Abstract
Purpose
To test the therapeutic effectiveness of voclosporin against experimental autoimmune uveoretinitis (EAU) in rats and to evaluate its effect on human T cells.
Methods
EAU was induced by immunization with a uveitogenic protein. Voclosporin administration, by subcutaneous injection, began on day (d) 0 or d7 after immunization. Treatment effectiveness was evaluated in vivo using clinical EAU scoring (d7–d13) and histopathologic evaluation of enucleated eyes after experimental termination. Rodent lymphocytes were harvested from lymph nodes on d14 for antigen-specific proliferation assays. The effect of voclosporin on human T-cell proliferation and cytokine secretion was examined in vitro.
Results
Voclosporin prevented EAU development in rats receiving medium and high preventive doses, whereas high-dose voclosporin administration effectively treated EAU. Lymphocytes from animals treated with voclosporin had decreased antigen-specific proliferation in vitro compared with lymphocytes from untreated animals. No evidence of abnormal ocular histopathology was found in the eyes from animals that received high doses of therapeutic voclosporin. Using human T cells, voclosporin inhibited human T-cell proliferation up to 100-fold. Furthermore, voclosporin treatment of human T cells significantly reduced pan T-cell effector responses.
Conclusions
Voclosporin effectively suppressed uveoretinitis in an animal model that imitates the human inflammatory ocular disease by inhibiting lymphocyte proliferation. In addition, voclosporin effectively inhibited human T-cell proliferation and function in vitro. The authors report the first evidence supporting the application of voclosporin to treat intraocular inflammation.
Uveitis causes 10% of the cases of blindness in the United States.1 The core treatment of endogenous, noninfectious uveitis includes topical, periocular, or systemic corticosteroid delivery.2 However, additional anti-inflammatory and immunosuppressive agents are often used when the disease is refractory to corticosteroids.3 Calcineurin inhibitors (CNIs) are effective agents for the treatment of refractory uveitis3-9 and other immune-mediated ocular conditions.10,11
Cyclosporine (CsA; Calbiochem, La Jolla, CA) is a potent immunosuppressant that partially functions through calcineurin inhibition. CNIs have widespread clinical use for the prevention of host-organ transplant rejection, and they show promise for treating a variety of autoimmune disorders, including uveitis.3-5 Unfortunately, the clinical advantages of calcineurin inhibition are overshadowed by its well-known side effects, including nephrotoxicity, neurotoxicity, hyperlipidemia, diabetes, and hypertension, usually limiting the duration of treatment. Probable causes for toxicity include calcineurin inhibition in nonlymphoid tissues12,13 and calcineurin-independent effects (activation of TGF-β14 or inhibition of mitochondrial high-energy phosphate metabolism).15,16 Investigators think toxicity may be abridged by the development of CNIs that directly target lymphocyte calcineurin.
Voclosporin, originally designated ISA247/LX211, is a novel CNI that has shown greater potency and less toxicity than CsA in in vitro and in vivo studies.17-21 Early work used a formulation containing a mixture of two isomers (55:45 trans/cis isomers, designated mix-ISA247). In the current formulation, the trans (E) isomer content was increased to 90%, and this change was found to improve bioavailability, absorption, and pharmacokinetic predictability.22 In addition, results from human clinical trials indicate that voclosporin is safe, efficacious, and well tolerated.23
Experimental autoimmune uveoretinitis (EAU), an established animal model, is often used to evaluate investigational agents for the treatment of uveitis. It is a predominantly T-cell-mediated disease produced by immunization using one of a variety of retinal antigens.17 Because calcineurin inhibition effectively prevents and treats EAU,24-28 we wanted to test the efficacy of the less toxic CNI in the rodent EAU model. Furthermore, we aimed to translate the observations in the rodent model to application in human cells. Therefore, we also examined the effect of voclosporin on human T-cell proliferation and function (cytokine secretion) in vitro. We report the successful use of voclosporin for the prevention and treatment of EAU and the effective inhibition of human T-cell proliferation and function.
Methods
Induction of Uveoretinitis
Male Lewis rats (6–8 weeks old) from Charles River Laboratories (Wilmington, MA) were immunized with interphotoreceptor retinoid-binding protein (IRBP), prepared as described.29 IRBP was emulsified 1:1 with complete Freund adjuvant (CFA; Sigma Chemical, St. Louis, MO) supplemented with 2.5 mg/mL Mycobacterium tuberculosis strain H37RA (Difco Laboratories, Lawrence, KS). Emulsion (100 μL) was injected into the base of the tail. Pertussis toxin (Sigma Chemical), which was obtained after isolation and purification as previously described,30 was injected intraperitoneally. All animal experiments were performed under the protocols approved by the National Eye Institute Institutional Animal Care and Use Committee. Rats were treated according to the ARVO Statement for the Use of Animals in Ophthalmic and Vision Research.
Therapeutic Agents and Treatment Schedule
CsA was solubilized in olive oil at 25 mg/mL. Voclosporin (labeled ISATX247), in powder form, was dissolved in miglyol 812 oil at three different concentrations (1.56, 6.25, and 25 mg/mL). Samples of each concentration were sent to Isotechnika (Edmonton, AB, Canada) to be analyzed by high-performance liquid chromatography. Dose analyses were performed at three intervals during the study to ensure stability of administered doses.
Therapeutic agents were voclosporin (administered in low [2.5 mg/kg], medium [10 mg/kg], and high [40 mg/kg] doses), CsA (positive control, 40 mg/kg), and vehicle (negative control, miglyol 812 oil). Rats were divided into two main treatment groups, preventive and therapeutic. Preventively treated rats received daily subcutaneous injections of CsA or voclosporin in the dorsal back area from day (d) 0 to d13. Treatments in the therapeutic group were delivered daily in the same manner, but treatments began on d7 with respect to IRBP immunization. Each treatment group consisted of three to five rats. Experiments were performed twice; eight rats were used for each treatment.
Clinical Scoring
To analyze treatment effectiveness against uveitis, daily clinical scores were assigned to each rat starting on d7. Each eye was graded, without masking, according to a previously described scoring system: 0, normal; 0.5, dilated blood vessels in the iris; 1, abnormal pupil contraction; 2, hazy anterior chamber; 3, moderately opaque anterior chamber with dull red reflex; 4, opaque anterior chamber, absent red reflex, and proptosis.31 There was no masking during this portion of the experiment because clinical scoring was recorded during the administration of therapy.
Lymphocyte Proliferation Assay and In Vitro Suppression
Draining lymph nodes were harvested after experimental termination, and lymphocytes were prepared by pushing lymph nodes through 40-μM disposable strainers. Cells were washed twice in RPMI 1640 medium and resuspended in assay medium (RPMI 1640 supplemented with 1 μM β-mercaptoethanol, 2 mM glutamine, 1 mM sodium pyruvate, 0.1 mM nonessential amino acids, 50 μg/mL gentamicin, 0.25 μg/mL amphotericin B, 20 mg/mL α-methyl mannopyranoside, and 1% normal rat serum). Triplicate cultures (2 × 105 cells/well) were stimulated with IRBP (5 μg/mL) in 96-well plates. To monitor the effects on cell proliferation, cultures were incubated for 7 days and were pulsed with 1 μCi [3H] thymidine for the last 16 hours.
Histopathology
Eyes from euthanatized rats were enucleated and immersed for 1 hour in 4% glutaraldehyde at room temperature and then transferred into 10% formaldehyde until processed. Fixed tissues were dehydrated, embedded in methacrylate, sectioned (4–6 μm) through the papillary-optic nerve plane, and stained with hematoxylin and eosin. Severity of EAU was scored in a masked fashion by an ophthalmic pathologist according to a previously described semiquantitative scale.32 No information about the experimental design or treatments was provided to the pathologist.
Human PBMCs and T-Cell Proliferation Assays
Normal donor human peripheral blood mononuclear cells (PBMCs) were isolated (Ficoll-Paque Plus; GE Healthcare, Uppsala, Sweden). T cells were purified by negative selection, according to the manufacturer's instructions (Pan T-Cell Isolation Kit II human: Miltenyi Biotec, Auburn, CA). T cells or PBMCs were stimulated for 30 minutes with 2 μg/mL anti-human CD3 and anti-human CD28 (BD Biosciences, Bedford, MA), followed by cross-linking with 8 μg/mL mouse anti-human IgG (Sigma, St. Louis, MO). Cells were incubated at 37°C in 5% CO2 for 3 to5 days in the presence of CsA (10 – 100 nM) or voclosporin (0.1–100 nM) and were pulsed with 1 μCi [3H] thymidine for the last 16 hours. Supernatants in duplicate cultures were collected at d2 and submitted to ThermoFisher Scientific (Woburn, MA) for Pierce (Rockford, IL) SearchLight cytokine testing services.
Results
EAU Model: Clinical Scoring
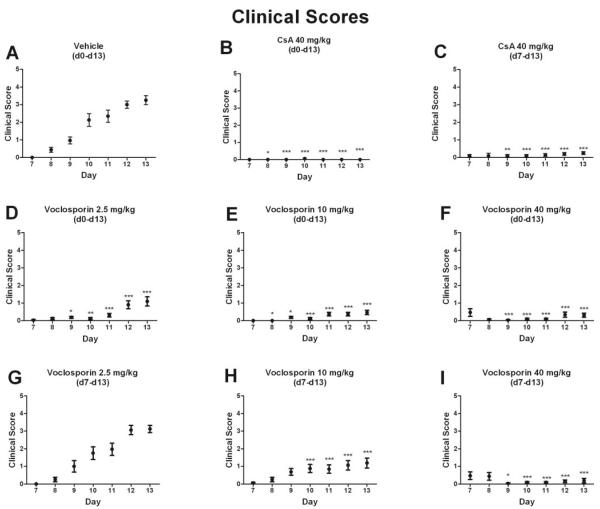
Daily subcutaneous voclosporin injections prevented grossly visible clinical signs of EAU development in comparison with vehicle treatment (Figs. 1A, 1D–1I). Doses of voclosporin from the preventive group were effective at preventing clinically significant uveitis (score >2), though the medium and high doses provided more statistically significant protection than the low dose (Figs. 1D–1F). More important, we observed dose-dependent effectiveness of voclosporin in animals that received subcutaneous injections after disease onset (d7 – d13; Figs. 1G–1I). Notably, the highest dose in the therapeutic arm reversed clinical signs of ocular inflammation (Fig. 1I).
Figure 1.
High-dose (40 mg/kg) subcutaneous voclosporin treatment of rats, starting at the earliest clinical sign of uveitis (d7 after induction), reversed clinical signs of ocular disease. Rats were immunized with IRBP (d0) to induce uveitis and were injected subcutaneously (d0–d13 [preventive] or d7–d13 [therapeutic]) with CsA (40 mg/kg) or voclosporin (low dose, 2.5 mg/kg; medium dose, 10 mg/kg; high dose, 40 mg/kg). At the time of injection, mean clinical scores were recorded (d7–d13) and are represented as mean clinical scores ± SEM (n = 16 eyes; two independent experiments). The values recorded on a given day for each treatment group were compared with the values recorded for other treatment groups (A-I). Specifically, one-way analysis of variance (ANOVA) with repeated measures was performed (Friedman test, P < 0.05), followed by Dunn multiple comparison test to determine differences among groups. Statistical significance compared with vehicle is indicated by asterisks (*P < 0.05; **P < 0.01; ***P < 0.001).
EAU Model: Histopathology
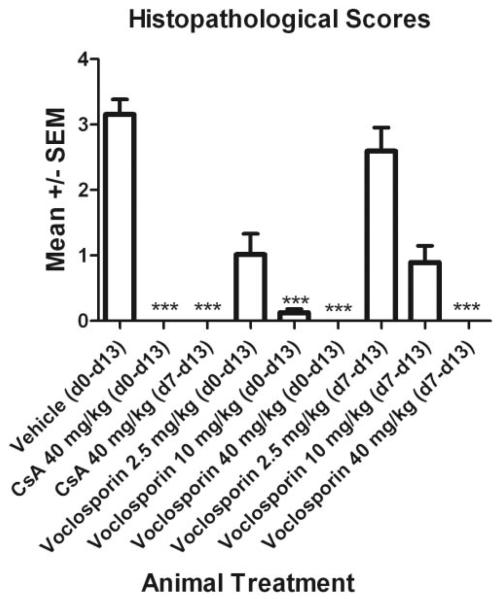
Masked evaluation of histopathology after experimental termination of in vivo experiments also revealed dose-dependent efficacy of voclosporin (Figs. 2, 3). Extensive ocular inflammation and photoreceptor destruction was observed in eyes of the vehicle-treated rats (Fig. 3A), which had the highest combined anterior and posterior histopathologic scores (Fig. 2). Retinas from vehicle-treated rats had marked inflammatory infiltration, focal destruction of the nuclear layers that were more prominent in the outer nuclear layer, and total loss of inner and outer photoreceptor segments (Fig. 3A). Compared with vehicle-treated controls, increasingly significant reductions in median EAU scores were found in eyes from the low, medium, and high voclosporin preventively treated rats (Fig. 2). Evidence of uveitis (0.25–3.0) was found in 8 of 16 eyes from the low-dose voclosporin preventive treatment group. Mild histopathology (0.5) was found in 4 of 16 rat eyes from the medium voclosporin preventive treatment group (Fig. 2). No visible histopathologic abnormality was reported in any of the 16 eyes taken from rats in the high-dose voclosporin preventive treatment group (Fig. 2).
Figure 2.
No visible signs of histopathology were found in rats that received daily subcutaneous 40 mg/kg voclosporin treatments, starting at the earliest sign of clinical disease (d7). Rats were immunized with IRBP (d0) to induce uveitis and were injected subcutaneously (d0 – d13 [preventive] or d7 – d13 [therapeutic]) with CsA (40 mg/kg) or voclosporin (low dose, 2.5 mg/kg; medium dose, 10 mg/kg; high dose, 40 mg/kg). At the termination of the experiment, eyes were fixed and embedded in methacrylate. Evidence of histopathologic findings in hematoxylin and eosin tissue sections was scored by a masked ocular histopathologist. Mean ± SEM values are represented (n =16 eyes; two independent experiments). Data from each treatment group were statistically compared by performing Kruskal-Wallis ANOVA using Gaussian approximation of P values, followed by Dunn multiple comparison test to determine differences among groups (***P < 0.001).
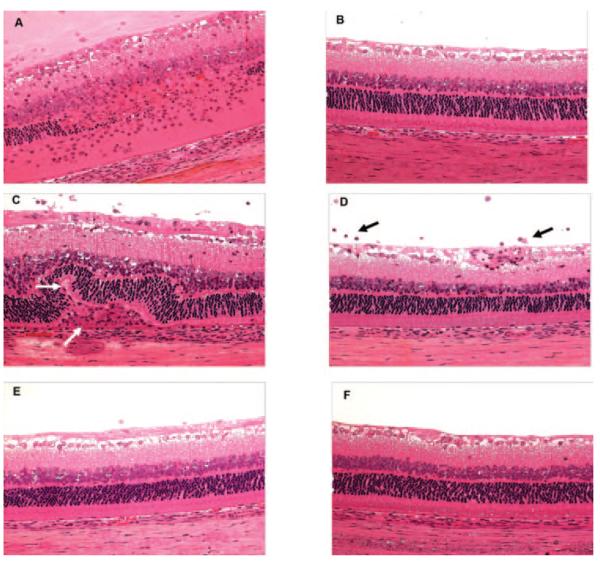
Figure 3.
High-dose therapeutic and preventive treatment with voclosporin preserved the retinal architecture in IRBP-immunized rats. At d0, rats were immunized with IRBP to induce uveitis. Subcutaneous preventive (d0 – d13) or therapeutic (d7 – d13) injections of CsA or voclosporin were administered. Animals were humanely euthanatized on d14, and ocular tissues were histologically processed. Retinal tissue sections (×100) stained with hematoxylin and eosin. (A) Vehicle, miglyol 812 oil. (B) CsA (high-dose preventive, 40 mg/kg). (C) Voclosporin (low-dose therapeutic, 2.5 mg/kg). (D) Voclosporin (medium-dose therapeutic, 10 mg/kg). (E) Voclosporin (high-dose therapeutic, 40 mg/kg). (F) Voclosporin (high-dose preventive, 40 mg/kg). White arrows: retinal folds and small granuloma formation. Black arrows: inflammatory cellular infiltrates surrounding retinal vessels and in the vitreous.
All animals in the low-dose voclosporin therapeutic treatment group exhibited uveitis (1.5–5.0), including retinal folds and small granuloma formation (Fig. 3C, white arrows). Mild uveitis (0.25–3.0) was found in 12 of 16 rat eyes from the medium-dose voclosporin therapeutic treatment group (Fig. 2), including inflammatory cellular infiltration surrounding the retinal vessels and in the vitreous (Fig. 3D, black arrows). No abnormality was present in any of the 16 eyes taken from rats in the high-dose voclosporin treatment group (Figs. 2, 3E).
Voclosporin Suppression of Lymphocyte Proliferation
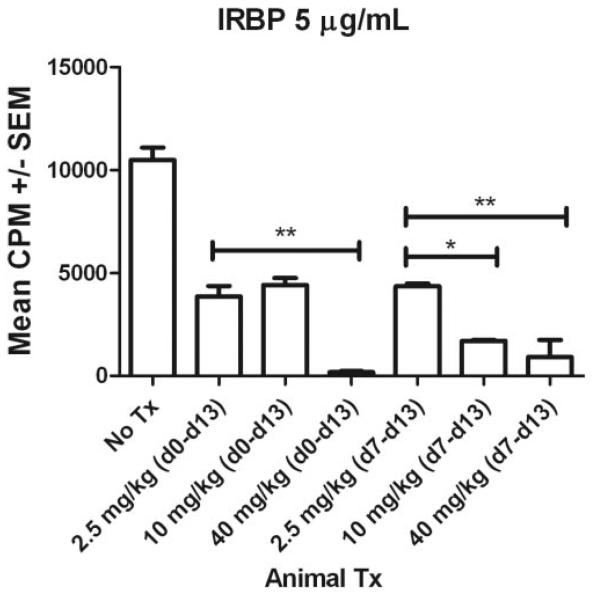
Along with suppression of intraocular inflammation (Figs. 2, 3), in vitro proliferation of lymphocytes taken from rats treated with voclosporin was significantly suppressed in cultures stimulated with IRBP (Fig. 4).
Figure 4.
Lymphocytes from animals treated with voclosporin underwent decreased antigen-specific proliferation. Rats were immunized with IRBP (d0) to induce uveitis and were injected subcutaneously (d0 – d13 [preventive] or d7 – d13 [therapeutic]) with voclosporin (low dose, 2.5 mg/kg; medium dose, 10 mg/kg; high dose, 40 mg/kg). At the termination of the experiment on d14 after immunization, triplicate cultures of lymphocytes (2 × 105 cells/well) were stimulated with IRBP (5 μg/mL) in 96-well plates. To monitor the effects on cell proliferation, cultures were incubated for 7 days and were pulsed with 1 μCi [3H] thymidine for the last 16 hours. Data are represented as mean CPM ± SEM. Data were analyzed with the use of ANOVA followed by Bonferroni multiple comparison test to identify differences among groups (*P < 0.05; **P < 0.01).
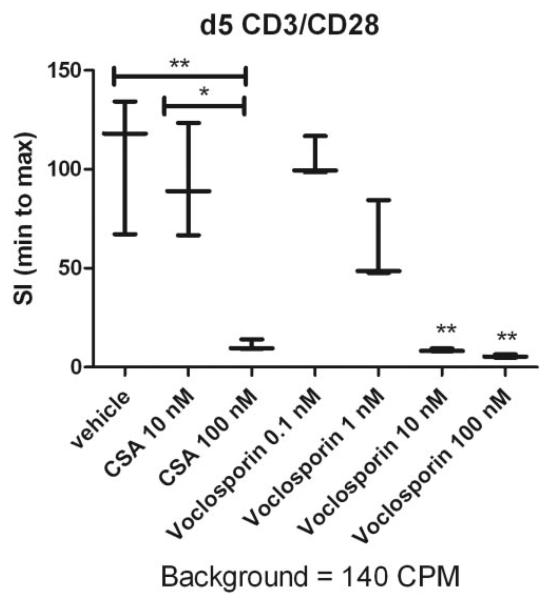
In addition, when analyzing the effect of voclosporin on human lymphocyte proliferation in vitro, dose-dependent inhibition of lymphocyte proliferation was found in cells from nine healthy donors. A representative graph from three independent experiments is shown in Figure 5. A dose-dependent reduction of CD3/CD28-stimulated T-cell proliferation was observed (Fig. 5).
Figure 5.
Voclosporin suppresses human lymphocyte proliferation. Purified human T cells (200,000 cells/well) in a 96-well plate were stimulated with anti-CD28 and anti-CD3 antibodies for 30 minutes, followed by cross-linking with mouse anti-human IgG. Cells were pulsed with 3H-thymidine for 16 hours, and proliferation in the presence of 0.1 to 100 nM voclosporin or 10 to 100 nM CsA was analyzed on d5. Data are plotted as stimulation index (SI) with an indication for minimum, median, and maximum values. The denominator used for calculation of SI (background CPM) is indicated below each graph. Kruskal-Wallis ANOVA was performed, followed by Dunn multiple comparison test to determine differences among all groups (*P < 0.05; **P < 0.01).
Voclosporin Suppression of Human T Cell Cytokine Secretion
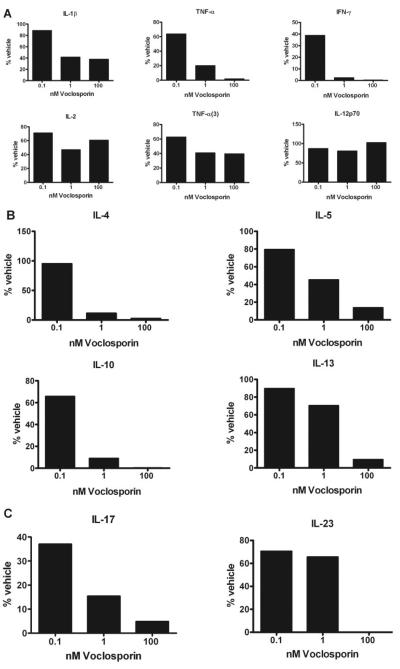
Compared with cytokine secretion from vehicle-treated cells, CD3/CD28-stimulated human T cells, incubated with various levels of voclosporin, had decreased Th1, Th2, and Th17 cytokine release proportional to the voclosporin dosage. Specifically, Th1 cytokine levels for IFN-γ (vehicle, 2084 ± 701 pg/mL; 100 nM voclosporin, 22 ± 13 pg/mL) and TNF-α (vehicle, 787 ± 127 pg/mL; 100 nM voclosporin, 20 ± 8 pg/mL) were reduced up to 300-fold and 58-fold, respectively (Fig. 6A). Th2 cytokine levels for IL-4 (vehicle, 49 ± 6 pg/mL; 100 nM voclosporin, 0.9 ± 0.3 pg/mL), IL-5 (vehicle, 154 ± 23 pg/mL; 100 nM voclosporin, 18.9 ± 0.7 pg/mL), IL-10 (vehicle, 1048 ± 83 pg/mL; 100 nM voclosporin, 8.6 ± 5.6 pg/mL), and IL-13 (vehicle, 312 ± 24 pg/mL; 100 nM voclosporin, 50.8 ± 23 pg/mL) were reduced up to 37-fold, 7-fold, 322-fold, and 10-fold, respectively (Fig. 6B). Th17 (IL-17) levels (vehicle, 1077 ± 158 pg/mL; 100 nM voclosporin, 72.6 ± 12.2 pg/mL) were reduced up to 20.5-fold, whereas IL-23 levels were completely inhibited (vehicle, 14 ± 14 pg/mL; 100 nM voclosporin, 0 ± 0 pg/mL; Fig. 6C).
Figure 6.
Voclosporin reduces the secretion of human Th1 (A), Th2 (B), and Th17 (C) cytokines. Purified human T cells (200,000 cells/well) in a 96-well plate were stimulated with anti-CD28 and anti-CD3 antibodies for 30 minutes, followed by cross-linking with mouse anti-human IgG. Cells were cultured in the presence of 0.1 to 100 nM voclosporin. Supernatants were collected on d2, and levels of cytokines were analyzed. (A-C) Data represent mean percentage of vehicle. TNF-α(3) refers to trimeric TNF-α. Mean values (pg/mL) for the analyzed cytokines ± SEM are represented in Results.
Discussion
CNIs have demonstrated efficacy in the treatment of refractory uveitis. CsA and FK-506 (tacrolimus) have been effective in preventing the development of uveitis in the EAU rodent model.26,28 Voclosporin (LX-211) is a novel immunosuppressive agent that also acts through calcineurin inhibition. Its efficacy for other autoimmune diseases has been demonstrated in previous animal studies. With the use of a collagen-induced animal model for arthritis, Maksymowych et al.33 demonstrated that ISATX247 (voclosporin) significantly decreased the development of joint erosions in treated animals compared with controls. Voclosporin has also demonstrated prolonged renal allograft survival in non-human primates20 and has demonstrated efficacy in clinical studies for moderate to severe plaque psoriasis.23
To our knowledge, the efficacy of voclosporin has not been previously evaluated for ocular inflammatory diseases. In this article, we have reported the efficacy of voclosporin in the prevention and treatment of ocular inflammation in a rat model of EAU. Voclosporin was effective in preventing the development of severe, clinical EAU with doses as low as 2.5 mg/kg when administered daily from the day of immunization with IRBP. In the therapeutic group of voclosporin (animals receiving voclosporin injections d7 – d13 after immunization with IRBP), a notable decrease in clinical and histologic evidence of intraocular inflammation in animals treated with the 10-mg/kg dose was observed compared with untreated control animals. The amelioration of intraocular inflammation, supported by histopathologic evidence of this effect, suggests that voclosporin may be a useful therapy for ocular inflammatory conditions.
CSA was used as the positive control because of its proven efficacy in preventing EAU formation and treating established EAU in the rodent model.28 The dosage of CsA used in this study, 40 mg/kg, was based on the results from the earlier study, which demonstrated this dose was able to inhibit clinical manifestations of uveitis and to decrease clinical signs of uveitis when administered 7 days after immunization with IRBP. As in earlier studies, CsA was successful in the prevention and treatment of uveitis in all rats treated with CsA in this study. Based on the clinical and histopathologic findings in this study, the therapeutic efficacy of voclosporin appears to compare favorably with cyclosporine.
The in vitro effects of voclosporin on lymphocytes, from rodents and humans, suggest that its efficacy may be mediated through the inhibition of T-cell effector responses. Lymphocytes harvested from animals treated with voclosporin demonstrated significant decreases in antigen proliferation assays compared with untreated control animals. This finding was observed with the subcutaneous administration of 2.5 mg/kg/d dosing and with higher doses of medication. Interestingly, voclosporin also demonstrated a dose-dependent ability to inhibit the proliferation of stimulated human T lymphocytes. Th1, Th2, and Th17 cytokines were also decreased in human T-cell cultures treated with voclosporin, suggesting that voclosporin inhibits pan T-cell effector responses. Pro-inflammatory Th1 and Th17 immune responses have previously been shown to be salient features of uveitis and scleritis.34-37 The ability of voclosporin to downregulate proinflammatory Th1 and Th17 effector responses, combined with its efficacy in the prevention and treatment of EAU, supports further investigation of this novel CNI for the treatment of ocular inflammatory disease.
Acknowledgments
The authors thank Seth Pantanelli for assistance with animal experiments and Dan Trepanier for performing HPLC analysis of voclosporin during the experiments.
Supported by National Institutes of Health Intramural Research and by Lux Biosciences.
Footnotes
Disclosure: M.A. Cunningham, None; B.A. Austin, None; Z. Li, None; B. Liu, None; S. Yeh, None; C.-C. Chan, None; E. Anglade, Lux Biosciences (E); P. Velagaleti, Lux Biosciences (E); R.B. Nussenblatt, None
References
- 1.Suttorp-Schulten MS, Rothova A. The possible impact of uveitis in blindness: a literature survey. Br J Ophthalmol. 1996;80:844–848. doi: 10.1136/bjo.80.9.844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nussenblatt RB, Whitcup SM, Palestine AG. Uveitis: Fundamentals and Clinical Practice. 2nd ed Mosby; St. Louis: 1996. [Google Scholar]
- 3.Kulkarni P. Review: uveitis and immunosuppressive drugs. J Ocul Pharmacol Ther. 2001;17:181–187. doi: 10.1089/10807680151125537. [DOI] [PubMed] [Google Scholar]
- 4.Hogan AC, McAvoy CE, Dick AD, Lee RW. Long-term efficacy and tolerance of tacrolimus for the treatment of uveitis. Ophthalmology. 2007;114(5):1000–1006. doi: 10.1016/j.ophtha.2007.01.026. [DOI] [PubMed] [Google Scholar]
- 5.Nussenblatt RB, Palestine AG, Chan CC. Cyclosporine therapy for uveitis: long-term follow-up. J Ocul Pharmacol. 1985;1(4):369–382. doi: 10.1089/jop.1985.1.369. [DOI] [PubMed] [Google Scholar]
- 6.BenEzra D, Cohen E, Rakotomalala M, et al. Treatment of endogenous uveitis with cyclosporine A. Transplant Proc. 1988;20:122–127. [PubMed] [Google Scholar]
- 7.Sloper CM, Powell RJ, Dua HS. Tacrolimus (FK506) in the treatment of posterior uveitis refractory to cyclosporine. Ophthalmology. 1999;106(4):723–728. doi: 10.1016/S0161-6420(99)90156-2. [DOI] [PubMed] [Google Scholar]
- 8.Walton RC, Nussenblatt RB, Whitcup SM. Cyclosporine therapy for severe sight-threatening uveitis in children and adolescents. Ophthalmology. 1998;105(11):2028–2034. doi: 10.1016/S0161-6420(98)91120-4. [DOI] [PubMed] [Google Scholar]
- 9.Whitcup SM, Salvo EC, Jr, Nussenblatt RB. Combined cyclosporine and corticosteroid therapy for sight-threatening uveitis in Behçet's disease. Am J Ophthalmol. 1994;118(1):39–45. doi: 10.1016/s0002-9394(14)72840-5. [DOI] [PubMed] [Google Scholar]
- 10.Wang Y, Ogawa Y, Dogru M, et al. Ocular surface and tear functions after topical cyclosporine treatment in dry eye patients with chronic graft-versus-host disease. Bone Marrow Transplant. 2007;41(3):293–302. doi: 10.1038/sj.bmt.1705900. [DOI] [PubMed] [Google Scholar]
- 11.Foulks GN. Topical cyclosporine for treatment of ocular surface disease. Int Ophthalmol Clin. 2006;46(4):105–122. doi: 10.1097/01.iio.0000212135.77675.6a. [DOI] [PubMed] [Google Scholar]
- 12.Cardenas ME, Zhu D, Heitman J. Molecular mechanisms of immunosuppression by cyclosporine, FK506, and rapamycin. Curr Opin Nephrol Hypertens. 1995;4(6):472–477. doi: 10.1097/00041552-199511000-00002. [DOI] [PubMed] [Google Scholar]
- 13.Su Q, Weber L, Le Hir M, et al. Nephrotoxicity of cyclosporin A and FK506: inhibition of calcineurin phosphatase. Ren Physiol Biochem. 1995;18(3):128–139. doi: 10.1159/000173910. [DOI] [PubMed] [Google Scholar]
- 14.Redondo-Horcajo M, Lamas S. Oxidative and nitrosative stress in kidney disease: a case for cyclosporine A. J Nephrol. 2005;18(4):453–457. [PubMed] [Google Scholar]
- 15.Serkova NJ, Christians U, Benet LZ. Biochemical mechanisms of cyclosporine neurotoxicity. Mol Interv. 2004;4(2):97–107. doi: 10.1124/mi.4.2.7. [DOI] [PubMed] [Google Scholar]
- 16.Henke W, Nickel E, Jung K. Cyclosporine A inhibits ATP net uptake of rat kidney mitochondria. Biochem Pharmacol. 1992;43(5):1021–1024. doi: 10.1016/0006-2952(92)90608-l. [DOI] [PubMed] [Google Scholar]
- 17.Aspeslet L, Freitag D, Trepanier D, et al. ISA(TX)247: a novel calcineurin inhibitor. Transplant Proc. 2001;33(1–2):1048–1051. doi: 10.1016/s0041-1345(00)02325-3. [DOI] [PubMed] [Google Scholar]
- 18.Birsan T, Dambrin C, Freitag DG, et al. The novel calcineurin inhibitor ISA247: a more potent immunosuppressant than cyclosporine in vitro. Transpl Int. 2005;17(12):767–771. doi: 10.1007/s00147-004-0799-z. [DOI] [PubMed] [Google Scholar]
- 19.Stalder M, Birsan T, Hubble RW, et al. In vivo evaluation of the novel calcineurin inhibitor ISATX247 in non-human primates. J Heart Lung Transplant. 2003;22(12):1343–1352. doi: 10.1016/s1053-2498(03)00033-0. [DOI] [PubMed] [Google Scholar]
- 20.Gregory CR, Kyles AE, Bernsteen L, et al. Compared with cyclosporine, ISATX247 significantly prolongs renal-allograft survival in a nonhuman primate model. Transplantation. 2004;78(5):681–685. doi: 10.1097/01.tp.0000131950.75697.71. [DOI] [PubMed] [Google Scholar]
- 21.Abel MD, Aspeslet LJ, Freitag DG, et al. ISATX247: a novel calcineurin inhibitor. J Heart Lung Transplant. 2001;20(2):161. doi: 10.1016/s1053-2498(00)00290-4. [DOI] [PubMed] [Google Scholar]
- 22.Anglade E, Yatscoff R, Foster R, Grau U. Next-generation calcineurin inhibitors for ophthalmic indications. Expert Opin Investig Drugs. 2007;16(10):1525–1540. doi: 10.1517/13543784.16.10.1525. [DOI] [PubMed] [Google Scholar]
- 23.Bissonnette R, Papp K, Poulin Y, et al. A randomized, multicenter, double-blind, placebo-controlled phase 2 trial of ISA247 in patients with chronic plaque psoriasis. J Am Acad Dermatol. 2006;54(3):472–478. doi: 10.1016/j.jaad.2005.10.061. [DOI] [PubMed] [Google Scholar]
- 24.Oh-i K, Keino H, Goto H, et al. Intravitreal injection of tacrolimus (FK506) suppresses ongoing experimental autoimmune uveoretinitis in Rats. Br J Ophthalmol. 2007;91(2):237–242. doi: 10.1136/bjo.2006.103168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ni M, Chan CC, Nussenblatt RB, Mochizuki M. FR900506 (FK506) and 15-deoxyspergualin (15-DSG) modulate the kinetics of infiltrating cells in eyes with experimental autoimmune uveoretinitis. Autoimmunity. 1990;8(1):43–51. doi: 10.3109/08916939008998431. [DOI] [PubMed] [Google Scholar]
- 26.Kawashima H, Fujino Y, Mochizuki M. Effects of a new immunosuppressive agent, FK506, on experimental autoimmune uveoretinitis in rats. Invest Ophthalmol Vis Sci. 1988;29(8):1265–1271. [PubMed] [Google Scholar]
- 27.Mochizuki M, Nussenblatt RB, Kuwabara T, Gery I. Effects of cyclosporine and other immunosuppressive drugs on experimental autoimmune uveoretinitis in rats. Invest Ophthalmol Vis Sci. 1985;26(2):226–232. [PubMed] [Google Scholar]
- 28.Nussenblatt RB, Rodrigues MM, Wacker WB, et al. Cyclosporin a: inhibition of experimental autoimmune uveitis in Lewis rats. J Clin Invest. 1981;67(4):1228–1231. doi: 10.1172/JCI110138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Redmond TM, Wiggert B, Robey FA, et al. Isolation and characterization of monkey interphotoreceptor retinoid-binding protein, a unique extracellular matrix component of the retina. Biochemistry. 1985;24:787–793. doi: 10.1021/bi00324a038. [DOI] [PubMed] [Google Scholar]
- 30.Pepperberg DR, Okajima TL, Ripps H, et al. Functional properties of interphotoreceptor retinoid-binding protein. Photochem Photobiol. 1991;54(6):1057–1060. doi: 10.1111/j.1751-1097.1991.tb02129.x. [DOI] [PubMed] [Google Scholar]
- 31.Agarwal RK, Caspi RR. Rodent models of experimental autoimmune uveitis. Methods Mol Med. 2004;102:395–419. doi: 10.1385/1-59259-805-6:395. [DOI] [PubMed] [Google Scholar]
- 32.Chan CC, Caspi RR, Ni M, et al. Pathology of experimental autoimmune uveoretinitis in mice. J Autoimmun. 1990;3(3):247–255. doi: 10.1016/0896-8411(90)90144-h. [DOI] [PubMed] [Google Scholar]
- 33.Maksymowych WP, Jhangri GS, Aspeslet L, et al. Amelioration of accelerated collagen induced arthritis by a novel calcineurin inhibitor, ISA(TX)247. J Rheumatol. 2002;29(8):1646–1652. [PubMed] [Google Scholar]
- 34.Amadi-Obi A, Yu CR, Liu X, et al. Th17 cells contribute to uveitis and scleritis and are expanded by IL-2 and inhibited by IL-27/STAT1. Nat Med. 2007;13(6):711–718. doi: 10.1038/nm1585. [DOI] [PubMed] [Google Scholar]
- 35.Murray PI, Clay CD, Mappin C, Salmon M. Molecular analysis of resolving immune responses in uveitis. Clin Exp Immunol. 1999;117(3):455–461. doi: 10.1046/j.1365-2249.1999.00993.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Murphy CC, Duncan L, Forrester JV, Dick AD. Systemic CD4(+) T cell phenotype and activation status in intermediate uveitis. Br J Ophthalmol. 2004;88(3):412–416. doi: 10.1136/bjo.2003.028506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Luger D, Silver PB, Tang J, et al. Either a Th17 or a Th1 effector response can drive autoimmunity: conditions of disease induction affect dominant effector category. J Exp Med. 2008;205(4):799–810. doi: 10.1084/jem.20071258. [DOI] [PMC free article] [PubMed] [Google Scholar]