Abstract
Recent studies have implicated the lipid mediator platelet-activating factor (PAF) in UVB-mediated systemic immunosuppression known to be a major cause for skin cancers. Previously, our group has demonstrated that UVB irradiation triggers the production of PAF and oxidized glycerophosphocholines that act as PAF-receptor (PAF-R) agonists. The present studies explored the mechanisms by which UVB generates PAF-R agonists. UVB irradiation of human epidermal KB cells resulted in both increased levels of reactive oxygen species (ROS) and PAF-R agonistic activity. Pretreatment of KB cells with antioxidants vitamin C and N-acetylcysteine or the pharmacological inhibitor PD168393 specific for the epidermal growth factor receptor all inhibited UVB-induced ROS as well as PAF-R agonists, yet had no effect on fMLP-mediated PAF-R agonist production. In addition, in vivo production of PAF-R agonists from UVB-irradiated mouse skin was blocked by both systemic vitamin C administration and topical PD168393 application. Moreover, both vitamin C and PD168393 abolished UVB-mediated but not the PAF-R agonist 1-hexadecyl-2-N-methylcarbamoyl glycerophosphocholine-mediated immunosuppression as measured by the inhibition of delayed type contact hypersensitivity to the chemical dinitrofluorobenzene. These studies suggest that UVB-induced systemic immunosuppression is due to epidermal growth factor receptor-mediated ROS which results in PAF-R agonist formation.
Ultraviolet B (290–320 nm) radiation found in sunlight has a spectrum of profound effects on human skin ranging from vitamin D metabolism to skin aging and carcinogenesis (1, 2). UVB exposure is believed to be the major cause for nonmelanoma skin cancer, the most common type of human cancer (3, 4). In addition to its ability to damage DNA, UVB is well known to exert an immunosuppressive effect via inhibiting cell-mediated immune responses that are indispensable for antitumor immunity (5, 6). As such, effort has been put forth to study the mechanisms underlying UVB-induced immunosuppression, and contact allergy to chemical haptens such as dinitrofluorobenzene (DNFB)3 has been widely used as a model (7).
Platelet-activating factor (1-alkyl-2-acetyl glycerophosphocholine, PAF) is a potent inflammatory lipid mediator, exerting its effects through a single specific G-protein-coupled receptor, the PAF receptor (8). PAF is synthesized enzymatically in response to diverse stimuli including cytokines, endotoxin, Ca2+ ionophores, and PAF itself (8–10). In addition, PAF and sn-2 short-chained acyl glycerophosphocholines (GPCs) with PAF-receptor (PAF-R) agonistic activity can also be produced through free radical-mediated damage (11). In contrast, PAF-acetylhydrolase (PAF-AH) inactivates PAF and oxidized GPCs by hydrolyzing the acetyl oxidatively modified sn-2 group of the glycerol backbone (12). Previous studies by our group (13–17) and others (18–20) have indicated that PAF plays pivotal roles in mediating not only the acute UVB-induced cytokine and apoptotic responses, but also UVB-mediated systemic immunosuppression. The latter was supported by several lines of evidence. First, PAF and PAF-like species are quickly synthesized by keratinocytes upon UVB-irradiation of skin keratinocytes (19). Second, intraperitoneal injection of PAF, PAF-R agonist 1-hexadecyl-2-N-methylcarbamoyl glycerophosphocholine (CPAF), or UVB-irradiated glycerophosphocholine all mimicked the immunosuppressive effect of UVB (18, 21). Third, UVB-induced immunosuppression was completely abolished in mice deficient in PAF-R or in wild-type mice pretreated with PAF-R antagonist (13, 18, 20). Given the importance of the PAF system in mediating UVB-induced systemic immunosuppression, it is critical to investigate how UVB stimulates PAF production.
Biological effects of UV irradiation occur as a consequence of absorption of electromagnetic energy by certain molecules within cells. The photochemical activation of molecular oxygen generates reactive oxygen species (ROS) that mediate many UV irradiation-induced responses including apoptosis (22–24). Interestingly, activation of the epidermal growth factor receptor (EGF-R) by UVB also results in the formation of ROS that subsequently induces the growth arrest and DNA damage-inducible gene (GADD45) (25). Moreover, a possible role of ROS in mediating the immunosuppressive effect of UVB has also been suggested as topical application of antioxidants such as N-acetylcysteine (NAC) and vitamins C and E abrogated UV-induced systemic immunosuppression (26–28). However, the underlying molecular mechanisms remain largely unknown.
In the present study, we demonstrate that UVB irradiation generates PAF-R agonists via induction of ROS both in vitro and in vivo. In addition, the EGF-R inhibitor PD168393 blocked both UVB-induced ROS and PAF-R agonists. More importantly, systemic administration of vitamin C or topical application of PD168393 abolished UVB-evoked but not CPAF-mediated systemic immunosuppression in C57BL/6 mice. Taken together, these studies suggest that UVB-induced systemic immunosuppression is due to EGF-R-mediated ROS which results in PAF-R agonist formation.
Materials and Methods
Reagents and UVB irradiation source
All chemicals were obtained from Sigma-Aldrich unless indicated otherwise. PD168393, a specific EGF-R inhibitor (25, 29), and diphenylene iodinium (DPI; NADPH oxidase inhibitor) were from Calbiochem. The pan-caspase inhibitor z-VAD-FMK was from Promega. The UV source was a Philips F20T12/UV-B lamp (270–390 nm; containing 2.6% UVC, 43.6% UVB, and 53.8% UVA). The intensity of the UVB source was measured before each experiment using an IL1700 radiometer and a SED240 UVB detector (International Light) at a distance of 8 cm from the UVB source to the monolayer of cells/mice. All chemicals used in the irradiation protocols were first tested to ensure there was no ability to absorb UVB (i.e., sunblock effect) by testing the intensity of UVB (as measured by detector) irradiation underneath a Kodacel membrane with/without application of the dose used in the in vitro or in vivo protocol.
Cells and mice
The human epidermoid cell line KB cells were grown in DMEM (Life Technologies) supplemented with 10% FBS (Intergen), 2 mM l-glutamine, and 100 µg/ml penicillin and streptomycin. A KB PAF-R model system was created by transduction of PAF-R-negative KB cells with the MSCV 2.1 retrovirus encoding the human leukocyte PAF receptor as described previously (15). KB cells stably transduced with the PAF receptor (designated as KBP cells) or with the fMLP receptor (designated as KBF cells) or control MSCV2.1 retrovirus (defined as KBM cells) were characterized by Southern blot, Northern blot, radioligand binding and by calcium transient studies that demonstrate the presence of a functional PAF or fMLP receptor signaling system in these cells.
C57BL/6 and SKH-1 hairless albino mice (age 6–8 wk), purchased from The Jackson Laboratory and Charles Rivers Laboratories, respectively, were housed under specific pathogen-free conditions at the Indiana University School of Medicine. All procedures were approved by the Animal Care and Use Committee of Indiana University School of Medicine.
Lipid extraction and PAF-R activity measurement
Cells grown on 10-cm plates were fed with 5 µM 1-hexadecyl-2-arachidonoyl-sn-glycero-3-phosphocholine (Avanti) overnight before being irradiated with UVB as previously described (14). Following UVB or fMLP treatment, the reactions were quenched with ice-cold methanol and total lipids extracted by the method of Bligh and Dyer (30). In some experiments, the lipid extract was treated with PAF-AH (10 mg), phospholipase A1 (PLA1, 5 mg), or PBS overnight at 37°C, and then lipids were re-extracted. The presence of PAF-R agonists in the lipid extracts were measured by their ability to induce an intracellular Ca2+ mobilization response in KBP cells as previously described (31). In brief, KBP cells were preloaded with the Ca2+-sensitive indicator, fura-2-AM (2 µM in HBSS) at 37°C for 90 min, followed by washing and resuspending and were maintained in HBSS at room temperature before use. Lipid extracts dissolved in ethanol (adjusted to 2.5 × 106 cells/20 µl) were added to an aliquot of KBP cells (1.0–1.5 × 106 cells/2 ml) in a cuvette at 37°C with constant stirring. Fura-2-AM fluorescence was monitored in a Hitachi F-4010 spectrophotometer with excitation and emission wavelengths of 331 and 410 nm, respectively. The Ca2+ influx was calculated as described (32) and shown as percentage of maximal peak calcium flux induced by 1 µM CPAF.
To measure the production of PAF-R agonist in vivo, shaved back skin of female C57BL/6 mice was UVB irradiated at 7500 J/m2 under anesthesia. One hour post UVB, mice were euthanized and the epidermal part of ~2 1.5 × 3 cm areas of shaved skin was scraped off using a curette after having any residual hairs further removed by depilatory agent (Nair) and the skin frozen with liquid nitrogen. The residual scraped skin was fixed in formalin and embedded in paraffin and H&E-stained sections were examined histologically to verify removal of the epidermis. Scraped epidermal tissue specimens were weighed and lipids extracted, after adding 0.1 N HCl to Nair-treated samples to adjust pH <4. PAF-R agonistic activity was measured as above and normalized by tissue weight. In some experiments, mice were treated with topical application of 50 µl of 4 mM PD168393, or vehicle alone (10% DMSO in ethanol) for 30 min before UVB irradiation. In other experiments, mice were given chow containing 10g/kg vitamin C (Research Diets) ad libitum for 10 days (33) before UVB irradiation. In SKH-1 hairless mice, PAF-R agonist production from UVB irradiated back skin was measured similarly as in C57BL/6 mice, albeit at a lower UVB dose of 1500 J/m2 without back shaving and Nair treatment.
ROS measurement
Intracellular levels of ROS were analyzed by flow cytometry using CMH2DCFDA (Invitrogen) as a fluorescent dye probe (34). Cells loaded with CM-H2DCFDA (5 µM, 30 min) were UVB irradiated after a recovery time of 45 min. In some experiment, cells were pretreated 30 min with vitamin C (2.5 mM), NAC (5 mM), z-VAD-FMK (10 µM), PD168393 (10 µM), or DPI (10 µM) before UVB exposure. Cells without dye loading were used as negative controls. Flow cytometric analysis was performed using a FACSCalibur and data were analyzed using CellQuest software (BD Biosciences) and presented as mean fluorescent intensity.
Contact hypersensitivity (CHS) reactions
CHS to DNFB was conducted as previously described (21) with minor modifications. In brief, to evaluate the effect of CPAF on sensitization reactions, mice were injected i.p. with 50 µl CPAF (250 ng), or 50 µl BSA vehicle alone. After 5 days, the back skin of mouse was shaved and 25 µl of 0.5% DNFB in acetone: olive oil (4:1, v/v) was applied to an area ~1 × 1 cm. Nine days later, one of the dorsal sides of ears was challenged with painting of 10 µl 0.5% DNFB and the other ear painted with vehicle. After 24 h, 5 mm punch biopsies were obtained from the ears and weighed. Our previous studies have demonstrated that measurement of weights of punch biopsies from ears correlated with measurement of ear thickness with calipers (35), and our laboratory prefers this methodology due to greater reproducibility in our hands. For studies assessing the ability of UVB to affect CHS, the back skin of mice was shaved, and the anesthetized mouse was draped to allow an area of 2.5 × 2.5 cm of distal back skin exposed to a single dose of UVB irradiation (7500 J/m2). This dose is comparable to those used by other groups to induce systemic immunosuppression in C57BL/6 mice (18, 36, 37). Five days later, an area of 1 × 1 cm of unirradiated back skin ~3 cm cephalic was sensitized with DNFB as outlined above. In some experiments, mice were pretreated with PAF-AH (5 mg/kg body weight, i.p.) or PD168393 (4 mM, 50 µl, topical painting of shaved back skin) or vehicle 30 min before CPAF treatment or UVB irradiation. To assess the effect of antioxidant on UVB- vs CPAF-mediated inhibition of CHS, mice were fed with regular chow or vitamin C-enriched (10 g/kg) chow for 10 days before experiments. In all experiments, vehicle or sham control groups were used as positive controls for CHS.
Statistical analysis
In the present study, at least five mice/group was used in all murine experiments. Differences between experimental and control groups were examined by a two-tailed Student’s t test. Statistical significance was defined as a p value <0.05.
Results
UVB irradiation of KB cells generates PAF-R agonists
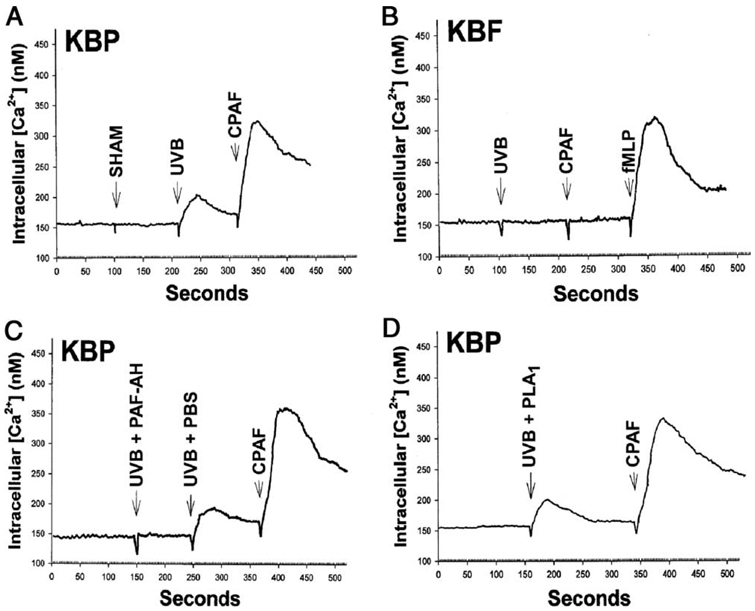
We first examined the ability of UVB irradiation of KB cells to produce PAF-R agonists. KB cells were used as this human epithelial cell line does not express PAF-R, the activation of which can stimulate further enzymatic synthesis of its ligand PAF (8, 15). Hence, the use of KB cells allows evaluation of PAF-R agonists derived from UVB directly. Our previous studies using mass spectrometry indicated that several PAF-R agonists produced in response to UVB in KB cells including 1-hexadecyl-2-acetyl-GPC (native PAF), butanoyl (C4-PAF), and butenoyl (C4:1-PAF) species (11, 14). These species were also measured upon direct UVB irradiation of purified lipid 1-hexadecyl-2-arachidonoyl-GPC (14). The generation of butanoyl and butenoyl C4-PAF analogues increases significantly with the increase of UVB dose, but their biological contributions are probably less important compared with native PAF, which is 100-fold more potent than C4-PAF product (14). In addition, there could be other yet uncharacterized sn-2 short-chained GPCs that act as PAF-R agonists. Therefore, the present study was designed to measure all PAF-R agonists as a whole in this complex mixture, using intracellular calcium mobilization, a sensitive and specific biochemical assay to measure PAF-R activity (31). As shown in Fig. 1A, lipid extracts derived from UVB-irradiated but not unirradiated KB cells triggered an intracellular calcium mobilization response in PAF-R-expressing KBP cells. Yet, treatment of KB cells transduced with the fMLP receptor (KBF) with lipid extracts derived from UVB-irradiated KB cells did not result in an intracellular calcium mobilization response (Fig. 1B). Of interest, UVB irradiation-induced PAF-R activity was ablated by pretreating the lipid extracts with PAF-AH (Fig. 1C) but not with PLA1 (Fig. 1D).
FIGURE 1.
Detection of PAF-R agonists by calcium mobilization. Stimulation with lipid extracts from UVB-irradiated (2000 J/m2, 1 h) but not un-irradiated (SHAM) KB cells results in calcium mobilization in KBP cells (A) but not in KBF cells (B). C and D, Lipid extracts from UVB-irradiated (2000 J/m2, 1 h) KB cells were pretreated with PBS (UVB plus PBS), PAF-acetylhydrolase (UVB plus PAF-AH) (C) or phospholipase A1 (UVB plus PLA1) (D) before PAF-R agonistic activity being measured in KBP cells. Stimulation with 1 µM CPAF or fMLP was used as control. The data pictured are representative of three independent experiments.
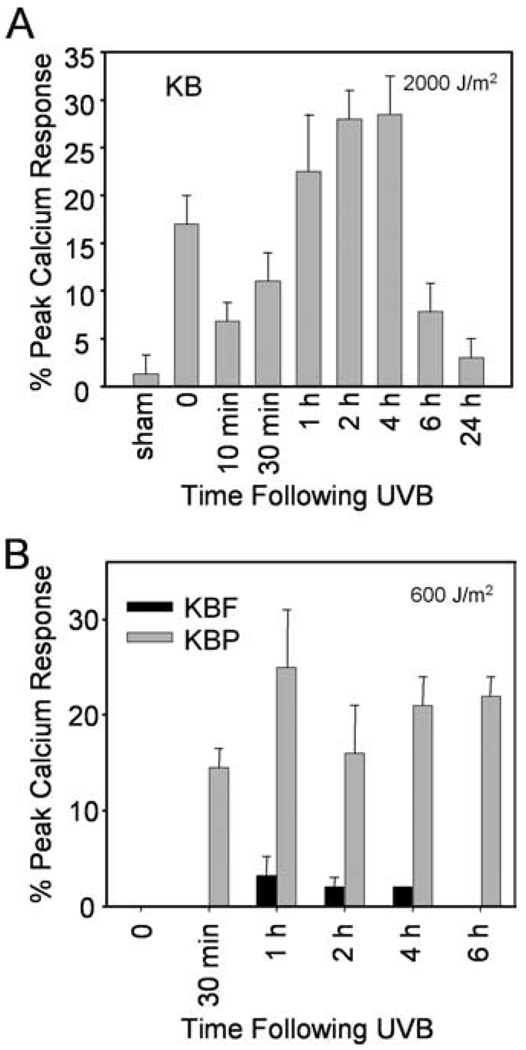
The next studies examined the time course of UVB-generated PAF-R agonists in KB cells. As shown in Fig. 2A, irradiation of KB cells with 2,000 J/m2 UVB resulted in a biphasic production of PAF-R agonists with an immediate (time = 0) and a later response which became apparent by 30 min. The second wave of PAF-R activity, which peaked at 2–4 h and almost came back to baseline levels by 6 h, was correlated with the onset of caspase-3 activation and apoptosis (16). Though significant levels of PAF-R agonists were measured using 2000 J/m2 UVB, very little was seen in response to 600 J/m2 (KBF controls; Fig. 2B). However, UVB irradiation of PAF-R-expressing KBP cells resulted in much greater levels of PAF-R agonists (Fig. 2B), presumably through the ability of small amounts of PAF-R agonists to trigger more PAF via PAF-R activation (8).
FIGURE 2.
Time course of UVB-mediated PAF-R agonist production. A, KB cells were sham or UVB-irradiated at 2000 J/m2. B, KBP and KBF cells were UVB-irradiated at 600 J/m2. Lipids were extracted at various time points post-UVB and tested for PAF-R agonist activity in KBP cells. Time “0” indicates immediately after UVB. Data are the mean ± SE from three independent experiments and shown as percentage of maximal peak calcium flux induced by 1 µM CPAF and adjusted for ~2.5 × 106 cells.
UVB-mediated production of ROS and PAF-R agonists
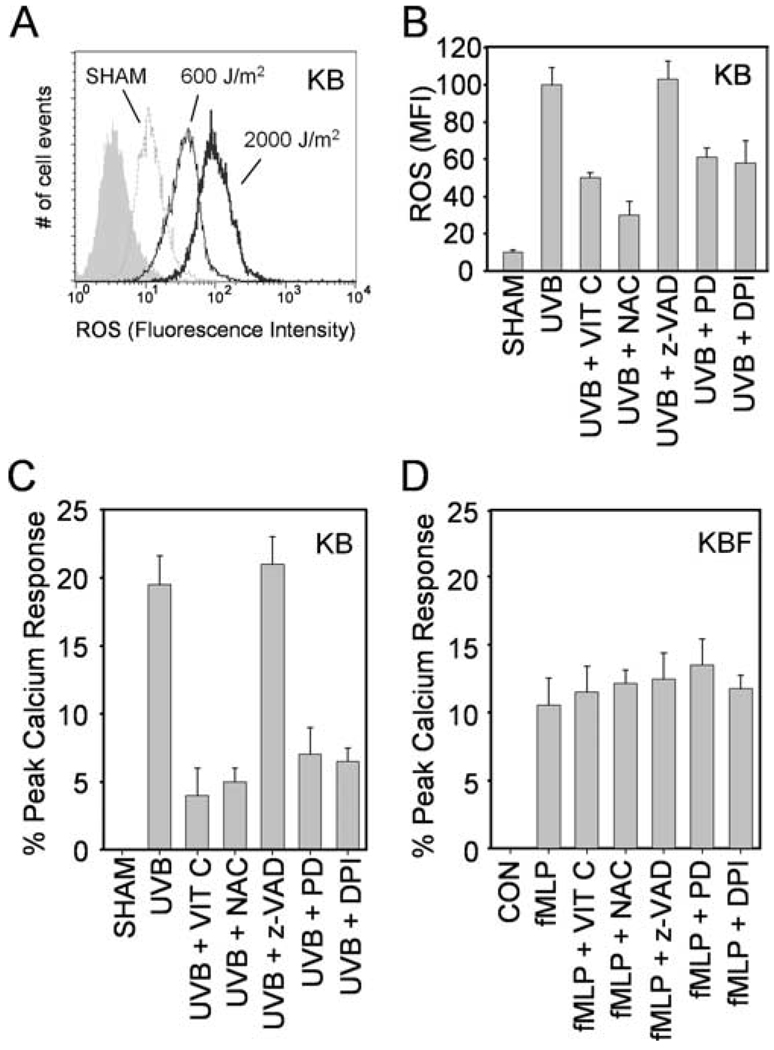
UVB irradiation is a potent inducer of ROS including superoxide radical, hydrogen peroxide and hydroxyl radical (23). We next tested the hypothesis that ROS generation was the reason for the PAF-R agonists produced in response to UVB in the KB cells. Intracellular levels of ROS were analyzed by flow cytometry using H2DCFDA as a fluorescent dye probe. UVB irradiation of KB cells resulted in increased levels of cellular ROS as early as 30 min (Fig. 3A), and was sustained for at least 2 h post-UVB (data not shown). As expected, more ROS were generated in KB cells in response to 2,000 J/m2 vs 600 J/m2 UVB.
FIGURE 3.
Effects of antioxidants and EGF-R inhibitor on UVB-mediated ROS and PAF-R agonist formation. A, Representative histograms showing ROS induction by UVB. KB cells were UVB-irradiated at 600 or 2000 J/m2. ROS levels were measured at 30 min post-UVB by flow cytometry using CM-H2DCFDA as a fluorescent dye probe. The gray filled histogram indicates negative control. B and C, KB cells were pretreated with vitamin C (VIT C, 2.5 mM), NAC (5 mM), z-VAD-FMK (z-VAD, 10 µM), PD168393 (PD, 10 µM) or DPI (10 µM) 30 min before UVB irradiation at 2000 J/m2 for 2 h. ROS levels (B) were measured by flow cytometry as in A and shown as mean fluorescent intensity (MFI). PAF-R agonistic activity (C) in lipid extracts were measured in KBP cells as in Fig. 2. D, KBF cells were pretreated with antioxidants or pharmacological inhibitors as in (C) 30 min before 1 µM fMLP stimulation. Lipids were extracted 10 min after and PAF-R agonists were measured and shown as percentage of maximal peak calcium flux induced by 1 µM CPAF and adjusted for 2.5 × 106 cells. Data are representative (A) or the mean ± SE (B–D) from three independent experiments.
Antioxidants vitamin C and NAC effectively scavenge a wide array of ROS and free radicals (26, 38). The next set of experiments assessed their ability to block UVB-mediated ROS and PAF-R agonistic activity. KB cells were pretreated with vitamin C or NAC before irradiation with 2,000 J/m2 UVB. As shown in Fig. 3, B and C, these antioxidants but not the pan-caspase inhibitor Z-VAD-FMK inhibited both ROS and PAF-R agonists produced by UVB irradiation. In contrast, these antioxidants did not affect PAF-R agonistic activity in KBF cells generated by 1 µM fMLP (Fig. 3D). These studies indicate that UVB-mediated PAF-R agonists are generated in direct response to ROS.
UVB-mediated ROS and PAF-R agonists involve the EGF-R
Previous studies have provided evidence that UV-mediated ROS in keratinocytes can involve the EGF-R and subsequent NADPH oxidase activation (23, 25, 39). Our next studies assessed whether this pathway is involved in UVB-mediated ROS and PAF-R agonist production. KB cells were preincubated with the EGF-R inhibitor PD168393 or the NADPH oxidase inhibitor DPI for 30 min before UVB irradiation (2000 J/m2). As shown in Fig. 3, B and C, both PD168393 and DPI inhibited ROS and PAF-R agonist formation in response to UVB at 2 h. However, these compounds did not affect PAF-R agonists generated immediately after UVB (data not shown). These studies indicate that the delayed UVB-mediated ROS and PAF-R agonist response in KB cells is due to a pathway involving the activation of the EGF-R.
UVB irradiation generates PAF-R agonists in murine skin in vivo
The next studies were designed to assess the ability of UVB to stimulate the production of PAF species in vivo. To that end, the back skin of immobilized C57BL6 mice was shaved and treated with UVB at a dose (7.5 kJ/m2) which our previous studies demonstrated induced systemic immunosuppression, a process that was dependent upon the PAF-R (13). At various times, the epidermis was removed from the irradiated area via a curette, and the tissue was weighed, then lipids extracted and tested for the ability to trigger a calcium mobilization response in KBP cells. PAF-R activity was not measured in unirradiated skin, but was found following UVB irradiation. A time course revealed significant levels by 1 h, which decreased by 4 h (data not shown). Again, lipid extracts from UVB-irradiated skin triggered a calcium response in KBP but not KBF cells (data not shown).
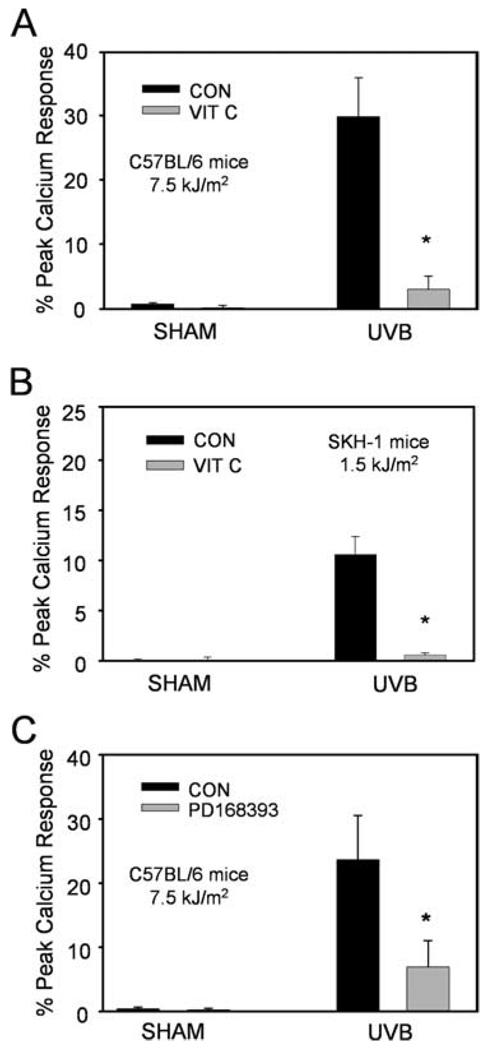
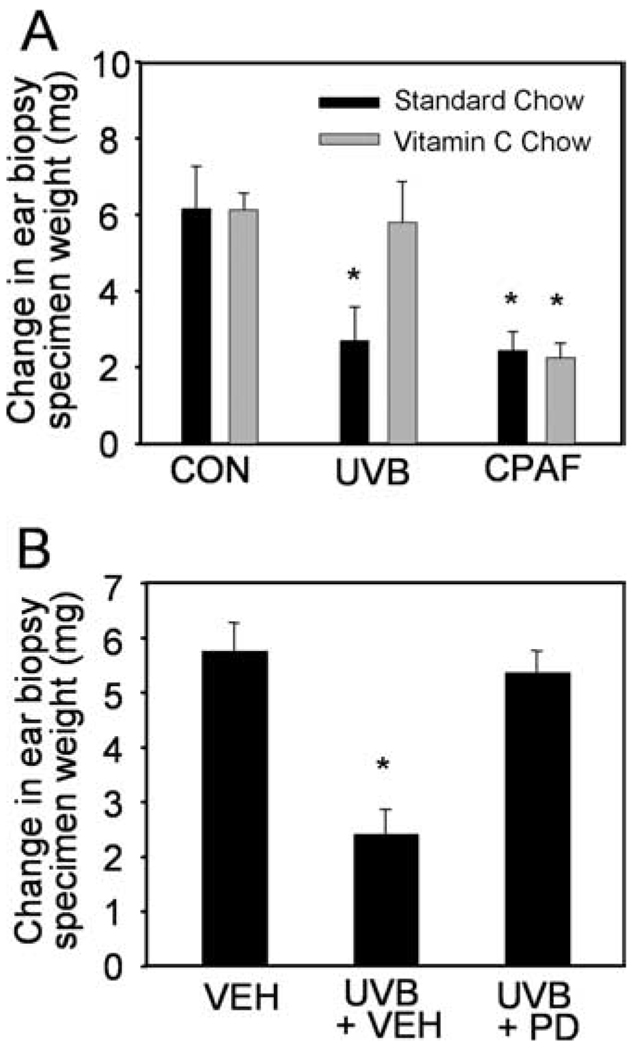
To assess whether UVB-generated PAF-R agonistic activity could be modulated by a systemic antioxidant, mice were fed vitamin C-enriched chow (10 g/kg) for 10 days before UVB irradiation. This strategy has been previously used successfully to inhibit PAF-R agonists generated in response to the potent pro-oxidative stimulus cigarette smoke (33). As shown in Fig. 4A, UVB irradiation of C57BL6 mice fed vitamin C-enriched chow resulted in significantly lower levels of skin PAF-R activity in comparison to standard chow. Similar results were obtained in SKH-1 hairless mice at a lower UVB dose of 1,500 J/m2 (Fig. 4B). Moreover, topical application of the EGF-R inhibitor PD168393 for 30 min before UVB irradiation (7500 J/m2) also inhibited UVB-mediated PAF-R agonist production in the skin of C57BL6 mice (Fig. 4C). These studies indicate that UVB generates PAF-R agonists via a process involving ROS and the EGF-R.
FIGURE 4.
Effects of vitamin C and PD168393 on PAF-R agonist formation in vivo. C57BL6 (A and C) or SKH-1 (B) mice were fed with standard (CON) or vitamin C-enriched (VIT C) chow for 10 days (A and B) or topically treated with PD168393 or vehicle for 30 min (C) before sham or UVB irradiation at indicated doses. Epidermis was removed 1 h after UVB via a curette, and the lipids were extracted from weighed tissue and tested for the ability to trigger a calcium mobilization response in KBP cells. Data are the mean ± SE percentage of maximal peak calcium flux induced by 1 µM CPAF and adjusted for 5 mg tissue weight from duplicate specimens obtained from five mice in each group. *, p < 0.05 compared with standard chow or vehicle control groups.
The effect of PAF-AH on UVB-mediated systemic immune suppression
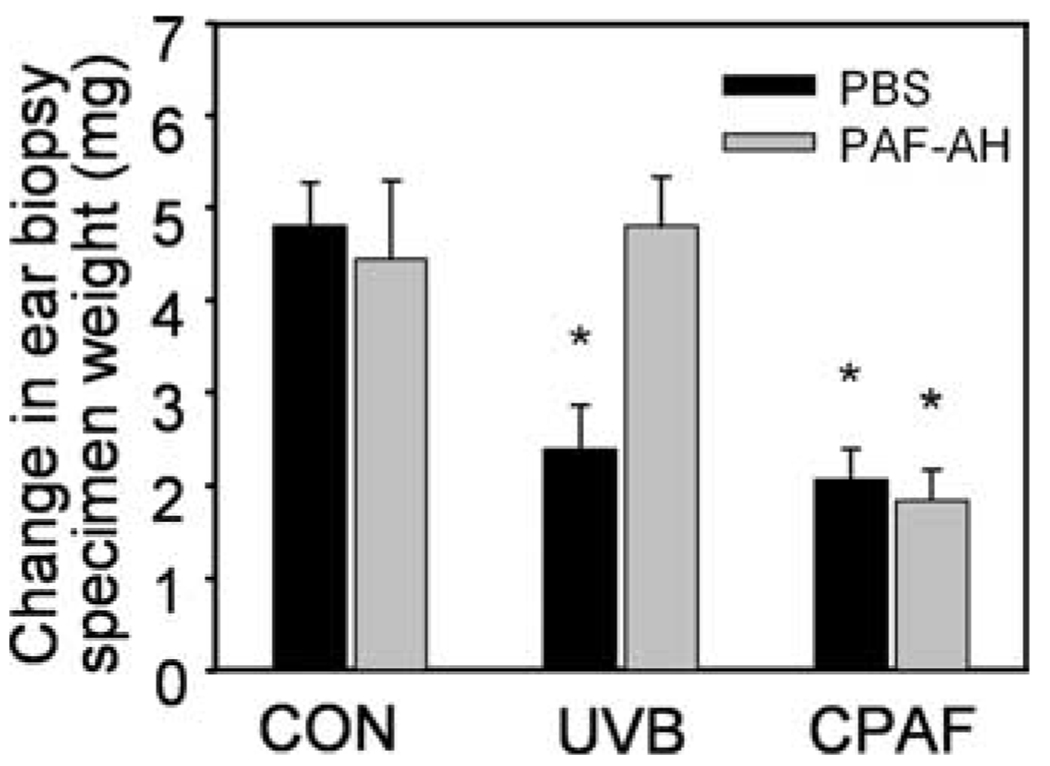
Previous studies have demonstrated that the PAF system is involved in UVB-mediated systemic immunosuppression (5, 18). In particular, we reported recently that UVB irradiation or i.p. injection of CPAF inhibits CHS to the chemical DNFB in wild-type but not PAF-R-deficient mice (13). It should be noted that our chimeric mice studies also demonstrated that PAF-R-expressing bone marrow-derived cells are necessary for UVB-mediated systemic immunosuppression (13), suggesting that UVB-irradiation of skin generates PAF-R agonists that act systemically. Given our finding that PAF-AH treatment of lipid extracts from UVB-irradiated KB cells ablated PAF agonistic activity (Fig. 1C), we next examined the ability of systemic delivery of PAF-AH to inhibit the immunosuppressive effects of UVB. Mice were given i.p. PAF-AH or PBS vehicle, then 30 min later UVB irradiated or underwent an i.p. injection with CPAF. Five days later, unirradiated back skin was sensitized to DNFB. Nine days later, one ear was treated with DNFB and the other was treated with vehicle. Twenty-four hours later, the mice were sacrificed and the ears removed. Five millimeter punch biopsies were obtained from the ears and inflammation was assessed by comparing ear weights of the biopsies. As shown in Fig. 5, PAF-AH treatment blocked the inhibition of CHS by UVB irradiation. However, administration of PAF-AH did not affect the immunosuppressive effects of CPAF, which contains a PAF-AH-insensitive N-methyl carbamoyl sn-2 bond. These studies confirm the importance of UVB-generated PAF-R agonists in UVB-mediated systemic immunosuppression.
FIGURE 5.
Effects of PAF-AH on UVB vs CPAF-induced systemic immunosuppression. C57BL6 mice were pretreated with PAF-AH or PBS i.p. 30 min before UVB irradiation (7500 J/m2), CPAF (250 ng i.p.), or BSA vehicle alone (CON) injections. Five days later, unirradiated back skin was sensitized to DNFB. Nine days later, one ear was treated with DNFB and the other was treated with vehicle. Five millimeter punch biopsies were obtained from the ears 24 h later and inflammation was assessed by comparing ear weights of the biopsies. Data are the mean ± SE difference in ear weights from five to eight mice in each group. *, p < 0.05 compared with vehicle control.
UVB-mediated systemic immune suppression is mediated by ROS-generated PAF-R agonists
Given our findings demonstrating that systemic vitamin C or topical EGF-R antagonist can block UVB-mediated PAF-R agonist production, the next experiments assessed the in vivo role of ROS and EGF-R in UVB-mediated inhibition of CHS to DNFB. Mice were fed with vitamin C-enriched chow or standard chow for 10 days or pretreated topically with PD168393 or vehicle for 30 min before being subjected to UVB irradiation or intraperitoneal injection of CPAF. Mice were then sensitized to DNFB 5 days later, and elicitation reactions were obtained on the ears. As shown in Fig. 6A, the inhibition of CHS by UVB was blocked in mice fed with the vitamin C-enriched chow. However, CPAF-mediated inhibition of CHS was not affected by systemic vitamin C supplementation. Similarly, PD168393 treatment abolished UVB-mediated inhibition of CHS to DNFB (Fig. 6B). However, topical treatment with the EGF-R inhibitor had no effect on the basal level or CPAF-mediated inhibition of CHS to DNFB (data not shown). These data fit with the notion that both vitamin C and PD168393 block the immunosuppressive effects of UVB by their ability to block PAF production. These studies confirm our in vitro findings that EGF-R activation and ROS formation mediate epidermal PAF-R agonist production by UVB.
FIGURE 6.
Effects of vitamin C and PD168393 on UVB-induced systemic immunosuppression. C57BL6 mice were fed with standard or vitamin C-enriched chow for 10 days (A) or topically treated with PD168393 (PD) or vehicle (VEH) for 30 min (B) before UVB irradiation (7500 J/m2), CPAF (250 ng, i.p.) or BSA vehicle alone (CON) injections. Delayed type hypersensitivity to DNFB was measured as in Fig. 5. Data are the mean ± SE difference in ear weights from five mice in each group. *, p < 0.05 compared with vehicle control.
Discussion
The role of PAF in mediating UVB-induced systemic immunosuppression has been greatly appreciated, but the molecular events governing its production in response to UVB are not well understood. In the present study, we provide evidence that UVB-generated PAF-R agonistic activity is almost entirely due to the ability of UVB to act as a pro-oxidative stressor. Moreover, activation of the EGF-R plays a pivotal role in the ability of UVB to generate PAF-R agonists.
It is well known that ROS generated by photochemical activation of molecular oxygen mediates many biological effects of UV irradiation including apoptosis (22, 40). Recent studies also suggested a possible role of ROS in mediating the immunosuppressive effect of UVB as topical application of antioxidants abrogated UV-induced systemic immunosuppression, but the possible sunscreen effect and the underlying molecular mechanisms were not addressed (26, 27). Our current study not only confirmed the role of the PAF system in UVB-mediated systemic immunosuppression but also revealed the importance of EGF-R-mediated ROS in the ability of UVB to generate PAF-R agonists. These notions are supported by the following: 1) antioxidants vitamin C and NAC blocked both UVB-induced ROS and PAF-R agonist production in vitro; 2) systemic administration of vitamin C by oral supplementation abolished PAF-R agonist production in UVB-irradiated mouse skin; 3) UVB- but not CPAF-mediated immunosuppression was blocked by oral supplementation of vitamin C. Our findings are in line with a previous study in which oral supplementation with vitamin C inhibited PAF species generation in response to the pro-oxidative stressor cigarette smoke in hamsters (33). Our in vitro data also suggested that PAF-R agonist generation and apoptosis might be parallel downstream effects of UVB-induced ROS formation because blocking apoptosis by a pan-caspase inhibitor did not affect PAF-R agonist production (Fig. 3C).
EGF-R phosphorylation is a well established response to UV exposure, which may be involved in photocarcinogenesis by both suppressing cell death and promoting cell proliferation (41). Previous studies have suggested that ROS mediates UVB-evoked EGF-R activation by oxidative inhibition of protein-tyrosine phosphatase (23, 42). There is also contradictory evidence that the activation of EGF-R precedes ROS formation (25, 39). Our data (Fig. 3B) indicated that EGF-R activation is required for the maximal ROS generation in response to UVB. Regardless the relationship between EGF-R activation and ROS formation, we report in this study for the first time, to our knowledge, that EGF-R activation and ROS formation are both involved in UVB-mediated PAF-R agonist production and subsequent systemic immunosuppression. Hence, we speculate that the pro-carcinogenic effect of EGF-R may also be mediated, at least in part, by the induction of PAF-R agonists that are immunosuppressive. This concept is supported by the recent report that systemic administration of a PAF-R antagonist inhibits UVB-mediated tumor formation in SKH-1 hairless mice (43).
Our findings that EGF-R mediates UVB-induced inhibition of contact hypersensitivity are in contrast to Mascia et al. (29) who reported that topical application of the EGF-R inhibitor suppresses the elicitation phase of contact hypersensitivity to DNFB. In that study indicating the anti-inflammatory effect of EGF-R inhibitor, UVB irradiation was not involved and the blockade of EGF-R occurred right before Ag challenge in previously sensitized mice. In the present study, the blockade of EGF-R most likely acts on UVB-induced PAF production as the EGF-R antagonist had no effect on CPAF-mediated immunosuppression.
The synthetic pathway for PAF consists of two enzymes: phospholipase A2 generates the lysolipid backbone by releasing the sn-2 fatty acyl residue from alkyl phosphatidylcholine and PAF acetyltransferase transfers anacetyl residue from acetyl-CoA to this newly generated lysolipid (8). The activity of these two enzymes is tightly regulated, with increased intracellular Ca2+ being the premier regulator. In addition, PAF-R agonists can also be produced through nonenzymatic oxidation, which is not subjected to cellular control (11). UVB irradiation generates a variety of ROS that oxidizes phospholipids. Oxidation of esterified fatty acyl residues introduces oxy functions, rearranges bonds and fragments carbon-carbon bonds by β-scission that generate a myriad of phospholipid reaction products including PAF-R agonists (44, 45). In this regard, cellular membranes serve as the source of oxidized phospholipids and are thus the source of UVB-mediated PAF formation.
The source of the UVB-induced PAF-R agonists is most likely the keratinocyte, as UVB irradiation does not appreciably pass through the epidermis (46). In the present study, UVB irradiation of the human epidermal KB cells resulted in the production of PAF-R agonistic activity which was evident at a relatively high dose of UVB (2000 J/m2), yet was not found in appreciable amounts at a lower, more physiological dose of 600 J/m2 (Fig. 2). However, elevated levels of ROS were detected at 600 J/m2, albeit at much lower levels compared with 2,000 J/m2 (Fig. 3A). Although it is difficult to assess to what extent the high dose UVB contributes to direct vs indirect ROS formation, both doses of UVB can generate DNA damage indicated by cyclobutane pyrimidine dimmer formation and are cytotoxic indicated by caspase-3 activation (data not shown). In addition, both antioxidants and EGF-R antagonist almost completely abolished UVB-induced PAF-R agonistic activity but only partially blocked ROS formation (Fig. 3, B and C). These results suggested that a threshold of UVB-generated ROS might be required to trigger PAF production. It should be noted that the KB cells used in our in vitro studies do not express the PAF-R as do primary keratinocytes (15, 31). This is important because the use of KB cells allows evaluation of PAF agonists derived from UVB directly, considering the fact that PAF-R activation by a small amount of PAF will stimulate further PAF production (8). Indeed, PAF-R agonistic activity was detectable in PAF-R expressing KBP cells at 600 J/m2 (Fig. 2B). Although it is difficult to compare in vitro vs in vivo doses of UVB irradiation, PAF-R agonistic activity was detectable in lipid extracts from irradiated mouse skin at 7500 J/m2 (Fig. 4A), the same dose used to induce systemic immunosuppression (Fig. 6) (13).
In summary, these studies demonstrate that UVB-induced PAF-R agonist production involves EGF-R-mediated ROS formation. Future studies are warranted to elucidate how EGF-R and/or ROS stimulate PAF-R agonists. Given our findings that systemic vitamin C administration or topical EGF-R inhibitor blocks UVB-mediated systemic immunosuppression, a process implicated in carcinogenesis, future studies could define whether these novel strategies can protect against UVB-induced skin cancers.
Acknowledgments
We greatly appreciate Qiaofang Yi and Danielle Jernigan for technical assistance and Dr. Dan Spandau for helpful discussion and critical reading of this manuscript.
Footnotes
This work was supported in part by National Institutes of Health Grants R01HL062996 (to J.B.T.) and U19A1070448 539 (to J.B.T.), and a Veterans Affairs Merit Award Grant (to J.B.T.). J.E.W. was supported by the Riley Memorial Foundation.
Abbreviations used in this paper: DNFB, dinitrofluorobenzene; PAF, platelet-activating factor; GPC, glycerophosphocholine; PAF-R, PAF receptor; PAF-AH, PAF-acetylhydrolase; ROS, reactive oxygen species; EGF-R, epithelial growth factor-receptor; NAC, N-acetylcysteine; DPI, diphenylene iodinium; CHS, contact hypersensitivity; CPAF, 1-hexadecyl-2-N-methylcarbamoyl glycerophosphocholine.
Disclosures
The authors have no financial conflict of interest.
References
- 1.Boring CC, Squires TS, Tong T. Cancer statistics, 1992. CA Cancer J. Clin. 1992;42:19–38. doi: 10.3322/canjclin.42.1.19. [DOI] [PubMed] [Google Scholar]
- 2.Fisher GJ, Datta SC, Talwar HS, Wang ZQ, Varani J, Kang S, Voorhees JJ. Molecular basis of sun-induced premature skin ageing and retinoid antagonism. Nature. 1996;379:335–339. doi: 10.1038/379335a0. [DOI] [PubMed] [Google Scholar]
- 3.Kripke ML. Latency, histology, and antigenicity of tumors induced by ultraviolet light in three inbred mouse strains. Cancer Res. 1977;37:1395–1400. [PubMed] [Google Scholar]
- 4.Ullrich SE. Photoimmune suppression and photocarcinogenesis. Front. Biosci. 2002;7:d684–d703. doi: 10.2741/A804. [DOI] [PubMed] [Google Scholar]
- 5.Ullrich SE. Mechanisms underlying UV-induced immune suppression. Mutat. Res. 2005;571:185–205. doi: 10.1016/j.mrfmmm.2004.06.059. [DOI] [PubMed] [Google Scholar]
- 6.Fisher MS, Kripke ML. Suppressor T lymphocytes control the development of primary skin cancers in ultraviolet-irradiated mice. Science. 1982;216:1133–1134. doi: 10.1126/science.6210958. [DOI] [PubMed] [Google Scholar]
- 7.Noonan FP, De Fabo EC, Kripke ML. Suppression of contact hypersensitivity by ultraviolet radiation: an experimental model. Springer Semin. Immunopathol. 1981;4:293–304. doi: 10.1007/BF01892183. [DOI] [PubMed] [Google Scholar]
- 8.Prescott SM, Zimmerman GA, Stafforini DM, McIntyre TM. Platelet-activating factor and related lipid mediators. Annu. Rev. Biochem. 2000;69:419–445. doi: 10.1146/annurev.biochem.69.1.419. [DOI] [PubMed] [Google Scholar]
- 9.Izumi T, Shimizu T. Platelet-activating factor receptor: gene expression and signal transduction. Biochim. Biophys. Acta. 1995;1259:317–333. doi: 10.1016/0005-2760(95)00171-9. [DOI] [PubMed] [Google Scholar]
- 10.Travers JB, Harrison KA, Johnson CA, Clay KL, Morelli JG. Platelet-activating factor biosynthesis induced by various stimuli in human Ha-CaT keratinocytes. J. Invest. Dermatol. 1996;107:88–94. doi: 10.1111/1523-1747.ep12298295. [DOI] [PubMed] [Google Scholar]
- 11.Marathe GK, Harrison KA, Murphy RC, Prescott SM, Zimmerman GA, McIntyre TM. Bioactive phospholipid oxidation products. Free Radical Biol. Med. 2000;28:1762–1770. doi: 10.1016/s0891-5849(00)00234-3. [DOI] [PubMed] [Google Scholar]
- 12.Stafforini DM, McIntyre TM, Zimmerman GA, Prescott SM. Platelet-activating factor acetylhydrolases. J. Biol. Chem. 1997;272:17895–17898. doi: 10.1074/jbc.272.29.17895. [DOI] [PubMed] [Google Scholar]
- 13.Zhang Q, Yao Y, Konger RL, Sinn AL, Cai S, Pollok KE, Travers JB. UVB radiation-mediated inhibition of contact hypersensitivity reactions is dependent on the platelet-activating factor system. J. Invest. Dermatol. 2008;128:1780–1787. doi: 10.1038/sj.jid.5701251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Marathe GK, Johnson C, Billings SD, Southall MD, Pei Y, Spandau D, Murphy RC, Zimmerman GA, McIntyre TM, Travers JB. Ultraviolet B radiation generates platelet-activating factor-like phospholipids underlying cutaneous damage. J. Biol. Chem. 2005;280:35448–35457. doi: 10.1074/jbc.M503811200. [DOI] [PubMed] [Google Scholar]
- 15.Pei Y, Barber LA, Murphy RC, Johnson CA, Kelley SW, Dy LC, Fertel RH, Nguyen TM, Williams DA, Travers JB. Activation of the epidermal platelet-activating factor receptor results in cytokine and cyclooxygenase-2 biosynthesis. J. Immunol. 1998;161:1954–1961. [PubMed] [Google Scholar]
- 16.Barber LA, Spandau DF, Rathman SC, Murphy RC, Johnson CA, Kelley SW, Hurwitz SA, Travers JB. Expression of the platelet-activating factor receptor results in enhanced ultraviolet B radiation-induced apoptosis in a human epidermal cell line. J. Biol. Chem. 1998;273:18891–18897. doi: 10.1074/jbc.273.30.18891. [DOI] [PubMed] [Google Scholar]
- 17.Travers JB, Edenberg HJ, Zhang Q, Al-Hassani M, Yi Q, Baskaran S, Konger RL. Augmentation of UVB radiation-mediated early gene expression by the epidermal platelet-activating factor receptor. J. Invest. Dermatol. 2008;128:455–460. doi: 10.1038/sj.jid.5701083. [DOI] [PubMed] [Google Scholar]
- 18.Walterscheid JP, Ullrich SE, Nghiem DX. Platelet-activating factor, a molecular sensor for cellular damage, activates systemic immune suppression. J. Exp. Med. 2002;195:171–179. doi: 10.1084/jem.20011450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Alappatt C, Johnson CA, Clay KL, Travers JB. Acute keratinocyte damage stimulates platelet-activating factor production. Arch. Dermatol. Res. 2000;292:256–259. doi: 10.1007/s004030050483. [DOI] [PubMed] [Google Scholar]
- 20.Wolf P, Nghiem DX, Walterscheid JP, Byrne S, Matsumura Y, Matsumura Y, Bucana C, Ananthaswamy HN, Ullrich SE. Platelet-activating factor is crucial in psoralen and ultraviolet A-induced immune suppression, inflammation, and apoptosis. Am. J. Pathol. 2006;169:795–805. doi: 10.2353/ajpath.2006.060079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhang Q, Mousdicas N, Yi Q, Al-Hassani M, Billings SD, Perkins SM, Howard KM, Ishii S, Shimizu T, Travers JB. Staphylococcal lipoteichoic acid inhibits delayed-type hypersensitivity reactions via the platelet-activating factor receptor. J. Clin. Invest. 2005;115:2855–2861. doi: 10.1172/JCI25429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kulms D, Schwarz T. Independent contribution of three different pathways to ultraviolet-B-induced apoptosis. Biochem. Pharmacol. 2002;64:837–841. doi: 10.1016/s0006-2952(02)01146-2. [DOI] [PubMed] [Google Scholar]
- 23.Peus D, Vasa RA, Meves A, Pott M, Beyerle A, Squillace K, Pittelkow MR. H2O2 is an important mediator of UVB-induced EGF-receptor phosphorylation in cultured keratinocytes. J. Invest. Dermatol. 1998;110:966–971. doi: 10.1046/j.1523-1747.1998.00210.x. [DOI] [PubMed] [Google Scholar]
- 24.Herrlich P, Bohmer FD. Redox regulation of signal transduction in mammalian cells. Biochem. Pharmacol. 2000;59:35–41. doi: 10.1016/s0006-2952(99)00298-1. [DOI] [PubMed] [Google Scholar]
- 25.Wan Y, Wang Z, Shao Y, Xu Y, Voorhees J, Fisher G. UV-induced expression of GADD45 is mediated by an oxidant sensitive pathway in cultured human keratinocytes and in human skin in vivo. Int. J. Mol. Med. 2000;6:683–688. doi: 10.3892/ijmm.6.6.683. [DOI] [PubMed] [Google Scholar]
- 26.Nakamura T, Pinnell SR, Darr D, Kurimoto I, Itami S, Yoshikawa K, Streilein JW. Vitamin C abrogates the deleterious effects of UVB radiation on cutaneous immunity by a mechanism that does not depend on TNF-α. J. Invest. Dermatol. 1997;109:20–24. doi: 10.1111/1523-1747.ep12276349. [DOI] [PubMed] [Google Scholar]
- 27.Steenvoorden DP, Beijersbergen van Henegouwen G. Protection against UV-induced systemic immunosuppression in mice by a single topical application of the antioxidant vitamins C and E. Int. J. Radiat. Biol. 1999;75:747–755. doi: 10.1080/095530099140096. [DOI] [PubMed] [Google Scholar]
- 28.Steenvoorden DP, Beijersburgen van Henegouwen GM. Glutathione synthesis is not involved in protection by N-acetylcysteine against UVB-induced systemic immunosuppression in mice. Photochem. Photobiol. 1998;68:97–100. [PubMed] [Google Scholar]
- 29.Mascia F, Mariani V, Girolomoni G, Pastore S. Blockade of the EGF receptor induces a deranged chemokine expression in keratinocytes leading to enhanced skin inflammation. Am. J. Pathol. 2003;163:303–312. doi: 10.1016/S0002-9440(10)63654-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bligh EG, Dyer WJ. A rapid method of total lipid extraction and purification. Can. J. Biochem. Physiol. 1959;37:911–917. doi: 10.1139/o59-099. [DOI] [PubMed] [Google Scholar]
- 31.Travers JB, Huff JC, Rola-Pleszczynski M, Gelfand EW, Morelli JG, Murphy RC. Identification of functional platelet-activating factor receptors on human keratinocytes. J. Invest. Dermatol. 1995;105:816–823. doi: 10.1111/1523-1747.ep12326581. [DOI] [PubMed] [Google Scholar]
- 32.MacDougall SL, Grinstein S, Gelfand EW. Detection of ligand-activated conductive Ca2+ channels in human B lymphocytes. Cell. 1988;54:229–234. doi: 10.1016/0092-8674(88)90555-7. [DOI] [PubMed] [Google Scholar]
- 33.Lehr HA, Weyrich AS, Saetzler RK, Jurek A, Arfors KE, Zimmerman GA, Prescott SM, McIntyre TM. Vitamin C blocks inflammatory platelet-activating factor mimetics created by cigarette smoking. J. Clin. Invest. 1997;99:2358–2364. doi: 10.1172/JCI119417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wang H, Joseph JA. Quantifying cellular oxidative stress by dichlorofluorescein assay using microplate reader. Free Radical Biol. Med. 1999;27:612–616. doi: 10.1016/s0891-5849(99)00107-0. [DOI] [PubMed] [Google Scholar]
- 35.Petersen JE, Hiran TS, Goebel WS, Johnson C, Murphy RC, Azmi FH, Hood AF, Travers JB, Dinauer MC. Enhanced cutaneous inflammatory reactions to Aspergillus fumigatus in a murine model of chronic granulomatous disease. J. Invest. Dermatol. 2002;118:424–429. doi: 10.1046/j.0022-202x.2001.01691.x. [DOI] [PubMed] [Google Scholar]
- 36.Rockel N, Esser C, Grether-Beck S, Warskulat U, Flogel U, Schwarz A, Schwarz T, Yarosh D, Haussinger D, Krutmann J. The osmolyte taurine protects against ultraviolet B radiation-induced immunosuppression. J. Immunol. 2007;179:3604–3612. doi: 10.4049/jimmunol.179.6.3604. [DOI] [PubMed] [Google Scholar]
- 37.Matsumura Y, Byrne SN, Nghiem DX, Miyahara Y, Ullrich SE. A role for inflammatory mediators in the induction of immunoregulatory B cells. J. Immunol. 2006;177:4810–4817. doi: 10.4049/jimmunol.177.7.4810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Aruoma OI, Halliwell B, Hoey BM, Butler J. The antioxidant action of N-acetylcysteine: its reaction with hydrogen peroxide, hydroxyl radical, superoxide, and hypochlorous acid. Free Radical Biol. Med. 1989;6:593–597. doi: 10.1016/0891-5849(89)90066-x. [DOI] [PubMed] [Google Scholar]
- 39.Halvey PJ, Watson WH, Hansen JM, Go YM, Samali A, Jones DP. Compartmental oxidation of thiol-disulphide redox couples during epidermal growth factor signalling. Biochem. J. 2005;386:215–219. doi: 10.1042/BJ20041829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bertling CJ, Lin F, Girotti AW. Role of hydrogen peroxide in the cytotoxic effects of UVA/B radiation on mammalian cells. Photochem. Photobiol. 1996;64:137–142. doi: 10.1111/j.1751-1097.1996.tb02433.x. [DOI] [PubMed] [Google Scholar]
- 41.Wang HQ, Quan T, He T, Franke TF, Voorhees JJ, Fisher GJ. Epidermal growth factor receptor-dependent, NF-κB-independent activation of the phosphatidylinositol 3-kinase/Akt pathway inhibits ultraviolet irradiation-induced caspases−3, −8, and −9 in human keratinocytes. J. Biol. Chem. 2003;278:45737–45745. doi: 10.1074/jbc.M300574200. [DOI] [PubMed] [Google Scholar]
- 42.Xu Y, Shao Y, Voorhees JJ, Fisher GJ. Oxidative inhibition of receptor-type protein-tyrosine phosphatase κ by ultraviolet irradiation activates epidermal growth factor receptor in human keratinocytes. J. Biol. Chem. 2006;281:27389–27397. doi: 10.1074/jbc.M602355200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sreevidya CS, Khaskhely NM, Fukunaga A, Khaskina P, Ullrich SE. Inhibition of photocarcinogenesis by platelet-activating factor or serotonin receptor antagonists. Cancer Res. 2008;68:3978–3984. doi: 10.1158/0008-5472.CAN-07-6132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Frankel EN. Chemistry of free radical and singlet oxidation of lipids. Prog. Lipid Res. 1984;23:197–221. doi: 10.1016/0163-7827(84)90011-0. [DOI] [PubMed] [Google Scholar]
- 45.Heery JM, Kozak M, Stafforini DM, Jones DA, Zimmerman GA, McIntyre TM, Prescott SM. Oxidatively modified LDL contains phospholipids with platelet-activating factor-like activity and stimulates the growth of smooth muscle cells. J. Clin. Invest. 1995;96:2322–2330. doi: 10.1172/JCI118288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Schade N, Esser C, Krutmann J. Ultraviolet B radiation-induced immunosuppression: molecular mechanisms and cellular alterations. Photochem. Photobiol. Sci. 2005;4:699–708. doi: 10.1039/b418378a. [DOI] [PubMed] [Google Scholar]