Abstract
In late-onset Tay-Sachs disease (LOTS), saccades are interrupted by one or more transient decelerations. Some saccades reaccelerate and continue on before eye velocity reaches zero, even in darkness. Intervals between successive decelerations are not regularly spaced. Peak decelerations of horizontal and vertical components of oblique saccades in LOTS is more synchronous than those in control subjects. We hypothesize that these decelerations are caused by dysregulation of the fastigial nuclei (FN) of the cerebellum, which fire brain stem inhibitory burst neurons (IBNs).
Keywords: fastigial nucleus, omnipause neurons, burst neurons, latch circuit
Introduction
Late-onset Tay-Sachs disease (LOTS) is an auto-somal recessive disorder of sphingolipid metabolism, caused by deficiency of the enzyme hexosaminidase A, which leads to accumulation of GM2 ganglioside (inclusions) in the nervous system. Patients with LOTS typically make a series of small saccades in response to a target jump, but their peak speeds seem normal (Rucker et al., 2004). LOTS saccades are interrupted by transient decelerations, not always coming to a standstill, after which a new saccade occurs. In a mouse model of LOTS, GM2 storage is prominent in the motor cortex and cerebellum (granular cells and Purkinje cells), along with atrophy in the brainstem (Chern et al., 1976). Recent neuropathological studies of LOTS (Rucker et al., this volume) have demonstrated inclusions and reduction in metabolism in omnipause neurons (OPN), inclusions in fastigial nuclei (FN), and complete loss of Purkinje cells in the dorsal cerebellar vermis. We asked three questions about LOTS saccades to understand how these losses cause the saccade’s interruptions: 1. Are saccades truncated if the target is turned off after it jumps? 2. Is there any periodicity to the decelerations? 3. Is the onset of saccade braking different for horizontal and vertical components of oblique saccades?
Methods
We used eye movements recorded with coils in 14 patients diagnosed with LOTS and 10 healthy control subjects (for details, see Rucker et al., 2004). Horizontal saccades were simulated in Matlab (Mathworks, Natick, MA).
Results
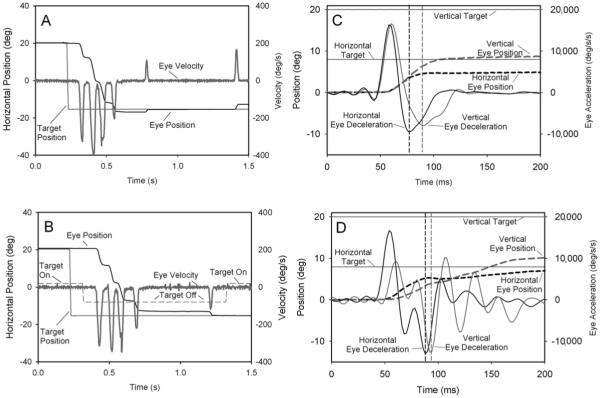
First, we found no difference in any patient between the accuracy of saccades made with the visual stimulus turned off 88 ms after each target jump (complete darkness) versus saccades made with the target continuously visible (Fig. 1A, B). Thus, vision was not needed to restart the saccade.
Fig. 1.
(A) Horizontal saccades made by a LOTS patient to an illuminated target. (B) Target was turned off for 1 s, 88 ms after its jump. The patient’s responses were similar under both conditions. (Note the lack of periodicity of the successive saccadic pulses.) (C) Horizontal and vertical components of a saccade made by a control subject to an oblique target jump (20° up and 8° right) show near-synchronous peak acceleration of both components, but peak decelerations (dashed vertical lines) occur 12 ms apart. (D) Saccade made by a LOTS patient (stimulus as in C) shows asynchronous peak acceleration of both components, but peak decelerations occurred within 5 ms. Note different axes. Upward and rightward movements are positive.
Second, we found no regularity to the timing of successive dips in peak velocity. This suggests they are not due to oscillations in the closed-loop circuit in the brain stem among inhibitory burst neurons (IBNs) and excitatory burst neurons (EBNs) (cf., Fig. 2A).
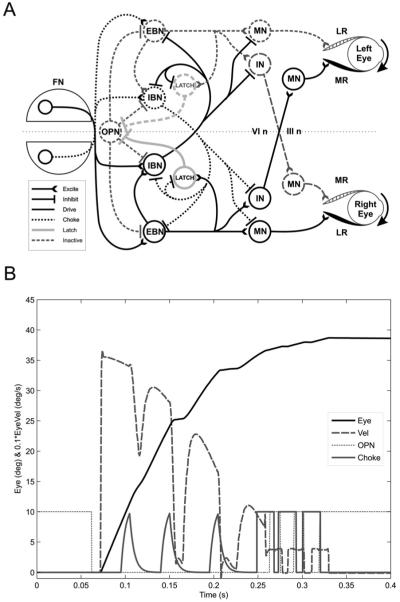
Fig. 2.
Simulation of interrupted saccades in LOTS patients. (A) Model. EBNs provide drive for ipsilateral movement. IBNs cross over and prevent activity on the contralateral side. Choke signal on contralateral IBNs (triggered by ipsilateral FN) stops saccade and opens latch. (B) Simulation. OPN turns off, allowing a saccade to the target. EBNs drive Latch neurons, holding off OPN. Eye velocity dips are preceded by bursts in the choke signal. After 0.25 s, the saccade is near its end, and the OPN turns back on. The last two movements are normal corrective saccades (note OPN and choke cycling on and off).
Third, we measured the asynchrony between the time of peak deceleration of the horizontal and vertical components of oblique saccades. LOTS patients were less asynchronous than controls, and the difference was significant (p<0.001) for oblique saccades with large vertical and small horizontal components (Fig. 1C, D). Normally, the components of an oblique saccade stop when that component is over. Although there may be some stretching of the duration of the shorter saccade, it is not always enough, and so the two components can stop asynchronously. However, in LOTS, the two components of the decelerations end more synchronously than in controls (e.g., Fig. 1D). If the OPNs or choke (which inhibit both horizontal and vertical bursters) restarted prematurely, both components would decelerate simultaneously.
Discussion
Normally, OPNs do not fire during saccades because they are held off by a latch circuit (Fig. 2A) (Keller and Missal, 2003). If EBN firing were greatly reduced during the deceleration because the OPN reactivated, one would expect the latch circuit to open and the saccade to stop. However, even when speed almost reaches zero, the saccade still restarts (Fig. 1A).
A hypothesis more consistent with our data is that contralateral IBNs activate too early, choking off the drive of the ipsilateral EBNs. Premature firing of the ipsilateral FN could generate this choke signal. The key difference between activating IBNs instead of OPNs is that in the former case the ipsilateral EBNs do not have to shut down, as their output can be choked off at the motor neuron (Fig. 2A). Thus, the OPN latch circuit would not be affected and the closed-loop saccadic system would drive the eye to the target.
We modelled lesions in the cerebellar vermis of LOTS patients by reducing their inhibition on FNs several times during a single saccade (Fig. 2B). FNs always receive excitatory inputs from mossy and climbing fibres, and will fire unless inhibited by the vermis. The loss of this inhibition has no effect on the FNs contralateral to the movement, because they normally start firing near the beginning of the movement. However, the ipsilateral FNs normally fire late to stop the saccade (Optican and Quaia, 2002). If they activate prematurely, they will turn on the contralateral IBN in the brain stem, decelerating the saccade.
Acknowledgements
This research was supported by the Office of Research and Development, Medical Research Service, Department of Veterans Affairs; National Eye Institute grants EY06717 and EY08060; the Evenor Armington Fund; and the Intramural Division of the National Eye Institute, NIH, DHHS.
References
- Chern J, Beutler E, Kuhl W, Gilbert F, Mellman WJ, Croce CM. Characterization of heteropolymeric hexosaminidase A in human X mouse hybrid cells. Proc. Natl. Acad. Sci. U.S.A. 1976;73(10):3637–3640. doi: 10.1073/pnas.73.10.3637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keller EL, Missal M. Shared brainstem pathways for saccades and smooth-pursuit eye movements. Ann. N.Y. Acad. Sci. 2003;1004:29–39. doi: 10.1196/annals.1303.004. [DOI] [PubMed] [Google Scholar]
- Optican LM, Quaia C. Distributed model of collicular and cerebellar function during saccades. Ann. N.Y. Acad. Sci. 2002;956:164–177. doi: 10.1111/j.1749-6632.2002.tb02817.x. [DOI] [PubMed] [Google Scholar]
- Rucker JC, Shapiro BE, Han YH, Kumar AN, Garbutt S, Keller EL, Leigh RJ. Neuro-ophthalmology of late-onset Tay-Sachs disease (LOTS) Neurology. 2004;63(10):1918–1926. doi: 10.1212/01.wnl.0000144275.76658.f4. [DOI] [PubMed] [Google Scholar]