Abstract
The anatomy and neurophysiology of the saccadic eye movement system have been well studied, but the roles of certain key neurons in this system are not fully appreciated. Important clues about the functional interactions in the saccadic system can be gleaned from the histochemistry of different saccadic neurons. The most prominent inhibitory neurons in the circuit are the omnidirectional pause neurons (OPN), which inhibit the premotor burst neurons that drive the eye. Most inhibitory neurons in the brain transmit γ-aminobutyric acid (GABA), but OPN transmit glycine (Gly). It is interesting to ask whether the saccadic system would work any differently if OPN were GABA-ergic. Gly and GABA receptors both provide a channel for a hyperpolarizing Cl- current that inhibits its target neuron. Depolarizing currents that excite the neurons come through several channels, including the NMDA receptor (NMDAR). The NMDAR is unique among receptors in that it has active sites for two different neurotransmitters, glutamate (Glu) and Gly. Gly is a co-agonist that acts to amplify the current produced by Glu. We have proposed a model of the saccadic brain stem circuitry that exploits this dual role of Gly to produce both inhibition of the saccadic circuit during fixation, and to increase its responsiveness, or gain, during movements. This suggests that OPNs act more as a regulator of the saccadic circuit’s gain, rather than as a gate for allowing saccades. We propose a new hypothesis: the OPNs play a general role as a modulator of arousal in orienting subsystems, such as saccades, pursuit, head movements, etc.
Keywords: glycine, burst neurons, brainstem, saccades
Introduction
Saccades are rapid, voluntary movements that reorient the retinal region of highest acuity (fovea) towards images of interest. As acuity is greatly impaired during these movements, good vision requires that saccades be fast and accurate. In engineering terms, a fast and accurate controller must have high gain and negative feedback, but having both properties makes a circuit prone to instability. Oscillations around the target at the end of a movement would interfere with vision just as much as a slower, more stable saccade. Thus, the brain must trade-off the speed, accuracy, and stability of the saccade. Indeed, this tradeoff must exist for all orienting subsystems (e.g., eye and head), which suggests the need for a general arousal system that could modulate all orienting behavior to increase sensitivity and maintain stability.
One of the best-studied orienting subsystems, the one for saccadic eye movements, is assumed to have high-gain premotor burst neurons (PBNs, providing the necessary sensitivity to changes) and a class of cells called omnipause neurons (OPN, cf., Fig. 1A), that fire except during saccades, inhibiting PBNs and ensuring stability of the saccadic system. Here I propose a single function for OPNs that gives them a larger role to play in arousing all systems that orient the animal towards targets of interest.
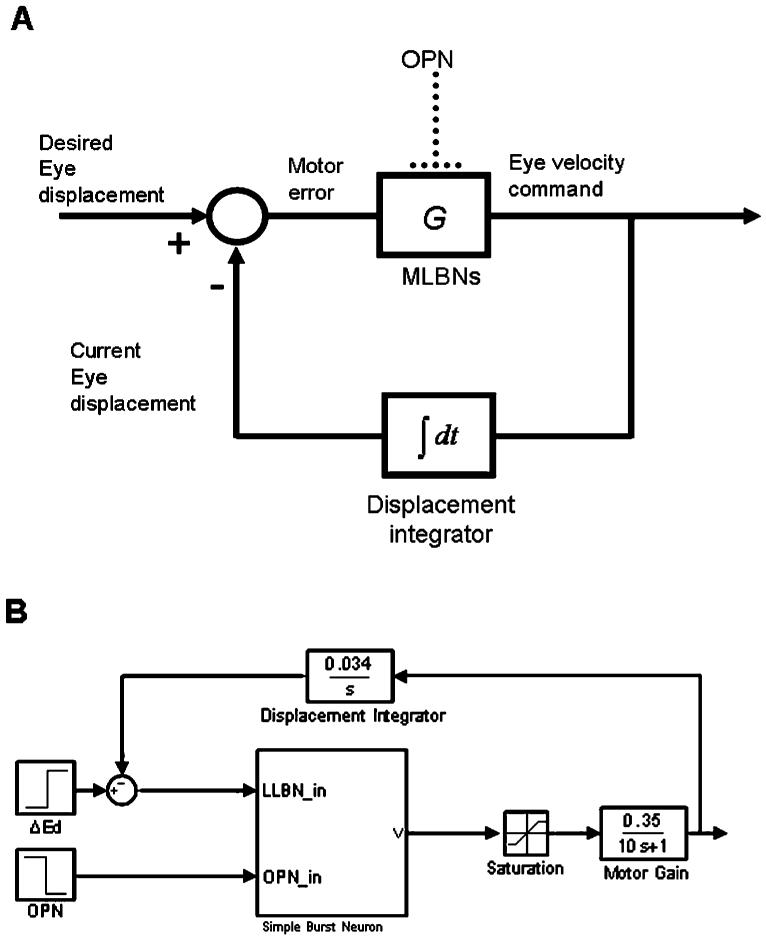
Fig. 1.
Central core of local feedback loop pulse generator in a saccadic system. (A) Block diagram showing gating of premotor burst neurons (MLBN) by OPN. (B) Simplified implementation of model in A in SIMULINK. Simple burst neuron is a block that models a single-compartment, spiking neuron. The output of this block is clipped (saturation) and low-pass filtered (motor gain) to produce an eye velocity command. The displacement integrator converts this command into an efference copy of current eye displacement during the saccade, which is subtracted from the desired eye displacement (ΔEd) to compute motor error.
OPNs were discovered in the brain stem of monkeys (Cohen and Henn, 1972; Luschei and Fuchs, 1972; Keller, 1974) and cats (Evinger et al., 1982). These neurons fire steadily during fixation and pause during saccades. About half the neurons pause for saccades in one direction, and the rest pause for saccades in all directions (i.e., omni-directional pause units, hence the name, omnipause). The OPNs make monosynaptic inhibitory connections (Nakao et al., 1980; Furuya and Markham, 1982; Strassman et al., 1987) to excitatory and inhibitory premotor burst neurons (EBN and IBN) in the brain stem (which connect to horizontal motor neurons) and midbrain (which connect to vertical/torsional motor neurons). The OPNs lie in the midbrain very close to the midline near the rostral pole of the abducens nucleus, in the raphe interpositus nucleus (RIP, Buttner-Ennever et al., 1988; Langer and Kaneko, 1990; Horn et al., 1994).
Most inhibitory neurons in the brain transmit either γ-aminobutyric acid (GABA) or glycine (Gly). OPNs happen to be glycinergic (Spencer et al., 1989; Horn et al., 1994). We could ask whether there is any significance to this fact, as the function of OPNs as a gate for saccades would still be possible if they used GABA. Gly differs from GABA in one important aspect. Although both GABA and Gly are inhibitory at their receptors (e.g., the GABAA receptor and the strychnine-sensitive Gly receptor), Gly also has another action at a different receptor. Gly acts as an excitatory modulator of glutamate (Glu) action at the co-agonist sites on the N-methyl d-aspartate receptor (NMDAR) (Johnson and Ascher, 1987). Below we explore some of the consequences of this dual action of Gly on the role of the OPNs.
Methods
We simulated a highly simplified model of the saccadic system (Fig. 1B), with essentially one excitatory premotor burst neuron (EBN) and no delays (SIMULINK/MATLAB, The Mathworks, Natick, MA). The EBN was modelled with differential equations for a single-compartment, conductance-based, spiking neuron (Miura and Optican, 2006). The equation for the membrane potential is given by summing the currents through each ionic channel:
| (1) |
In this test, the model EBN had only one or the other of the Gly or GABAA receptors (Figs. 2A, B). Previous studies have demonstrated that the Gly receptors act in a rapid manner when near body temperature. Specifically, a deactivation time constant in the range of 1.3-5.4 ms has been suggested (see discussion in Harty and Manis, 1998). The dynamics of the current, IGly, are given by:
| (2) |
| (3) |
where gGly (1 mS/cm2) and EGly (-80 mV) denote the conductance and reversal potential of this channel, respectively, and αGly and τGly are constants. αGly was set to 0.05, and the decay time constant, τGly, was set to 2 ms in the simulations.
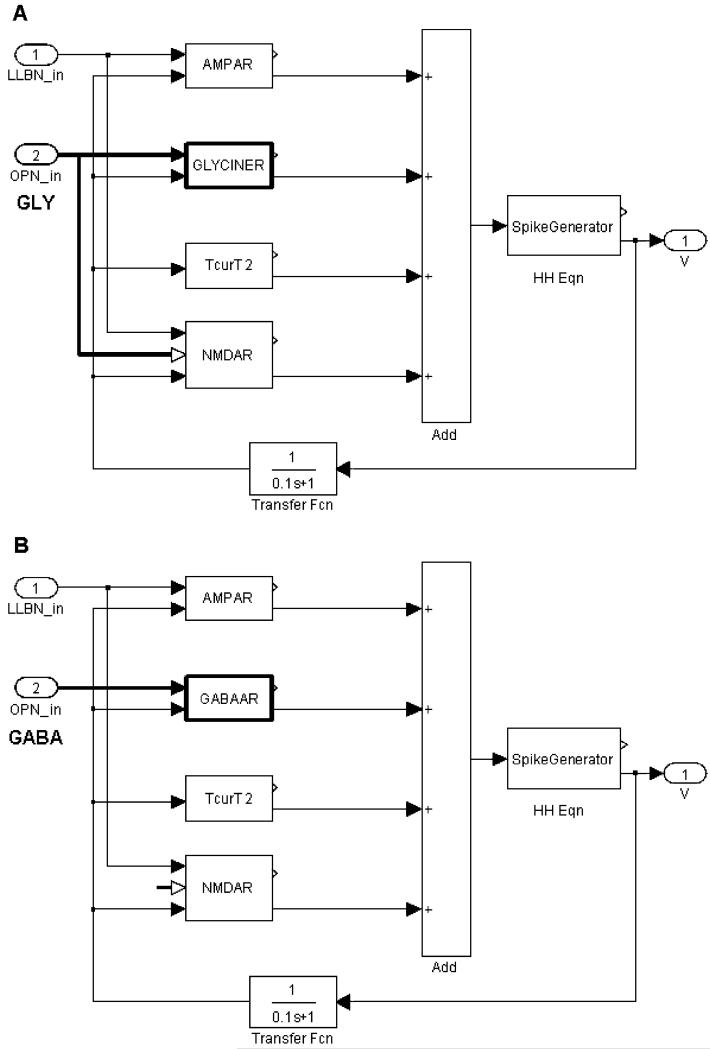
Fig. 2.
Inside the simple burst neuron block. (A) A single-compartment neuron represented by adding currents through each ligand-binding receptor (for AMPA, Gly, NMDA, and a T-type Ca++ channel) to the Hodgkin-Huxley equations for the voltage-gated channels (for details, see Miura and Optican, 2006). Note that Gly acts at both the strychnine-sensitive glycine receptor (GLYCINER) and the co-agonist cite of the NMDA receptor. (B) Burst neuron simulating GABA-ergic OPNs. Note that OPN output goes only to GABAAR, and not to the co-agonist site on the NMDAR.
The dynamics of the channel for the GABAA receptor are more complicated than the first-order equations for GlyR, and its decay is usually described by two or three exponential functions (Rossi and Hamann, 1998; Dumoulin et al., 2001). For the purposes of this paper, the kinetics were simplified to first order and described by the same equations as the GlyR. The range of time constants for the decay of current in the GABAA channel is quite large (about 40-200 ms in rat cerebellar slice or dorsal root ganglion, Rossi and Hamann, 1998; Dumoulin et al., 2001). Whether or not there are faster subtypes of GABAA receptor proteins in the oculomotor neurons is simply not known. The effects of switching from Gly to GABAA in the OPN model depend almost entirely on the choice of this time constant. For this simulation, we chose a decay time constant of 50 ms; the other parameters were the same as for GlyR.
Results
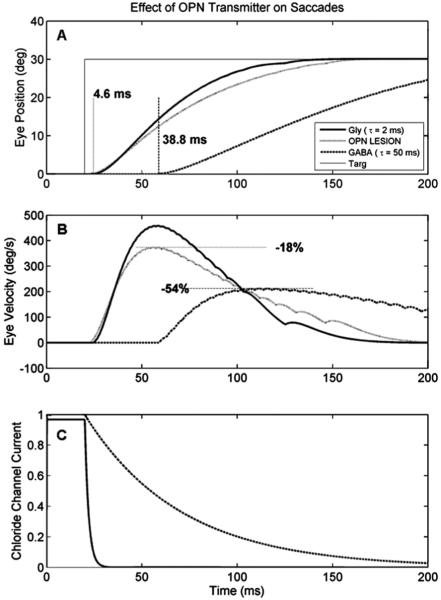
The first model used a glycinergic OPN and generated a normal saccade (Fig. 3, solid lines). The effects of an OPN lesion were then simulated by turning off the OPN completely, so that no Gly was transmitted (Fig. 3, dotted lines). The effect was to reduce the speed of the saccade, but not its amplitude (because of the feedback loop). In this case, the loss of the Gly co-agonist on the NMDA channel caused the peak speed to drop by 18%. The latency (4.6 ms) was not affected, because it is determined by the threshold of the EBN, not the offset of the OPN (Miura and Optican, 2006).
Fig. 3.
Effect of OPN transmitter type on saccades. (A) and (B) show simulated position and velocity traces of a single saccade. When OPNs are glycinergic, the saccade (thick line) is fast (peak speed about 450 deg/s), and starts with a short latency (4.6 ms). After OPN lesion (dotted gray line), the saccade latency and accuracy are unaffected, but saccade slows down by about 18% because the loss of the co-agonist at the NMDAR reduces the gain of the Glu input. When OPNs are made GABA-ergic, saccade (dashed line) starts much later (38.8 ms) and is much slower (by 54%). This difference between the GABA traces and the OPN lesion traces are due to the much slower time course of decay of the GABAA receptor (C).
The effect of an OPN using GABA on a saccade is shown in Fig. 2 (dashed line). First, the peak speed of the movement is greatly reduced (by 54%). This is a much greater drop than can be accounted for by the loss of Gly at the co-agonist site on the NMDAR ( 18%). Thus, the slow recovery of the GABAAR (Fig. 3C) has decreased the acceleration of the EBN firing rate, and thus of the saccade.
Discussion
Here, we propose a new hypothesis about the role and function of OPNs. Evidence shows that OPNs are not simply a gate for saccades, nor are they controlling saccade duration (Busettini and Mays, 2003). Pursuit eye movements slow OPN firing, and OPNs inhibit (but do not stop) pursuit neurons (Missal and Keller, 2002). When OPNs are not active (either during sleep or following lesions of the RIP), saccades are slow, but do not oscillate (Henn et al., 1984; Kaneko, 1996; Soetedjo et al., 2002). We infer from this that OPNs arouse the orienting systems in the brain by increasing their local supply of Gly. Thus, OPN activity signals the brain’s expectation that useful information, which may require orientation towards a new target of interest, is about to arrive. When a new orienting movement is ongoing, the OPN signal is reduced (or stopped) because the brain no longer expects any useful information to arrive during the self-motion. This is consistent with the finding that movements are often preceded by a trigger signal and accompanied by a latch signal that prevent OPNs from firing (Yoshida et al., 1999).
How the expectation signal provided by the OPNs is utilized depends upon the downstream neuron. The use of Gly as the OPN transmitter admits two mechanisms. First, the ratio of NMDA to non-NMDA excitatory receptors could be adjusted to allow the OPN signal to modulate the gain (sensitivity) of the downstream neuron. Second, the ratio of strychnine-sensitive to GABA inhibitory receptors could be adjusted to allow the OPN signal to decrease the sensitivity or even gate the activity of the downstream neuron. Presumably, neurons in circuits that are unstable would have more strychnine-sensitive receptors than those that were intrinsically stable. This predicts that saccadic premotor burst neurons would have more strychnine-sensitive receptors than pursuit neurons.
Reduction in OPN activity during pursuit (Missal and Keller, 2002) can then be seen to have two benefits. First, the threshold for a catch-up saccade may be lowered (less Gly at strychnine-sensitive GlyR). Second, pursuit can only follow one object in a complex visual world. Arousal of other subsystems may be lowered (less Gly at NMDA co-agonist sites), preventing distracting stimuli from interfering with the pursuit of the chosen target.
According to this hypothesis, if OPNs were part of a general arousal system for orienting movements, OPNs would be off or slowed during self-motion, because the expectation that a new stimulus would indicate a required reorientation would be low. This suggests that other systems, in addition to pursuit and saccades, should also have an inhibitory effect on OPNs.
Acknowledgement
This work was supported by the Intramural Research Program of the NEI.
References
- Busettini C, Mays LE. Pontine omnipause activity during conjugate and disconjugate eye movements in macaques. J. Neurophysiol. 2003;90:3838–3853. doi: 10.1152/jn.00858.2002. [DOI] [PubMed] [Google Scholar]
- Buttner-Ennever JA, Cohen B, Pause M, Fries W. Raphe nucleus of the pons containing omnipause neurons of the oculomotor system in the monkey, and its homologue in man. J. Comp. Neurol. 1988;267:307–321. doi: 10.1002/cne.902670302. [DOI] [PubMed] [Google Scholar]
- Cohen B, Henn V. Unit activity in the pontine reticular formation associated with eye movements. Brain Res. 1972;46:403–410. doi: 10.1016/0006-8993(72)90030-3. [DOI] [PubMed] [Google Scholar]
- Dumoulin A, Triller A, Dieudonne S. IPSC kinetics at identified GABAergic and mixed GABAergic and glycinergic synapses onto cerebellar Golgi cells. J. Neurosci. 2001;21:6045–6057. doi: 10.1523/JNEUROSCI.21-16-06045.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evinger C, Kaneko CR, Fuchs AF. Activity of omnipause neurons in alert cats during saccadic eye movements and visual stimuli. J. Neurophysiol. 1982;47:827–844. doi: 10.1152/jn.1982.47.5.827. [DOI] [PubMed] [Google Scholar]
- Furuya N, Markham CH. Direct inhibitory synaptic linkage of pause neurons with burst inhibitory neurons. Brain Res. 1982;245:139–143. doi: 10.1016/0006-8993(82)90348-1. [DOI] [PubMed] [Google Scholar]
- Harty TP, Manis PB. Kinetic analysis of glycine receptor currents in ventral cochlear nucleus. J. Neurophysiol. 1998;79:1891–1901. doi: 10.1152/jn.1998.79.4.1891. [DOI] [PubMed] [Google Scholar]
- Henn V, Baloh RW, Hepp K. The sleep-wake transition in the oculomotor system. Exp. Brain Res. 1984;54:166–176. doi: 10.1007/BF00235828. [DOI] [PubMed] [Google Scholar]
- Horn AK, Buttner-Ennever JA, Wahle P, Reichenberger I. Neurotransmitter profile of saccadic omnipause neurons in nucleus raphe interpositus. J. Neurosci. 1994;14:2032–2046. doi: 10.1523/JNEUROSCI.14-04-02032.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson JW, Ascher P. Glycine potentiates the NMDA response in cultured mouse brain neurons. Nature. 1987;325:529–531. doi: 10.1038/325529a0. [DOI] [PubMed] [Google Scholar]
- Kaneko CR. Effect of ibotenic acid lesions of the omnipause neurons on saccadic eye movements in rhesus macaques. J. Neurophysiol. 1996;75:2229–2242. doi: 10.1152/jn.1996.75.6.2229. [DOI] [PubMed] [Google Scholar]
- Keller EL. Participation of medial pontine reticular formation in eye movement generation in monkey. J. Neurophysiol. 1974;37:316–332. doi: 10.1152/jn.1974.37.2.316. [DOI] [PubMed] [Google Scholar]
- Langer TP, Kaneko CR. Brainstem afferents to the oculomotor omnipause neurons in monkey. J. Comp. Neurol. 1990;295:413–427. doi: 10.1002/cne.902950306. [DOI] [PubMed] [Google Scholar]
- Luschei ES, Fuchs AF. Activity of brain stem neurons during eye movements of alert monkeys. J. Neurophysiol. 1972;35:445–461. doi: 10.1152/jn.1972.35.4.445. [DOI] [PubMed] [Google Scholar]
- Missal M, Keller EL. Common inhibitory mechanism for saccades and smooth-pursuit eye movements. J. Neurophysiol. 2002;88:1880–1892. doi: 10.1152/jn.2002.88.4.1880. [DOI] [PubMed] [Google Scholar]
- Miura K, Optican LM. Membrane channel properties of premotor excitatory burst neurons may underlie saccade slowing after lesions of omnipause neurons. J. Comp. Neurosci. 2006;20:25–41. doi: 10.1007/s10827-006-4258-y. [DOI] [PubMed] [Google Scholar]
- Nakao S, Curthoys IS, Markham CH. Direct inhibitory projection of pause neurons to nystagmus-related pontomedullary reticular burst neurons in the cat. Exp. Brain Res. 1980;40:283–293. doi: 10.1007/BF00237793. [DOI] [PubMed] [Google Scholar]
- Rossi DJ, Hamann M. Spillover-mediated transmission at inhibitory synapses promoted by high affinity alpha6 subunit GABA(A) receptors and glomerular geometry. Neuron. 1998;20:783–795. doi: 10.1016/s0896-6273(00)81016-8. [DOI] [PubMed] [Google Scholar]
- Soetedjo R, Kaneko CR, Fuchs AF. Evidence that the superior colliculus participates in the feedback control of saccadic eye movements. J. Neurophysiol. 2002;87:679–695. doi: 10.1152/jn.00886.2000. [DOI] [PubMed] [Google Scholar]
- Spencer RF, Wenthold RJ, Baker R. Evidence for glycine as an inhibitory neurotransmitter of vestibular, reticular, and prepositus hypoglossi neurons that project to the cat abducens nucleus. J. Neurosci. 1989;9:2718–2736. doi: 10.1523/JNEUROSCI.09-08-02718.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strassman A, Evinger C, McCrea RA, Baker RG, Highstein SM. Anatomy and physiology of intracellularly labelled omnipause neurons in the cat and squirrel monkey. Exp. Brain Res. 1987;67:436–440. doi: 10.1007/BF00248565. [DOI] [PubMed] [Google Scholar]
- Yoshida K, Iwamoto Y, Chimoto S, Shimazu H. Saccade-related inhibitory input to pontine omnipause neurons: an intracellular study in alert cats. J. Neurophysiol. 1999;82:1198–1208. doi: 10.1152/jn.1999.82.3.1198. [DOI] [PubMed] [Google Scholar]