Abstract
Purpose
TRA-8 is an agonistic mouse monoclonal antibody that binds to TRAIL death receptor 5 (DR5) which induces apoptosis in cancer cells through a caspase-8 dependent mechanism. We investigated the ability of TRA-8 to augment radiation therapy (RT) and chemotherapy response of human glioma cells in vitro and in vivo.
Methods and Materials
In vitro cytotoxicity of TRA-8 and temozolomide (Tmz) or RT was examined using ATP dependent viability and clonogenic survival assays with 5 glioma cell lines. DR5 expression was determined by flow cytometry. In vivo studies included subcutaneous and intracranial xenograft models testing various combination treatments, including RT, Tmz, and TRA-8.
Results
TRA-8 combined with Tmz or RT produced enhanced cytotoxicity against 5 glioma cell lines compared to individual agents. DR5 upregulation occurred in response to RT. Complete tumor regressions in subcutaneous experiments were most common in animals that received combination therapy with TRA-8/Tmz/RT. TRA-8 enhanced tumor growth delay in combination with RT or Tmz. TRA-8 alone had limited activity against intracranial tumors, whereas median survival of mice treated with TRA-8/Tmz/RT was significantly greater than control or TRA-8 alone treated mice. Median survival of mice treated with TRA-8/Tmz/RT or chemoradiation only was significantly greater than control or TRA-8 treated mice. A trend towards improved survival was observed comparing TRA-8/Tmz/RT treated animals vs. Tmz/RT.
Conclusions
These preliminary findings support the hypothesis that TRA-8 will augment RT and chemotherapy response in gliomas. A humanized version of TRA-8 is being evaluated in a Phase II clinical trial.
Keywords: TRAIL, apoptosis, glioblastoma multiforme, radiation therapy, temozolomide
INTRODUCTION
Radiation therapy (RT) and chemotherapy modestly improve overall survival after surgical resection for malignant glioma, but newer therapies are clearly needed (1, 2). Targeting cell survival or apoptotic pathways is one strategy to improve overall survival for patients with cancer. Tumor necrosis factor-related apoptosis-inducing ligand (TRAIL, Apo2L) is a member of the TNF superfamily that induces apoptosis after binding to membrane bound death receptors through a caspase-8 to -3 dependent mechanism (extrinsic pathway) or through caspase-8 to depolarization of mitochondria and release of cytochrome c (intrinsic pathway) (3). TRAIL plays a role in the neutralization of activated lymphocytes, killing of virally infected cells, immune mediated tumor cell death, and tissue turnover during embryogenesis. Five death receptors have been described for TRAIL including two pro-apoptotic receptors (DR4 and DR5) and three decoy receptors. The therapeutic efficacy of systemic TRAIL may be limited by the induction of apoptosis in some normal tissues including hepatocytes. To further improve upon the specificity of TRAIL-based anticancer therapies, pro-apoptotic monoclonal antibodies against TRAIL death receptors have been developed. TRA-8 is one such antibody directed against DR5. These antibodies may have clinical applications in cancer and autoimmune or inflammatory diseases (4–6). TRAIL and DR5 agonistic antibodies have activity both as pro-apoptotic agents, but also in stimulating immune-mediated tumor surveillance (7).
Preclinical studies have demonstrated that TRA-8 does not induce apoptosis in normal human hepatocytes and does not competitively bind to decoy receptors, suggesting that it may have greater anti-tumor specificity than TRAIL (4). The expression of decoy receptors is tumor grade dependent in gliomas and thus the specificity of TRA-8 may be of clinical relevance in this disease (8). Preclinical studies have shown anti-tumor activity of TRA-8 against a variety of tumor types as monotherapy and enhanced tumor cell kill in combination with both RT and chemotherapy (9–14). TRAIL mediated cell death occurs independent of oxygenation level, which may be particularly relevant in necrotic tumors such as glioblastoma multiforme (15). Temozolomide (Tmz) chemotherapy has recently been shown to improve overall survival in newly diagnosed glioblastoma multiforme when administered concurrently with and after conventional RT (16). In the current study, the preclinical therapeutic efficacy of TRA-8 alone and in combination with RT and Tmz chemotherapy was evaluated in vitro using 5 glioma cell lines and in s.c. and intracranial models using D54MG xenografts.
METHODS AND MATERIALS
Cells and reagents
Human glioblastoma cell lines were cultured at 37°C and 5% CO2 atmosphere in DMEM:F12 medium with 7% FBS (D54MG, CH235MG, U87MG), EMEM with nonessential amino acids and 10% FBS (U373MG), or RPMI 1640 medium with 10 mM HEPES, 1 mM sodium pyruvate, 4.5 g/L glucose, and 10% FBS (U251MG). Purified TRA-8 (IgG1) monoclonal antibody used for in vitro studies was produced and purified as previously described (4). Daiichi Sankyo (Tokyo, Japan) provided preparations used for in vivo studies. Temozolomide (Temodar; Schering Corp., Kenilworth, NJ) was obtained from the University of Alabama at Birmingham Hospital Pharmacy (Birmingham, AL) as 5 mg tablets which were dissolved in DMSO and centrifuged to remove insoluble material. Final DMSO concentration in culture medium was <0.1%. Isotype-specific IgG1 control antibody and goat anti-mouse IgG1-phycoerythrin (PE) were from Southern Biotechnology Associates (Birmingham, AL). Collagenase type 11 and protease inhibitor cocktail were from Sigma Chemical Co. (St. Louis, MO). Antibodies for Western blot analysis were as follows: caspase 3 and XIAP (Stressgen, Ann Arbor, MI); caspase 8 (BD Pharmingen, San Jose, CA); Bax (Southern Biotechnology Associates); Bid and Bcl-xl, (Cell Signaling Technologies, Beverly, MA); p53 (Calbiochem, San Diego, CA); and actin (Sigma Chemical Co.). HRP-conjugated goat anti-mouse IgG and anti-rabbit IgG were from Bio-Rad. ECL enhanced chemiluminescence reagents were from GE Healthcare (Piscataway, NJ).
Cell viability assays using ATPLite
The cytotoxicity of TRA-8 was compared to soluble TRAIL plus anti-Flag crosslinker (Alexis, San Diego, CA) using 10 glioma cell lines. Cells were plated (1,000 cells/well) in 96-well black plates and incubated overnight before starting treatments. For combination treatments, cells were pretreated with Tmz, RT, or Tmz followed 1 h later by RT. TRA-8 was added 24 h later and cell viability was assessed 5 days after starting treatments using ATPLite assay (Perkin Elmer Biosciences, Meriden, CT). Samples were assayed in quadruplicate and reported as mean ± SD from representative experiments, which were performed at least twice.
Clonogenic survival (CS) assay
D54MG cells were seeded in 24-well plates at 10,000 cells/well and incubated overnight. TRA-8 (39, 78, and 155 ng/ml) and Tmz (2 or 4 μM) were added to the media for 18 h. The cells were washed, trypsinized and replated in 6-well plates at 1,000 cells/well. Relative surviving fraction was determined 11 days later by counting colonies of >50 cells. There were 2 to 4 replicates per data point.
Curve fitting to the experimental data was done with CurveExpert 1.3 software (http://curveexpert.webhop.biz/; Daniel G. Hyams, Hixson, TN). Inhibition concentration at 50% (IC50) was calculated from regression models. Results of CS assays were analyzed for interaction between modalities, using CombiTool software (Biocomputing Institut für Molekulare Biotechnologie, Jena, Germany). Analysis of synergy/antagonism of TRA-8 and Tmz was performed by comparing theoretical and experimental effects. A theoretical dose response surface was generated based on the results obtained from the separate treatments with each individual agent. It represents the calculated additive effect of combined doses of TRA-8 and Tmz. If experimental data points map above the theoretical surface, coincide with it, or fall below it, the interaction of modalities is defined as synergistic, additive or antagonistic respectively (17). This method assigns a “synergy index” to each experimental data point to quantify synergy. Zero interaction is defined when index (I) = 1. I < 1 represents synergism, and I > 1 represents antagonism. The results of a representative experiment are shown.
Indirect immunofluorescence and flow cytometry analysis of DR5 expression
DR5 cell surface expression was determined using flow cytometry (FACScan and CellQuest software, Becton Dickinson, San Jose, CA) as previously described (13). To examine the effect of Tmz or RT on DR5 expression, glioma cancer cell lines were treated with 10 μM Tmz, 2 Gy RT, or 10 μM Tmz followed 1 h later by 2 Gy RT. Cells were harvested 24 h after starting treatment, incubated with TRA-8 and PE-conjugated goat anti-mouse IgG1, and analyzed by flow cytometry.
Mitochondrial depolarization assay
D54MG cells were seeded in 96-well plates at 10,000 cells/well and incubated overnight. Cells were loaded for 30 min with 10 μg/mL JC-1 (5,5′,6,6′-tetrachloror-1,1′,3,3′-tetraethylbenzimidazolylcarbocyanine iodide) (Molecular Probes, Inc. Eugene, OR), a cationic dye that exists as green-fluorescent monomers at low membrane potential or red-fluorescent “J-aggregates” at higher concentrations associated with higher membrane potentials. Immediately after loading, cells were washed in PBS and treated with TRA-8, Tmz, and RT. JC-1 dual fluorescence (excitation/emission, Ex/Em = 485 nm/528 nm and Ex/Em = 485 nm/590 nm) was monitored with a Synergy HT plate reader (Bio-Tek Instruments, Winooski, VT). Change in 590 nm to 528 nm fluorescence ratio represents the leakage of JC-1 from mitochondria.
Western blot analysis of glioma cell lines treated with TRA-8 and Tmz or radiation
Cell lines were trypsinized, plated in 6-well plates (5 × 105 cells/well), and incubated overnight before starting treatments. Cells were treated with 30 μM Tmz or 5 Gy radiation for 21 h at 37°C, then TRA-8 was added (U373MG, 5 or 25 ng/mL; CH235MG and D54MG, 25 or 125 ng/mL; U251MG and U87MG, 125 or 1,000 ng/mL). Whole cell lysates were prepared after 3 h treatment with TRA-8 and analyzed as described previously (14).
Subcutaneous therapy experiments
Athymic nude mice were injected s.c. with 2×107 D54MG cells. When tumors reached approximately 0.2 cm3 in volume as determined from calculation (0.4 × width2 × length) using two perpendicular measurements with a vernier caliper, mice were randomized into treatment groups and treatments begun. Eight treatment groups of 7 mice each were compared: untreated control, TRA-8 alone, RT alone, Tmz alone, RT plus TRA-8, RT plus Tmz, TRA-8 plus Tmz, and TRA-8 plus Tmz plus RT (triple therapy). Mice were injected i.p. with 200 μg TRA-8 on days 15, 18, 22, 25, 29, and 32 after tumor cell injection. Mice received 50 mg/kg Tmz by gavage on days 16, 18, 21, 23, and 25. Tumors were irradiated with five 5 Gy fractions (25 Gy total) of 60Co RT given 1 h after each Tmz dose. RT was delivered to the tumor region using 8 cm lead shielding to minimize whole body exposure. In a second s.c. experiment, 8 treatment groups with 8 animals per group were compared but the total dose of chemoradiation was lowered. Temozolomide was administered at 50 mg/kg on days 14, 16, and 19, and RT was given in 3 fractions of 2 Gy 1 h after Tmz administration. The primary endpoints of s.c. experiments were 1) tumor response, as determined from volume measurement described above, and 2) regrowth, to initial treatment volume, if tumor regression occurred. All mouse studies were conducted under Institutional Animal Care and Use Committee approval (APN 070106018).
Intracranial therapy experiments
D54MG tumors were established by stereotactic intracranial injection of mice and treated with TRA-8 alone, Tmz plus RT, or TRA-8 plus Tmz plus RT vs. untreated control. Eight to eleven animals were treated per group in each experiment. The 60Co RT was administered in an 8×32 cm collimated beam to 12 animals arranged on a 5 cm backscatter board in a head-to-head manner, with the bodies shielded from the back of the ears caudally with 8 cm thick cerrobend lead shielding. The primary endpoint of intracranial experiments was survival. When mice became moribund as indicated by loss of normal feeding, grooming and avoidance behavior (neurologic signs of increasing tumor burden) or experienced a 20% decline in initial body weight, they were killed and that date was used to determine survival from tumor induction. All mice were necropsied to ascertain that the cause of death was due to intracranial tumor. In the first study, 5 × 105 D54MG cells were injected. Due to observed weight loss during treatment, only partial therapy was administered: 200 μg TRA-8 given by i.p. injection on days 10, 14, and 17 after tumor cell injection; 3 doses of 3 Gy RT given on days 11, 14, and 16; and 50 mg/kg Tmz given 1 h prior to RT. Animals were then followed after treatment was discontinued. In the second intracranial experiment, the number of injected tumor cells was lowered to 2.5 × 105. At this lower tumor burden, weight loss was not observed and all animals were able to complete treatment. TRA-8 was administered on days 7, 9, 13, 16, 20, and 23 after tumor cell injection. RT was given at 3 Gy on days 7, 9, 12, 14, and 16 at 1 h after administration of Tmz (50 mg/kg) by gavage. Survival was estimated utilizing the Kaplan-Meier method and comparisons between groups were performed by log-rank test.
RESULTS
In vitro cytotoxicity of TRA-8 in combination with Tmz and radiation
The in vitro cytotoxicity of TRAIL was compared to TRA-8 using an ATP dependent viability assay against 10 different glioma cell lines. Figure 1 shows that TRA-8 produced equal or greater cytotoxicity compared to TRAIL in all cell lines. The in vitro efficacy of TRA-8 in combination with Tmz, RT, or Tmz plus RT was examined using 5 human glioma cell lines of varying sensitivity to TRA-8, including sensitive (U373MG), intermediate (D54MG, CH235MG), and resistant (U251MG, U87MG) cell lines. Figure 2 shows that combination treatment with TRA-8 and Tmz produced increased killing of all 5 glioma cell lines after 24 h pretreatment with Tmz followed by 96 h treatment with Tmz plus TRA-8. Figure 3 shows that increased killing also occurred in the 5 glioma cell lines treated with 2, 5 or 10 Gy RT followed 24 h later by treatment with TRA-8 for 96 h. The effect of chemoradiation was investigated in 5 glioma cell lines treated with Tmz and RT in combination with TRA-8. The results indicated enhanced cytotoxicity of combination treatment with all 3 modalities over the individual treatments or combinations of two modalities (Fig. 4).
Figure 1.
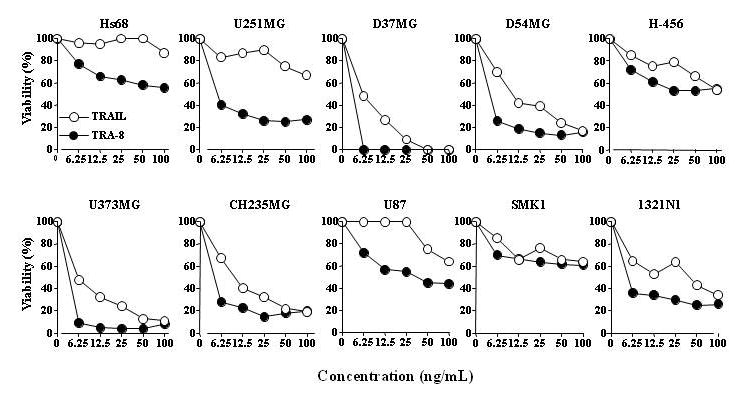
Relative cytotoxicity of TRA-8 in glioma cell lines compared to TRAIL. Cells were treated with various concentrations of recombinant TRAIL plus anti-FLAG crosslinker or TRA-8 overnight. Cell viability was determined by the ATPLite assay.
Figure 2.
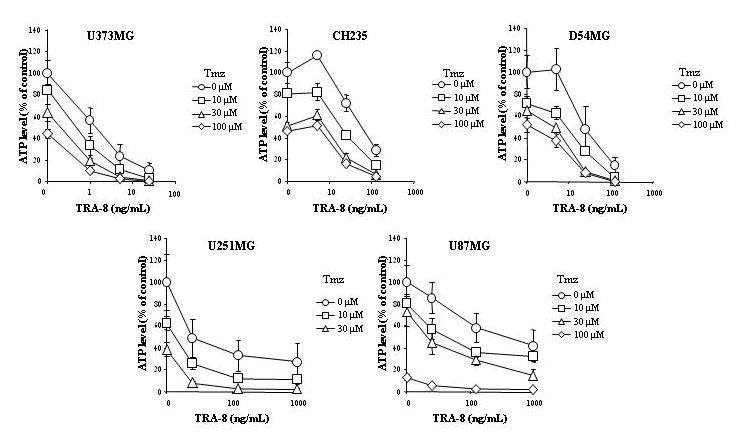
Cytotoxicity of TRA-8 in combination with Tmz treatment of glioma cell lines. Cells were treated with 0–100 μM Tmz for 24 h followed by 96 h treatment with TRA-8 alone, Tmz alone, or TRA-8 plus Tmz. ATP levels were determined 5 d after the start of treatments.
Figure 3.
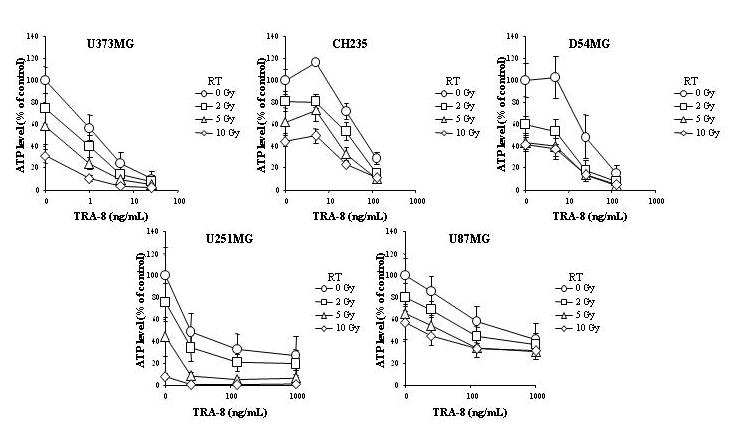
Cytotoxicity of TRA-8 in combination with radiation treatment of glioma cell lines. Cells were treated with 0–10 Gy RT, and TRA-8 was added 24 h later. ATP levels were determined after 5 d treatment.
Figure 4.
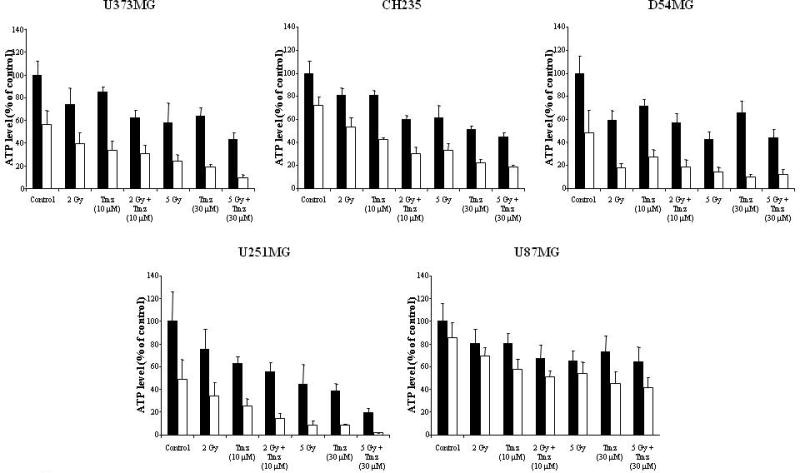
Cytotoxicity of TRA-8 in combination with Tmz and radiation treatment of glioma cell lines. Cells were treated with 10 μM Tmz followed 1 h later by 2 Gy RT or treated with 30 μM Tmz followed by 5 Gy, then TRA-8 was added 24 h after RT. ATP levels were determined after 5 d treatment. Closed bars represent cells incubated without TRA-8. Open bars represent cells treated with TRA-8 at 1 ng/mL (U373MG), 25 ng/mL (CH235MG, D54MG, U251MG), or 1,000 ng/mL (U87MG).
Clonogenic survival assays were also used to detect enhanced in vitro killing of D54MG cells using TRA-8 in combination with Tmz, as shown in Figure 5. The TRA-8 alone dose response curve from Figure 5 was fitted to a linear model [y=a+bx] (SE = 0.034, R = 0.994). The Tmz alone curve was fitted to a sinusoidal model [y=a+b*cos(cx+d)] (SE = 0, R = 1), and the combination of TRA-8 and 2 μM Tmz curve was fitted to an exponential association model [y=a(b−exp(−cx))] (SE = 0.021, R = 0.999). Analysis of dose response curves from Figure 5 revealed that adding 2 μM Tmz to TRA-8 resulted in lowering TRA-8 IC50 values from 126.8 ng/ml to 9.8 ng/ml, whereas the IC50 value for Tmz alone was 3.2 μM. An analysis of synergy or antagonism of TRA-8 and Tmz combination treatment presented in Figure 5 was performed, and an interaction index (I) was assigned to each experimental data point. For TRA-8 (39, 78, 156 ng/ml) in combination with 2 μM Tmz, values of I = 0.570, 0.608 and 0.694 were calculated, respectively, indicating that the strongest synergistic interaction occurred at the lowest TRA-8 dose.
Figure 5.
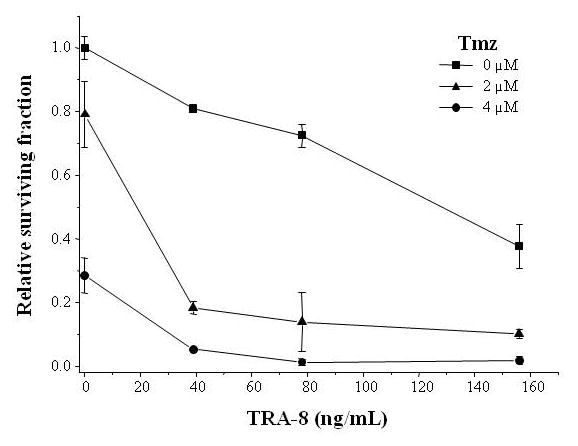
Effect of TRA-8 and Tmz on clonogenic survival of D54MG cells. Cells were treated with TRA-8 and Tmz for 18 h then trypsinized and replated. Viable colonies (>50 cells/colony) were counted after 11 days of growth. Surviving fraction was calculated by normalizing the number of colonies present after Tmz treatment to the untreated control.
Mechanisms of chemotherapy and radiation enhancement of TRA-8 induced cytotoxicity
The induction of apoptosis is associated with a disruption in mitochondrial membrane integrity and subsequent reduction in transmembrane potential. Mitochondrial membrane depolarization was investigated in response to treatment with TRA-8 alone and in combination with Tmz or RT using the fluorescent dye JC-1, which differentially stains aggregates of the dye red under conditions of high mitochondrial potential and stains monomeric JC-1 molecules green under conditions of low membrane potential. The ratio of JC-1 aggregates to monomers in D54MG cells was reduced by 21 h treatment with TRA-8, indicating a loss in mitochondrial membrane potential, as shown in Figure 6. Neither 100 μM Tmz nor 3 Gy RT treatment contributed to the reduction of mitochondrial membrane potential relative to TRA-8 alone.
Figure 6.
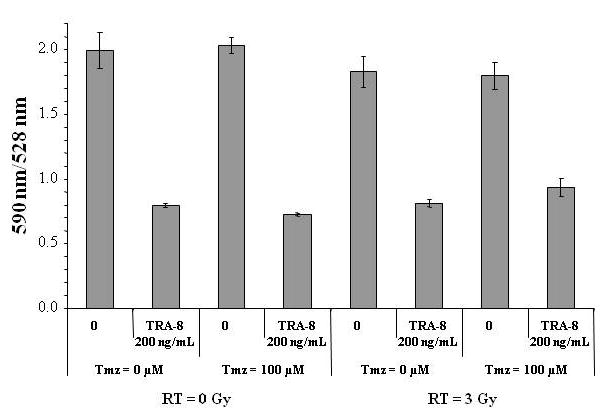
TRA-8 induced depolarization of mitochondria in D54MG cells. Cells were treated with 200 ng/mL TRA-8, 100 μM Tmz, 3 Gy RT, or combinations of TRA-8, Tmz and RT. The ratio of JC-1 aggregates (red fluorescence) to monomers (green fluorescence) was determined 21 h after the start of treatment.
Chemotherapeutic or radiation induced enhancement of death receptor-mediated apoptosis may occur through several mechanisms, including upregulation of DR5 expression. Flow cytometry was used to examine DR5 expression in glioma cell lines after treatment with Tmz, RT, or Tmz plus RT. DR5 expression was not increased in D54MG, U251MG or CH235MG cells after 24 h treatment with 10 or 30 μM Tmz (Fig. 7 and data not shown). Treatment with 2 or 5 Gy RT modestly increased DR5 expression in D54MG cells, but DR5 levels were not increased by the combination of Tmz and RT compared to treatment with RT alone. Little or no change in DR5 expression was detected in U251MG, CH235MG or U87MG cells 24 h after treatment with RT or Tmz followed by RT (data not shown).
Figure 7.
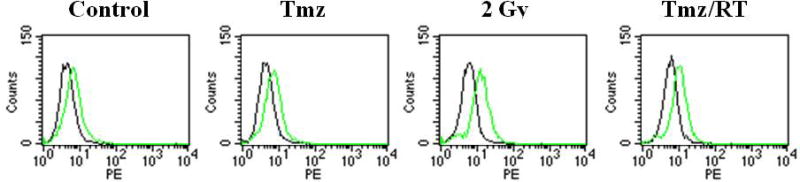
DR5 cell surface expression in D54MG glioma cells treated with Tmz and/or RT. Cells were treated with 10 μM Tmz, 2 Gy RT, or Tmz followed 2 h later by 2 Gy RT. Cells were harvested 24 h after starting treatments, stained with TRA-8 (green lines) or mouse IgG1 isotype control antibody (black lines) followed by goat anti-mouse IgG1-PE, and analyzed by flow cytometry.
Alterations in other apoptotic regulatory molecules were investigated in 5 glioma cell lines to further explore mechanisms of response to TRA-8 in combination with Tmz or RT. Caspase 8 cleavage has been shown to be a critical event in the induction of DR5 mediated apoptosis resulting in the activation of downstream molecules, including caspase 3. Treatment with TRA-8 alone for 3 h resulted in cleavage of caspase 8 and caspase 3 in all 5 cell lines, although the required dose of TRA-8 and the levels of caspase cleavage products varied with the sensitivity of the cell lines to TRA-8, as shown in Figure 8a and 8b. Treatment with 5 Gy RT increased caspase 8 cleavage as shown by a reduction in procaspase 8 levels in CH235MG and U87MG glioma cells compared to treatment with TRA-8 alone. Treatment with TRA-8 alone also reduced Bid levels, presumably due to caspase 8 dependent cleavage, whereas treatment with 5 Gy RT alone upregulated Bid in 3 glioma cell lines. A modest reduction in anti-apoptotic Bcl-xl protein levels was detected in U251MG and U87MG cells after treatment with TRA-8 alone and in combination with Tmz or RT, whereas Bcl-xl levels were very high in CH235MG and D54MG cells (Fig. 8a and 8b). Slightly increased Bax levels were detected in U373MG, CH235MG and D54MG cells treated with TRA-8 and RT. However, p53 levels did not change appreciably after treatment with TRA-8, Tmz or RT, suggesting that p53 upregulation does not contribute significantly to the enhanced killing by TRA-8.
Figure 8.
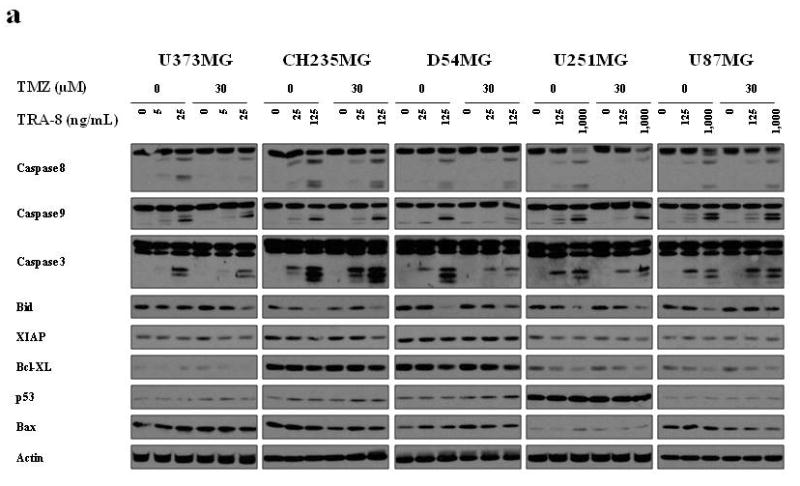
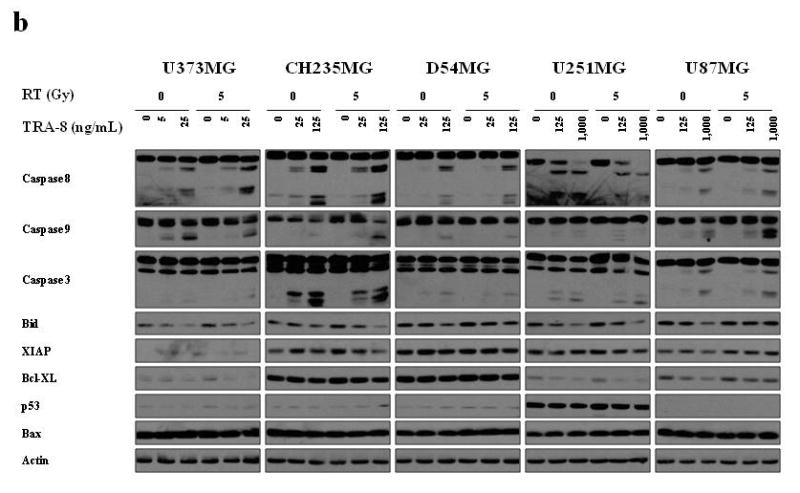
Alterations in the levels of pro-and anti-apoptotic proteins in glioma cell lines treated with TRA-8 in combination with Tmz or RT. Cells were treated with (a) 0 or 30 μM Tmz and (b) 0 or 5 Gy RT. TRA-8 was added 21 h after Tmz or RT treatment. Whole cell lysates were prepared after 3 h treatment with TRA-8 and analyzed by Western blot.
Subcutaneous in vivo therapy experiments
The anti-tumor efficacy of various combinations of TRA-8, Tmz, and RT was evaluated as compared to untreated controls in an eight arm experiment using D54MG s.c. xenografts. The mean tumor size in each treatment group at the initiation of treatment was similar (8 mm in diameter). The only tumors to completely regress received TRA-8, including all seven tumors treated with triple therapy of TRA-8, Tmz, and RT, as shown in Figure 9a. The mean time to complete regression of the tumors was 71 days (range 33–99 days), even though all therapy was completed by day 33. TRA-8 alone had limited activity as a single agent in this model. Results were similar in a second s.c. experiment utilizing a lower chemoradiation dose (Fig. 9b). Combining both s.c. experiments, complete tumor regressions were most common in animals that received the triple therapy combination of TRA-8/Tmz/RT (12/15 animals) compared to those that did not (4/112 animals), p<0.001. Three of the four complete regressions that did not receive triple therapy received TRA-8/Tmz.
Figure 9.
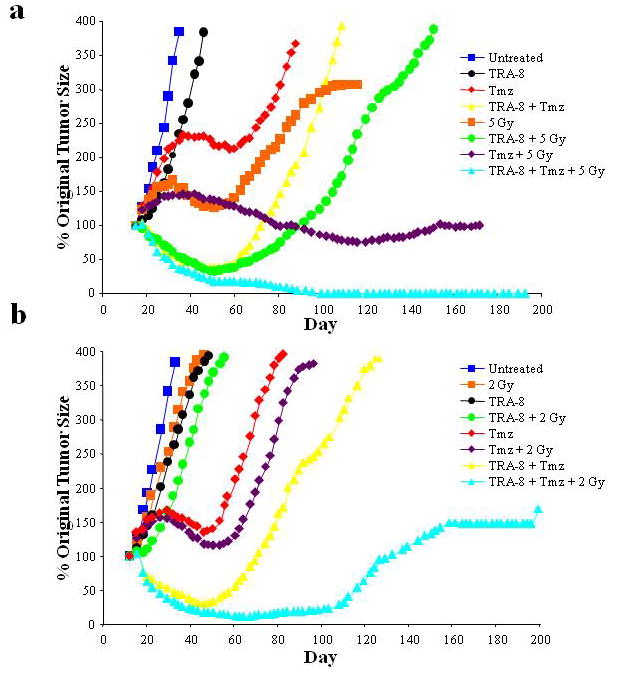
TRA-8 induced D54MG tumor regression and regrowth in s.c. tumor models. (a) Athymic nude mice were injected s.c. with 2×107 D54MG cells. Eight treatment groups were compared: untreated control, TRA-8 alone, RT alone, Tmz alone, RT plus TRA-8, RT plus Tmz, TRA-8 plus Tmz, and TRA-8 plus Tmz plus RT (triple therapy). The primary endpoints were tumor response and regrowth. (b) A repeat of the experiment was performed using a lower chemotherapy and RT dose.
The addition of TRA-8 also enhanced tumor growth delay in combination with either RT or Tmz alone. Tumor growth curves are shown in Figure 9 for the two s.c. experiments. The addition of TRA-8 to either RT or chemotherapy delayed tumor growth compared to RT or chemotherapy alone. Upon long-term follow-up in the first s.c. experiment (Fig. 9a), selected tumors that did not regrow after treatment with chemoradiation underwent biopsy and were found to be sterile despite what appeared to be residual gross tumor. Thus, this first s.c. experiment did not confirm that triple therapy including TRA-8 was better than Tmz plus RT without TRA-8. TRA-8 with Tmz or RT did enhance the inhibition of tumor growth compared to Tmz and RT individually. However, in the second s.c. experiment utilizing a lower chemoradiation dose, there was a difference observed in tumor regrowth between chemoradiation alone and chemoradiation therapy with the antibody (Fig. 9b). The sample size of this experiment was insufficient for statistical analysis.
Intracranial in vivo therapy experiments
The interaction of TRA-8, Tmz, and RT were evaluated in a D54MG intracranial xenograft model with overall survival as the primary endpoint. In intracranial glioma experiments, TRA-8 alone had marginal activity, with significantly different median survival in studies in which different numbers of tumor cells were injected. In both studies, the median survival of triple treated or chemoradiation only treated mice were significantly greater from that of control or TRA-8 alone treated mice (Fig. 10). While median survival of triple-treated mice (107, 219 days) were greater in both studies than those of mice receiving chemoradiation without TRA-8 (75, 166 days), these differences were not statistically significant by log-rank analysis of Kaplan-Meier survival estimations (p=0.10 and p=0.17).
Figure 10.
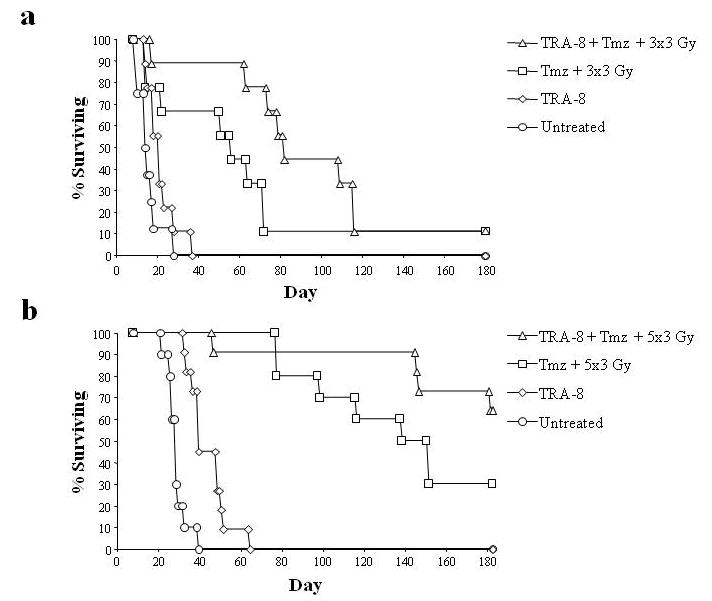
Overall survival in the D54MG intracranial model. (a) Tumors were established by stereotactic intracranial injection of 5×105 D54MG cells in mice and treated with TRA-8 alone, Tmz plus RT, or TRA-8 plus Tmz plus RT (3 Gy × 3) vs. untreated control. The primary endpoint was survival. (b) In the second intracranial experiment the burden of injected tumors cells was lowered to 2.5 × 105.
DISCUSSION
Targeted pro-apoptotic therapies hold great promise in the treatment of cancer. As early as 1999, intracranial experiments suggested that locally administered TRAIL may be efficacious in malignant gliomas (18). Systemic TRAIL administration may not be feasible because of potential toxicity, but this has been debated as different preparations of recombinant TRAIL have differential hepatocyte toxicity (4, 19–21). This potential limitation led to the development of pro-apoptotic monoclonal antibodies against individual death receptors (22–29), including TRA-8, which binds to DR5 (4). TRA-8 has potential application in several tumor types where enhancement of chemotherapy and RT has been observed preclinically (9–14). A humanized version of TRA-8 was evaluated in a Phase I study and is currently being evaluated in a Phase II clinical trial.
The in vitro studies presented here demonstrate that combination treatment with Tmz or RT increased TRA-8 cytotoxicity against a panel of established glioma cell lines. The in vivo experiments support the hypothesis that TRA-8 would enhance both RT and Tmz chemotherapy in a human glioma xenograft model, although a larger sample size will be required to demonstrate statistical significance to these observations. RT increased DR5 expression on D54MG cells as detected by TRA-8 binding and flow cytometry, but no change in DR5 expression was detected in 3 other glioma cell lines treated with Tmz or RT, indicating that other mechanisms contribute to the enhanced TRA-8 activity. TRA-8 induced cleavage of caspase 8 was detected in all 5 glioma cell lines, which correlated with reduced levels of Bid, presumably due to cleavage by caspase 8, an event linked to activation of the intrinsic mitochondrial apoptotic pathway (30). RT induced a modest increase in Bid levels in 3 glioma cell lines, which may contribute to activation of the intrinsic pathway. RT also increased Bax levels in U373MG, CH235MG, and D54MG cells, which may enhance TRA-8 killing, as Bax is important for TRAIL mediated apoptosis (31), but no direct correlation between TRA-8 response and the levels of p53 or Bax was observed in the 5 glioma cell lines. Likewise, a modest reduction in levels of anti-apoptotic Bcl-xl protein was detected in glioma cells treated with TRA-8 alone and in combination with Tmz or RT, but no direct correlation between basal levels of Bcl-xl and TRA-8 response was observed, as CH235MG and D54MG cells expressed high levels of Bcl-xl but were sensitive to TRA-8. These results indicate that more detailed mechanistic studies will be required to identify factors responsible for sensitivity or resistance to TRA-8 mediated apoptosis.
Previous investigations on TRAIL resistance reported no correlation in DR4 or DR5 expression between sensitive or resistant glioma cell lines (32). TRAIL sensitivity has been correlated with levels or localization of various proteins, including Akt, FLIP and c-myc (33, 34). Several investigators demonstrated increased activity of TRAIL-based therapies in combination with alkylating agents clinically used to treat malignant gliomas. Rohn et al. found that several chemotherapeutic drugs, including CCNU and Tmz, in combination with TRAIL in U87MG glioma cells synergistically enhanced cytochrome c release from mitochondria, suggesting activation of the intrinsic apoptotic pathway (32). Tmz treatment also increased caspase-3 activation without changing caspase-8 levels compared to TRAIL alone, which is consistent with intrinsic pathway activation (35). Several other reports suggested that RT augments TRAIL-based therapies by various mechanisms, including upregulation of DR5, Bak, Bax, and inhibition of bcl-2 (36–38). RT induced p53-dependent upregulation of DR5 in breast cancer cell lines (39), and increased Bax expression enhanced TRA-8 apoptosis in glioma cell lines (40). RT and TRAIL were synergistic in a lymphoma cell line that over-expressed anti-apoptotic bcl-2 protein, but only additive in a cell line without bcl-2 over-expression (41). It is unclear whether RT will sensitize normal cells to TRAIL to any degree.
The clinical application of monoclonal antibodies such as TRA-8 in malignant glioma may be limited by antibody penetration into the tumor. Contrast-enhancing tumors such as glioblastoma multiforme typically have a disturbed blood brain barrier such that macromolecules may cross in clinically relevant concentrations. If systemically administered antibodies do not localize in the tumor radiographically, another option is local administration with convective enhanced delivery (CED) or intratumoral injection. Intracranial animal studies of TRAIL administered with CED show enhanced survival in combination with Tmz (35), as we have observed with systemic administration of TRA-8. Challenges to the development of TRA-8 and other TRAIL-based therapies for gliomas include tumor delivery, optimal timing with chemotherapy agents and RT, and molecular identification of resistant tumors that may benefit from combination with other targeted therapies. Future work will extend this s.c. model to other malignant glioma xenografts and expand the intracranial model to improve statistical power.
Acknowledgments
Sponsored in part by Accelerate Brain Cancer Cure (ABC2) and Daiichi Sankyo
Footnotes
Presented in part at October 2004 meeting of American Society for Therapeutic Radiology and Oncology.
Conflict of Interest: TZ and DJB have intellectual property interest related to the TRA-8 antibody.
References
- 1.Walker MD, Alexander E, Jr, Hunt WE, et al. Evaluation of BCNU and/or radiotherapy in the treatment of anaplastic gliomas. A cooperative clinical trial. J Neurosurg. 1978;49:333–343. doi: 10.3171/jns.1978.49.3.0333. [DOI] [PubMed] [Google Scholar]
- 2.Fine HA, Dear KB, Loeffler JS, Black PM, Canellos GP. Meta-analysis of radiation therapy with and without adjuvant chemotherapy for malignant gliomas in adults. Cancer. 1993;71:2585–2597. doi: 10.1002/1097-0142(19930415)71:8<2585::aid-cncr2820710825>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- 3.Rowinsky EK. Targeted induction of apoptosis in cancer management: the emerging role of tumor necrosis factor-related apoptosis-inducing ligand receptor activating agents. J Clin Oncol. 2005;23:9394–9407. doi: 10.1200/JCO.2005.02.2889. [DOI] [PubMed] [Google Scholar]
- 4.Ichikawa K, Liu W, Zhao L, et al. Tumoricidal activity of a novel anti-human DR5 monoclonal antibody without hepatocyte cytotoxicity. Nat Med. 2001;7:954–960. doi: 10.1038/91000. [DOI] [PubMed] [Google Scholar]
- 5.Ichikawa K, Liu W, Fleck M, et al. TRAIL-R2 (DR5) mediates apoptosis of synovial fibroblasts in rheumatoid arthritis. J Immunol. 2003;171:1061–1069. doi: 10.4049/jimmunol.171.2.1061. [DOI] [PubMed] [Google Scholar]
- 6.Buchsbaum DJ, Forero-Torres A, LoBuglio AF. TRAIL receptor antibodies as a potential cancer treatment. Future Oncol. 2007;3:405–409. doi: 10.2217/14796694.3.4.405. [DOI] [PubMed] [Google Scholar]
- 7.Takeda K, Yamaguchi N, Akiba H, et al. Induction of tumor-specific T cell immunity by anti-DR5 antibody therapy. J Exp Med. 2004;199:437–448. doi: 10.1084/jem.20031457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Roth W, Isenmann S, Nakamura M, et al. Soluble decoy receptor 3 is expressed by malignant gliomas and suppresses CD95 ligand-induced apoptosis and chemotaxis. Cancer Res. 2001;61:2759–2765. [PubMed] [Google Scholar]
- 9.Buchsbaum DJ, Zhou T, Grizzle WE, et al. Antitumor efficacy of TRA-8 anti-DR5 monoclonal antibody alone or in combination with chemotherapy and/or radiation therapy in a human breast cancer model. Clin Cancer Res. 2003;9:3731–3741. [PubMed] [Google Scholar]
- 10.Ohtsuka T, Buchsbaum D, Oliver P, et al. Synergistic induction of tumor cell apoptosis by death receptor antibody and chemotherapy agent through JNK/p38 and mitochondrial death pathway. Oncogene. 2003;22:2034–2044. doi: 10.1038/sj.onc.1206290. [DOI] [PubMed] [Google Scholar]
- 11.Straughn JM, Jr, Oliver PG, Zhou T, et al. Anti-tumor activity of TRA-8 anti-death receptor 5 (DR5) monoclonal antibody in combination with chemotherapy and radiation therapy in a cervical cancer model. Gynecol Oncol. 2006;101:46–54. doi: 10.1016/j.ygyno.2005.09.053. [DOI] [PubMed] [Google Scholar]
- 12.DeRosier LC, Buchsbaum DJ, Oliver PG, et al. Combination treatment with TRA-8 anti-death receptor-5 antibody and CPT-11 induces tumor regression in an orthotopic model of pancreatic cancer. Clin Cancer Res. 2007;13:5535s–5543s. doi: 10.1158/1078-0432.CCR-07-1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.DeRosier LC, Vickers SM, Zinn KR, et al. TRA-8 anti-DR5 monoclonal antibody and gemcitabine induce apoptosis and inhibit radiologically validated orthotopic pancreatic tumor growth. Mol Cancer Ther. 2007;6:3198–3207. doi: 10.1158/1535-7163.MCT-07-0299. [DOI] [PubMed] [Google Scholar]
- 14.Oliver PG, LoBuglio AF, Zinn KR, et al. Clin Cancer Res. 2008. Treatment of human colon cancer xenografts with TRA-8 anti-death receptor 5 antibody alone or in combination with CPT-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Weinmann M, Marini P, Jendrossek V, et al. Influence of hypoxia on TRAIL-induced apoptosis in tumor cells. Int J Radiat Oncol Biol Phys. 2004;58:386–396. doi: 10.1016/j.ijrobp.2003.09.052. [DOI] [PubMed] [Google Scholar]
- 16.Stupp R, Mason WP, van den Bent MJ, et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med. 2005;352:987–996. doi: 10.1056/NEJMoa043330. [DOI] [PubMed] [Google Scholar]
- 17.Dressler V, Muller G, Suhnel J. CombiTool--a new computer program for analyzing combination experiments with biologically active agents. Comput Biomed Res. 1999;32:145–160. doi: 10.1006/cbmr.1999.1509. [DOI] [PubMed] [Google Scholar]
- 18.Roth W, Isenmann S, Naumann U, et al. Locoregional Apo2L/TRAIL eradicates intracranial human malignant glioma xenografts in athymic mice in the absence of neurotoxicity. Biochem Biophys Res Commun. 1999;265:479–483. doi: 10.1006/bbrc.1999.1693. [DOI] [PubMed] [Google Scholar]
- 19.Ashkenazi A, Pai RC, Fong S, et al. Safety and antitumor activity of recombinant soluble Apo2 ligand. J Clin Invest. 1999;104:155–162. doi: 10.1172/JCI6926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mori E, Thomas M, Motoki K, et al. Human normal hepatocytes are susceptible to apoptosis signal mediated by both TRAIL-R1 and TRAIL-R2. Cell Death Differ. 2004;11:203–207. doi: 10.1038/sj.cdd.4401331. [DOI] [PubMed] [Google Scholar]
- 21.Zheng SJ, Wang P, Tsabary G, Chen YH. Critical roles of TRAIL in hepatic cell death and hepatic inflammation. J Clin Invest. 2004;113:58–64. doi: 10.1172/JCI200419255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Humpreys R. HGS-TR2J, a human, agonistic, TRAIL Receptor-2 monoclonal antibody, induces apoptosis, tumor regression and growth inhibition as a single agent in diverse human solid tumor cell lines. Proc Am Assoc Cancer Res. 2004:45. [Google Scholar]
- 23.Bonomi P, Greco FA, Crawford J, et al. Results of a phase 2 trial of HGS-ETR1 (agonistic human monoclonal antibody to TRAIL receptor 1) in subjects with relapsed/recurrent non-small cell lung cancer (NSCLC). 11th World Conference on Lung Cancer; Barcelona, Spain. 2005. [Google Scholar]
- 24.Georgakis GV, Li Y, Humphreys R, et al. Activity of selective fully human agonistic antibodies to the TRAIL death receptors TRAIL-R1 and TRAIL-R2 in primary and cultured lymphoma cells: induction of apoptosis and enhancement of doxorubicin- and bortezomib-induced cell death. Br J Haematol. 2005;130:501–510. doi: 10.1111/j.1365-2141.2005.05656.x. [DOI] [PubMed] [Google Scholar]
- 25.Pukac L, Kanakaraj P, Humphreys R, et al. HGS-ETR1, a fully human TRAIL-receptor 1 monoclonal antibody, induces cell death in multiple tumour types in vitro and in vivo. Br J Cancer. 2005;92:1430–1441. doi: 10.1038/sj.bjc.6602487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tolcher AW, Wakelee H, Mita M, et al. A phase I clinical study of HGS-ETR2, a fully-human monoclonal antibody to TRAIL-R2 in patients with advanced solid tumors. Proc Am Assoc Cancer Res. 2005:46. [Google Scholar]
- 27.Marini P, Denzinger S, Schiller D, et al. Combined treatment of colorectal tumours with agonistic TRAIL receptor antibodies HGS-ETR1 and HGS-ETR2 and radiotherapy: enhanced effects in vitro and dose-dependent growth delay in vivo. Oncogene. 2006;25:5145–5154. doi: 10.1038/sj.onc.1209516. [DOI] [PubMed] [Google Scholar]
- 28.Tolcher AW, Mita M, Meropol NJ, et al. Phase I pharmacokinetic and biologic correlative study of mapatumumab, a fully human monoclonal antibody with agonist activity to tumor necrosis factor-related apoptosis-inducing ligand receptor-1. J Clin Oncol. 2007;25:1390–1395. doi: 10.1200/JCO.2006.08.8898. [DOI] [PubMed] [Google Scholar]
- 29.Plummer R, Attard G, Pacey S, et al. Phase 1 and pharmacokinetic study of lexatumumab in patients with advanced cancers. Clin Cancer Res. 2007;13:6187–6194. doi: 10.1158/1078-0432.CCR-07-0950. [DOI] [PubMed] [Google Scholar]
- 30.Lacour S, Micheau O, Hammann A, et al. Chemotherapy enhances TNF-related apoptosis-inducing ligand DISC assembly in HT29 human colon cancer cells. Oncogene. 2003;22:1807–1816. doi: 10.1038/sj.onc.1206127. [DOI] [PubMed] [Google Scholar]
- 31.LeBlanc H, Lawrence D, Varfolomeev E, et al. Tumor-cell resistance to death receptor–induced apoptosis through mutational inactivation of the proapoptotic Bcl-2 homolog Bax. Nat Med. 2002;8:274–281. doi: 10.1038/nm0302-274. [DOI] [PubMed] [Google Scholar]
- 32.Röhn TA, Wagenknecht B, Roth W, et al. CCNU-dependent potentiation of TRAIL/Apo2L-induced apoptosis in human glioma cells is p53-independent but may involve enhanced cytochrome c release. Oncogene. 2001;20:4128–4137. doi: 10.1038/sj.onc.1204534. [DOI] [PubMed] [Google Scholar]
- 33.Ricci MS, Jin Z, Dews M, et al. Direct repression of FLIP expression by c-myc is a major determinant of TRAIL sensitivity. Mol Cell Biol. 2004;24:8541–8555. doi: 10.1128/MCB.24.19.8541-8555.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Panner A, James CD, Berger MS, Pieper RO. mTOR controls FLIPS translation and TRAIL sensitivity in glioblastoma multiforme cells. Mol Cell Biol. 2005;25:8809–8823. doi: 10.1128/MCB.25.20.8809-8823.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Saito R, Bringas JR, Panner A, et al. Convection-enhanced delivery of tumor necrosis factor-related apoptosis-inducing ligand with systemic administration of temozolomide prolongs survival in an intracranial glioblastoma xenograft model. Cancer Res. 2004;64:6858–6862. doi: 10.1158/0008-5472.CAN-04-1683. [DOI] [PubMed] [Google Scholar]
- 36.Belka C, Jendrossek V, Pruschy M, et al. Apoptosis-modulating agents in combination with radiotherapy-current status and outlook. Int J Radiat Oncol Biol Phys. 2004;58:542–554. doi: 10.1016/j.ijrobp.2003.09.067. [DOI] [PubMed] [Google Scholar]
- 37.Shankar S, Srivastava RK. Enhancement of therapeutic potential of TRAIL by cancer chemotherapy and irradiation: mechanisms and clinical implications. Drug Resist Updat. 2004;7:139–156. doi: 10.1016/j.drup.2004.03.002. [DOI] [PubMed] [Google Scholar]
- 38.Verbrugge I, de Vries E, Tait SW, et al. Ionizing radiation modulates the TRAIL death-inducing signaling complex, allowing bypass of the mitochondrial apoptosis pathway. Oncogene. 2007 doi: 10.1038/sj.onc.1210696. [DOI] [PubMed] [Google Scholar]
- 39.Chinnaiyan AM, Prasad U, Shankar S, et al. Combined effect of tumor necrosis factor-related apoptosis-inducing ligand and ionizing radiation in breast cancer therapy. Proc Natl Acad Sci U S A. 2000;97:1754–1759. doi: 10.1073/pnas.030545097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kaliberov S, Stackhouse MA, Kaliberova L, Zhou T, Buchsbaum DJ. Enhanced apoptosis following treatment with TRA-8 anti-human DR5 monoclonal antibody and overexpression of exogenous Bax in human glioma cells. Gene Ther. 2004;11:658–667. doi: 10.1038/sj.gt.3302215. [DOI] [PubMed] [Google Scholar]
- 41.Belka C, Schmid B, Marini P, et al. Sensitization of resistant lymphoma cells to irradiation-induced apoptosis by the death ligand TRAIL. Oncogene. 2001;20:2190–2196. doi: 10.1038/sj.onc.1204318. [DOI] [PubMed] [Google Scholar]


