ, Chang DA, West GH, Brunet JF, Mulkey DK, Bayliss DA, Guyenet PG. Expression of Phox2b by brainstem neurons involved in chemosensory integration in the adult rat. J Neurosci. 2006;26(40):10305–14. doi: 10.1523/JNEUROSCI.2917-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takakura ACT, Moreira TS, Colombari E, West GH, Stornetta RL, Guyenet PG. Peripheral chemoreceptor inputs to retrotrapezoid nucleus (RTN) CO2-sensitive neurons in rats. J Physiol. 2006;572:503–523. doi: 10.1113/jphysiol.2005.103788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas JA, Buchsbaum RN, Zimniak A, Racker E. Intracellular pH measurement in ehrlich ascites tumor cells utilizing spectroscopic probes generated in situ. Biochemistry. 1979;81:2210–2218. doi: 10.1021/bi00578a012. [DOI] [PubMed] [Google Scholar]
- Villafuerte FC, Cárdenas-Alayza R, Macarlupú JL, Monge CC, León-Velarde F. Ventilatory response to acute hypoxia in transgenic mice over-expressing erythropoietin: effect of acclimation to 3-week hypobaric hypoxia. Respir Physiol Neurobiol. 2007;158(23):243–50. doi: 10.1016/j.resp.2007.06.010. [DOI] [PubMed] [Google Scholar]
- Vizek M, Pickett CK, Weil JV. Increased carotid body hypoxic sensitivity during acclimatization to hypobaric hypoxia. J Appl Physiol. 1987;63:2403–2410. doi: 10.1152/jappl.1987.63.6.2403. [DOI] [PubMed] [Google Scholar]
- Wang W, Richerson GB. Development of chemosensitivity of rat medullary raphé neurons. Neuroscience. 1999;90(3):1001–1011. doi: 10.1016/s0306-4522(98)00505-3. [DOI] [PubMed] [Google Scholar]
- Weil JV. Ventilatory control at high altitude. In: Cherniack NS, Widdicombe JG, editors. Handbook of Physiology, Section 3: The Respiratory System, Vol II: Control of Breathing part: 2. American Physiological Society; Bethesda, MD: 1986. pp. 703–728. [Google Scholar]
- Wilkinson KA, Nichols NL, Putnam RW, Powell FL. Chronic hypoxia decreases response to central chemoreceptor stimulation in the nucleus tractus solitarius (NTS) FASEB J. 2009 Program # 621.16. [Google Scholar]
- Zhang W, Mifflin SW. Modulation of synaptic transmission to second-order peripheral chemoreceptor neurons in caudal nucleus tractus solitarius by alpha1-adrenoreceptors. J Pharmacol Exp Ther. 2007;320:670–677. doi: 10.1124/jpet.106.114033. [DOI] [PubMed] [Google Scholar]