Abstract
The tandem N-arylation/carboamination of γ-amino alkenes with two different aryl bromides provides rapid entry to differentially arylated N-aryl-2-benzyl pyrrolidine derivatives in good yields with good to excellent levels of diastereoselectivity. The selective diarylation is achieved in a one-pot process by an in situ modification of the palladium catalyst via phosphine ligand exchange.
In recent years much effort has been devoted to the development of tandem or sequential reactions that effect the formation of several different bonds, stereocenters, and/or rings in one-pot transformations.1 These types of reactions lead to the conversion of simple starting materials to relatively complex molecules in a rapid and efficient manner, and have considerable potential utility for the construction of diverse libraries of compounds. Transition metal catalysis has played a central role in the development of many of these processes.1, 2
Transformations effected through tandem metal-catalyzed reactions can be broadly grouped into two categories: 1) sequences that involve multiple iterations of the same reaction,2 and 2) sequences that involve two or more fundamentally different reactions.3 The latter tend to be more challenging as the efficiency and selectivity of many metal-catalyzed reactions is highly dependent on catalyst structure.
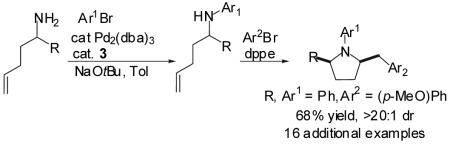
We have recently described a new method for the stereoselective synthesis of N-aryl-2-benzylpyrrolidines via Pd-catalyzed reactions of γ-(N-arylamino)alkenes with aryl bromides (Scheme 1, eq 1).4,5,6 In our initial studies, several of the substrates for these transformations were prepared by Pd-catalyzed N-arylation of primary amines.7 Since both the carboamination and the N-arylation reactions were effected using palladium/phosphine catalysts, we reasoned that a modular synthesis of N-aryl-2-benzylpyrrolidines might be achieved in a one-pot, Pd-catalyzed process via sequential treatment of a 4-pentenylamine derivative with two different aryl bromides (Scheme 1, eq 2). A transformation of this type would lead to the formation of two C-N bonds, one C-C bond, one ring, and one stereocenter in a single operation. Additionally, this protocol could potentially be employed in the conversion of readily available starting materials into pyrrolidine libraries in which two groups on the heterocycle could be easily varied.8 Our preliminary studies on the development of this one-pot reaction sequence are described herein.
Scheme 1.
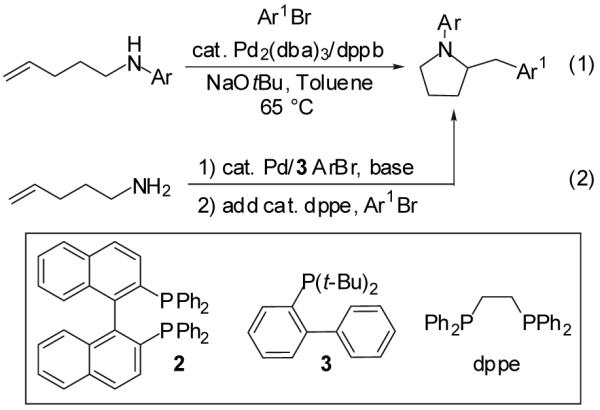
Both palladium-catalyzed N-arylations of amines and Pd-catalyzed carboamination reactions are known to be very sensitive to catalyst structure.4,7 A search of the literature revealed that relatively few ligands are highly effective and selective for the Pd-catalyzed monoarylation of primary aliphatic amines; (rac)-BINAP (2)9 and 2-di-tert-butylphosphinobiphenyl (3) appeared to be the most general with respect to substrate scope.7 Our previous studies indicated that 2 was only marginally effective for the conversion of γ-(N-arylamino)alkenes to N-aryl-2-benzylpyrrolidines, whereas 3 provided low yields in all reactions examined.4,10 Thus, we felt that it would be necessary to carry out the desired sequential transformation by modifying the palladium catalyst following the N-arylation reaction. This would allow for maximum efficiency of each reaction in the sequence, and could be achieved via an in situ ligand exchange that would not require the use of additional palladium or workup/purification between steps. We chose to employ the monodentate ligand 3 for the first step of the reaction sequence, as we felt that chelating ligand 2 would be more difficult to replace with a different phosphine prior to the second step of the sequence. The chelating ligand dppe9 was employed for the second reaction of the sequence, as we have found this ligand to be effective in a broad range of carboamination processes.4
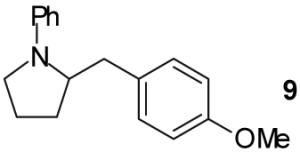
In a representative experiment, a 0.25 M toluene solution of 4-pentenylamine (1) and one equivalent of bromobenzene were added to a dry, argon-filled Schlenk tube containing catalytic amounts of Pd2(dba)3 (1 mol %) and 3 (2 mol %) and 2.4 equiv of NaOtBu. The reaction mixture was heated to 60 °C with stirring and monitored periodically by GC analysis. When the bromobenzene had been completely consumed (c.a. 30-90 min), a 0.005 M toluene solution of dppe (2 mol %) was added and the reaction mixture was heated to 110 °C for 15 min to allow the exchange process to occur. 4-Bromoanisole (1.2 equiv) was then added and heating was continued until the intermediate N-aryl alkylamine was completely consumed. Upon aqueous workup and chromatographic purification the desired N-phenyl-2-(4-methoxybenzyl)pyrrolidine product (9) was obtained in 67% yield (Table 1, entry 1, Method A).
Table 1.
(a) Palladium-Catalyzed Stereoselective Synthesis of N-Aryl-2-Benzylpyrrolidine Derivatives
| entry | amine | product | method | dr | yield(c) |
|---|---|---|---|---|---|
| 1 |

|
|
A | - | 67%(d) |
| 2 | B | - | 70%(d) | ||
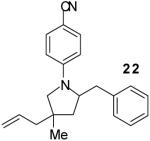
| 3 | 1 |
|
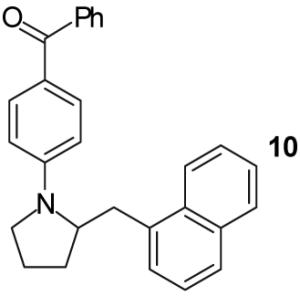
A | - | 72%(d) |
| 4 | B | - | 92%(d) | ||
| 5 | 1 |
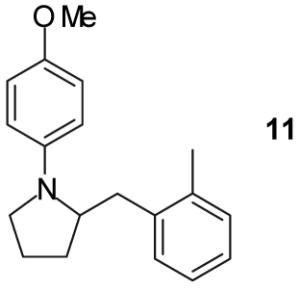
|
A | - | 17%(d) |
| 6 | B | - | 0% | ||
| 7 |
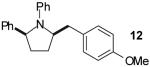
|
|
A | >20:1 | 68%(d) |
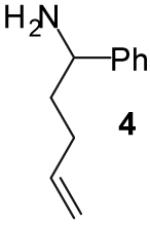
| 8 | 4 |
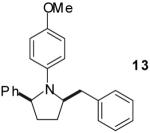
|
A | >20:1 | 67%(d) |
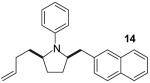
| 9 |
|
|
A | >20:1 | 70% |
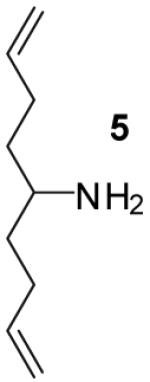
| 10 | 5 |
|
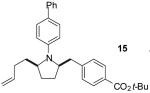
A | >20:1 | 65%(d) |
| 11 |
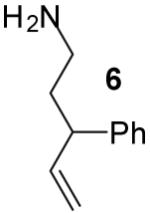
|
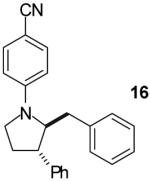
|
A | >20:1 | 56% |
| 12 | 6 |
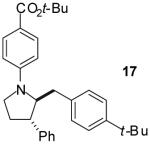
|
A | >20:1 | 41% |
| 13 | 6 |
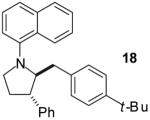
|
A | >20:1 | 51% |
| 14 |
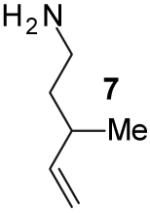
|
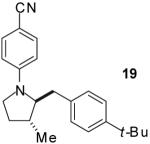
|
A | 9:1 | 71% |
| 15 | 7 |
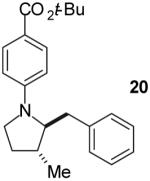
|
A | 9:1 | 42% |
| 16 |
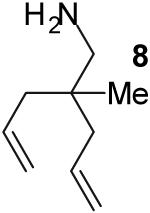
|
|
A | 3:2 | 49% |
| 17 | 8 |
|
A | 3:2 | 63% |
Method A: 1.0 equiv amine, 1.0 equiv ArBr, 2.4 equiv NaOtBu, 1 mol % Pd2(dba)3, 2 mol % 3, toluene (0.125 M), 60 °C then 2 mol % dppe, 1.2 equiv Ar1Br, 110 °C. Method B: 1.0 equiv amine, 1.0 equiv ArBr, 2.4 equiv NaOtBu, 1 mol % Pd2(dba)3, 2 mol % (rac)-BINAP (2), toluene (0.125 M), 80 °C then 1.2 equiv Ar1Br, 80 °C.
Yields represent average isolated yields from two or more experiments.
This product contained a small amount (c.a. 5-10%) of an inseparable regioisomer.
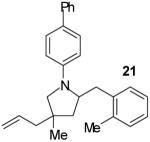
As shown in Table 1, this method is effective for the conversion of a variety of γ-aminoalkenes to N-aryl-2-benzylpyrrolidine derivatives.11 In general, the best results are obtained when electron-neutral or -deficient aryl bromides are employed in the first step of the tandem transformation. Use of electron-rich aryl halides such as 4-bromoanisole as the first coupling partner often resulted in slow reactions and provided only modest yields of the desired, differentially arylated products due to the formation of N-aryl-2-benzylpyrrolidine side products that had incorporated two equivalents of the electron-rich arene (entry 5). However, the reaction of substrate 4, which bears a phenyl substituent at the 1-position proceeded in good yield when 4-bromoanisole was employed as the first coupling partner (entry 7). Electron-neutral or -rich aryl bromides provide the best yields in the second step of the sequence; use of electron-deficient halides as the second coupling partner often led to the formation of substantial amounts of N,N-diarylated side products.
The tandem transformations of substrates 4-7, which bear substituents at the 1- or 3-position, proceeded with good to excellent levels of diastereoselectivity.12 Reactions of 1-substituted aminoalkenes 4 and 5 afforded 2,5-cis-disubstituted pyrrolidines with >20:1 dr (entries 7-10). Transformations of 3-phenyl substituted amine 6 (entries 11-13) also proceeded with excellent stereocontrol (>20:1 dr), and the analogous 3-methyl substituted substrate 7 was transformed with good (c.a. 10:1) diastereoselectivity for the formation of 2,3-trans-disubstituted products (entries 14-15). In contrast, the reactions of 8 proceeded with low stereoselectivity (entries 16-17). The stereochemical outcome of these reactions parallels that observed in previously described reactions of γ-(N-arylamino)alkenes with aryl bromides.4
The use of BINAP as ligand for both steps of the tandem transformation was briefly examined and was found to be effective in some reactions of the unsubstituted 4-pentenylamine (1) (Table 1, entries 2 and 4). However, these conditions were not effective for reactions of the more substituted amines 4-8.13
In summary, the Pd-catalyzed tandem N-arylation/carboamination of primary aliphatic amines proceeds in moderate to good yield to afford differentially arylated N-aryl-2-benzylpyrrolidine derivatives. Although each of the individual catalytic reactions requires a different phosphine ligand to achieve optimal yields, the two reactions can be conducted sequentially in one-pot via an in situ ligand exchange protocol that allows modification of the catalyst structure after the first arylation. Further studies on the use of in situ ligand exchange in sequential one-pot catalytic processes and efforts to extend the scope of this transformation to allow for the synthesis of six- and seven-membered nitrogen heterocycles are currently underway.
Supplementary Material
Acknowledgment
The authors thank the University of Michigan and the NIH-NIGMS (GM 071650) for financial support of this work. JPW thanks the Camille and Henry Dreyfus Foundation for a new faculty award, and Research Corporation for an innovation award. JEN acknowledges the University of Michigan for a Regents Fellowship and Pfizer for a Graduate Research Fellowship. Additional unrestricted support was provided by Amgen, Eli Lilly, and 3M.
References
- 1.(a) Tietze LF, Rackelmann N. Pure Appl. Chem. 2004;76:1967. [Google Scholar]; (b) Nicolaou KC, Montagnon T, Snyder SA. Chem. Commun. 2003:551. doi: 10.1039/b209440c. [DOI] [PubMed] [Google Scholar]; (c) Parsons PJ, Penkett CS, Shell AJ. Chem. Rev. 1996;96:195. doi: 10.1021/cr950023+. [DOI] [PubMed] [Google Scholar]; (d) Tietze LF. Chem. Rev. 1996;96:115. doi: 10.1021/cr950027e. [DOI] [PubMed] [Google Scholar]
- 2.(a) Heumann A, Reglier M. Tetrahedron. 1996;52:9289. [Google Scholar]; (b) Malacria M. Chem. Rev. 1996;96:289. doi: 10.1021/cr9500186. [DOI] [PubMed] [Google Scholar]
- 3.For recent examples, see: Ajamian A, Gleason JL. Angew. Chem., Int. Ed. 2004;43:3754. doi: 10.1002/anie.200301727. Yamamoto Y, Nakagai Y, Itoh K. Chem. Eur. J. 2004;10:231. doi: 10.1002/chem.200305340. Cossy J, Bargiggia F, BouzBouz S. Org. Lett. 2003;5:459. doi: 10.1021/ol027347m. Thadani AN, Rawal VH. Org. Lett. 2002;4:4317. doi: 10.1021/ol0269594. Son SU, Park KH, Chung YK. J. Am. Chem. Soc. 2002;124:6838. doi: 10.1021/ja0260420. Louie J, Bielawski CW, Grubbs RH. J. Am. Chem. Soc. 2001;123:11312. doi: 10.1021/ja016431e. Evans PA, Robinson JE. J. Am. Chem. Soc. 2001;123:4609. doi: 10.1021/ja015531h.
- 4.Ney JE, Wolfe JP. Angew. Chem., Int. Ed. 2004;43:3605. doi: 10.1002/anie.200460060. [DOI] [PubMed] [Google Scholar]
- 5.For related syntheses of N-acyl- and N-boc-2-benzylpyrrolidines, see: Bertrand MB, Wolfe JP. Tetrahedron. 2005 In Press.
- 6.For related syntheses of tetrahydrofurans, see: Wolfe JP, Rossi MA. J. Am. Chem. Soc. 2004;126:1620. doi: 10.1021/ja0394838. Hay MB, Hardin AR, Wolfe JP. J. Org. Chem. 2005;70:3099. doi: 10.1021/jo050022+.
- 7.For recent reviews on Pd-catalyzed N-arylation reactions see: Muci AR, Buchwald SL. Top. Curr. Chem. 2002;219:131. Hartwig JF. In: Modern Arene Chemistry. Didier A, editor. Wiley-VCH; Weinheim: 2002. p. 107. Schlummer B, Scholz U. Adv. Synth. Catal. 2004;346:1599.
- 8.For related transformations of 2-allylanilines to N-aryl-2-benzylindolines, see: Lira R, Wolfe JP. J. Am. Chem. Soc. 2004;126:13906. doi: 10.1021/ja0460920.
- 9.BINAP = 2,2′-diphenylphosphino-1,1′-binaphthyl, dppe = 1,2-bis(diphenylphosphino)ethane, dpe-phos = bis(2-diphenylphosphinophenyl)ether.
- 10.The Pd2(dba)3/3 catalyzed reaction of N-(4-pentenyl)aniline with 2-bromonaphthalene afforded <5% yield of the desired pyrrolidine; use of 2 in place of 3 provided a 65% GC yield of the pyrrolidine product. However, the Pd2(dba)3/2 catalyzed reaction of 5-(N-phenylamino)-1,8-nonadiene with 2-bromonaphthalene afforded only 41% isolated yield of the desired 2,5-disubstituted pyrrolidine.
- 11.Small amounts (c.a. 5-10%) of 2-methyl-3-arylpyrrolidine derivatives were also formed in some of these transformations. See the Supporting Information for further details.
- 12.Diastereomeric ratios were determined by 1H NMR and/or GC analysis. The relative stereochemistry of the products was assigned based on the results of 1H NMR nOe experiments or by comparison of spectra to related compounds of known configuration. See the Supporting Information for further details.
- 13.Use of BINAP in the second step of the tandem reaction led to the formation of increased amounts of N,N-diarylamino alkenes bearing two different N-aryl substituents.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.