Abstract
Guided by a combination of nuclear magnetic resonance binding assays and computational docking studies, we synthesized a library of 5, 5’ substituted Apogossypol derivatives as potent Bcl-XL antagonists. Each compound was subsequently tested for its ability to inhibit Bcl-XL in an in vitro fluorescence polarization competition assay and to exert single-agent proapoptotic activity in human cancer cell lines. The most potent compound, BI79D10, binds to Bc1-XL, Bcl-2 and Mcl-1 with IC50 values of 190 nM, 360 nM and 520 nM, respectively, and potently inhibits cell growth in the H460 human lung cancer cell line with an EC50 value of 680 nM, which express high level of Bcl-2. BI79D10 also effectively induces apoptosis of the RS11846 human lymphoma cell line in a dose-dependent manner and shows little cytotoxicity against bax−/− bak−/− mouse embryonic fibroblast cells in which anti-apoptotic Bcl-2 family proteins lack a cytoprotective phenotype, implying that BI79D10 has little off-target effects. BI79D10 displays in vivo efficacy in transgenic mice in which Bcl-2 is over-expressed in splenic B-cells. Together with its improved plasma and microsomal stability relative to Apogossypol, BI79D10 represents a lead compound for the development of novel apoptosis-based therapies for cancer.
Introduction
Programmed cell-death (apoptosis) plays critical roles in normal tissue homeostasis, ensuring a proper balance of cell production and cell loss (1, 2). Defects in the regulation of programmed cell death promote tumorgenesis, and also contribute significantly to chemoresistance (3, 4). Bcl-2 (B-cell lymphoma/leukemia-2) family proteins are central regulators of apoptosis (5–7). In humans, six anti-apoptotic members of the Bcl-2 family have been identified and characterized, including Bcl-2, Bcl-XL, Mcl-1, Bfl-1, Bcl-W and Bcl-B. Over-expression of anti-apoptotic Bcl-2 family proteins occurs in many human cancers and leukemias, and therefore these proteins are very attractive targets for the development of novel anticancer agents (8–11). Members of the Bcl-2 family proteins also include proapoptotic effectors such as Bak, Bax, Bad, Bim and Bid. Anti-apoptotic and pro-apoptotic Bcl-2 family proteins dimerize and negate each other’s functions (3). Structural studies have elucidated a hydrophobic crevice on the surface of anti-apoptotic Bcl-2 family proteins that binds the BH3 dimerization domain of pro-apoptotic family members (10). Thus, molecules that mimic the BH3 domain of pro-apoptotic proteins induce apoptosis and/or abrogate the ability of anti-apoptotic Bcl-2 proteins to inhibit cancer cell death.
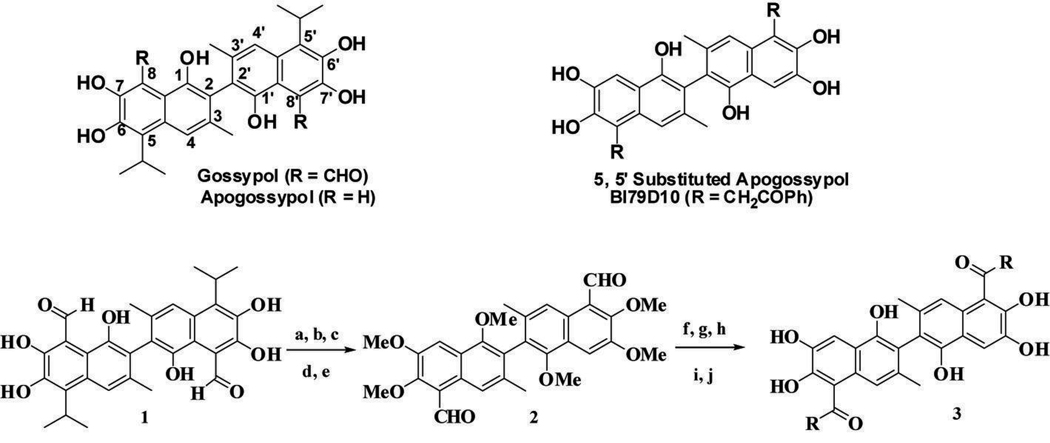
We and others have reported that the natural product Gossypol (Fig. 1A) is a potent inhibitor of Bcl-2, Bcl-XL and Mcl-1, functioning as a BH3 mimic (12–15). (−) Gossypol is currently in clinical trails, displaying single-agent antitumor activity in patients with advanced malignancies (14). Given that Gossypol has toxicity problems likely due to two reactive aldehyde groups (16), we designed Apogossypol (Fig. 1A), a compound that lacks these aldehydes, but retains activity against anti-apoptotic Bcl-2 family proteins in vitro and in cells (17). Recently, we further compared the efficacy and toxicity in mice of Gossypol and Apogossypol. Our preclinical in vivo data show that Apogossypol has superior efficacy and markedly reduced toxicity compared to Gossypol (18). We also evaluated the single-dose pharmacokinetic characteristics of Apogossypol in mice. Apogossypol displayed superior blood concentrations over time compared to Gossypol, due to slower clearance (19). These observations indicate that Apogossypol is a promising lead compound for cancer therapy. Recently, we reported the separation and characterization of Apogossypol atropoisomers (15). These studies revealed that the racemic Apogossypol is as effective as its individual isomers (15). In this current work we focused our attention on preparing and evaluating activities of novel 5, 5’ substituted Apogossypol derivatives (Fig. 1B).
Figure 1.
A, Structure of Gossypol and Apogossypol. B, Structure of 5, 5’ substituted Apogossypol derivatives and BI79D10. C, Synthesis of 5, 5’ substituted Apogossypol derivatives: a) NaOH, H2O; b) H2SO4; c) DMS, K2CO3; d) TiCl4, Cl2CHOCH3; e) HCl, H2O; f) RMgBr or RL; g) NH4Cl, H2O; h) PCC, CH2Cl2; i) BBr3; j) HCl.
Materials and Methods
Molecular Modeling
Molecular modeling studies were conducted on a Linux workstation and a 64 3.2-GHz CPUs Linux cluster. Docking studies were performed using the crystal structure of Bcl-XL in complex with Bakderived peptide (20) (Protein Data Bank code 1BXL). The Bak-derived peptide was extracted from the protein structure and was used to define the binding site for small molecules. Apogossypol and its derivatives were docked into the Bcl-XL protein by the GOLD (21) docking program using ChemScore (22) as the scoring function. The active site radius was set at 10 Å and 10 GA solutions were generated for each molecule. The GA docking procedure in GOLD (21) allowed the small molecules to flexibly explore the best fit conformations in the binding pocket whereas the protein structure was static. The binding poses of apogossypol and its derivatives were compared to prioritize the synthesis. The protein surface was prepared with the program MOLCAD (23) as implemented in Sybyl (Tripos, St. Louis) and was used to analyze the binding poses for studied small molecules.
Chemicals
The synthesis for 5, 5’ substituted Apogossypol derivatives is outlined in Fig. 1C. Briefly, Gossypol 1 was treated with NaOH solution followed by dimethyl sulfate to afford methyl Apogossypol. Reaction of methyl Apogossypol with TiCl4 and dichloromethyl methyl ether resulted in loss of isopropyl groups and simultaneous bisformylation to give aldehyde 2 (24). The compound 2 was treated with different Grignard or lithium reagents to afford a secondary alcohol, which was oxidized to the phenone using pyridinium chlorochromate. Subsequent demethylation of the phenone afforded compound 3. Characterizations of the compound BI79D10 (Fig. 1B) are given as an example. The detail experiments and characterization of all other compounds are in supporting information. 1H NMR (CD3OD) δ 7.61 (s, 2H), 7.30 (m, 8H), 7.22 (m, 2H), 6.97 (s, 2H), 4.40 (dd, J1 = 15.6 Hz, J2= 22.8 Hz, 4H), 1.87 (s, 6H); 13C NMR (CD3)2SO) δ 204.6, 149.4, 144.8, 144.5, 135.4, 134.2, 130.5, 128.6, 126.9, 126.3, 122.6, 119.4, 116.8, 115.0, 107.1, 51.0, 21.1; HRMS calcd for [C38H30O8 + H] 615.2019; Found 615.2013. HPLC is 99% pure.
NMR Experiments
NMR-based binding assays have been conducted by acquiring one-dimensional 1H experiments with 500 µL solution of Bcl-XL at 25 µM concentration, in absence and presence of added compounds, each at 200 µM concentration. By observing the aliphatic region of the spectra, binding could be readily detected due to chemical shift changes in active site methyl groups of Ile, Leu, Thr, Val or Ala (region between −0.8 and 0.3 ppm). The binding mode was characterized by recording [15N, 1H]-HSQC experiments with 500 µL solution of uniformly 15N-labeled Bcl-XL (125 µM concentration) in absence and presence of added compounds, each at 500 µM concentration. 15N and unlabeled Bcl-XL samples were prepared and purified as described previously (20). All experiments were performed with a 600 MHz spectrometer Bruker Avance 600 equipped with four rf channels and z-axis pulse-field gradients.
Fluorescence Polarization Assays (FPAs)
A Bak BH3 peptide (F-BakBH3) (GQVGRQLAIIGDDINR) was labeled at the N-terminus with fluorescein isothiocyanate (FITC) (Molecular Probes) and purified by HPLC. For competitive binding assays, 100 nM GST–Bcl-XL ΔTM protein was preincubated with the tested compound at varying concentrations in 47.5 µL PBS (pH=7.4) in 96-well black plates at room temperature for 10 min, then 2.5 µL of 100 nM FITC-labeled Bak BH3 peptide was added to produce a final volume of 50 µL. The wild-type and mutant Bak BH3 peptides were included in each assay plate as positive and negative controls, respectively. After 30 min incubation at room temperature, the polarization values in millipolarization units (20) were measured at excitation/emission wavelengths of 480/535 nm with a multilabel plate reader (PerkinElmer). IC50 was determined by fitting the experimental data to a sigmoidal dose-response nonlinear regression model (SigmaPlot 10.0.1, Systat Software, Inc., San Jose, CA, USA). Data reported are mean of three independent experiments ± standard error (SE). Performance of Bcl-2 and Mcl-1 FPA are similar. Briefly, 50 nM of GST-Bcl-2 or -Mcl-1 were incubated with various concentrations of Apogossypol, or its 5, 5’ substituted derivatives for 2 min, then 15 nM FITC-conjugated-Bim BH3 peptide (25) was added in PBS buffer. Fluorescence polarization was measured after 10 min.
Isothermal Titration Calorimetry Assays (ITC)
Titrations were performed using a VP-ITC or ITC200 calorimeter from Microcal (Northampton, MA). Bcl-XL was used at concentrations between 25 and 100 µM in 20 mM sodium phosphate buffer (pH 7.4) and 5–10% DMSO. Titrants were used at concentrations 10–15x of the protein in the same buffer. Titrations were carried out at 25 °C. Data were analyzed using Microcal Origin software provided by the ITC manufacturer (Microcal, Northampton, MA).
Cell Viability Assays
The activity of the compounds against human cancer cell lines (PC3ML, H460, H1299, RSI 1846) were assessed by using the ATP-LITE assay (PerkinElmer). All cells were seeded in either F12 or RPMI1640 medium with 5 mM L-glutamine supplemented with 5% fetal bovine serum (Mediatech Inc.), penicillin and streptomycin (Omega). For maintenance, cells were cultured in 5% FBS. Cells plated into 96 well plates at varying initial densities depending on doubling time. H460 and H1299 plated at 2000 cells / well, A549 and PC3 at 3000 cells / well, and RS118456S at 10,000 cells / well. Compounds were diluted to final concentrations with 0.1% DMSO. Prior to dispensing compounds onto cells, fresh 5% media was placed into wells. Administration of compounds occurred 24 hours after seeding into the fresh media. Cell viability was evaluated using ATP-LITE reagent (PerkinElmer) after 72 hours of treatment. Data were normalized to the DMSO control-treated cells using Prism version 5.01 (Graphpad Software).
The apoptotic activity of the compounds against RSl 1846 cells was assessed by staining with Annexin V- and propidium iodide (PI). Lymphoma cell line, RSl 1846, was cultured in RPMI 1640 medium (Mediatech Inc., Herndon, VA 20171) containing 10% fetal bovine serum (Mediatech Inc., Herndon, VA 20171) and Penicillin/Streptomycin (Mediatech Inc., Herndon, VA 20171). Cells were cultured with various concentrations of 5, 5’ substituted Apogossypol for 1 – 2 days. The percentage of viable cells was determined by FITC-Annexin V- and propidium iodide (PI)-labeling, using an Apoptosis Detection kit (BioVision Inc.), and analyzing stained cells by flow cytometry (FACSort; Bectin-Dickinson, Inc.; Mountain View, CA). Cells that were annexin-V-negative and PI-negative were considered viable.
The apoptotic activity of the compound BI79D10 against mouse embryonic fibroblast wild-type cells (MEF/WT) and mouse embryonic fibroblast BAX/Bak double knockout cells (MEF/DKO) was assessed by staining with Annexin V- and propidium iodide (PI). MEF/WT and MEF/DKO cells were seeded in 24-well plate at a seeding density of half a million per well (in 1 ml of DMEM medium supplemented by 10% FCS). Next day, BI79D10 was added to wild-type and DKO cells at final concentration of 0, 2.5, 5.0, 7.5 and 10 µM. On the following day, floating cells were pooled with adherent cells harvested after brief incubation with 0.25% Trypsin/EDTA solution (Gibco/In-Vitrogen Inc.). Cells were centrifuged and supernatant was discarded, and cell pellet was re-suspended with 0.2 ml of Annexin-V binding buffer, followed by addition of 1 µl Annexin-FITC and 1 µl PI (propidium iodide). The percentage of viable cells was determined by a 3-color FACSort instrument and data analyzed by Flow-Jo program, scoring Annexin V-negative, Pi-negative as viable cells.
In Vitro ADME Studies
Liver Microsomal Stability
Pooled rat liver microsomes (BD Biosciences, # 452701) were preincubated with test compounds at 37.5 °C for 5 min in the absence of NADPH. The reaction was initiated by addition of NADPH and then incubated under the same conditions. The final incubation concentrations were 4 µM test compound, 2 mM NADPH, and 1 mg/mL (total protein) liver microsomes in phosphate-buffered saline (PBS) at pH 7.4. One aliquot (100 µL) of the incubation mixture was withdrawn at 0, 15, 30, and 60 min and combined immediately with 200 µL of ACN/MeOH containing an internal standard. After mixing, the sample was centrifuged at approximately 13,000 rpm for 12 min. The supernatant was transferred into an autosampler vial and the amount of test compound was quantified using the Shimadzu LCMS 2010EV mass spectrometer. The change of the AUC (area under the curve) of the parent compound as function of time was used as a measure of microsomal stability.
Plasma Stability
A 20 µL aliquot of a 10 mM solution in DMSO of the test compound was added to 2.0 mL of heparinized rat plasma (Lampire, P1-150N) to obtain a 100 µM final solution. The mixture was incubated for 1 h at 37.5 °C. Aliquots of 100 µL were taken (0, 30 min, 1 h) and diluted with 200 µL of MeOH containing internal standard. After mixing, the sample was centrifuged at approximately 13,000 rpm for 12 min. The supernatant was transferred into an autosampler vial and the amount of test compound was quantified using the Shimadzu LCMS-2010EV system. The change of the AUC (area under the curve) of the parent compound as function of time was used as a measure of microsomal stability.
PAMPA (parallel artificial membrane permeation assay)
A 96-well microtiter plate (Millipore, # MSSACCEPTOR) was completely filled with aqueous buffer solution (pH 7.2) and covered with a microtiter filterplate (Millipore, # MAPBMN310). The hydrophobic filter material was impregnated with a 10% solution of hexadecane in hexane and the organic solvent was allowed to completely evaporate. Permeation studies were started by the transfer of 200 µL of a 100 µM test compound solution on top of the filterplate. In general phosphate buffer at pH 7.2 buffer was used. The maximum DMSO content of the stock solutions was <5%. In parallel, an equilibrium solution lacking a membrane was prepared using the exact concentrations and specifications but lacking the membrane. The concentrations of the acceptor and equilibrium solutions were determined using the Shimadzu LCMS-2010EV and AUC methods. The permeation of a compound through the membrane layer is described by the percentage permeation (% flux). The flux values were calculated considering the concentration of the acceptor compartment after 8 h and that of a reference well with the same concentration containing no membrane barrier.
Bcl-2 Transgenic Mice Studies
Transgenic mice expressing Bcl-2 have been described as the B6 line (26). The BCL-2 transgene represents a minigene version of a t(14;18) translocation in which the human BCL-2 gene is fused with the immunoglobulin heavy-chain (IgH) locus and associated IgH enhancer. The transgene was propagated on the Balb/c background. These mice develop polyclonal B-cell hyperplasia with asynchronous transformation to monoclonal aggressive lymphomas beginning at approximately 6 months of age, with approximately 90% of mice undergoing transformation by the age of 12 to 24 months. All animals used here had not yet developed aggressive lymphoma.
Mouse Experiments
Compounds dissolved in 500 µL of solution (Ethanol: Cremophor EL: Saline = 10: 10: 80) were injected intraperitoneally to age- and sex-matched B6Bcl2 mouse, while control-mice were injected intraperitoneally with 500 µL of the same formulation without compound. After 24 hours, B6Bcl2 mice were sacrificed by intraperitoneal injection of lethal dose of Avertin. Spleen was removed and weighed. The spleen weight of mice is used as an end-point for assessing activity as we determined that spleen weight is highly consistent in age- and sex-matched Bcl-2-transgenic mice in preliminary studies (18). Variability of spleen weight was within ±2% among control-treated age-matched, sex-matched B6Bcl2 mice. Spleen tissue was fixed in z-FIX for 3 days and rinsed in PBS, and saved for histological analysis of spleen (H&E staining and TUNEL assay).
Results and Discussion
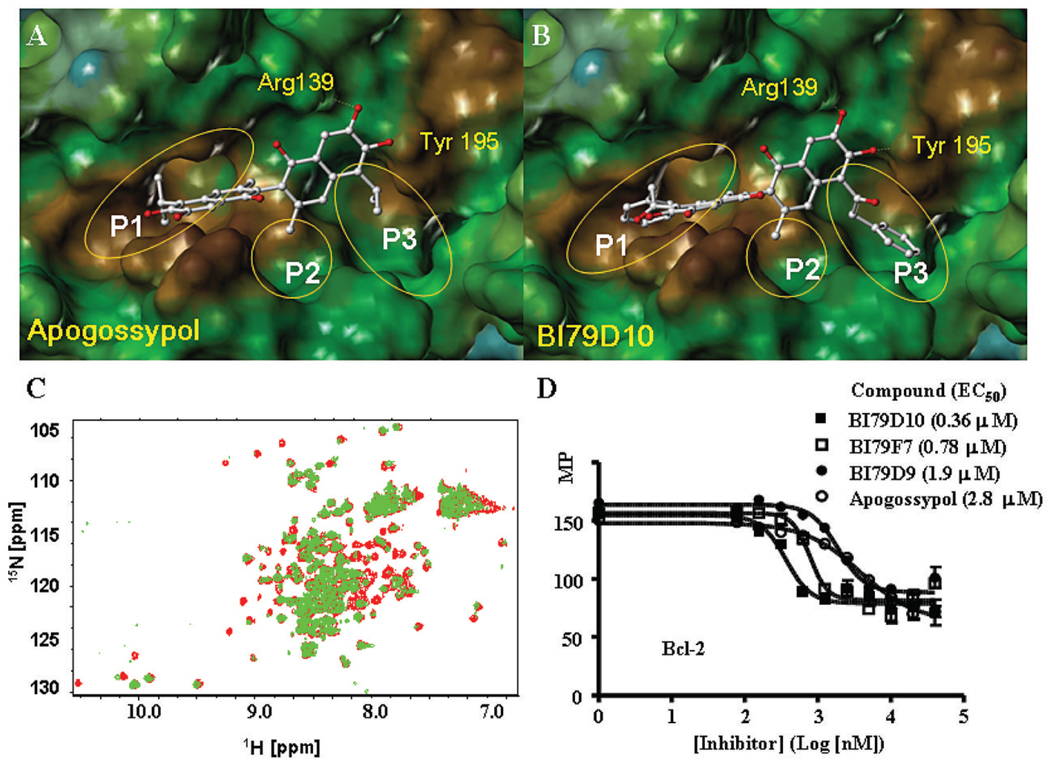
We have recently reported that Apogossypol is a promising inhibitor of Bcl-XL with improved in vivo efficacy and reduced toxicity compared to Gossypol (17, 18). Molecular docking studies of Apogossypol into the BH3 binding groove in Bcl-XL (Fig. 2A) suggest that Apogossypol forms two hydrogen bonds with residues Arg 139 and Tyr 195 in Bcl-XL through adjacent sixth and seventh hydroxyl groups on the right naphthalene ring. The isopropyl group on the left naphthalene ring inserts into the first hydrophobic pocket (Pl) in Bcl-XL (Fig. 2A), while the methyl group and the isopropyl group on the right naphthalene ring insert into the adjacent two hydrophobic pockets, P2 and P3, respectively (Fig. 2A). Analysis of the predicted binding models indicates that while the overall core structure of Apogossypol fits rather well into BH3 binding groove of Bcl-XL, the two isopropyl groups do not apparently fully occupy the hydrophobic pockets Pl and P3. Therefore, a library of 5, 5’ substituted Apogossypol derivatives (Fig. 1B) that replace the isopropyl groups with larger hydrophobic substituents was designed with the aim of deriving novel molecules that could occupy the hydrophobic pockets on Bcl-XL more efficiently.
Figure 2.
A and B, Molecular docking studies. Stereo views of docked structures of Apogossypol (A) and BI79D10 (B) into the BH3 peptide binding groove in Bcl-XL. C, Superposition of [15N, 1H]-TROSY spectra of free Bcl-XL (125 µM, red) and spectra after addition of BI79D10 (green, 500 µM). D, Fluorescence polarization-based competitive binding curves of BI79D10 (solid squares), BI79F7 (hollow squares), BI79D9 (solid dots) and Apogossypol (hollow dots) to Bcl-2.
The designed 5, 5’ substituted Apogossypol derivatives were synthesized (Fig. 1C and Supplementary 1–2) and evaluated by nuclear magnetic resonance spectroscopy (NMR) binding assays, competitive fluorescence polarization assays (FPA), and cell viability assays (Table 1). Compound BI79D10 (Fig. 1B) displayed high affinity for Bcl-XL in these assays. It induced significant chemical shift changes in active site methyl groups (region between −0.3 and 0.8 ppm) in the one-dimensional 1H-NMR spectra of Bcl-XL (Supplementary Fig. 3A) and also has an IC50 value of 0.19 µM in the FP displacement assays, which is almost 20 times more effective than Apogossypol (Table 1 and Supplementary Fig. 3B). To confirm the result from the one-dimensional 1H-NMR binding assay, we also produced uniformly 15N-labeled Bcl-XL protein and measured 2D [15N,1H]-TROSY correlation spectra in absence and presence of the compound BI79D10 (Fig. 2C). BI79D10 displayed strong binding with Bcl-XL, as qualitatively evaluated by the nature of the shifts at the ligand/protein ratio of 4:1. A group of compounds, such as BI79D7, D8, D9, F4, F6 and F7 also displayed high binding affinity to Bcl-XLin the FP assays with IC50 values ranging from 0.14 to 0.45 µM and induced chemical shift changes in the one-dimensional 1H-NMR spectra of Bcl-XL (Table 1 and Supplementary Fig. 3C and D). To confirm results of the NMR binding data and the FP assays, we further evaluated the binding affinity of BI79D10 and other compounds for Bcl-XL using ITC (Isothermal Titration Calorimetry) (Table 2). In agreement with NMR binding and FPA data, BI79D10 and its para-methyl substituted derivative BI79F7, displayed potent binding affinity to Bcl-XL with Kd values of 0.17 and 0.04 µM, respectively, which is 10 and 40 times more potent than Apogossypol (Kd =1.7 µM) in the same assay. Molecular docking studies of BI79D10 in the BH3 binding groove of Bcl-XL (Fig. 2B) demonstrated that 5, 5’ benzyl groups insert deeper into hydrophobic pockets (P1 and P3) in Bcl-XL hence occupying these regions more efficiently compared to isopropyl groups of Apogossypol (Fig. 2B). The chemscore value for BI79D10 was 33.9, improved from 24 of apogossypol. Consistent with NMR binding, FPA, and ITC data, compounds such as BI79D10 and F7 display significant efficacy in inhibiting cell growth in PC3ML cells, which express high levels of Bcl-XL. Their EC50 values ranged from 1.9 to 4.6 µM, hence 2–5 fold more potent than Apogossypol (EC50= 10.3 µM).
Table 1.
Evaluation of 5, 5’ substituted Apogossypol derivatives using a combination of 1D 1H-NMR binding assays, competitive fluorescence polarization assays and cell viability assays
 | ||||||||
|---|---|---|---|---|---|---|---|---|
| Compd. (BI79-) | R | 1D 1H-NMR Binding Assay a* (Bcl-XL) |
FPA IC50 (µM) (Bcl-XL) |
PC3MLb* EC50 (µM) |
H460b* EC50 (µM) |
H1299b* EC50 (µM) |
RS11846b* EC50 (µM) |
RS11846c* EC50 (µM) |
| Gossypol | ++ | 2.72 | 3.1 | 3.0 | 6.0 | 2.2 | 4.23 | |
| Apogossypol | ++ | 3.69 | 10.3 | 5.8 | 3.4 | 5.0 | 8.6 | |
| F5 | + | NR | 12.6 | 10.1 | 13.4 | 10.0 | 24.7 | |
| D1 | 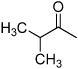 |
++ | NR | 3.9 | 1.5 | 4.8 | 15 | 14.7 |
| D3 | 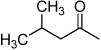 |
+ | 1.30 | 7.5 | 1.1 | 3.6 | 10 | 13.7 |
| D11 | + | 1.29 | 3.0 | 1.5 | 3.0 | 2.8 | 6.6 | |
| D8 | 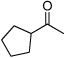 |
+ | 0.45 | 3.4 | 1.1 | 3.1 | 4.0 | 4.5 |
| D6 | 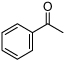 |
+ | 2.9 | 3.6 | 0.31 | 4.2 | NR | 18.3 |
| D9 | 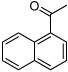 |
+ | 0.16 | 3.0 | 0.59 | 2.4 | 1.8 | 4.2 |
| D12 | 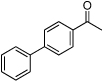 |
− | NR | 7.7 | 8.2 | 9.6 | 2.8 | 25.9 |
| F3 | 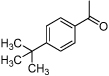 |
− | NR | 2.8 | 3.6 | 4.8 | 2.3 | 13.4 |
| F4 | 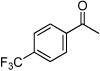 |
+ | 0.25 | 2.9 | 2.2 | 2.0 | 2.5 | 3.8 |
| D10 | +++ | 0.19 | 4.6 | 0.68 | 3.5 | 2.6 | 4.9 | |
| F7 | ++ | 0.32 | 2.5 | 0.82 | 1.7 | 2.2 | 3.0 | |
| F11 | ++ | 1.31 | 3.1 | 2.7 | 2.6 | 8.4 | 5.3 | |
| F12 | ++ | 1.30 | 1.9 | 3.3 | 3.9 | 1.8 | 6.2 | |
| F8 | + | NR | 1.9 | 1.8 | 2.1 | 2 | 5.2 | |
| F6 | 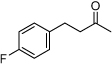 |
++ | 0.14 | 2.8 | 1.5 | 2.2 | 2.3 | 3.1 |
| D7 | + | 0.39 | 5.2 | 1.4 | 5.8 | 2.9 | 7 | |
| D2 | 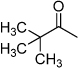 |
++ | NR | NR | NR | NR | NR | 14.7 |
| D5 | 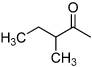 |
+ | NR | NR | NR | NR | NR | 17.1 |
| D4 | + | NR | NR | NR | NR | NR | 11.7 | |
4-point-rating scale: +++: Very Active; ++: Active; +: Mild; −: Weak
Compounds against RS11846 cell line using ATP-LITE assay
Compounds against RS11846 cell line using Annexin V-FITC and propidium iodide assay
Table 2.
Cross-activity of selected 5, 5’ substituted Apogossypol derivatives against Bcl-XL, Bcl-2, and Mcl-1.
 | |||||
|---|---|---|---|---|---|
| Compd. (BI79-) | R = | EC50 (µM) FPA | Kd (µM) ITC | ||
| Bcl-XL | Bcl-2 | Mcl-1 | Bcl-XL | ||
| Apogossypol | 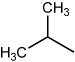 |
3.69 | 2.80 | 2.60 | 1.70 |
| D10 | 0.19 | 0.36 | 0.52 | 0.17 | |
| F7 | 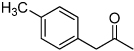 |
0.32 | 0.78 | 1.10 | 0.04 |
| D9 | 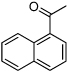 |
0.16 | 1.90 | 2.20 | 2.75 |
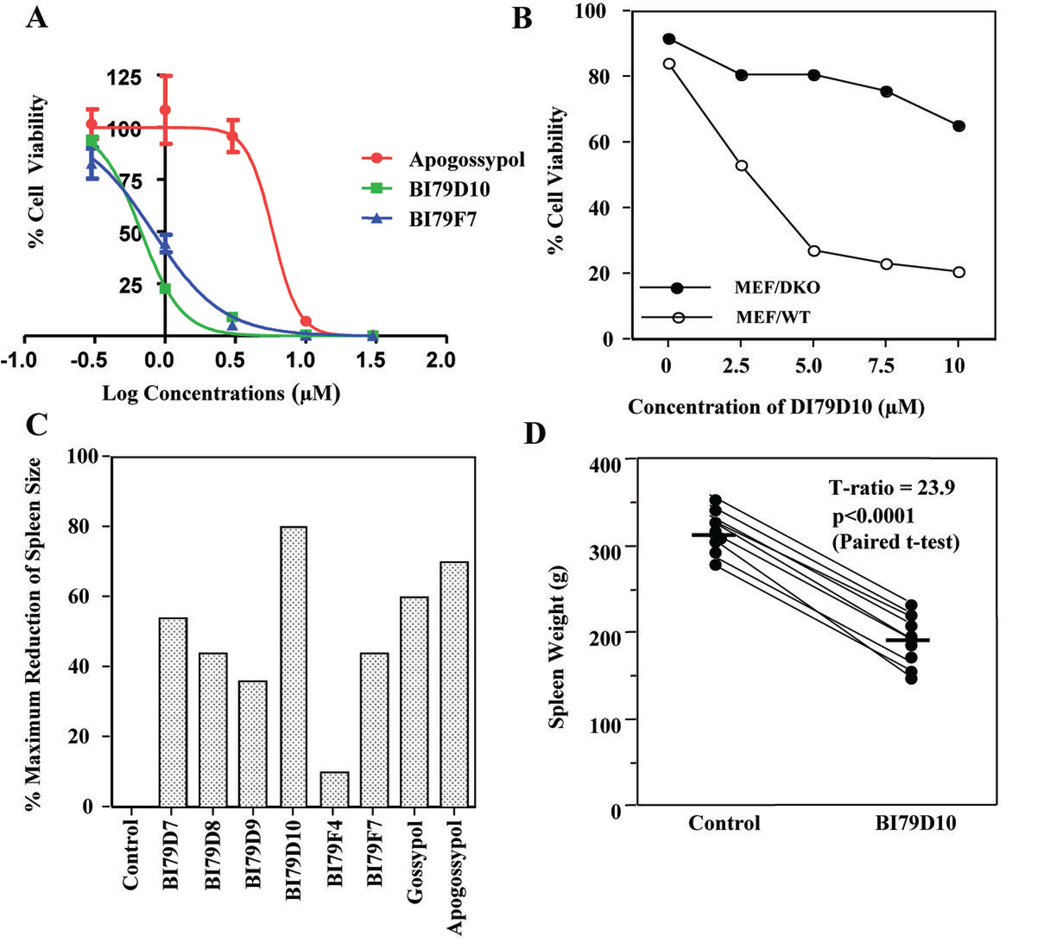
To evaluate the binding properties and specificity of 5, 5’ substituted Apogossypol derivatives to other anti-apoptotic Bcl-2 family proteins, we evaluated selected Bcl-XL active compounds against Bcl-2 and Mcl-1 using FP assays (Table 2 and Fig. 2D). These potent Bcl-XL inhibitors also displayed strong binding affinity to Bcl-2 and Mcl-1. The most potent compound, BI79D10, binds to Bcl-2 and Mcl-1 with EC50 values of 0.36 and 0.52 µM, respectively, which are approximately 8 and 5 fold more potent than Apogossypol (EC50 = 2.8 µM). The compound BI79F7 is slightly less active than the compound BI79D10, while the compound BI79D9 has similar activity as Apogossypol. Since BI79D10 and F7 displayed strong binding affinities to Bcl-2 and Mcl-1 in FP assay, we further evaluated all 5, 5’ substituted Apogossypol derivatives against H460 and H1299 cell lines, which express high levels of Bcl-2 and Mcl-1, respectively (Table 1). In agreement with FPA data, compounds BI79D10 and F7 potently inhibited growth of the H460 cell line with EC50 values of 0.68 and 0.82 µM, respectively, which are approximately 7–8 times more potent than Apogossypol (EC50 = 5.8 µM) (Fig. 3A and Supplementary Fig. 3E). Compounds BI79D6 and D9 having similar structure as BI79D10 also strongly inhibited cell growth in the H460 cell line with EC50 values of 0.30 and 0.59 µM, respectively. Most of the tested 5, 5’ substituted Apogossypol derivatives also showed potent cell activity in the H460 and H1299 cell lines with EC50 values ranging from 1 to 4 µM. In contrast, the compound BI79F5 (Table 1), the negative control compound with only hydrogen atoms on 5, 5’ positions, displayed weak cell growth inhibition activity in both H460 (EC50 = 10.1 µM) and H1299 (EC50 = 13.4 µM) cell lines indicating 5, 5’ substituted groups are necessary for strong inhibition. This observation is in agreement with reports for the potent Bcl-XL antagonist ABT-737 (27), which is not effective against Mcl-1 (27) and consequentially is not effective in killing Mcl-1 overexpressing cell lines such as the H1299 (28).
Figure 3.
A, Inhibition of cell growth by Apogossypol derivatives in the H460 human lung cancer cell line. Cells were treated for 3 days and cell viability was evaluated using ATP-LITE assay. B, Mouse embryonic fibroblast cells with wild-type (MEF/WT; white symbols) or bax−/−bak−/− double knockout (black symbols) genotypes were treated with BI79D10 at various concentrations. C, Effects of 5, 5’ substituted Apogossypol derivatives on shrinkage of Bcl-2 mouse spleen at a single intraperitoneal injection dose of 0.06 mmol/kg. All shrinkage data are percentage of maximum reduction of mice spleen size. D, Effects of BI79D10 on shrinkage of spleen of six Bcl-2 mice at a single intraperitoneal injection dose of 0.06 mmol/kg.
We further tested 5, 5’ substituted Apogossypol derivatives for their ability to induce apoptosis of the human lymphoma RS11846 cell line, which expresses high levels of Bcl-2 and Bcl-XL. For these assays, we used Annexin V-FITC and propidium iodide (PI) double staining, followed by flow-cytometry analysis (Table 1). Most of synthesized Apogossypol derivatives effectively induced apoptosis of the RS11846 cell line in a dose-dependent manner (Table 1 and Supplementary Fig. 4). In particular, compounds BI79D10, D9, F4, F7 are most effective with EC50 values ranging from 3.0 to 5.5 µM, which is consistent with previous results in human cancer PC3ML and H460 cell lines. Again, the negative control compound BI79F5 induced weak apoptosis (EC50 = 24.7) of the RS11846 cell line, consistent with its poor anti-Bcl-2 activity.
We next explored whether the compound BI79D10, the most potent 5, 5’ substituted Apogossypol derivative had cytotoxic activity against Bak/Bak double knockout mouse embryonic fibroblast cells in which antiapoptotic Bcl-2 family proteins lack a cytoprotective phenotype (29, 30). The compound BI79D10 only displayed slight toxicity in Bak/Bak double knockout mouse embryonic fibroblast cells while it kill 80% wild type mouse embryonic fibroblast cells at 10 µM using FITC-Annexin V/PI assays (Fig. 3B), implying that the compound BI79D10 only displayed slight off-target effects.
To test the pharmacological properties of 5, 5’ substituted Apogossypol derivatives, we determined their in vitro plasma stability, microsomal stability, and cell membrane permeability (Table 3). From these studies, we could conclude that our synthesized compounds displayed superior plasma stability and overall are more stable than Apogossypol. BI79D10 only degraded 15% after 1 hour incubation in rat plasma. In addition, BI79D10 and F7 showed similar or improved microsomal stability compared to Apogossypol, while BI79D2 and D8 degraded faster than Apogossypol in rat hepatocytes microsomal preparations. BI79D10 and F7 also displayed improved cell membrane permeability compared to Apogossypol.
Table 3.
Plasma stability, microsomal stability, and cell permeability of selected 5, 5’ substituted Apogossypol derivatives.
| Compd. (BI79-) | R = | Plasma Stability (T=1 hr) |
Microsomal Stability (T=1 hr) |
Cell Permeability |
|---|---|---|---|---|
| Apogossypol | 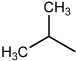 |
53% | 60% | Low |
| D7 | 90% | 68% | Medium | |
| D8 | 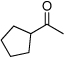 |
79% | 27% | Low |
| D9 | 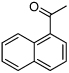 |
62% | 52% | Low |
| D10 | 85% | 64% | Medium | |
| F4 | 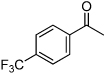 |
NR | 41% | Low |
| F7 | 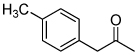 |
72% | 92% | Medium |
| D2 | 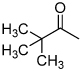 |
90% | 30% | Medium |
Hence, using a combination of lD 1H-NMR binding assays, FP assays, ITC assays, cytotoxicity assays and preliminary in vitro ADME data, compounds such as BI79D10 and F7 were selected for further in vivo studies using B6Bcl-2 transgenic mice. B-cells of the B6Bcl-2 transgenic mice overexpress Bcl-2 and accumulate in the spleen of mice. The spleen weight is used as an end-point for assessing in vivo activity as we have determined that the spleen weight is highly consistent in age- and sex-matched Bcl-2-transgenic mice and variability was within ±2% among control-treated age-matched, sex-matched B6Bcl2 mice (18). We first screened the in vivo activities of compounds such as BI79D10 and F7 side by side with Apogossypol and Gossypol in a single Bcl-2 transgenic mouse at 60 µmol/kg. All tested compounds induced spleen weight reduction of mice (Fig. 3C) and the BI79D10 displayed best efficiency causing 40% reduction in spleen weight and showed mild GI toxicity (Supplementary 5). Since the maximum spleen shrinkage would be no more than 50% in this experimental model, the in vivo effect of BI79D10 induced near maximal (80%) biological activity at 60 µmol/kg (36 mg/kg). To confirm the result from a single mouse experiment, we next evaluated the in vivo activity of BI79D10 in groups of six mice each. In agreement with the single mouse experiment, BI79D10 treatment of these mice resulted in a significant (~40%) reduction of spleen weight (P<0.0001), compared to the control group of six mice (Fig. 3D). All mice tolerated the treatment well with no macroscopic toxicity; the maximal weight loss was 4% during the course of study of BI79D10.
Conclusions
In summary, a library of 5, 5’ substituted Apogossypol derivatives was synthesized and evaluated in a variety of in vitro and in vivo assays. The most potent compound, BI79D10, was found to bind to Bcl-XL, Bcl-2 and Mcl-1 with IC50 values of 190 nM, 360 nM and 520 nM, respectively, in FPA and also potently inhibited growth in culture of the PC3ML, H460, H1299 cancer cell lines, which express Bcl-XL, Bcl-2 and Mcl-1, respectively, with EC50 values in the submicromolar to nanomolar range. BI79D10 effectively induced apoptosis of the RS11846 human lymphoma cell line in a dose-dependent manner and show little cytotoxicity against Bax/Bak double knockout mouse embryonic fibroblast cells in which antiapoptotic Bcl-2 family proteins lack a cytoprotective phenotype, implying that BI79D10 has little off-target effects. Finally, BI79D10 showed favorable in vitro ADME properties and superior in vivo efficacy in Bcl-2 transgenic mice in which Bcl-2 is overexpressed in B-cells compared to Apogossypol. Considering the critical roles of anti-apoptotic Bcl-2 family proteins in tumorgenesis, chemoresistance, and the potent inhibitory activity of BI79D10 against anti-apoptotic Bcl-2 family proteins, BI79D10 represents a lead compound for the development of novel apoptosis-based cancer therapies.
Supplementary Material
Acknowledgments
We thank NIH (Grant CA113318 to MP and JCR) and Coronado Biosciences (CSRA #08-02) for financial support.
References
- 1.Vaux DL, Korsmeyer SJ. Cell death in development. Cell. 1999;96:245–254. doi: 10.1016/s0092-8674(00)80564-4. [DOI] [PubMed] [Google Scholar]
- 2.Reed JC. Dysregulation of apoptosis in cancer. J Clin Oncol. 1999;17:2941–2953. doi: 10.1200/JCO.1999.17.9.2941. [DOI] [PubMed] [Google Scholar]
- 3.Johnstone RW, Ruefli AA, Lowe SW. Apoptosis: a link between cancer genetics and chemotherapy. Cell. 2002;108:153–164. doi: 10.1016/s0092-8674(02)00625-6. [DOI] [PubMed] [Google Scholar]
- 4.Reed JC. Apoptosis-based therapies. Nature reviews Drug discovery. 2002;1:111–121. doi: 10.1038/nrd726. [DOI] [PubMed] [Google Scholar]
- 5.Reed JC. Molecular biology of chronic lymphocytic leukemia: implications for therapy. Seminars in hematology. 1998;35:3–13. [PubMed] [Google Scholar]
- 6.Adams JM, Cory S. The Bcl-2 protein family: arbiters of cell survival. Science (New York, NY) 1998;281:1322–1326. doi: 10.1126/science.281.5381.1322. [DOI] [PubMed] [Google Scholar]
- 7.Gross A, McDonnell JM, Korsmeyer SJ. BCL-2 family members and the mitochondria in apoptosis. Genes & development. 1999;13:1899–1911. doi: 10.1101/gad.13.15.1899. [DOI] [PubMed] [Google Scholar]
- 8.Wang JL, Liu D, Zhang ZJ, et al. Structure-based discovery of an organic compound that binds Bcl-2 protein and induces apoptosis of tumor cells. Proceedings of the National Academy of Sciences of the United States of America. 2000;97:7124–7129. doi: 10.1073/pnas.97.13.7124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Degterev A, Lugovskoy A, Cardone M, et al. Identification of small-molecule inhibitors of interaction between the BH3 domain and Bcl-xL. Nat Cell Biol. 2001;3:173–182. doi: 10.1038/35055085. [DOI] [PubMed] [Google Scholar]
- 10.Reed JC. Bcl-2 family proteins. Oncogene. 1998;17:3225–3236. doi: 10.1038/sj.onc.1202591. [DOI] [PubMed] [Google Scholar]
- 11.Reed JC. Bcl-2 family proteins: strategies for overcoming chemoresistance in cancer. Advances in pharmacology (San Diego, Calif) 1997;41:501–532. doi: 10.1016/s1054-3589(08)61070-4. [DOI] [PubMed] [Google Scholar]
- 12.Kitada S, Leone M, Sareth S, Zhai D, Reed JC, Pellecchia M. Discovery, characterization, and structure-activity relationships studies of proapoptotic polyphenols targeting B-cell lymphocyte/leukemia-2 proteins. Journal of medicinal chemistry. 2003;46:4259–4264. doi: 10.1021/jm030190z. [DOI] [PubMed] [Google Scholar]
- 13.Zhang M, Liu H, Guo R, et al. Molecular mechanism of gossypol-induced cell growth inhibition and cell death of HT-29 human colon carcinoma cells. Biochemical pharmacology. 2003;66:93–103. doi: 10.1016/s0006-2952(03)00248-x. [DOI] [PubMed] [Google Scholar]
- 14.Wang G, Nikolovska-Coleska Z, Yang C-Y, et al. Structure-based design of potent small-molecule inhibitors of anti-apoptotic Bcl-2 proteins. Journal of medicinal chemistry. 2006;49:6139–6142. doi: 10.1021/jm060460o. [DOI] [PubMed] [Google Scholar]
- 15.Wei J, Rega M, Kitada S, et al. Synthesis and evaluation of Apogossypol atropisomers as potential Bcl-xL antagonists. Cancer Letters. 2009;273:107–113. doi: 10.1016/j.canlet.2008.07.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shelley MD, Hartley L, Groundwater PW, Fish RG. Structure-activity studies on gossypol in tumor cell lines. Anti-cancer drugs. 2000;11:209–216. doi: 10.1097/00001813-200003000-00009. [DOI] [PubMed] [Google Scholar]
- 17.Becattini B, Kitada S, Leone M, et al. Rational design and real time, in-cell detection of the proapoptotic activity of a novel compound targeting Bcl-X(L) Chemistry & biology. 2004;11:389–395. doi: 10.1016/j.chembiol.2004.02.020. [DOI] [PubMed] [Google Scholar]
- 18.Kitada S, Kress CL, Krajewska M, Jia L, Pellecchia M, Reed JC. Bcl-2 antagonist apogossypol (NSC736630) displays single-agent activity in Bcl-2-transgenic mice and has superior efficacy with less toxicity compared with gossypol (NSC19048) Blood. 2008;111:3211–3219. doi: 10.1182/blood-2007-09-113647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Coward L, Gorman G, Noker P, et al. Quantitative determination of apogossypol, a pro apoptotic analog of gossypol, in mouse plasma using LC/MS/MS. Journal of pharmaceutical and biomedical analysis. 2006;42:581–586. doi: 10.1016/j.jpba.2006.05.020. [DOI] [PubMed] [Google Scholar]
- 20.Sattler M, Liang H, Nettesheim D, et al. Structure of Bcl-xL-Bak peptide complex: recognition between regulators of apoptosis. Science (New York, N Y) 1997;275:983–986. doi: 10.1126/science.275.5302.983. [DOI] [PubMed] [Google Scholar]
- 21.Jones G, Willett P, Glen RC, Leach AR, Taylor R. Development and validation of a genetic algorithm for flexible docking. Journal of molecular biology. 1997;267:727–748. doi: 10.1006/jmbi.1996.0897. [DOI] [PubMed] [Google Scholar]
- 22.Eldridge MD, Murray CW, Auton TR, Paolini GV, Mee RP. Empirical scoring functions: I. The development of a fast empirical scoring function to estimate the binding affinity of ligands in receptor complexes. J Comput Aided Mol Des. 1997;11:425–445. doi: 10.1023/a:1007996124545. [DOI] [PubMed] [Google Scholar]
- 23.Teschner M, Henn C, Vollhardt H, Reiling S, Brickmann J. Texture mapping: a new tool for molecular graphics. J Mol Graph. 1994;12:98–105. doi: 10.1016/0263-7855(94)80074-x. [DOI] [PubMed] [Google Scholar]
- 24.Meltzer PC, Bickford HP, Lambert GJ. A Regioselective Route to Gossypol Analogues: The Synthesis of Gossypol and 5,5'-Didesisopropyl-5,5'-diethylgossypol. J Org Chem. 1985;50:3121–3124. [Google Scholar]
- 25.Ramjaun AR, Tomlinson S, Eddaoudi A, Downward J. Upregulation of two BH3-only proteins, Bmf and Bim, during TGF beta-induced apoptosis. Oncogene. 2007;26:970–981. doi: 10.1038/sj.onc.1209852. [DOI] [PubMed] [Google Scholar]
- 26.Katsumata M, Siegel RM, Louie DC, et al. Differential effects of Bcl-2 on T and B cells in transgenic mice. Proc Natl Acad Sci U S A. 1992;89:11376–11380. doi: 10.1073/pnas.89.23.11376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Oltersdorf T, Elmore SW, Shoemaker AR, et al. An inhibitor of Bcl-2 family proteins induces regression of solid tumours. Nature. 2005;435:677–681. doi: 10.1038/nature03579. [DOI] [PubMed] [Google Scholar]
- 28.Cory S, Adams JM. Killing cancer cells by flipping the Bcl-2/Bax switch. Cancer cell. 2005;8:5–6. doi: 10.1016/j.ccr.2005.06.012. [DOI] [PubMed] [Google Scholar]
- 29.Wei MC, Zong WX, Cheng EH, et al. Proapoptotic BAX and BAK: a requisite gateway to mitochondrial dysfunction and death. Science (New York, N Y) 2001;292:727–730. doi: 10.1126/science.1059108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhai D, Jin C, Shiau CW, Kitada S, Satterthwait AC, Reed JC. Gambogic acid is an antagonist of antiapoptotic Bcl-2 family proteins. Mol Cancer Ther. 2008;7:1639–1646. doi: 10.1158/1535-7163.MCT-07-2373. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.