Abstract
SOCS1-1 is crucial for control of immune cell cytokine expression, including those cytokines known to enable memory T cell formation and homeostasis. In this study, we found that immunization with SOCS1-downregulated bone marrow-derived dendritic cells generated increased antigen-specific CD8+ T memory cells and antigen-specific responses, as measured by ELISPOT, CTL assays, serum ELISAs, and T-cell proliferation assays. Bone marrow-derived dendritic cells in which SOCS1 was downregulated expressed increased levels of surface IL-15Ra and thymic leukemia (TL) antigen, both of which support memory cell development. This work supports a crucial role for SOCS1 in regulation of dendritic cell-directed generation of memory T cell responses.
1. Introduction
Successful immune responses utilize a memory phase that recalls immune cells trained in the first exposure to tumor or pathogen. In response to a second exposure, memory cells proliferate and recruit other cells to create a barrage of effectors that act quickly upon the tumor or invading pathogen. Key players in this second response are memory CD4+ and CD8+ T cells. These memory cells originate from a portion of T cells activated in the initial response that avoid programmed cell death at the end of the primary response, and then differentiate into long-lived memory T cells [1–3].
Several cytokines are crucial for development and maintenance of memory T cells. IL-2, for example, promotes the generation of CD4+ T cell memory [4]. IL-7 is important for CD8+ T cell survival and CD4+ memory cell homeostasis [5, 6]. IL-15 is crucial for antigen-specific CD8+ T cell proliferation [5, 7, 8]. IL-12 conditions CD8+ T cells for long-term immunity by increasing sensitivity to IL-7 and IL-15 [9]. These and other cytokines work together to generate and maintain memory CD4+ and CD8+ T cells.
The suppressor of cytokine signaling 1 molecule (SOCS1) controls signaling of many cytokines, including IFN-γ, IFN-α, IL-2, IL-4, IL-6, IL-7, IL-10, IL-12, IL-15, and IL-21 utilizing a feedback loop mechanism [10–19]. First, binding of a cytokine to its receptor upregulates expression of SOCS1. SOCS1 then inhibits JAK tyrosine kinase activity by binding to the catalytic site of Janus kinase tyrosine kinase (JAK), and by binding to and recruiting the ubiquitin-transferase complex to target JAK for proteasomal degradation [20, 21]. SOCS1 also controls STAT and, indirectly, TLR signaling [22].
Previous studies in our lab showed that silencing of SOCS1 enhances antigen presentation by dendritic cells and antigen-specific anti-tumor immunity [23]. Since IL-2, IL-7, IL-12, and IL-15 are important cytokines for memory T cell production and homeostasis [24, 25], we hypothesized that downregulating the production of SOCS1 in dendritic cells might allow increased signaling to T cells by these and other cytokines, resulting in expanded memory T-cell populations. Enhanced antigen presentation by SOCS1-downregulated dendritic cells should also boost memory T cell production.
In this study, we tested the ability of SOCS1-downregulated bone marrow-derived dendritic cells (BMDCs) to produce antigen-specific CD8+ T memory cells. Our analysis showed enhanced production of ovalbumin-specific CD8+ memory cells in mice that received SOCS1-downregulated, ovalbumin-pulsed BMDCs. These findings have important implications for vaccine development.
2. Materials and Methods
2.1 Mice
C57BL/6, H-2Kb/OT-I-TCR (OT-I), and H-2Kb/OT-II-TCR (OT-II) transgenic mice were purchased from Jackson Laboratories (Bar Harbor, Maine, USA) and maintained in a pathogen-free mouse facility at Baylor College of Medicine (Houston, TX, USA) according to institutional guidelines.
2.2 Peptides and cell lines
H2-Kb-restricted TRP2 (VYDFFVWL), H2-Kb-restricted OT-I (chicken ovalbumin [OVA] peptide 257–264, SIINFEKL) and OT-II chicken (OVA peptide 323–339, ISQAVHAAHAEINEAGR), were synthesized and purified by HPLC to >95% purity by Genemed Synthesis Inc. (South San Francisco, CA, USA). All peptides were dissolved in DMSO before final dilution in endotoxin-free PBS (Sigma). EL4 and EG7 (mouse lymphoma cell lines) were purchased from ATCC (American Type Tissue Culture).
2.3 BMDC transduction
Bone marrow cells from 6–8 weeks-old naïve C57/BL6 female mice were treated with ACK lysis buffer (0.15 M NH4Cl, 1 mM KHCO3, 0.1 mM NaEDTA), washed to remove RBCs, and cultured at 1.5×106 cells/ml in 24-well plates for 6 days in RPMI/10% FBS/antibiotics plus rmGM-CSF and rmIL-4 (20 ng/ml each, Peprotech). Medium was changed every 2 days. On day 5 of culture, BMDCs were centrifuged at 350 × g for 5 minutes, and medium was removed. The cells were then resuspended in 0.25 mls/0.5×106 cells of transduction medium (RPMI/10% FBS/antibiotics/GM-CSF/IL-4/polybrene/β-mercaptoethanol) and lentivirus at a MOI of 5. The cells were centrifuged at 1000 × g for 45 minutes at room temperature, and then incubated overnight at 37°C, 5% CO2. 80–90% of the cells expressed characteristic DC-specific markers as determined by FACS. On day 6, 0.75 mls medium/well was added, and cells were then pulsed for at least three hours with ovalbumin protein (20 ug/ml) to facilitate uptake and antigen processing of ovalbumin by the immature DCs. The cells were then allowed to mature overnight in the presence of LPS (100 ng/ml). On day 7, the BMDCs were washed and injected.
2.4 Lentiviral vector construction and production
The HIV self-inactivating (SIN) vector used in this study was pTRIP-H1-BY-W modified from pTRIPΔU3 CMV eGFP, which contains a 178-bp fragment encompassing the central polypurine tract (cPPT), a H1 RNA promoter and a bicistronic blasticidin resistance/eYFP selection marker. Mouse SOCS1 small hairpin interfering RNA sequence was inserted into pTRIP-H1-BY-W to generate pTRIP-SOCS1-BY-W (LV-SOCS1-siRNA). Recombinant pseudotyped lentiviral vectors were generated by co-transfection of three plasmids into 293T cells and concentrated by ultracentrifugation, as described previously [23]. The control LV-siGFP was generated as described previously [23]. BMDCs transfected with SOCS1 siRNA-encoding lentivirus were designated as LV-SOCS1-siRNA-DCs, while control BMDCs transfected with the GFP siRNA-encoding lentivirus were designated as LV-GFP-siRNA-DCs. LV-SOCS1-siRNA-DCs exhibit a decrease of ~60% SOCS1 transcription, as measured by RT-PCR in a previous study [23]. Each batch of lentivirus was checked for proper titer using flow cytometric detection of the YFP (yellow fluorescent protein) expression. Lentivirus batches with >4 × 107 ifu/ml titers yielded the SOCS1 knockdown seen in the previous study. Any batches with lower titers were not used. In all experiments, a third group of BMDCs were mock transfected (no lentivirus).
2.5 BMDC injection and boost
Female C57/Bl6 mice, 6–8 weeks of age, were inoculated twice, on days 0 and 7, via footpad injection with 1×106 mock-transduced BMDCs or BMDCs transduced with LV-SOCS1-siRNA or LV-GFP-siRNA, followed by i.p. injections of LPS (30 ug/mouse, i.p.) on days 1 and 3 after each BMDC injection. Mice were boosted with footpad injections of ovalbumin peptides (100 ug each OT-1 and OT-2 per mouse) suspended in incomplete Freund’s adjuvant two weeks before analysis, which occurred two to four months after DC injection.
2.6 Serum OVA ELISA
Blood was collected from three mice from each treatment group, and spun at 1500 × g for 10 minutes. The serum samples were isolated from individual mice and analyzed separately. Serum (supernatant) was diluted in BDOptEIA assay diluent (BD Biosciences) and applied to ovalbumin-coated (20 ug/ml) ELISA plates (Nunc). After overnight incubation at 4°C, the plates were washed with wash solution (KPL, Gaithersburg, MD), and diluted serum samples were added, in triplicate. After 2 hours incubation, the plates were washed, and incubated with either biotin anti-mouse IgG1 (clone A85-1) or biotin anti-mouse IgG2a (clone R19-15) pre-mixed with avidin-HRP (catalog #554058, BD Biosciences). After 1 hour incubation at room temperature, the plates were washed, and BD OptEIA substrate (BD Biosciences) was applied. Color development was stopped by addition of 2M sulfuric acid, and O.D. at 450 nm, with a reference of 570 nm, was measured using a Perkin Elmer HTS 7000 Plus Bioassay reader. All samples were tested in triplicate with repeated assays.
2.7 Flow cytometry
Splenocytes were gently pushed through cell strainers to obtain single-cell suspensions. Cells were centrifuged and resuspended in hypotonic (ACK) lysis buffer, and washed. Single cell suspensions of spleen were first incubated for 15 minutes at 4°C with CD16/CD32 (BDPharmingen). Cells were then stained with FITC- or PE-labeled antibodies to CD4, CD8, or mouse Vb 5.1, 5.2 TCR (OT-2 TCR, H2-Kb)(all from BDPharmingen) or iTag MHC tetramer to SIINFEKL (Beckman Coulter) and analyzed by flow cytometry on a Becton Dickinson FACScan instrument. FlowJo software (Treestar, Inc.) was used for analysis and histogram generation.
2.8 ELISPOT analysis
2×105 splenocytes (prepared as above)/well in triplicate were plated in 96-well plates. Splenocytes were isolated from three individual mice from each treatment group and analyzed separately. Stimulating peptide (OT-1, OT-2, TRP2 [irrelevant control], or no peptide) was added at a concentration of 10 ug/ml. After incubation at 37°C, 5%CO2 for 20 hours, the plates were washed and treated with secondary antibody, and developed. Spots/well were counted by Zellnet Consulting (Fort Lee, NJ). Results shown are representative of three similar experiments.
2.9 CTL assays
CD8+ CTL responses were assessed with a standard chromium release assay, which measures the ability of in vitro-restimulated splenocytes to lyse target cells. Splenocytes from immunized mice were restimulated in vitro in RPMI-1640 containing OT-I and OT-II peptides (10 μg/ml) and IL-2 (20 U/ml) for 3–4 days. Splenocytes from three individual mice from each treatment group were analyzed separately. Target cells and control cells (EG-7 and EL4 mouse lymphoma cell lines, respectively) were labeled with sodium chromate (51Cr). EG-7 cells express the OT-1 peptide. After 4–6 hours of coculture with different effector:target ratios, supernatants were collected using SCS harvesting frames (Molecular Devices) and were counted with a gamma counter (Beckman). Percent lysis was calculated as (experimental release – spontaneous release)/(maximum release – spontaneous release) × 100%.
2.10 Proliferation assay
5×105 splenocytes/well were cultured on a CD3-coated 96-well plate with OT-1 or OT-2 peptides in complete RPMI (RPMI 1640 medium supplemented with 10% FCS, 100 U/ml penicillin and streptomycin, 10 mM HEPES, 1 mM sodium pyruvate, and 5 μM 2-ME) for two days. Splenocytes were isolated from three individual mice from each treatment group and analyzed separately. Cpm/well were measured by a Packard TopCount NXT counter after addition of 1 μCi [3H] thymidine/well for 18 hours and harvested on a Packard Filtermate harvester.
2.11 Anti-mIL15R staining of BMDCs
BMDCs (prepared as described above) were mock transduced or transduced with either LV-GFP or LV-SOCS1, incubated overnight with or without LPS (100 ng/ml), and washed with PBS. The cells were incubated with human IgG for 30 minutes at 4°C. After washing, 3 ug/ml goat anti-mouse IL-15Rα (catalog #AF551, R&D Systems) was added to cells for 30 minutes at 4°C. The cells were then washed two times with PBS, and then incubated with 10 ug/ml PE-rabbit anti-goat IgG (Jackson Immunoresearch), washed, and then analyzed on a BectonDickinson FACScan using FloJo software (TreeStar).
2.12 Anti-TL staining of BMDCs
BMDCs (prepared as described above) were mock transduced or transduced with either LV-GFP or LV-SOCS1, incubated overnight with or without LPS (100 ng/ml), and washed with PBS. The cells were incubated first with 5% mouse serum, then anti-TL antibody (rat IgG2a, prepared from mouse hybridoma cell line HD168 [ATCC #HB-252], which we received as a generous gift from Dr. Lloyd Old at the Ludwig Institute for Cancer Research-New York Branch [Memorial Sloan-Kettering Cancer Center]). HD168 staining was followed by incubation with biotinylated F(ab′)2 fragment of mouse anti-rat IgG (Jackson Immunoresearch) and streptavidin-PECy5 (Pharmingen), and analyzed on a BectonDickinson FACScan using FloJo software (TreeStar).
2.13 Statistical Analysis
For statistical analysis, we used t′ (Welch’s approximate t) with the critical value of Student’s t, or the Mann-Whitney (rank) test for non-parametric testing (Fig. 5). Results are typically presented as means ± standard errors (SE).
Figure 5.
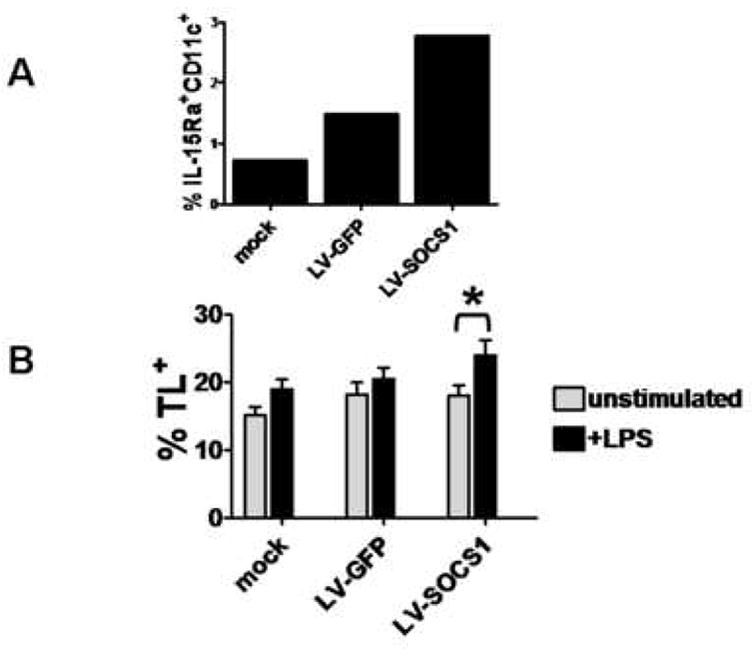
A) Percentages of CD11c+mIL-15Rα+ BMDCs for mock transfected BMDCs, LV-GFP-siRNA-DCs, and LV-SOCS1-siRNA-DCs. Representative of two trials. B) Percentages of CD11c+TL+ BMDCs, with and without LPS (100 ng/ml) stimulation. Data from three separate trials. *p<0.0025.
3. Results
3.1 Vaccination with SOCS1-downregulated BMDCs induced memory anti-OVA serum antibody responses
Mice were inoculated with LV-GFP-siRNA-DCs, LV-SOCS1-siRNA-DCs, or mock transfected BMDCs twice in two weeks, followed by LPS or poly I:C injections on days 1, 3 and 5 after each vaccination. Two to four months later, mice were boosted with the ovalbumin peptides, OT-I and OT-II, mixed with Freund’s Incomplete Adjuvant (Fig. 1). Two weeks later, serum samples were analyzed for IgG1 and IgG2a antibodies to ovalbumin (OVA). Mice vaccinated with LV-SOCS1-siRNA-DCs showed significantly higher levels of both IgG1 and IgG2a antibodies to OVA (Fig. 2), indicating that these mice had developed more memory cells (both Th1-directing and Th2-directing CD4+ cells) than the mice vaccinated with LV-GFP-siRNA-DCs or mock transfected BMDCs.
Figure 1.
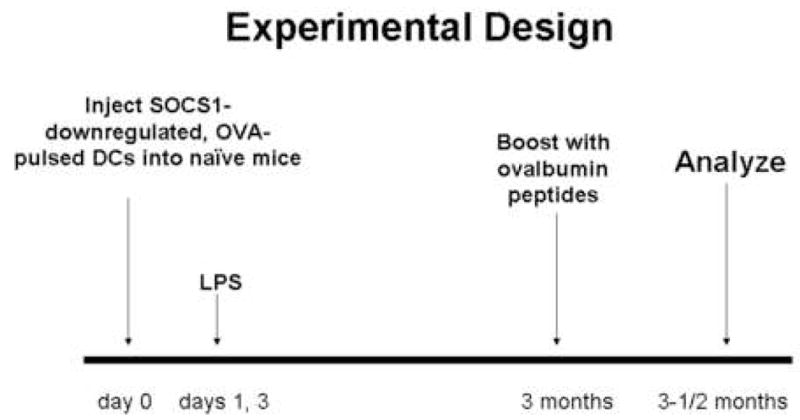
Inoculation scheme for generating ovalbumin-specific memory T cell responses. C57BL/6 mice (female, 6–8 weeks old) were inoculated via footpad with LV-SOCS1-siRNA-DCs, LV-GFP-siRNA-DCs, or mock transfected DCs on days 0 and 7. LPS (30 ug/mouse) was injected i.p. on days 1, 3, 8, and 10. Two to four months later, the mice were boosted with the ovalbumin peptides, OT-1 and OT-II (100 μg/mouse each), and two weeks after this boost, were analyzed for memory responses.
Figure 2.
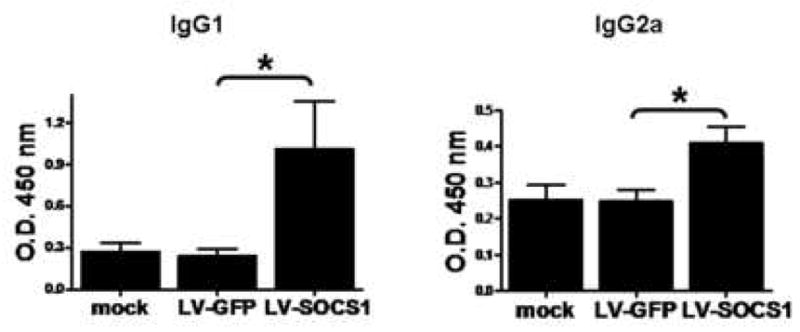
Levels of IgG1 and IgG2a anti-ovalbumin antibodies in vaccinated mouse serum. Four months after initial vaccination, serum samples were analyzed by ELISA for IgG1 and IgG2a (representing Th 2 and Th 1 responses, respectively) anti-ovalbumin antibodies. n=3, *p<0.05.
3.2 Vaccination with SOCS1-downregulated BMDCs enhanced OVA-specific CD8+ memory cells
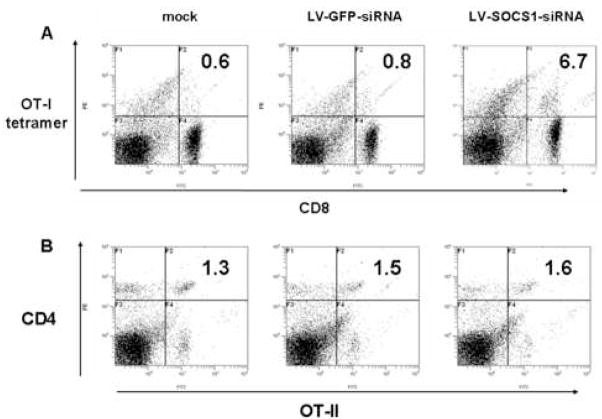
Using the same vaccination and boosting strategy described above, we identified more CD8+OT-1 tetramer+ cells in spleen of mice inoculated with LV-SOCS1-siRNA-DCs as compared to mice receiving mock transfected BMDCs or LV-GFP-siRNA-DCs (Fig. 3A). We did not detect more CD4+OT-2+ cells in the same mice, though (Fig. 3B). Since IL-7R and CD8αα have been reported to be early markers of memory CD8+ T cells [26–31], IL-7R and CD8aa expression were tracked using flow cytometry on CD8+ T cells after the initial inoculation. No increases in CD8+IL-7R+ or CD8αα+ cells were detected in blood or spleen in any of the mice we tested over the first 14 days after the second initial vaccination (data not shown).
Figure 3.
Flow cytometric analysis of antigen-specific CD8+ and CD4+ cells in spleen four months after initial BMDC vaccination. Percentages of CD8+ OT-1 tetramer+ or CD4+OT-2+ cells are shown in histograms. Results shown are representative of three trials.
3.3 Vaccination with SOCS1-downregulated BMDCs enhanced memory IFN-γ production
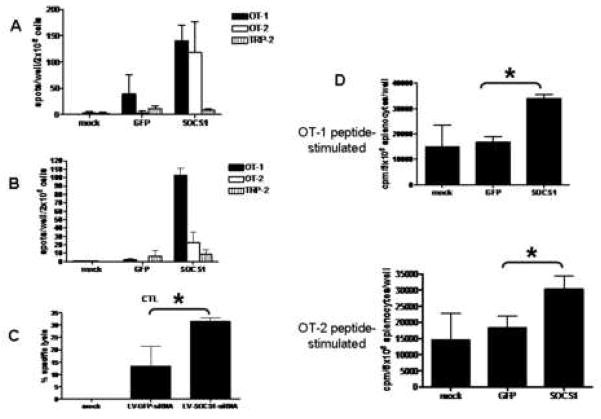
IFN-γ ELISPOT activity of splenocytes from mice vaccinated with LV-SOCS1-siRNA-DCs showed a significantly enhanced response by both OT-I and OT-2 peptide-stimulated splenic T cells, at two months and four months after the initial vaccinations (Figs. 4A and B).
Figure 4.
A) IFN-γ ELISPOT results of splenocytes from mice two months after vaccination. B) IFN-γ ELISPOT results of splenocytes from mice four months after vaccination. n=3–4 mice for each group. C) Percent lysis of target cells (100:1 target:effector ratio) in CTL assay of splenocytes from mice four months after vaccination. n=3 mice per group. *p<0.05. D) Proliferation of peptide-stimulated splenocytes from mice four months after vaccination. cpm/5×105 splenocytes, n=3–4 mice per group, *p<0.05.
3.4 Vaccination with SOCS1-downregulated BMDCs enhanced memory CTL activity
CTL activity directed against the mouse lymphoma cell line, EG7 (EL4 mouse lymphoma line stably transfected with ovalbumin), was significantly increased in splenocytes from mice vaccinated with LV-SOCS1-siRNA-DCs four months before the assay, as compared to controls (Fig. 4C).
3.5 OT-1- and OT-2-stimulated memory splenic T-cell proliferation was significantly higher in mice vaccinated with LV-SOCS1-siRNA-DC
Splenocytes from mice vaccinated with LV-SOCS1-siRNA-DCs four months before the proliferation assay proliferated at a higher rate than splenocytes from control vaccinated mice when stimulated with OT-1 or OT-2 peptide (Fig. 4D). This result indicated a very long-term memory response in LV-SOCS1-siRNA-DC-vaccinated mice.
3.6 LV-SOCS1-siRNA-DCs expressed higher levels of surface mIL-15Ra than LV-GFP-siRNA-DCs and nontransfected DCs
IL-15 contributes to memory T cell development [7, 8]. IL-15 complexed to IL-15Rα is expressed on DC cell surfaces. This IL-15/IL-15Rα complex then can interact with IL-2Rγ on T cells to enable IL-15-directed signaling for growth, proliferation, and memory cell formation [7]. We analyzed surface levels of IL-15Ra on LV-SOCS1-siRNA-DCs using flow cytometry. Cultured LV-SOCS1-siRNA-DCs exhibited higher expression of IL-15Rα than control DCs after in vitro LPS stimulation (Fig. 5A).
3.7 LV-SOCS1-siRNA-DCs expressed higher levels of surface TL than LV-GFP-siRNA-DCs and nontransfected DCs
TL (thymic leukemia antigen), a nonclassical major histocompatibility complex (MHC) class I molecule, expressed on antigen presenting cells (APCs) is a ligand for CD8αα on T cells. Interaction of the two molecules triggers signaling that results in a CD8+ T cell becoming a memory T cell [29]. We analyzed levels of TL on DCs using an anti-TL antibody and flow cytometry, and we found that LV-SOCS1-siRNA-DCs, upon stimulation with LPS, increased expression of surface TL significantly more than control DCs (Fig. 5B).
Discussion
In this study, murine bone marrow dendritic cells in which SOCS1 was stably downregulated by lentiviral-encoded siRNA directed generation of enhanced memory T-cell responses. CD8+ OT-1 tetramer+ memory cells appeared in greater percentages in blood of mice vaccinated with LV-SOCS1-siRNA-DCs, compared to control DCs. Anti-ovalbumin antibody titers were higher in the LV-SOCS1-siRNA-DC-treated mice, as well. Memory T cells from LV-SOCS1-siRNA-DC-vaccinated mice proliferated in response to antigen stimulation at a greater rate than those from control mice. ELISPOT analysis showed greatly increased numbers of IFN-γ-producing memory cells, and CTL analysis displayed enhanced targeted lysis activity by memory cells, as well. All of these data indicated enhanced memory responses due to SOCS1 downregulation in DCs.
SOCS1 and other family members play important roles in immune cell responses [22]. SOCS1 is particularly important for T cell homeostasis regulation [12]. For instance, SOCS1 regulates IL-7 and IL-15 functions during T lymphocyte development and homeostasis [18]. IL-7 is essential for CD4+ and CD8+ memory cell generation [32], and IL-15 is a requirement for regulating CD8+-cell homeostasis (Tan 34). Additionally, IL-15 is critical for proliferation and survival of peripheral memory CD8+ T cells [33]. IL-15Rα and IL-15 are coordinately expressed, and IL-15Rα on DCs presents IL-15 in trans to responding T cells that express IL-2/15Rβ and γ [7, 34]. When we analyzed IL-15Rα surface expression on LV-SOCS1-siRNA-DCs using flow cytometry, these cells exhibited elevated IL-15Rα levels compared to control DCs. These cells may then present IL-15 to memory CD8+ T cells to promote proliferation and long-term survival.
Previous work has shown that IL-12 signaling is enhanced when SOCS1 is downregulated in T cells and DCs [17, 35]. IL-12 is required for induction but not maintenance of memory T1 (type 1 T cell) responses [24]. Based on our data, we suspect that LV-SOCS1-siRNA-DCs produced more IL-12 in a feedback loop-enhanced manner to help induce the memory responses we saw.
Blood levels of CD8+OT-1 tetramer+ cells were increased in LV-SOCS1-siRNA-DC-vaccinated mice, but levels of CD4+OT-2+ cells were not, in our studies. The increased anti-ovalbumin antibody response, though, would suggest at least a memory CD4+ T cell functional increase. Our observation of CD8+, but not CD4+, antigen-specific memory T cells may have a basis in the effects of SOCS1 downregulation on T cells. Increased IL-12 signaling (which could result from SOCS1 downregulation) can contribute to an increased CD8+ number in thymus [36], and decreased CD4:CD8 cell ratios were observed in SOCS1−/−IFNγ−/− mice, as well as SOCS1−/−STAT4−/− mice (that have no IL-12 signaling)[35], so perhaps perturbation of T cell ratios can occur when SOCS1 is missing only from DCs. Also, not all CD4+ memory T cells are long-lived [37], so we may have been able to detect the effects of CD4+ memory cells, but not the cells themselves, at 14 days after antigenic rechallenge.
Our attempts to track early memory cell development after initial vaccination by using IL-7R and CD8αα as markers in vivo were not successful. Although studies using LCMV-infected mice have shown that these two molecules can mark CD8+ memory cell precursors [26–28, 31], other studies using peptide-pulsed DCs or peptide immunization have shown that IL-7R is not a marker for such precursors [38–41]. Antigen-presenting cells expressing thymic leukemia antigen TL can boost CD8αα expression by T cells, and CD8αα expression induces IL-7Rα expression [29]. Our work showed elevated expression of TL on LV-SOCS1-siRNA-DCs compared to control DCs, so we would expect to see increased expression of IL-7R and CD8αα. Perhaps in vitro studies could show this. The in vivo detection method we used may have been too insensitive, or the time course for memory cell generation of our peptide-pulsed DC vaccination strategy may differ from that of virus-induced memory T cells. Further studies could elucidate this discrepancy.
SOCS1 downregulation in DCs may have quantitatively different effects on Th1 and Th2 antibody production as compared to Th1 and Th2 cell effector functions, such as IFN-γ production and proliferation in response to Th1 vs. Th2 (OT-1 or OT-2 peptide) stimulation. Our results show a greater Th2 antibody response, similar Th1 and Th2 IFN-γ production at two months post-immunization, greater Th1 versus Th2 IFN-g production at four months post-immunization, yet similar Th1 and Th2-stimulated T cell proliferation. SOCS1 downregulation in DC, then, certainly may affect Th1 and Th2 responses differently. Th1 and Th2 antibody and cell effector functions may also peak at different timepoints, which we did not investigate in this study. Intracellular staining of Th1 and Th2 cytokines, together with staining for long-term memory/effector T cell markers, such as CCR7, CD62L, CD27, and CD28, would help establish Th1 and Th2 differences more specifically.
The differences seen in extracellular expression of TL and IL-15Ra were small, but consistent. It is probable that only a small percentage of the SOCS1-downregulated DCs direct production of memory T cells, and these DCs may be the only ones to express TL and/or IL-15/IL-15Ra. Also, our measurements were conducted on in vitro cultured DCs, which may not receive the benefit of other, in vivo, effectors that help develop the memory response, so fewer TL/IL-15Ra-expressing DCs could result in our in vitro system.
In summary, our work supports a crucial role for SOCS1 in regulation of dendritic cell-directed generation of memory T cell responses. These results have important implications for future vaccine design.
Acknowledgments
We thank the members of Chen lab for technical assistance and valuable suggestions. This work was supported by grants from the National Institute of Health (T32-AI007495, R01AI68472, and R01CA116677).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Swain SL, Agrewala JN, Brown DM, Jelley-Gibbs DM, Golech S, Huston G, et al. CD4+ T-cell memory: generation and multi-faceted roles for CD4+ T cells in protective immunity to influenza. Immunol Rev. 2006;211:8–22. doi: 10.1111/j.0105-2896.2006.00388.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lefrancois L. Development, trafficking, and function of memory T-cell subsets. Immunol Rev. 2006;211:93–103. doi: 10.1111/j.0105-2896.2006.00393.x. [DOI] [PubMed] [Google Scholar]
- 3.Williams MA, Holmes BJ, Sun JC, Bevan MJ. Developing and maintaining protective CD8+ memory T cells. Immunol Rev. 2006;211:146–53. doi: 10.1111/j.0105-2896.2006.00389.x. [DOI] [PubMed] [Google Scholar]
- 4.Dooms H, Wolslegel K, Lin P, Abbas AK. Interleukin-2 enhances CD4+ T cell memory by promoting the generation of IL-7R alpha-expressing cells. J Exp Med. 2007;204(3):547–57. doi: 10.1084/jem.20062381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Surh CD, Boyman O, Purton JF, Sprent J. Homeostasis of memory T cells. Immunol Rev. 2006;211:154–63. doi: 10.1111/j.0105-2896.2006.00401.x. [DOI] [PubMed] [Google Scholar]
- 6.Bradley LM, Haynes L, Swain SL. IL-7: maintaining T-cell memory and achieving homeostasis. Trends Immunol. 2005;26(3):172–6. doi: 10.1016/j.it.2005.01.004. [DOI] [PubMed] [Google Scholar]
- 7.Burkett PR, Koka R, Chien M, Chai S, Boone DL, Ma A. Coordinate expression and trans presentation of interleukin (IL)-15Ralpha and IL-15 supports natural killer cell and memory CD8+ T cell homeostasis. J Exp Med. 2004;200(7):825–34. doi: 10.1084/jem.20041389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sato N, Patel HJ, Waldmann TA, Tagaya Y. The IL-15/IL-15Ralpha on cell surfaces enables sustained IL-15 activity and contributes to the long survival of CD8 memory T cells. Proc Natl Acad Sci U S A. 2007;104(2):588–93. doi: 10.1073/pnas.0610115104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Li Q, Eppolito C, Odunsi K, Shrikant PA. IL-12-programmed long-term CD8+ T cell responses require STAT4. J Immunol. 2006;177(11):7618–25. doi: 10.4049/jimmunol.177.11.7618. [DOI] [PubMed] [Google Scholar]
- 10.Ilangumaran S, Ramanathan S, Ning T, La Rose J, Reinhart B, Poussier P, et al. Suppressor of cytokine signaling 1 attenuates IL-15 receptor signaling in CD8+ thymocytes. Blood. 2003;102(12):4115–22. doi: 10.1182/blood-2003-01-0175. [DOI] [PubMed] [Google Scholar]
- 11.Ilangumaran S, Ramanathan S, La Rose J, Poussier P, Rottapel R. Suppressor of cytokine signaling 1 regulates IL-15 receptor signaling in CD8+CD44high memory T lymphocytes. J Immunol. 2003;171(5):2435–45. doi: 10.4049/jimmunol.171.5.2435. [DOI] [PubMed] [Google Scholar]
- 12.Cornish AL, Chong MM, Davey GM, Darwiche R, Nicola NA, Hilton DJ, et al. Suppressor of cytokine signaling-1 regulates signaling in response to interleukin-2 and other gamma c-dependent cytokines in peripheral T cells. J Biol Chem. 2003;278(25):22755–61. doi: 10.1074/jbc.M303021200. [DOI] [PubMed] [Google Scholar]
- 13.Gagnon J, Ramanathan S, Leblanc C, Ilangumaran S. Regulation of IL-21 signaling by suppressor of cytokine signaling-1 (SOCS1) in CD8(+) T lymphocytes. Cell Signal. 2007;19(4):806–16. doi: 10.1016/j.cellsig.2006.10.003. [DOI] [PubMed] [Google Scholar]
- 14.Wormald S, Zhang JG, Krebs DL, Mielke LA, Silver J, Alexander WS, et al. The comparative roles of suppressor of cytokine signaling-1 and -3 in the inhibition and desensitization of cytokine signaling. J Biol Chem. 2006;281(16):11135–43. doi: 10.1074/jbc.M509595200. [DOI] [PubMed] [Google Scholar]
- 15.Ding Y, Chen D, Tarcsafalvi A, Su R, Qin L, Bromberg JS. Suppressor of cytokine signaling 1 inhibits IL-10-mediated immune responses. J Immunol. 2003;170(3):1383–91. doi: 10.4049/jimmunol.170.3.1383. [DOI] [PubMed] [Google Scholar]
- 16.Dickensheets H, Vazquez N, Sheikh F, Gingras S, Murray PJ, Ryan JJ, et al. Suppressor of cytokine signaling-1 is an IL-4-inducible gene in macrophages and feedback inhibits IL-4 signaling. Genes Immun. 2007;8(1):21–7. doi: 10.1038/sj.gene.6364352. [DOI] [PubMed] [Google Scholar]
- 17.Evel-Kabler K, Song XT, Aldrich M, Huang XF, Chen SY. SOCS1 restricts dendritic cells’ ability to break self tolerance and induce antitumor immunity by regulating IL-12 production and signaling. J Clin Invest. 2006;116(1):90–100. doi: 10.1172/JCI26169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ramanathan S, Gagnon J, Leblanc C, Rottapel R, Ilangumaran S. Suppressor of cytokine signaling 1 stringently regulates distinct functions of IL-7 and IL-15 in vivo during T lymphocyte development and homeostasis. J Immunol. 2006;176(7):4029–41. doi: 10.4049/jimmunol.176.7.4029. [DOI] [PubMed] [Google Scholar]
- 19.Zimmerer JM, Lesinski GB, Kondadasula SV, Karpa VI, Lehman A, Raychaudhury A, et al. IFN-alpha-induced signal transduction, gene expression, and antitumor activity of immune effector cells are negatively regulated by suppressor of cytokine signaling proteins. J Immunol. 2007;178(8):4832–45. doi: 10.4049/jimmunol.178.8.4832. [DOI] [PubMed] [Google Scholar]
- 20.Kobayashi T, Yoshimura A. Keeping DCs awake by putting SOCS1 to sleep. Trends Immunol. 2005;26(4):177–9. doi: 10.1016/j.it.2005.02.004. [DOI] [PubMed] [Google Scholar]
- 21.Waiboci LW, Ahmed CM, Mujtaba MG, Flowers LO, Martin JP, Haider MI, et al. Both the suppressor of cytokine signaling 1 (SOCS-1) kinase inhibitory region and SOCS-1 mimetic bind to JAK2 autophosphorylation site: implications for the development of a SOCS-1 antagonist. J Immunol. 2007;178(8):5058–68. doi: 10.4049/jimmunol.178.8.5058. [DOI] [PubMed] [Google Scholar]
- 22.Alexander WS, Hilton DJ. The role of suppressors of cytokine signaling (SOCS) proteins in regulation of the immune response. Annu Rev Immunol. 2004;22:503–29. doi: 10.1146/annurev.immunol.22.091003.090312. [DOI] [PubMed] [Google Scholar]
- 23.Shen L, Evel-Kabler K, Strube R, Chen SY. Silencing of SOCS1 enhances antigen presentation by dendritic cells and antigen-specific anti-tumor immunity. Nat Biotechnol. 2004;22(12):1546–53. doi: 10.1038/nbt1035. [DOI] [PubMed] [Google Scholar]
- 24.Wuthrich M, Warner T, Klein BS. IL-12 is required for induction but not maintenance of protective, memory responses to Blastomyces dermatitidis: implications for vaccine development in immune-deficient hosts. J Immunol. 2005;175(8):5288–97. doi: 10.4049/jimmunol.175.8.5288. [DOI] [PubMed] [Google Scholar]
- 25.Berard M, Brandt K, Bulfone-Paus S, Tough DF. IL-15 promotes the survival of naive and memory phenotype CD8+ T cells. J Immunol. 2003;170(10):5018–26. doi: 10.4049/jimmunol.170.10.5018. [DOI] [PubMed] [Google Scholar]
- 26.Kaech SM, Tan JT, Wherry EJ, Konieczny BT, Surh CD, Ahmed R. Selective expression of the interleukin 7 receptor identifies effector CD8 T cells that give rise to long-lived memory cells. Nat Immunol. 2003;4(12):1191–8. doi: 10.1038/ni1009. [DOI] [PubMed] [Google Scholar]
- 27.Liu N, Phillips T, Zhang M, Wang Y, Opferman JT, Shah R, et al. Serine protease inhibitor 2A is a protective factor for memory T cell development. Nat Immunol. 2004;5(9):919–26. doi: 10.1038/ni1107. [DOI] [PubMed] [Google Scholar]
- 28.Wang XZ, Brehm MA, Welsh RM. Preapoptotic phenotype of viral epitope-specific CD8 T cells precludes memory development and is an intrinsic property of the epitope. J Immunol. 2004;173(8):5138–47. doi: 10.4049/jimmunol.173.8.5138. [DOI] [PubMed] [Google Scholar]
- 29.Madakamutil LT, Christen U, Lena CJ, Wang-Zhu Y, Attinger A, Sundarrajan M, et al. CD8alphaalpha-mediated survival and differentiation of CD8 memory T cell precursors. Science. 2004;304(5670):590–3. doi: 10.1126/science.1092316. [DOI] [PubMed] [Google Scholar]
- 30.Kim SV, Flavell RA. Immunology. CD8alphaalpha and T cell memory. Science. 2004;304(5670):529–30. doi: 10.1126/science.1097678. [DOI] [PubMed] [Google Scholar]
- 31.Huster KM, Busch V, Schiemann M, Linkemann K, Kerksiek KM, Wagner H, et al. Selective expression of IL-7 receptor on memory T cells identifies early CD40L-dependent generation of distinct CD8+ memory T cell subsets. Proc Natl Acad Sci U S A. 2004;101(15):5610–5. doi: 10.1073/pnas.0308054101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Schluns KS, Kieper WC, Jameson SC, Lefrancois L. Interleukin-7 mediates the homeostasis of naive and memory CD8 T cells in vivo. Nat Immunol. 2000;1(5):426–32. doi: 10.1038/80868. [DOI] [PubMed] [Google Scholar]
- 33.Kennedy MK, Glaccum M, Brown SN, Butz EA, Viney JL, Embers M, et al. Reversible defects in natural killer and memory CD8 T cell lineages in interleukin 15-deficient mice. J Exp Med. 2000;191(5):771–80. doi: 10.1084/jem.191.5.771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Waldmann TA. The biology of interleukin-2 and interleukin-15: implications for cancer therapy and vaccine design. Nat Rev Immunol. 2006;6(8):595–601. doi: 10.1038/nri1901. [DOI] [PubMed] [Google Scholar]
- 35.Eyles JL, Metcalf D, Grusby MJ, Hilton DJ, Starr R. Negative regulation of interleukin-12 signaling by suppressor of cytokine signaling-1. J Biol Chem. 2002;277(46):43735–40. doi: 10.1074/jbc.M208586200. [DOI] [PubMed] [Google Scholar]
- 36.Godfrey DI, Kennedy J, Gately MK, Hakimi J, Hubbard BR, Zlotnik A. IL-12 influences intrathymic T cell development. J Immunol. 1994;152(6):2729–35. [PubMed] [Google Scholar]
- 37.Robertson JM, MacLeod M, Marsden VS, Kappler JW, Marrack P. Not all CD4+ memory T cells are long lived. Immunol Rev. 2006;211:49–57. doi: 10.1111/j.0105-2896.2006.00383.x. [DOI] [PubMed] [Google Scholar]
- 38.Lacombe MH, Hardy MP, Rooney J, Labrecque N. IL-7 receptor expression levels do not identify CD8+ memory T lymphocyte precursors following peptide immunization. J Immunol. 2005;175(7):4400–7. doi: 10.4049/jimmunol.175.7.4400. [DOI] [PubMed] [Google Scholar]
- 39.Klonowski KD, Williams KJ, Marzo AL, Lefrancois L. Cutting edge: IL-7-independent regulation of IL-7 receptor alpha expression and memory CD8 T cell development. J Immunol. 2006;177(7):4247–51. doi: 10.4049/jimmunol.177.7.4247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sun JC, Lehar SM, Bevan MJ. Augmented IL-7 signaling during viral infection drives greater expansion of effector T cells but does not enhance memory. J Immunol. 2006;177(7):4458–63. doi: 10.4049/jimmunol.177.7.4458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Buentke E, Mathiot A, Tolaini M, Di Santo J, Zamoyska R, Seddon B. Do CD8 effector cells need IL-7R expression to become resting memory cells? Blood. 2006;108(6):1949–56. doi: 10.1182/blood-2006-04-016857. [DOI] [PubMed] [Google Scholar]