Abstract
The phosphatase and tensin homolog (PTEN) exerts its function, in part, by negatively regulating the well-known phosphatidylinositol-3-kinase/AKT signaling pathway. Previous histological work has suggested that alterations in the nuclear/cytoplasmic compartmentalization of PTEN may play a role in the development and progression of melanoma. In this study, we examined the nuclear/cytoplasmic compartmentalization of PTEN in melanoma cell lines and its correlation with the cell cycle. Studies were performed in melanoma cells lines using classic cell biological techniques. In contrast to breast cancer cell lines, we found that increased levels of nuclear PTEN levels correlate with G2 rather than with G1 arrest. In WM164 and SKmel28 cells, overexpression of PTEN protein did not significantly increase the number of cells in the G2 phase. Differential CDC2 phosphorylation levels in cells that overexpressed PTEN compared with those where PTEN was downregulated suggest some involvement of PTEN in G2 checkpoint regulation. The data suggest that although nuclear PTEN levels correlate with the G2 phase, the role of PTEN in modulating G2/M arrest is not limiting. Further, the specific cell cycle phase regulated by nuclear PTEN is cell-type dependent. Taken together, our observations suggest that in melanoma, nuclear PTEN is involved in G2 progression possibly through the modulation of CDC2, opening up a new arena for investigation.
Keywords: G2/M arrest and progression, PTEN
Introduction
PTEN (phosphatase and tensin homolog deleted on chromosome 10), the tumor suppressor gene located at 10q23.3, is known to participate in the pathogenesis of a wide variety of hereditary cancer syndromes and sporadic malignancies [1]. Germline mutations in PTEN have been described in hamartoma syndromes, including Cowden syndrome [2], Bannayan–Riley–Ruvalcaba syndrome [3], Proteus syndrome [4], and a Proteus-like syndrome [5]. Somatic mutations in PTEN, which result in loss-of-function, occur in multiple sporadic tumor types [1,6–10]. In cutaneous melanoma, PTEN is the second most frequently altered tumor suppressor [1], whereas in cultured melanoma cell lines, the frequency of intragenic mutations and homozygous deletions is 60% [11–14]. This may suggest that PTEN plays a role in melanoma progression. Indeed, when analyzing PTEN protein’s subcellular localization, we found that 30 of 30 metastatic melanomas had complete loss of nuclear PTEN protein expression [15]. This indicates that nuclear expression of PTEN is critical for the regulation of growth, an idea that has been supported in thyroid and breast carcinomas, pancreatic islet cell tumors, and uveal melanoma [16–19].
The PTEN protein is a dual activity phosphatase which dephosphorylates both protein and lipid substrates, particularly the second messenger phosphatidylinositol-(3,4,5)-triphosphate. As such, this antagonizes the phosphatidylinositol-3-kinase/AKT signal transduction pathway [20–23]. PTEN mediates G1 cell cycle arrest in breast cancer lines, induces apoptosis and inhibits cell migration, spreading and focal adhesion formation [24,25]. In the breast cancer cell line MCF-7, nuclear and cytoplasmic levels of PTEN fluctuate with the cell cycle and nuclear PTEN levels peak at G0–G1 indicating a role for nuclear PTEN as a cell cycle modulator [18,26,27]. Indeed, nuclear PTEN mediates growth suppression, independent of AKT downregulation, and is essential for maintaining chromosomal integrity through physical association with the kinetochore [28], whereas cytoplasmic PTEN mainly mediates apoptosis [26,29] further emphasizing the role of proper cellular localization in the regulation of PTEN function.
Although preliminary reports based on clinical correlative study suggest that the localization of PTEN plays a role in melanoma progression [30], it is not clear what that role may be. Work in breast cancer lines suggest that PTEN’s role may be in the regulation of the cell cycle. Therefore, we examined the role of PTEN localization on cell cycle progression in cultured melanoma cell lines.
Materials and methods
Cell lines, culture conditions, and reagents
SKMel28, A375S2, and C32TG (PTEN null) cutaneous melanoma lines were from American Type Culture Collection (Manassas, Virginia, USA). The WM164 cutaneous melanoma cell line was a generous gift from Dr Ernest T. Borden (Cleveland Clinic Taussig Cancer Institute, Cleveland, OH, USA). PTEN-overexpressing WM164 cells (WM164OE), PTEN-overexpressing SKmel28 (SKmel28OE), and PTEN-expressing C32TG cells (C32TGOE), were generated as described below and were grown in the presence of tetracycline (Tet), unless otherwise indicated. Cutaneous melanoma cell lines were cultured in high-glucose Dulbecco’s modified Eagle’s medium (Sigma, St Louis, Missouri, USA) supplemented with 10% fetal bovine serum and 100 units penicillin/streptomycin at 37°C with 5% CO2. Hydroxyurea (HU), Tet, propidium iodide, protease and phosphatase inhibitors, Ponceau S, and α-tubulin antibody were purchased from Sigma. The 5,6-dichlorobenzimidazole riboside (DRB) was purchased from Calbiochem (San Diego, California, USA). The α-CDC2 tyrosine-15 phosphorylation, α-CDC2 threonine-161 phosphorylation, α-CDC2, α-AKT, and α-phospho-AKT antibodies were from Cell Signaling Company (Beverly, Massachusetts, USA). OligofectAMINE and hygromycin were purchased from Invitrogen (Carlsbad, California, USA). Nuclear and cytoplasmic extraction reagents and bicinchoninic acid protein assay kits were from Pierce Biotechnology Inc. (Rockford, Illinois, USA). The α-PTEN antibody, clone 6H2.1, was from Cascade Biosciences (Winchester, Massachusetts, USA) and α-poly (ADP-ribose) polymerase-1 from Santa Cruz Antibodies (Santa Cruz, California, USA). ON-TARGETplus SMARTpool L-003023-00-0050 Human PTEN small interfering RNA (siRNA) and siCONTROL Nontargeting siRNA pool D-001206-13–20, was purchased from Dharmacon (Lafayette, Colorado, USA).
Generation of stable Tet-off cell lines
Stable cell lines expressing Tet-regulated PTEN were generated according to the manufacturer’s protocol (Clontech Protocol No. PT3001-1). Briefly, the pTetOff vector containing the tTA regulatory element was transfected into the parental line (WM164, SKmel28, and C32TG) and placed under G418 selection. Resistant colonies were isolated and expanded. Expanded clones were transiently transfected with pTRE2Hyg-luc in the presence or absence of Tet and luciferase levels were measured after 48λh. Clones showing an induction of luciferase activity in the absence of Tet were transfected with a select PTEN cDNA construct cloned into the pTRE2Hyg vector. Cells were grown under hygromycin selection in the presence of Tet and resistant colonies isolated and expanded. Tet-regulated PTEN expression was confirmed by western analysis on total protein lysates collected from cells grown in the presence or absence of Tet.
Cell cycle measurements
Cells were synchronized using HU as previously described [18]. Cells were seeded in 10λcm dishes and allowed to adhere for 24λh. The medium was then augmented with 3λmmol/l HU for 16–17λh, washed with phosphate buffer saline (PBS), and replenished with fresh media. For Tet-regulated experiments, the following modifications were applied: SKmel28OE and WM164OE cells were propagated in Tet-containing media. Before 24λh of HU augmentation, cells were split and reseeded as above and grown in the presence or absence of Tet, as indicated. For siRNA experiments, WM164, A375S2, and SKmel28 cells were grown and split 24λh before siRNA transfection with OligofectAMINE according to manufacturers instructions. Twenty hours later, HU was added; subsequent manipulation was identical to normal cells. At harvest, all cells ranged from 70–90% confluent.
Cell cycle analysis
At the indicated times, cells were washed with PBS, harvested through trypsinization, and pelleted by centrifugation. Cells were resuspended in 500λµl cold PBS and added drop wise to 70% ice-cold ethanol. Samples were stored at −20°C until analysis. Washed cells were stained in 0.1% Triton X-100 in PBS with 1λµg/ml propidium iodide. For the analysis of H3 phosphorylation, cells were treated with 1% Triton X-100 in PBS for 1λh and stained with antiphospho-Ser10 H3 antibodies, conjugated to Alexa Fluor 488, as previously described [31]. Flow cytometry was performed using a Beckman-Coulter elite flow cytometer using a 610 long pass filter for data collection. Data were filtered and cell phases were quantified using the ModFit program (Verity Software, Bowdoin, Maine, USA) and with FlowJo (Tree Star, Inc., Ashland, Oregon, USA) when gating for H3 signal.
Sodium dodecyl sulphate-polyacrylamide gel electrophoresis, immunoblotting, and antibodies
At indicated time points, medium was removed and cells were washed twice with ice-cold PBS. Cells were scraped into cold PBS and subjected to nuclear-cytosolic fractionation using the nuclear and cytoplasmic extraction reagents kit with Sigma protease and phosphatase inhibitors added to all buffers, both according to the manufacturers’ instructions. Protein concentration was determined by the bicinchoninic acid method using bovine serum albumin as a standard. Protein was fractionated on 10% sodium dodecyl sulphate-polyacrylamide gels, transferred to nitrocellulose membranes (Whatmann, Dassel, Germany) and probed using the relevant antibodies. Equal loading of lanes was evaluated by both Ponceau S treatment before analysis and western analysis for either: α-tubulin (cytoplasmic marker), poly (ADP-ribose) polymerase-1 (nuclear marker), or actin. Proteins were detected using either IRDye 680 goat-α-rabbit IgG or IRDye 800CW goat-α-mouse secondary antibodies, where appropriate, and using the Odyssey laser activated visualization system (LI-COR Biotechnology, Lincoln, Nebraska, Canada) according to manufacturer’s instructions. Simultaneous dual channel detection of anti-mouse and anti-rabbit antibodies was digitally captured in color and transformed into gray scale for each channel.
Results
Nuclear PTEN correlates with G2 but not G1 in melanoma cell lines
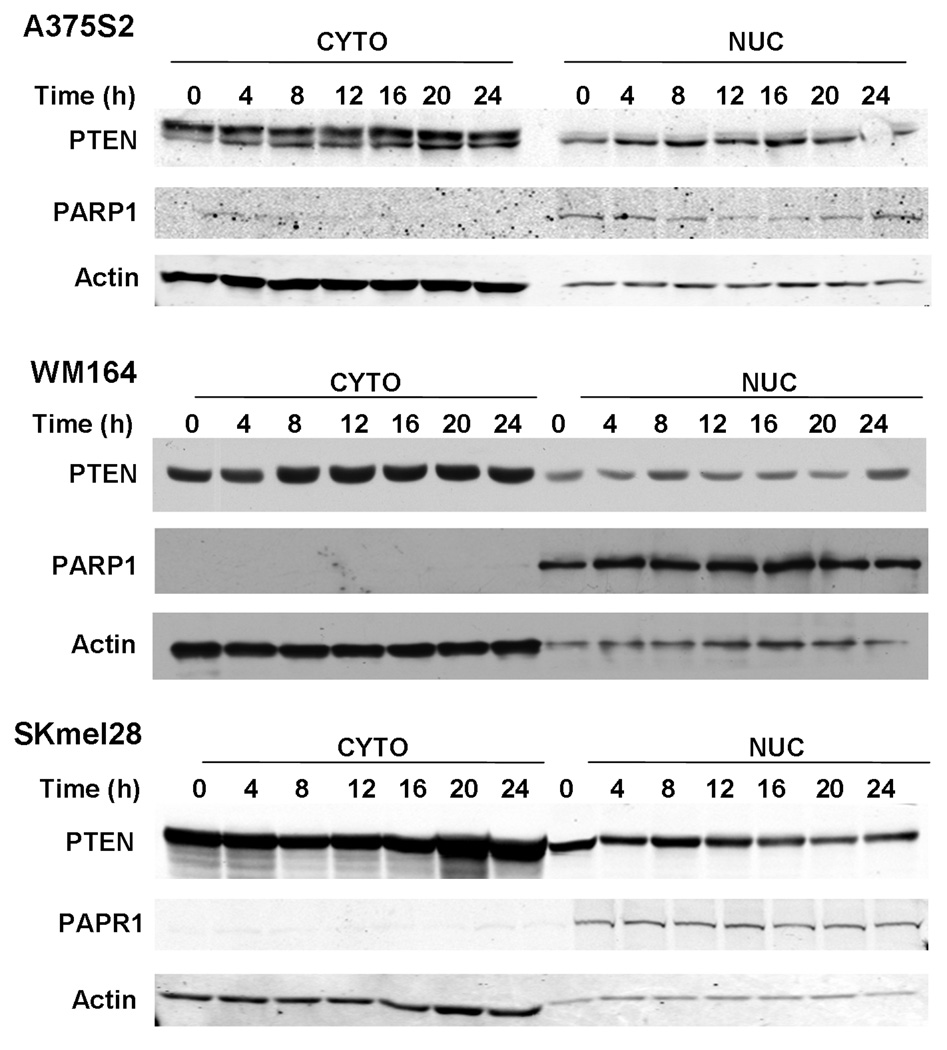
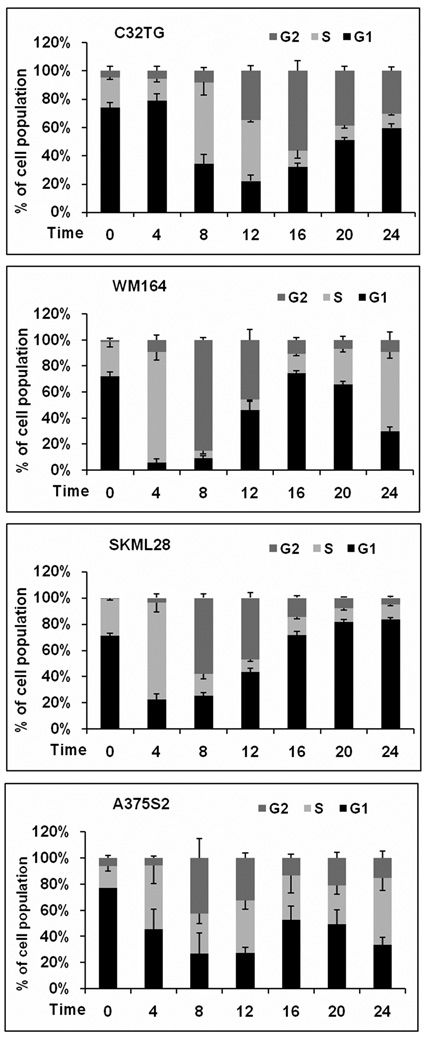
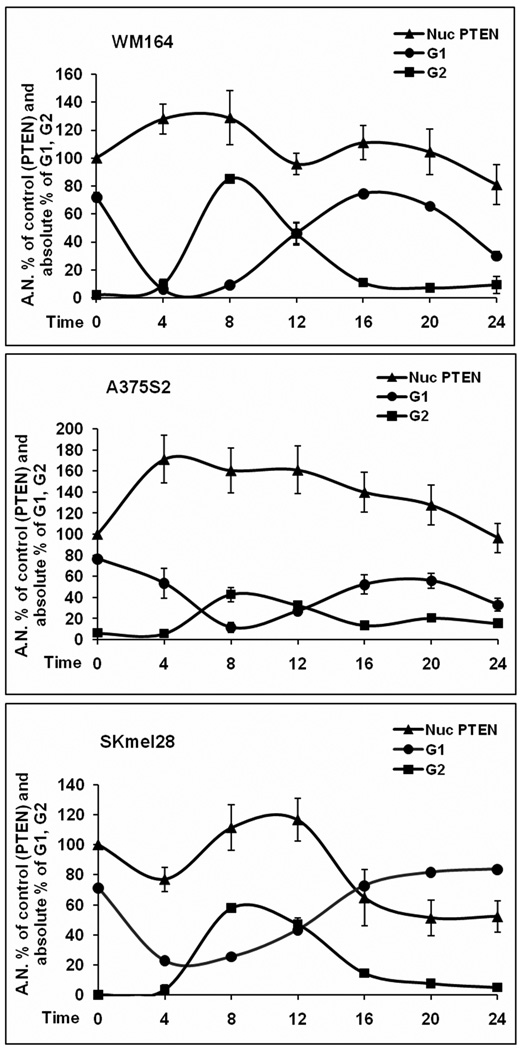
In breast cancer cell lines, nuclear PTEN protein levels correlate with G1 arrest [18], however, little is known about the role of nuclear PTEN in melanoma. We used four different melanoma cell lines. Three of the lines expressed PTEN at varying levels (SKML28, WM164, and A375S2) and one line was PTEN-null (C32TG). When examining the levels of both nuclear and cytosolic PTEN protein levels through western analysis, we found, as expected, that the levels of PTEN protein in each fraction fluctuated after HU release and cell cycle (Fig. 1). Although there was cell line variability, cell lines that expressed PTEN had maximal nuclear PTEN levels 8–12λh post-HU release (Fig. 1), whereas the lowest level of nuclear PTEN occurred 0–4λh post-HU release (Fig. 1). Concurrent with this analysis, we performed cell cycle analysis. The C32TG PTEN-null line had a delayed entry into G2 after HU release when compared with lines that express PTEN (Fig. 2), and by the time PTEN-expressing lines cycled back into G1, the null line was peaking in G2. This suggests that because of the absence of PTEN protein, C32TG cells do not progress to G2 as fast as cells with any PTEN protein, after HU release. Interestingly, in breast cancer cells, G1 arrest was correlated with an increase in PTEN, whereas here, in melanoma, we see decreased PTEN protein levels and apparent G2 delay. WM164 cells had the fastest entry into G2. Over 80% of WM164 cells were in G2 by 8λh. This correlated with the maximal nuclear PTEN protein levels. Approximately 60% of SKmel28 cells were in G2 by 8λh, whereas A375S2 cells only had 40% of cells in G2 at 8λh. It seems that WM164 cells, although having the fastest entry into G2, also have the lowest amount of nuclear PTEN when compared with the other lines. Nonetheless, we think it important to look at the individual pattern of expression of each cell line. Indeed, densitometric analysis of the western blot data indicates that the maximal PTEN nuclear levels occurred just before cells peaking in G2 (Fig. 3).
Fig. 1.
Nuclear (Nuc) and cytoplasmic (Cyto) PTEN levels change with cell cycle progression. Melanoma lines were grown to 60% confluence and synchronized with 3λmmol/l HU for 16–18λh. Cells were harvested at the time of release and at the indicated times, after release of HU. After subcellular fractionation, equal amounts of protein were separated on sodium dodecyl sulphate-polyacrylamide gel electrophoresis, transferred to membranes, and immunoblotted with specific anti-PTEN antibodies. Each blot is representative of five individual experiments. HU, hydroxyurea; PARP, poly (ADP-ribose) polymerase; PTEN, phosphatase and tensin homolog.
Fig. 2.
HU synchronized melanoma cells differ in G2 entrance. Cells of each melanoma cell line were grown to 60% confluence and treated with 3λmmol/l HU for 16–18λh. After HU release, cells were collected and analyzed by flow cytometry. Panels show the percent mean and SEM of cells in G1 (black bars, bottom), S (light gray bars), and G2 (dark gray bars, top) at each time point, for at least five individual experiments. HU, hydroxyurea.
Fig. 3.
Nuclear (Nuc) PTEN correlates with G2 population. After HU treatment and release, cells were subjected to subcellular fractionation and submitted to western blot or flow cytometry and quantitated. Shown are quantitated data from Figure 1, representing the nuclear fraction from 5–7 western blots, together with the cell cycle data from Figure 3, showing G1 and G2 from 5–7 experiments. For the western blots, time 0 was defined as control at 100%, and subsequent time points were calculated as percent of controls. Bars represent SEM and time in hours after HU release. HU, hydroxyurea.
PTEN modulates CDC2 tyrosine-15 and threonine-161 phosphorylation
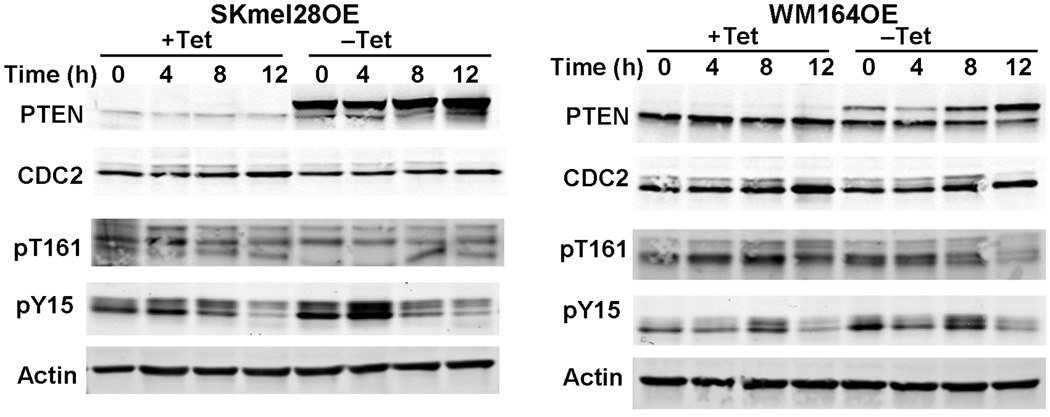
The results above led us to examine whether PTEN modulates the cellular regulators of the G2/M transition. CDC2 is a cyclin-dependent kinase that binds to cyclin B, activating the G2/M transition. CDC2-cyclin B binding and subsequent checkpoint activation is finely controlled by both phosphorylation and dephosphorylation events. Phosphorylation of tyrosine-15 of CDC2 inhibits cyclin B binding and subsequently diminishes entry into M phase [32]. Conversely, the phosphorylation of threonine-161 of CDC2 is required for progression into M phase [33]. Thus, we examined both of these phosphorylation events in WM164 and SKmel28 cells capable of overexpressing PTEN in the absence of Tet (WM164OE and SKmel28OE, respectively). Although there was not a statistically significant change in the cell cycle itself, we did find that PTEN expression did modulate the phosphorylation of CDC2. CDC2 threonine-161 phosphorylation levels were unchanged in SKmel28OE, compared with cells not overexpressing PTEN. WM164OE cells exhibited a slight decrease in CDC2 threonine-161 phosphorylation levels when PTEN expression increased. Overexpression of PTEN in the WM164 and SKmel28 lines resulted in a further increase in the phosphorylation of CDC2 tyrosine-15 when compared with cells not overexpressing PTEN (Fig. 4).
Fig. 4.
Overexpression of PTEN decreases CDC2 phosphorylation. Cells overexpressing PTEN were grown to 60% confluence and synchronized with 3λmmol/l hydroxyurea for 16–18λh. After release and at each time point, cells were harvested and equal amounts of protein were separated by sodium dodecyl sulphate-polyacrylamide gel electrophoresis, transferred to membranes, and immunoblotted with the indicated antibodies. The blots in the figure are representative of five individual experiments. PTEN, phosphatase and tensin homolog; Tet, tetracycline; Skmel28OE, PTEN-overexpressing SKmel28; WM164OE, PTEN-overexpressing WM164 cells.
Alterations in PTEN protein levels modulates CDC2 phosphorylation but not cell cycle
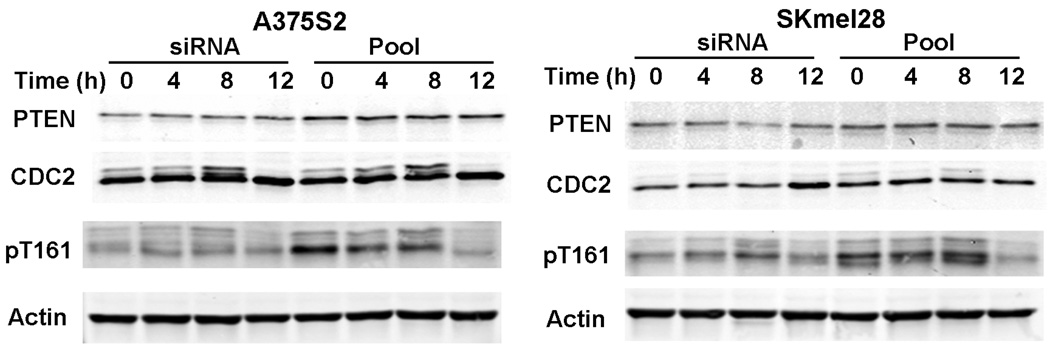
To further investigate the role in regulating G2, we downregulated PTEN protein levels through siRNA transfection. The siRNA treatment decreased PTEN protein levels in both A375S2 and SKmel28 cell lines when compared with control treated cells. However, despite multiple attempts, we were unable to decrease PTEN levels in WM164 cell lines (data not shown). The siRNA-treated A375S2 and SKmel28 cells showed no significant changes in cell cycle when compared with nontreated cells (data not shown). Despite this, we observed a decrease in CDC2 threonine-161 phosphorylation levels in both PTEN siRNA treated A375S2 and SKmel28 cells when compared with control cells (Fig. 5 and Fig. S2 for PTEN band quantification).
Fig. 5.
Downregulation of phosphatase and tensin homolog (PTEN) does not affect cell cycle. Cells treated with small interfering RNA (siRNA) were treated grown to 60% confluence and synchronized with 3λmmol/l hydroxyurea for 16–18λh. After release and at each time point, cells were harvested and equal amounts of protein were separated by sodium dodecyl sulphate-polyacrylamide gel electrophoresis, transferred to membranes, and immunoblotted with the indicated antibodies. The blots in this figure are representative of five individual experiments.
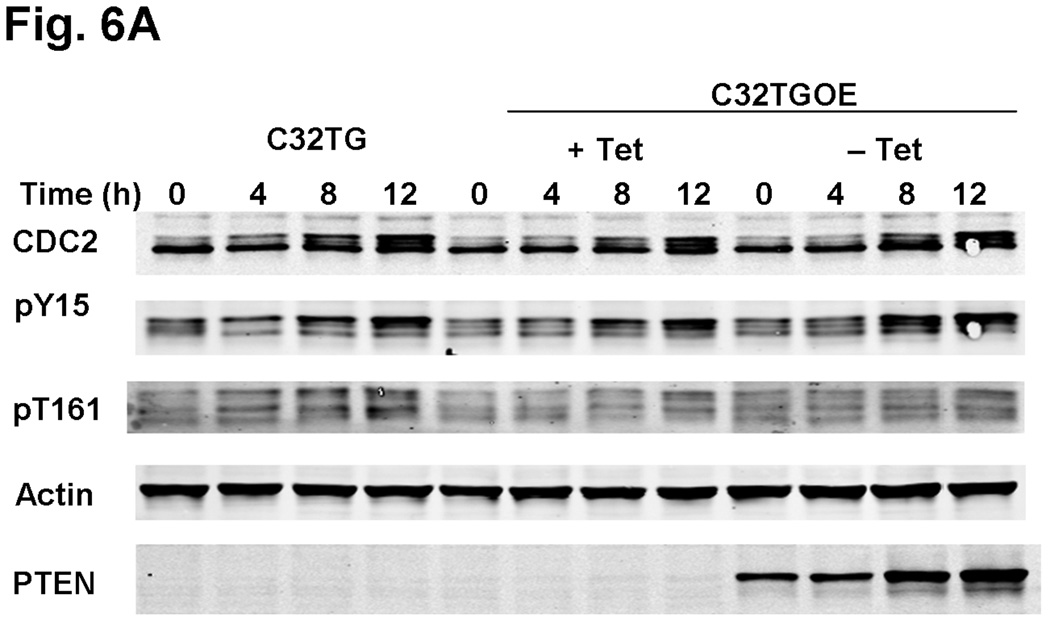
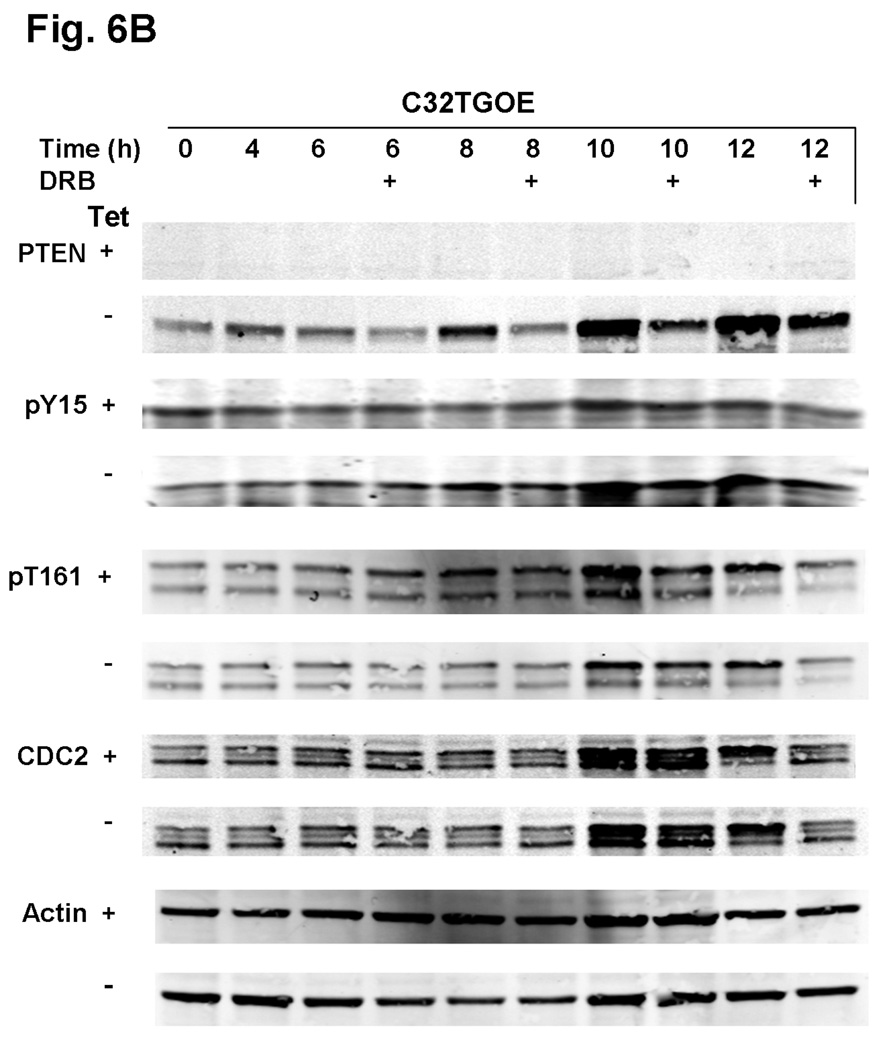
To examine further how PTEN modulates G2 checkpoint proteins, we generated a PTEN-expressing line using the native PTEN-null line, C32TG. When we expressed PTEN in C32TG cells, we found that there was an additional increase in CDC2 tyrosine-15 phosphorylation levels at 8 and 12λh, post-HU release, when compared with the control PTEN-null C32TG cells (Fig. 6a and Fig. S3 for pY15 band quantification). Similar to the results we found in A375S2 and SKmel28 cells, we did not see a significant change in CDC2 threonine-161 phosphorylation levels in the presence of PTEN expression. Inhibition of PTEN expression, by DRB treatment, modulated CDC2 tyrosine-15 phosphorylation levels. When PTEN protein levels in the C32TG PTEN-expressing cells was decreased by DRB, there was an increase in CDC2 tyrosine-15 phosphorylation levels, as compared with C32TG PTEN-expressing cells, in which the PTEN levels were not attenuated by DRB (Fig. 6b and Fig. S4 for pY15 band quantification).
Fig. 6.
PTEN overexpressing C32TG cells show increased CDC2 tyrosine-15 phosphorylation. (a) C32TG cells overexpressing PTEN were grown to 60% confluence and synchronized with 3λmmol/l HU for 16–18λh. After release and at each time point, cells were harvested and equal amounts of protein were separated by sodium dodecyl sulphate-polyacrylamide gel electrophoresis, transferred to membranes, and immunoblotted with the indicated antibodies. Figure is representative of five individual experiments. (b) Cells were treated as in (a) with the following modification: starting 4λh after HU release, indicated cells (+) were treated with DRB until cells were harvested. Arrows indicate changes in phosphorylation status due to DRB treatment. C32TGOE, PTEN-expressing C32TG cells; DRB, 5,6-dichlorobenzimidazole riboside; HU, hydroxyurea; PTEN, phosphatase and tensin homolog; Tet, tetracycline.
Discussion
Previous work has suggested that nuclear PTEN protein levels play an important role in the progression of melanoma [30], yet, a specific function or role for nuclear PTEN in melanoma has not been investigated. Based upon studies in breast cancer lines, one may suspect that nuclear PTEN levels would be associated with G1 arrest. [18,26,27]. We show here, that increasing nuclear PTEN levels in melanoma is associated with an increase in the number of cells in G2. Our analysis suggests that nuclear PTEN regulates, but is not the limiting factor or absolute requirement for, cell cycle progression in melanoma lines. In multiple melanoma cell lines, we found that there is a lag between increasing nuclear PTEN and the resulting increase in the G2 cell population, indicating a causal relationship between PTEN and G2/M. This may suggest that increasing nuclear PTEN levels occurs in anticipation of G2 entrance. Alternatively, PTEN may be involved in a negative feedback mechanism; however, this remains to be determined.
In pancreatic cancer, an increase in the melanoma differentiation-associated gene product, interleukin-24, induces an increase in PTEN expression and results in G2/M arrest [34], providing further evidence of a link between overall PTEN protein levels and G2. It is interesting to hypothesize, based upon our results, that in this system, increasing levels of interleukin-24 induces PTEN protein levels ultimately resulting in an increase in nuclear PTEN protein levels and that it is this fraction that influences G2/M arrest. This suggests that the nuclear-PTEN/G2 association may not be melanoma-specific. Previous results in breast cancer indicate that the role of nuclear PTEN in specific cancer types may be different. It might be that it is the intracellular or microenvironmental milieu that is pertinent to melanoma and perhaps pancreatic cancer.
In our hands, overexpression of PTEN did not dramatically affect cell cycle progression in melanoma cell lines (data not shown). Nonetheless, we clearly see an association between increased nuclear PTEN protein levels and G2/M. SKmel28 nuclear PTEN protein levels do reach maximal levels after the peak of G2. Nevertheless, before maximal G2 content, there is a 20% increase in nuclear PTEN protein levels suggesting that the increase in nuclear PTEN protein levels may be an important part of G2. These data indicate that PTEN is not the sole regulator of G2/M progression nor is present in limiting quantities in melanoma. Although our data suggest that endogenous levels of PTEN may be involved in these pathways, it also suggests that solely increasing cellular PTEN levels will not alter the cell cycle in melanoma.
Little is known about the role PTEN may have on CDC2 phosphorylation. Indeed, this is the first report linking PTEN function and CDC2 phosphorylation. Overexpression of PTEN does seem to affect the level of phosphorylation on individual residues of CDC2. Although not reaching significance, we found a clear and consistent trend that PTEN levels are inversely related to the level of CDC2 threonine-161 phosphorylation. Our data do suggest that PTEN modulates CDC2 tyrosine-15 phosphorylation levels. Interestingly, although PTEN seems to modulate this phosphorylation event, it does not seem to have a significant effect on G2 arrest in cells overexpressing PTEN. Whether this is because of insufficient crosstalk with other PTEN-dependent and independent limiting factors remains to be determined. Nonetheless, the data support the hypothesis that PTEN can modulate G2 progression in melanoma cells. Undoubtedly, more remains to be done to investigate the modulation of CDC2 phosphorylation events by PTEN. This should be an exciting area for future investigation. It is interesting to postulate that PTEN-associated CDC2 threonine-161 phosphorylation might be a cellular compensation mechanism, telling cells, in essence, to ‘keep to the program.’
On the basis of our results, one would expect that a decrease in PTEN protein levels would result in altered cell-cycle kinetics, yet this was not seen. PTEN reduction in A375S2 and SKmel28 cells resulted in no clear change in cell cycle kinetics. We were unable to decrease PTEN protein levels in WM164 cells, despite multiple attempts. It may be of interest to note that these cells have a rapid rate of division and low levels of AKT phosphorylation. Although the low AKT phosphorylation levels indicate a high basal level of PTEN protein, the rapid proliferation rate suggests that the phosphatidylinositol-3-kinase/AKT/PTEN pathway is not the major pathway modulating cellular proliferation in this cell, as high levels of PTEN should result in decreased proliferation [35–37]. Further, these observations likely indicate that there are a multitude of variables influencing the final state of cell cycle kinetics. Nevertheless, our data may suggest that even reduced amounts of PTEN are sufficient to drive the pathway.
On the basis of our results, we would expect to find a change in cell cycle in C32TG cells (PTEN null) when PTEN is introduced into the cell line. However, C32TG cells expressing PTEN did not exhibit the same cell cycle pattern as the PTEN-expressing WM164, SKmel28, and A375S2 cell lines. We would hypothesize that, as discussed above, like WM164 lines, alternative pathways for cellular proliferation are dominate in the C32TG cells. This may be due to natural adaptation of the cell or, more likely, additional genetic mutations. Despite a lack of significant cell cycle change in the presence of PTEN, we were, nonetheless, able to show that PTEN did affect CDC2 phosphorylation levels. Because CDC2 has a relatively long half-life [38] compared with PTEN’s shorter half-life [39,40], we were able to modulate PTEN levels without significantly decreasing CDC2 protein. We found that decreased PTEN protein levels in C32TG cells overexpressing PTEN correlated with a decrease in the levels of CDC2 tyrosine-15 phosphorylation. This provides further support that PTEN is able to modulate this regulator of G2 phase.
It is not readily clear why nuclear PTEN regulates G1 arrest in breast cancer and yet is involved in G2 in melanoma. It is well known that proteins are expressed differently in breast cancer and melanoma. For example, Metastasis Associated 1 [41] and Dickkopf-1 [42], are expressed in breast cancers yet absent in melanoma cells. Whether these two proteins play a role in PTEN-mediated G1 arrest in breast cancer remains to be determined and is beyond the scope of this paper. Kimura and co-workers [43] have reported that there is a decrease in PTEN expression in endometrioid endometrial adenocarcinoma, which have high levels of estrogen and progesterone receptors. In addition, they have correlated PTEN expression with G1 cell cycle regulators [43]. PTEN expression has also been shown to be reduced in hormone receptor-positive carcinomas [44]. This may suggest that PTEN has a different mode of action in hormone-responsive tissues and tumors. Although melanoma may express both progesterone and estrogen receptors [45], there is currently little understanding of their functional importance in melanoma. Our data here may suggest that these receptors function differently in various tumor types, at least with respect to the role of PTEN. This sets the stage for an interesting area of both research and therapeutics. Investigation into the differences in cell cycle regulation between these two cell types should be enlightening and further our understanding how cellular type and environment play a role in modulating PTEN-mediated events.
Acknowledgements
The authors would like to thank Drs Sarah Planchon Pope and Glenn Lobo for helpful discussions and Frank Mularo for able technical assistance.
Funding sources: This work was partially supported by 1R01CA118980-01A2 from the National Cancer Institute, Bethesda, MD (to C.E.). Charis Eng is a recipient of the Doris Duke Distinguished Clinical Scientist Award and is the Sondra J. and Stephen R. Hardis Endowed Chair of Cancer Genomic Medicine at the Cleveland Clinic.
Footnotes
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal’s Web site (http://www.lerner.ccf.org/gmi/igac/published_data.php)
Conflict of interest: All of the authors declare no conflict of interest.
References
- 1.Eng C. PTEN: one gene, many syndromes. Hum Mutat. 2003;22:183–198. doi: 10.1002/humu.10257. [DOI] [PubMed] [Google Scholar]
- 2.Liaw D, Marsh DJ, Li J, Dahia PL, Wang SI, Zheng Z, et al. Germline mutations of the PTEN gene in Cowden disease, an inherited breast and thyroid cancer syndrome. Nat Genet. 1997;16:64–67. doi: 10.1038/ng0597-64. [DOI] [PubMed] [Google Scholar]
- 3.Marsh DJ, Dahia PL, Zheng Z, Liaw D, Parsons R, Gorlin RJ, et al. Germline mutations in PTEN are present in Bannayan-Zonana syndrome. Nat Genet. 1997;16:333–334. doi: 10.1038/ng0897-333. [DOI] [PubMed] [Google Scholar]
- 4.Eng C, Thiele H, Zhou XP, Gorlin RJ, Hennekam RC, Winter RM. PTEN mutations and proteus syndrome. Lancet. 2001;358:2079–2080. doi: 10.1016/S0140-6736(01)07110-0. [DOI] [PubMed] [Google Scholar]
- 5.Zhou XP, Marsh DJ, Hampel H, Mulliken JB, Gimm O, Eng C. Germline and germline mosaic PTEN mutations associated with a Proteus-like syndrome of hemihypertrophy, lower limb asymmetry, arteriovenous malformations and lipomatosis. Hum Mol Genet. 2000;9:765–768. doi: 10.1093/hmg/9.5.765. [DOI] [PubMed] [Google Scholar]
- 6.Ali IU, Schriml LM, Dean M. Mutational spectra of PTEN/MMAC1 gene: a tumor suppressor with lipid phosphatase activity. J Natl Cancer Inst. 1999;91:1922–1932. doi: 10.1093/jnci/91.22.1922. [DOI] [PubMed] [Google Scholar]
- 7.Bonneau D, Longy M. Mutations of the human PTEN gene. Hum Mutat. 2000;16:109–122. doi: 10.1002/1098-1004(200008)16:2<109::AID-HUMU3>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- 8.Zbuk KM, Eng C. Cancer phenomics: RET and PTEN as illustrative models. Nat Rev Cancer. 2007;7:35–45. doi: 10.1038/nrc2037. [DOI] [PubMed] [Google Scholar]
- 9.Mutter GL, Lin MC, Fitzgerald JT, Kum JB, Baak JP, Lees JA, et al. Altered PTEN expression as a diagnostic marker for the earliest endometrial precancers. J Natl Cancer Inst. 2000;92:924–930. doi: 10.1093/jnci/92.11.924. [DOI] [PubMed] [Google Scholar]
- 10.Mutter GL, Lin MC, Fitzgerald JT, Kum JB, Eng C. Changes in endometrial PTEN expression throughout the human menstrual cycle. J Clin Endocrinol Metab. 2000;85:2334–2338. doi: 10.1210/jcem.85.6.6652. [DOI] [PubMed] [Google Scholar]
- 11.Boni R, Vortmeyer AO, Burg G, Hofbauer G, Zhuang Z. The PTEN tumour suppressor gene and malignant melanoma. Melanoma Res. 1998;8:300–302. doi: 10.1097/00008390-199808000-00002. [DOI] [PubMed] [Google Scholar]
- 12.Teng DH, Hu R, Lin H, Davis T, Iliev D, Frye C, et al. MMAC1/PTEN mutations in primary tumor specimens and tumor cell lines. Cancer Res. 1997;57:5221–5225. [PubMed] [Google Scholar]
- 13.Tsao H, Zhang X, Benoit E, Haluska FG. Identification of PTEN/MMAC1 alterations in uncultured melanomas and melanoma cell lines. Oncogene. 1998;16:3397–3402. doi: 10.1038/sj.onc.1201881. [DOI] [PubMed] [Google Scholar]
- 14.Reifenberger J, Wolter M, Bostrom J, Buschges R, Schulte KW, Megahed M, et al. Allelic losses on chromosome arm 10q and mutation of the PTEN (MMAC1) tumour suppressor gene in primary and metastatic malignant melanomas. Virchows Arch. 2000;436:487–493. doi: 10.1007/s004280050477. [DOI] [PubMed] [Google Scholar]
- 15.Zhou XP, Gimm O, Hampel H, Niemann T, Walker MJ, Eng C. Epigenetic PTEN silencing in malignant melanomas without PTEN mutation. Am J Pathol. 2000;157:1123–1128. doi: 10.1016/S0002-9440(10)64627-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Abdel-Rahman MH, Yang Y, Zhou XP, Craig EL, Davidorf FH, Eng C. High frequency of submicroscopic hemizygous deletion is a major mechanism of loss of expression of PTEN in uveal melanoma. J Clin Oncol. 2006;24:288–295. doi: 10.1200/JCO.2005.02.2418. [DOI] [PubMed] [Google Scholar]
- 17.Gimm O, Perren A, Weng LP, Marsh DJ, Yeh JJ, Ziebold U, et al. Differential nuclear and cytoplasmic expression of PTEN in normal thyroid tissue, and benign and malignant epithelial thyroid tumors. Am J Pathol. 2000;156:1693–1700. doi: 10.1016/s0002-9440(10)65040-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ginn-Pease ME, Eng C. Increased nuclear phosphatase and tensin homologue deleted on chromosome 10 is associated with G0-G1 in MCF-7 cells. Cancer Res. 2003;63:282–286. [PubMed] [Google Scholar]
- 19.Perren A, Komminoth P, Saremaslani P, Matter C, Feurer S, Lees JA, et al. Mutation and expression analyses reveal differential subcellular compartmentalization of PTEN in endocrine pancreatic tumors compared to normal islet cells. Am J Pathol. 2000;157:1097–1103. doi: 10.1016/S0002-9440(10)64624-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Engelman JA, Luo J, Cantley LC. The evolution of phosphatidylinositol 3-kinases as regulators of growth and metabolism. Nat Rev Genet. 2006;7:606–619. doi: 10.1038/nrg1879. [DOI] [PubMed] [Google Scholar]
- 21.Gu J, Tamura M, Yamada KM. Tumor suppressor PTEN inhibits integrin- and growth factor-mediated mitogen-activated protein (MAP) kinase signaling pathways. J Cell Biol. 1998;143:1375–1383. doi: 10.1083/jcb.143.5.1375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Maehama T. PTEN: its deregulation and tumorigenesis. Biol Pharm Bull. 2007;30:1624–1627. doi: 10.1248/bpb.30.1624. [DOI] [PubMed] [Google Scholar]
- 23.Maehama T, Dixon JE. The tumor suppressor, PTEN/MMAC1, dephosphorylates the lipid second messenger, phosphatidylinositol 3,4,5-trisphosphate. J Biol Chem. 1998;273:13375–13378. doi: 10.1074/jbc.273.22.13375. [DOI] [PubMed] [Google Scholar]
- 24.Nakamura N, Ramaswamy S, Vazquez F, Signoretti S, Loda M, Sellers WR. Forkhead transcription factors are critical effectors of cell death and cell cycle arrest downstream of PTEN. Mol Cell Biol. 2000;20:8969–8982. doi: 10.1128/mcb.20.23.8969-8982.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wu H, Goel V, Haluska FG. PTEN signaling pathways in melanoma. Oncogene. 2003;22:3113–3122. doi: 10.1038/sj.onc.1206451. [DOI] [PubMed] [Google Scholar]
- 26.Chung JH, Eng C. Nuclear-cytoplasmic partitioning of phosphatase and tensin homologue deleted on chromosome 10 (PTEN) differentially regulates the cell cycle and apoptosis. Cancer Res. 2005;65:8096–8100. doi: 10.1158/0008-5472.CAN-05-1888. [DOI] [PubMed] [Google Scholar]
- 27.Chung JH, Ostrowski MC, Romigh T, Minaguchi T, Waite KA, Eng C. The ERK1/2 pathway modulates nuclear PTEN-mediated cell cycle arrest by cyclin D1 transcriptional regulation. Hum Mol Genet. 2006;15:2553–2559. doi: 10.1093/hmg/ddl177. [DOI] [PubMed] [Google Scholar]
- 28.Shen WH, Balajee AS, Wang J, Wu H, Eng C, Pandolfi PP, et al. Essential role for nuclear PTEN in maintaining chromosomal integrity. Cell. 2007;128:157–170. doi: 10.1016/j.cell.2006.11.042. [DOI] [PubMed] [Google Scholar]
- 29.Liu JL, Sheng X, Hortobagyi ZK, Mao Z, Gallick GE, Yung WK. Nuclear PTEN-mediated growth suppression is independent of Akt down-regulation. Mol Cell Biol. 2005;25:6211–6224. doi: 10.1128/MCB.25.14.6211-6224.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Whiteman DC, Zhou XP, Cummings MC, Pavey S, Hayward NK, Eng C. Nuclear PTEN expression and clinicopathologic features in a population-based series of primary cutaneous melanoma. Int J Cancer. 2002;99:63–67. doi: 10.1002/ijc.10294. [DOI] [PubMed] [Google Scholar]
- 31.Taylor WR. FACS-based detection of phosphorylated histone H3 for the quantitation of mitotic cells. Methods Mol Biol. 2004;281:293–299. doi: 10.1385/1-59259-811-0:293. [DOI] [PubMed] [Google Scholar]
- 32.Taylor WR, Stark GR. Regulation of the G2/M transition by p53. Oncogene. 2001;20:1803–1815. doi: 10.1038/sj.onc.1204252. [DOI] [PubMed] [Google Scholar]
- 33.Pines J. Cyclins and cyclin-dependent kinases: a biochemical view. Biochem J. 1995;308(Pt 3):697–711. doi: 10.1042/bj3080697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chada S, Bocangel D, Ramesh R, Grimm EA, Mumm JB, Mhashilkar AM, et al. mda-7/IL24 kills pancreatic cancer cells by inhibition of the Wnt/PI3K signaling pathways: identification of IL-20 receptor-mediated bystander activity against pancreatic cancer. Mol Ther. 2005;11:724–733. doi: 10.1016/j.ymthe.2004.12.021. [DOI] [PubMed] [Google Scholar]
- 35.Dupont J, Renou JP, Shani M, Hennighausen L, LeRoith D. PTEN overexpression suppresses proliferation and differentiation and enhances apoptosis of the mouse mammary epithelium. J Clin Invest. 2002;110:815–825. doi: 10.1172/JCI13829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Waite KA, Sinden MR, Eng C. Phytoestrogen exposure elevates PTEN levels. Hum Mol Genet. 2005;14:1457–1463. doi: 10.1093/hmg/ddi155. [DOI] [PubMed] [Google Scholar]
- 37.Fang J, Ding M, Yang L, Liu LZ, Jiang BH. PI3K/PTEN/AKT signaling regulates prostate tumor angiogenesis. Cell Signal. 2007;19:2487–2497. doi: 10.1016/j.cellsig.2007.07.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Welch PJ, Wang JY. Coordinated synthesis and degradation of cdc2 in the mammalian cell cycle. Proc Natl Acad Sci U S A. 1992;89:3093–3097. doi: 10.1073/pnas.89.7.3093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tolkacheva T, Boddapati M, Sanfiz A, Tsuchida K, Kimmelman AC, Chan AM. Regulation of PTEN binding to MAGI-2 by two putative phosphorylation sites at threonine 382 and 383. Cancer Res. 2001;61:4985–4989. [PubMed] [Google Scholar]
- 40.Torres J, Pulido R. The tumor suppressor PTEN is phosphorylated by the protein kinase CK2 at its C terminus. Implications for PTEN stability to proteasome-mediated degradation. J Biol Chem. 2001;276:993–998. doi: 10.1074/jbc.M009134200. [DOI] [PubMed] [Google Scholar]
- 41.Nicolson GL, Nawa A, Toh Y, Taniguchi S, Nishimori K, Moustafa A. Tumor metastasis-associated human MTA1 gene and its MTA1 protein product: role in epithelial cancer cell invasion, proliferation and nuclear regulation. Clin Exp Metastasis. 2003;20:19–24. doi: 10.1023/a:1022534217769. [DOI] [PubMed] [Google Scholar]
- 42.Forget MA, Turcotte S, Beauseigle D, Godin-Ethier J, Pelletier S, Martin J, et al. The Wnt pathway regulator DKK1 is preferentially expressed in hormone-resistant breast tumours and in some common cancer types. Br J Cancer. 2007;96:646–653. doi: 10.1038/sj.bjc.6603579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kimura F, Watanabe J, Hata H, Fujisawa T, Kamata Y, Nishimura Y, et al. PTEN immunohistochemical expression is suppressed in G1 endometrioid adenocarcinoma of the uterine corpus. J Cancer Res Clin Oncol. 2004;130:161–168. doi: 10.1007/s00432-003-0517-8. [DOI] [PubMed] [Google Scholar]
- 44.Kappes H, Goemann C, Bamberger AM, Loning T, Milde-Langosch K. PTEN expression in breast and endometrial cancer: correlations with steroid hormone receptor status. Pathobiology. 2001;69:136–142. doi: 10.1159/000048768. [DOI] [PubMed] [Google Scholar]
- 45.Stoica A, Hoffman M, Marta L, Voiculetz N. Estradiol and progesterone receptors in human cutaneous melanoma. Neoplasma. 1991;38:137–145. [PubMed] [Google Scholar]